Fungal Metabolites with Antagonistic Activity against Fungi of Lithic Substrata
Abstract
1. Introduction
2. Materials and Methods
2.1. General Chemical Procedure
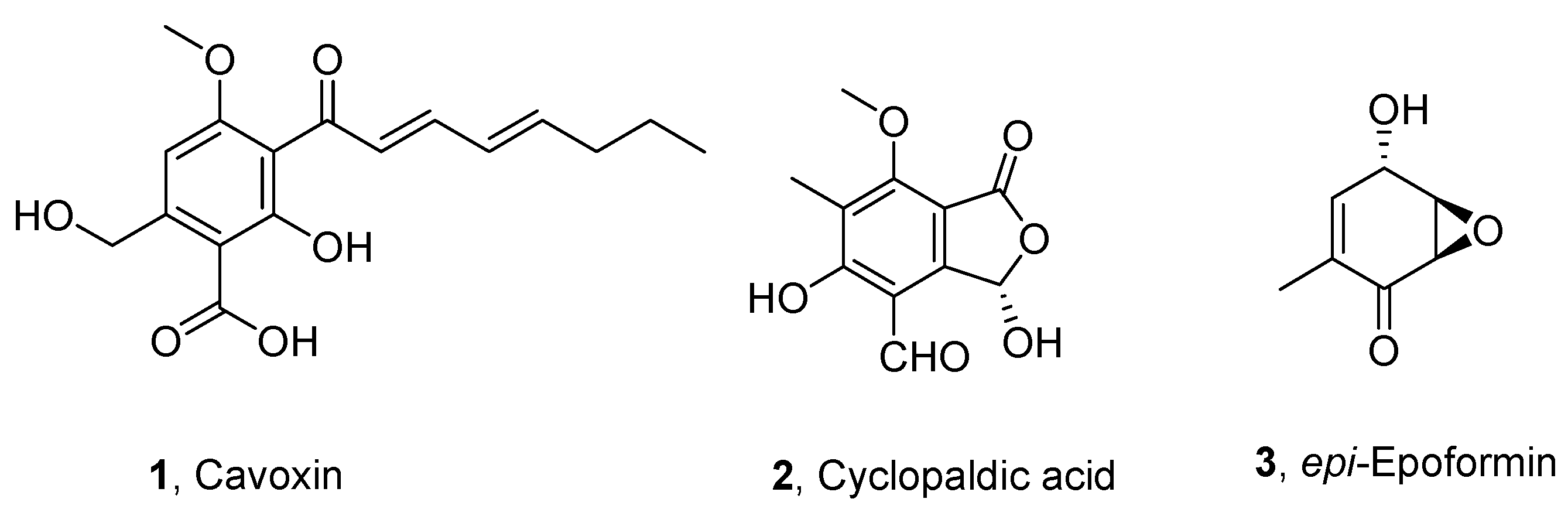
2.2. Fungal Metabolites
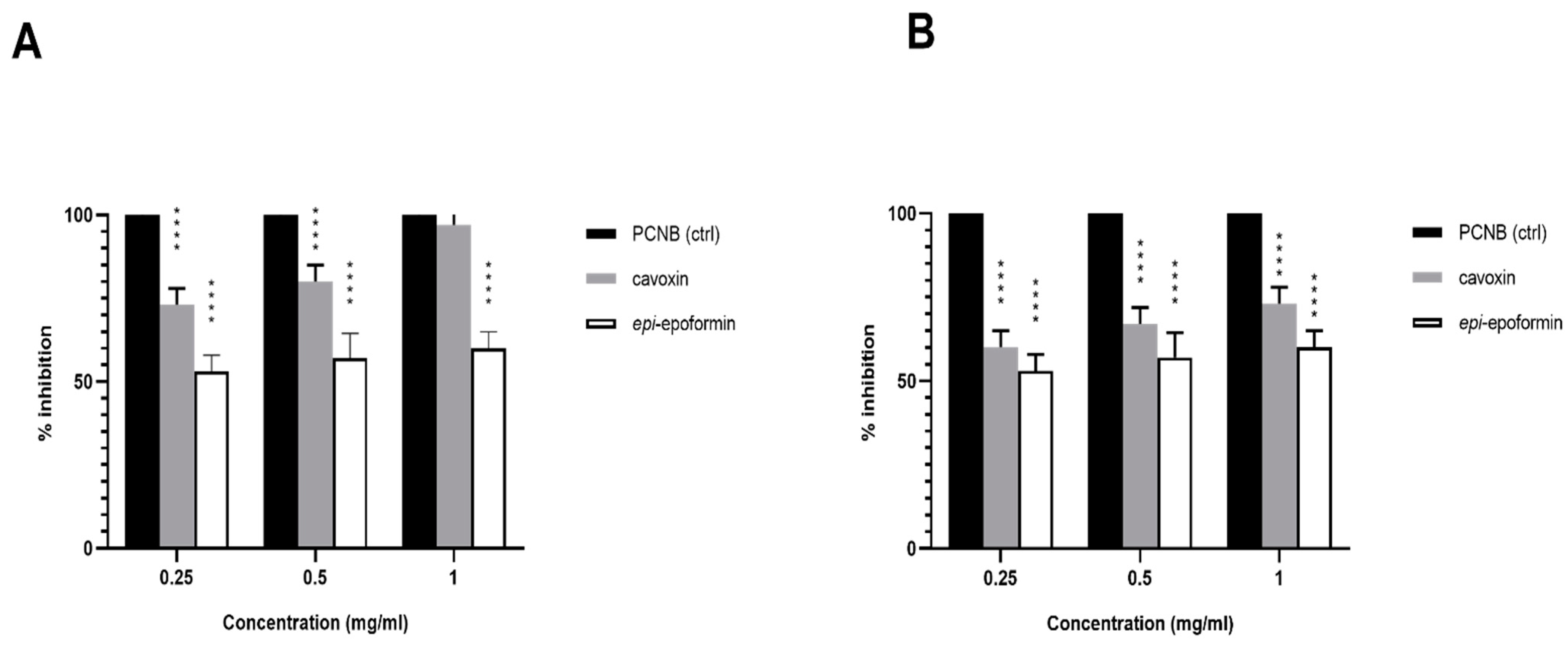
2.3. Antifungal Test-Well Diffusion Assay
2.4. Lithotypes and Microorganisms
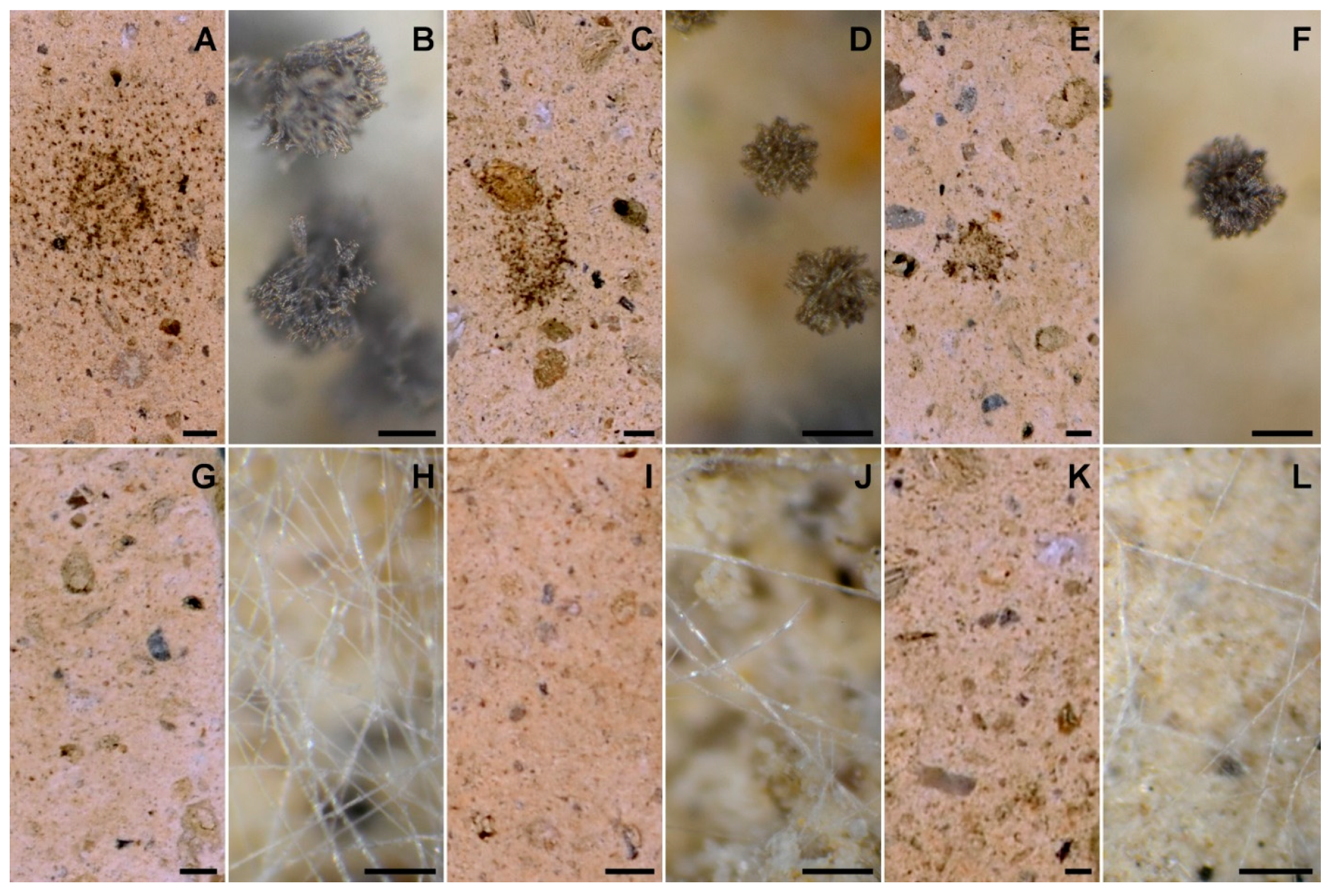
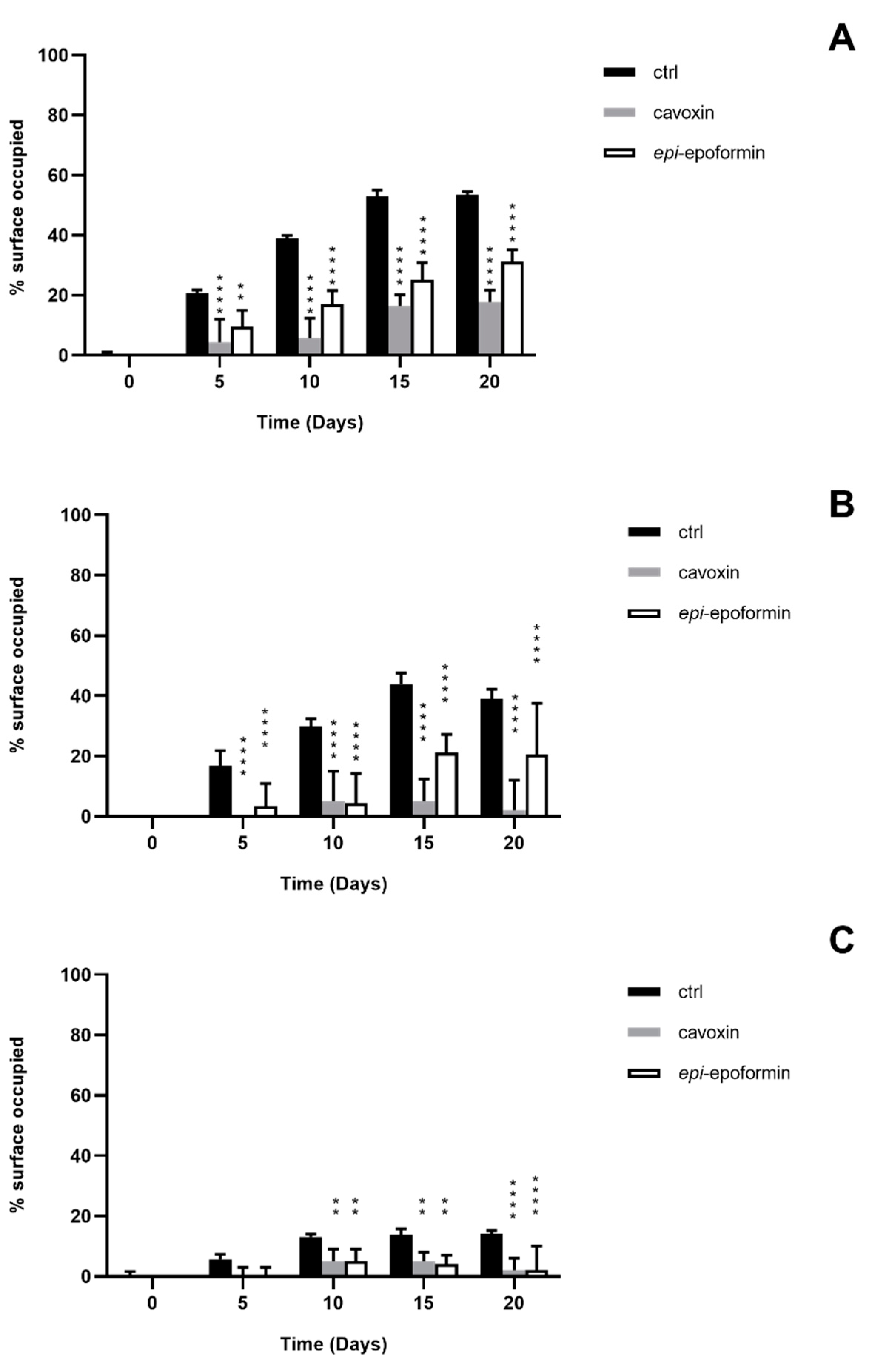
2.5. In Vitro Biodeterioration Test
2.6. Laboratory Strains and Culturing Conditions
2.7. Evaluation of Fungal Growth
2.8. Statistical Analysis
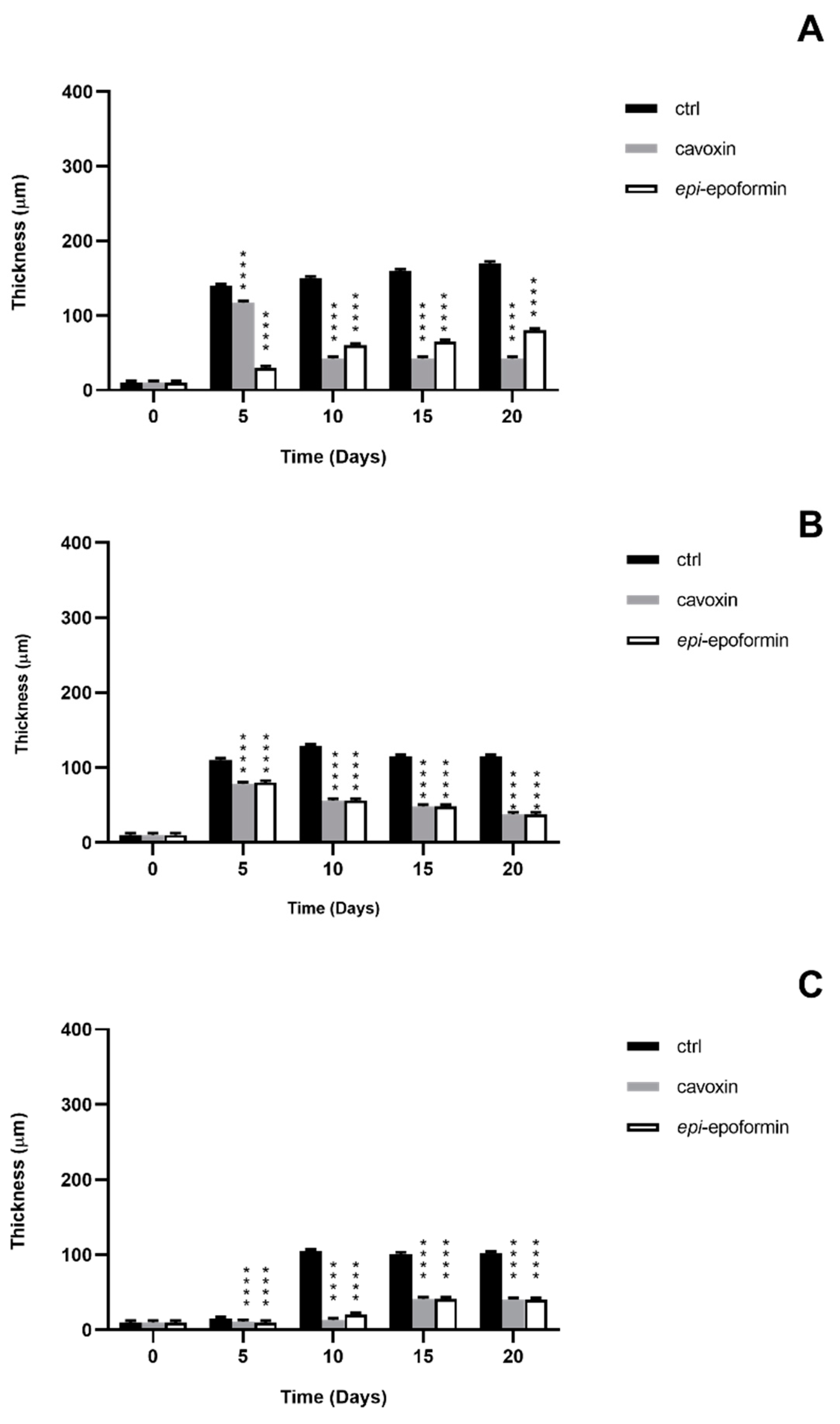
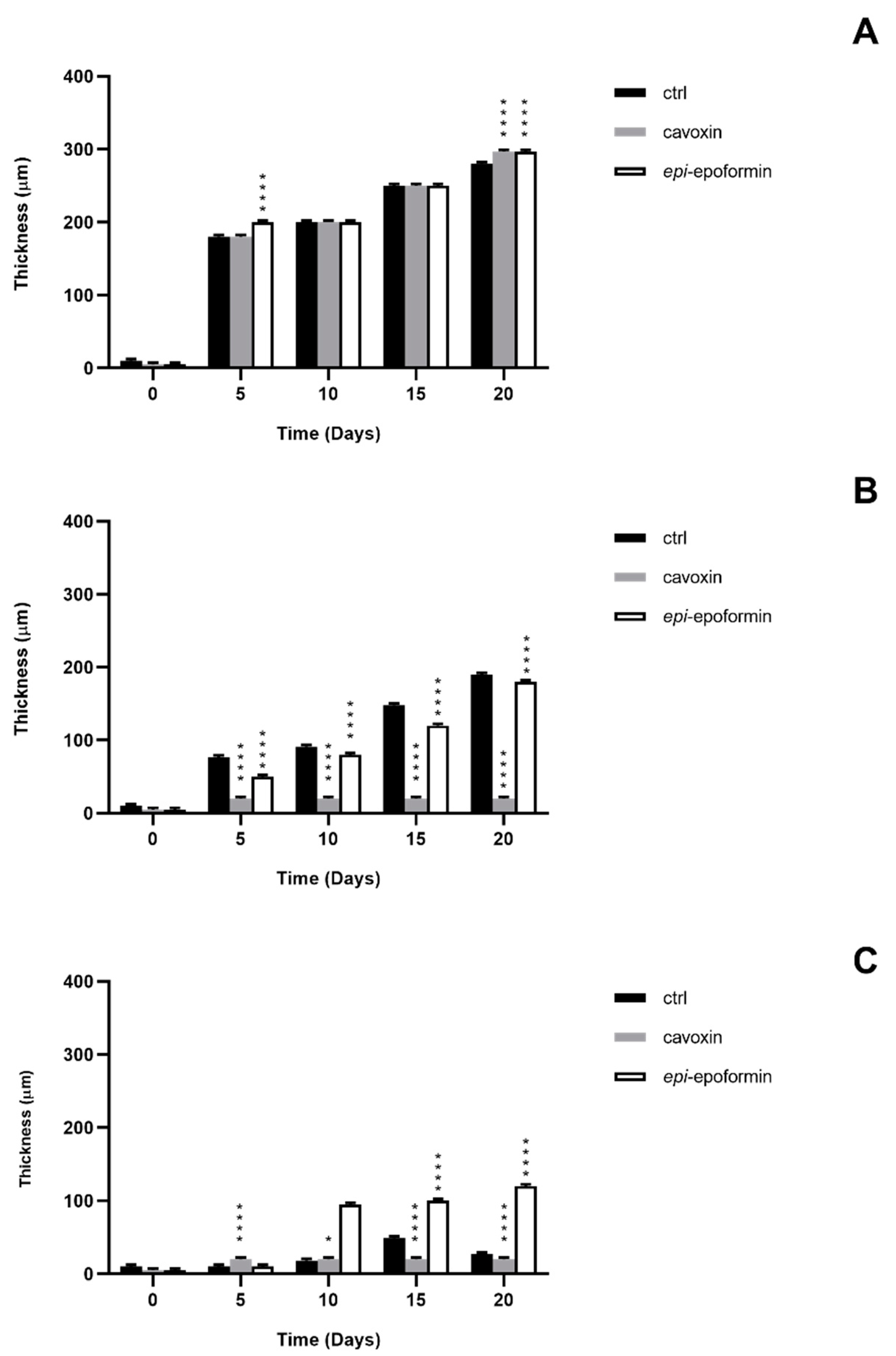
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Salvadori, O.; Municchia, A.C. The role of fungi and lichens in the biodeterioration of stone monuments. Open Conf. Proc. J. 2016, 7, 39–54. [Google Scholar] [CrossRef]
- Gadd, G.M. Geomicrobiology of the built environment. Nat. Microbiol. 2017, 2, 1–9. [Google Scholar] [CrossRef]
- Evidente, A.; Abouzeid, M.A.; Andolfi, A.; Cimmino, A. Recent achievements in the bio-control of Orobanche infesting important crops in the Mediterranean basin. J. Agric. Sci. Technol. A 2011, 1, 461–483. [Google Scholar]
- Cimmino, A.; Masi, M.; Evidente, M.; Superchi, S.; Evidente, A. Fungal phytotoxins with potential herbicidal activity: Chemical and biological characterization. Nat. Prod. Rep. 2015, 32, 1629–1653. [Google Scholar] [CrossRef]
- Matassini, C.; Parmeggiani, C.; Cardona, F. New frontiers on human safe insecticides and fungicides: An opinion on trehalase inhibitors. Molecules 2020, 25, 3013. [Google Scholar] [CrossRef]
- Marrone, P.G. Pesticidal natural products–status and future potential. Pest Man. Sci. 2019, 75, 2325–2340. [Google Scholar] [CrossRef]
- Turner, W.B.; Aldridge, D.C. Fungal Metabolites II; Academic Press: London, UK, 1983. [Google Scholar]
- Osbourn, A.E.; Lanzotti, V. Plant-Derived Products; Springer: Drdrecht, Germany, 2009. [Google Scholar]
- Dewick, P.M. Medicinanal Natural Products—A Biosynthetic Approach; Wiley and Sons Ltd.: Chicester, UK, 2009. [Google Scholar]
- Coleman, J.J.; Ghosh, S.; Okoli, I.; Mylonakis, E. Antifungal activity of microbial secondary metabolites. PLoS ONE 2011, 6, e25321. [Google Scholar] [CrossRef]
- Kumar, S.; Kaushik, N. Metabolites of endophytic fungi as novel source of biofungicide: A review. Phytochem. Rev. 2012, 11, 507–522. [Google Scholar] [CrossRef]
- Wang, Y.T.; Xue, Y.R.; Liu, C.H. A brief review of bioactive metabolites derived from deep-sea fungi. Mar. Drugs 2015, 13, 4594–4616. [Google Scholar] [CrossRef]
- Roscetto, E.; Masi, M.; Esposito, M.; Di Lecce, R.; Delicato, A.; Maddau, L.; Calabrò, V.; Evidente, A.; Catania, M.R. Anti-biofilm activity of the fungal phytotoxin sphaeropsidin A against clinical isolates of antibiotic-resistant bacteria. Toxins 2020, 12, 444. [Google Scholar] [CrossRef] [PubMed]
- Masi, M.; Maddau, L.; Linaldeddu, B.T.; Scanu, B.; Evidente, A.; Cimmino, A. Bioactive metabolites from pathogenic and endophytic fungi of forest trees. Curr. Med. Chem. 2018, 25, 208–252. [Google Scholar] [CrossRef] [PubMed]
- Hasan, S.; Ansari, M.I.; Ahmad, A.; Mishra, M. Major bioactive metabolites from marine fungi: A Review. Bioinformation 2015, 11, 176. [Google Scholar] [CrossRef] [PubMed]
- Reveglia, P.; Cimmino, A.; Masi, M.; Nocera, P.; Berova, N.; Ellestad, G.; Evidente, A. Pimarane diterpenes: Natural source, stereochemical configuration, and biological activity. Chirality 2018, 30, 1115–1134. [Google Scholar] [CrossRef] [PubMed]
- Kot, A.M.; Błażejak, S.; Kieliszek, M.; Gientka, I.; Bryś, J.; Reczek, L.; Pobiega, K. Effect of exogenous stress factors on the biosynthesis of carotenoids and lipids by Rhodotorula yeast strains in media containing agro-industrial waste. World J. Microbiol. Biotechnol. 2019, 35, 1–10. [Google Scholar] [CrossRef]
- Masi, M.; Evidente, A. Fungal bioactive anthraquinones and analogues. Toxins 2020, 12, 714. [Google Scholar] [CrossRef]
- Reveglia, P.; Masi, M.; Evidente, A. Melleins—Intriguing natural compounds. Biomolecules 2020, 10, 772. [Google Scholar] [CrossRef]
- Masi, M.; Meyer, S.; Clement, S.; Andolfi, A.; Cimmino, A.; Evidente, A. Spirostaphylotrichin W, a spirocyclic γ-lactam isolated from liquid culture of Pyrenophora semeniperda, a potential mycoherbicide for cheatgrass (Bromus tectorum) biocontrol. Tetrahedron 2014, 70, 1497–1501. [Google Scholar] [CrossRef]
- Evidente, A.; Randazzo, G.; Iacobellis, N.S.; Bottalico, A. Structure of cavoxin, a new phytotoxin from Phoma cava and cavoxone, its related chroman-4-one. J. Nat. Prod. 1985, 48, 916–923. [Google Scholar] [CrossRef]
- Graniti, A.; Sparapano, L.; Evidente, A. Cyclopaldic acid, a major phytotoxic metabolite of Seiridium cupressi, the pathogen of a canker disease of cypress. Plant Pathol. 1992, 41, 563–568. [Google Scholar] [CrossRef]
- Cala, A.; Masi, M.; Cimmino, A.; Molinillo, J.M.; Macias, F.A.; Evidente, A. (+)-epi-Epoformin, a phytotoxic fungal cyclohexenepoxide: Structure activity relationships. Molecules 2018, 23, 1529. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Chen, R.; Xing, R.; Liu, S.; Yu, H.; Wang, P.; Li, C.; Li, P. Novel derivatives of chitosan and their antifungal activities in vitro. Carb. Res. 2006, 341, 351–354. [Google Scholar] [CrossRef] [PubMed]
- D’Elia, L.; Del Mondo, A.; Santoro, M.; De Natale, A.; Pinto, G.; Pollio, A. Microorganisms from harsh and extreme environments: A collection of living strains at ACUF (Naples, Italy). Ecol. Quest. 2018, 29, 63–74. [Google Scholar] [CrossRef]
- Doyle, J.J.; Doyle, J.L. Isolation of plant DNA from fresh tissue. Focus 1990, 12, 13–15. [Google Scholar]
- Kastenmeier, P.; Di Maio, G.; Balassone, G.; Boni, M.; Joachimski, M.; Mondillo. N. The source of stone building materials from the Pompeii archaeological area and its surroundings. Period. Mineral. 2010, 39–58. [Google Scholar] [CrossRef]
- Del Mondo, A.; Pinto, G.; De Natale, A.; Pollio, A. In vitro colonization experiments for the assessment of mycelial growth on a tuff substratum by a Fusarium solani strain isolated from the oplontis (Naples, Italy) archaeological site. Int. J. Conserv. Sci. 2017, 8, 651–662. [Google Scholar]
- Nichols, H.W.; Bold, H.C. Trichosarcina polymorpha gen. et sp. Nov. J. Phycol. 1965, 1, 34–38. [Google Scholar] [CrossRef]
- Jeger, M.J.; Lamour, A.; Gilligan, C.A.; Otten, W. A fungal growth model fitted to carbonlimited dynamics of Rhizoctonia solani. New Phytol. 2008, 178, 625–633. [Google Scholar] [CrossRef]
- Vyas, N.; Sammons, R.L.; Addison, O.; Dehghani, H.; Walmsley, A.D. A quantitative method to measure biofilm removal efficiency from complex biomaterial surfaces using SEM and image analysis. Sci. Rep. 2016, 6, 32694. [Google Scholar]
- Arganda-Carreras, I.; Kaynig, V.; Rueden, C.; Eliceiri, K.W.; Schindelin, J.; Cardona, A.; Sebastian Seung, H. Trainable Weka Segmentation: A machine learning tool for microscopy pixel classification. Bioinformatics 2017, 33, 2424–2426. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biologica limage analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Bakke, R.; Olsson, P.Q. Biofilm thickness measurements by light microscopy. J. Microb. Methods 1986, 5, 93–98. [Google Scholar] [CrossRef]
- Santagata, G.; Valerio, F.; Cimmino, A.; Dal Poggetto, G.; Masi, M.; Di Biase, M.; Malinconico, M.; Lavermicocca, P.; Evidente, A. Chemico-physical and antifungal properties of poly (butylene succinate)/cavoxin blend: Study of a novel bioactive polymeric based system. Eur. Polym. J. 2017, 94, 230–247. [Google Scholar] [CrossRef]
- Sparapano, L.; Evidente, A.A. Biological activity of cyclopaldic acid, a major toxin of Seiridium cupressi, its six derivatives, and iso-cyclopaldic acid. Nat. Toxins 1995, 3, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Barilli, E.; Cimmino, A.; Masi, M.; Evidente, M.; Rubiales, D.; Evidente, A. Inhibition of early development stages of rust fungi by the two fungal metabolites cyclopaldic acid and epi-epoformin. Pest Man. Sci. 2017, 73, 1161–1168. [Google Scholar] [CrossRef]
- Mishra, N. Influence of temperature and relative humidity on fungal flora of some species in storage. Z. Lebensm. Unters. Forsch. 1981, 172, 30–31. [Google Scholar] [CrossRef]
- Siqueira, V.M.; Lima, N. Biofilm formation by filamentous fungi recovered from a water system. J. Mycol. 2013, 152941. [Google Scholar] [CrossRef]
- Shirakawa, M.A.; Beech, I.B.; Tapper, R.; Cincotto, M.A.; Gambale, W. The development of a method to evaluate bioreceptivity of indoor mortar plastering to fungal growth. Int. Biodeter. Biodegr. 2003, 51, 83–92. [Google Scholar] [CrossRef]
- Wiktor, V.; De Leo, F.; Urzì, C.; Guyonnet, R.; Grosseau, P.H.; Garcia-Diaz, E. Accelerated laboratory test to study fungal biodeterioration of cementitious matrix. Int. Biodeter. Biodegr. 2009, 63, 1061–1065. [Google Scholar] [CrossRef]
- Caneva, G.; Terscari, M. Stone biodeterioration: Treatments and preventive conservation. In Proceedings of the 2017 International Symposium of Stone Conservation, Conservation Technologies for Stone Cultural Heritages: Status and Future Prospects, Seoul, Korea, 21 September 2017; ISBN 978-89-299-1102-793600. [Google Scholar]
- De Leo, F.; Urzì, C. Fungal colonization on treated and untreated stone surfaces. In Molecular Biology and Cultural Heritage; Swets & Zeitlinger BV: Lisse, The Netherlands, 2003; pp. 213–218. [Google Scholar]
- Fidanza, M.R.; Caneva, G. Natural biocides for the conservation of stone cultural heritage: A review. J. Cult. Herit. 2019, 38, 271–286. [Google Scholar] [CrossRef]
- Young, M.E.; Alakomi, H.L.; Fortune, I.; Gorbushina, A.A.; Krumbein, W.E.; Maxwell, I.; McCullagh, C.; Robertson, P.; Saarela, M.; Valero, J.; et al. Development of a biocidal treatment regime to inhibit biological growths on cultural heritage: BIODAM. Environ. Geol. 2008, 56, 631–641. [Google Scholar] [CrossRef]
- Miller, A.Z.; Sanmartìn, P.; Pereira-Pardo, L.; Dionisio, A.; Macedo, M.F.; Priteo, B. Bioreceptivity of buildings stones: A review. Sci. Total Environ. 2012, 426, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bastian, F.; Jurado, V.; Novakova, A.; Alabouvette, C.; Saiz-Jimenez, C. The microbiology of lascaux cave. Microbiology 2010, 156, 644–652. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masi, M.; Petraretti, M.; De Natale, A.; Pollio, A.; Evidente, A. Fungal Metabolites with Antagonistic Activity against Fungi of Lithic Substrata. Biomolecules 2021, 11, 295. https://doi.org/10.3390/biom11020295
Masi M, Petraretti M, De Natale A, Pollio A, Evidente A. Fungal Metabolites with Antagonistic Activity against Fungi of Lithic Substrata. Biomolecules. 2021; 11(2):295. https://doi.org/10.3390/biom11020295
Chicago/Turabian StyleMasi, Marco, Mariagioia Petraretti, Antonino De Natale, Antonino Pollio, and Antonio Evidente. 2021. "Fungal Metabolites with Antagonistic Activity against Fungi of Lithic Substrata" Biomolecules 11, no. 2: 295. https://doi.org/10.3390/biom11020295
APA StyleMasi, M., Petraretti, M., De Natale, A., Pollio, A., & Evidente, A. (2021). Fungal Metabolites with Antagonistic Activity against Fungi of Lithic Substrata. Biomolecules, 11(2), 295. https://doi.org/10.3390/biom11020295







