Influence of Circadian Rhythm in the Eye: Significance of Melatonin in Glaucoma
Abstract
:1. Circadian Rhythm in the Eye
1.1. Melanopsin
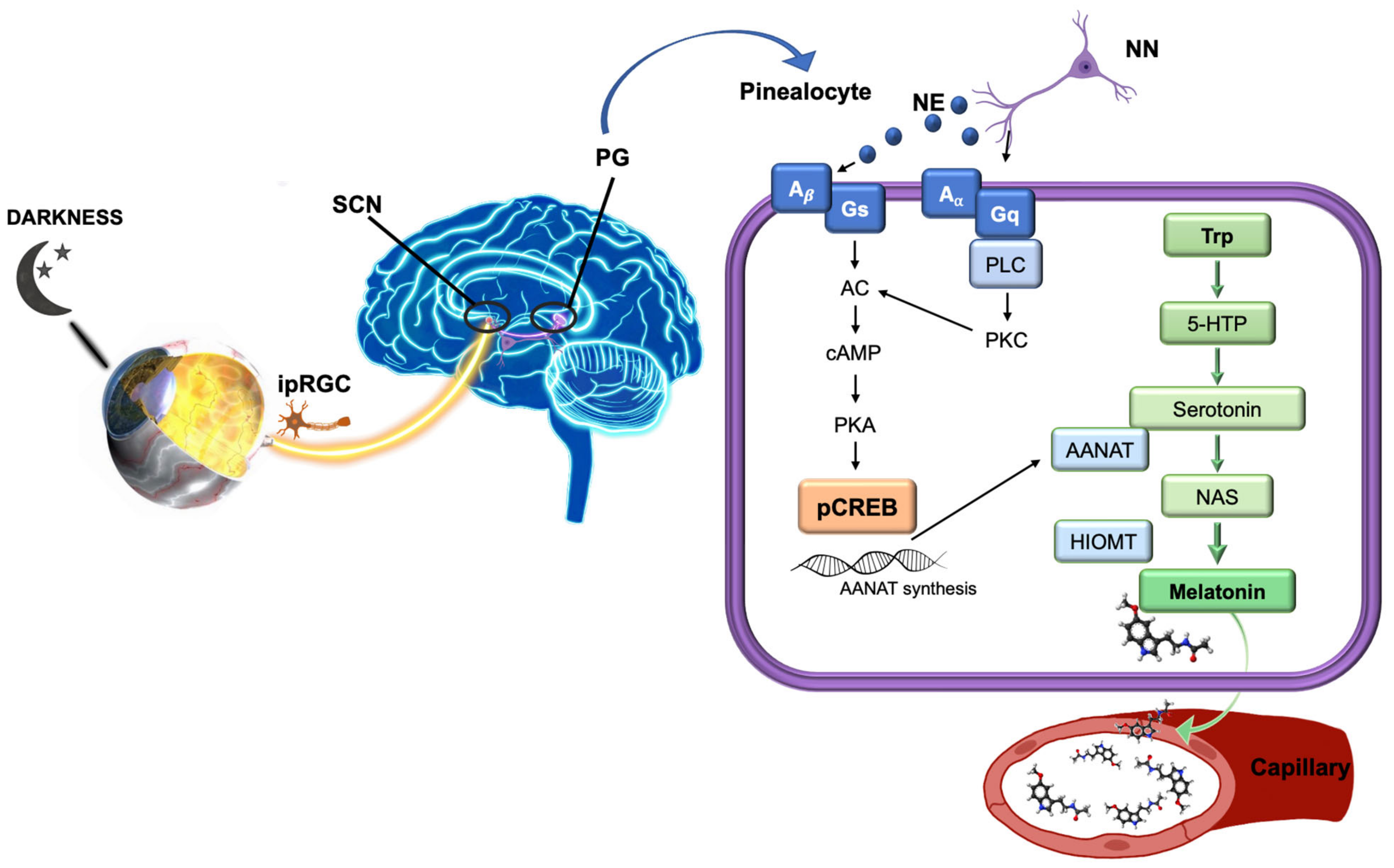
1.2. Melatonin
2. Ocular Pathologies Related to Circadian Rhythm
2.1. Dry Eye
2.2. Corneal Wound Healing
2.3. Myopia
2.4. Cataracts
2.5. Retinal Diseases
3. Relation of Circadian Rhythm with IOP Fluctuations and Glaucoma
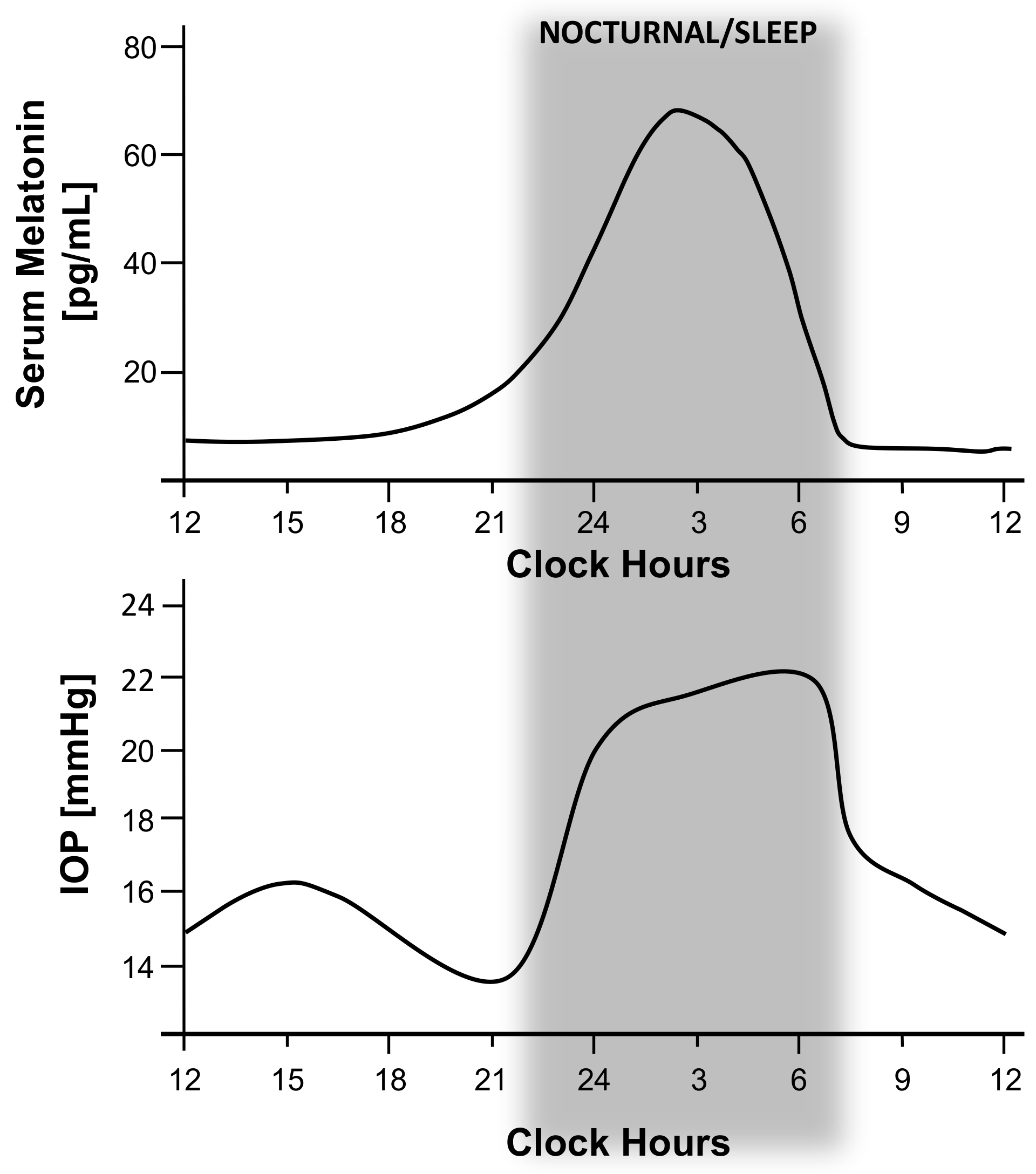
3.1. Circadian Rhythm of IOP and OPP
3.2. Circadian Rhythm of Melatonin and Glaucoma
4. Melatonin and Analogs: Its Function on IOP
5. Summary
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Martinez-O’Ferrall, J.A. Circadian rhythms. J. Occup. Med. 1968, 10, 305–315. [Google Scholar] [CrossRef]
- Emens, J.S.; Burgess, H.J. Effect of Light and Melatonin and Other Melatonin Receptor Agonists on Human Circadian Physiology. Sleep Med. Clin. 2015, 10, 435–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukuhara, C.; Aguzzi, J.; Bullock, N.; Tosini, G. Effect of long-term exposure to constant dim light on the circadian system of rats. Neurosignals 2005, 14, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Vandewalle, G.; Middleton, B.; Rajaratnam, S.M.; Stone, B.M.; Thorleifsdottir, B.; Arendt, J.; Dijk, D.J. Robust circadian rhythm in heart rate and its variability: Influence of exogenous melatonin and photoperiod. J. Sleep Res. 2007, 16, 148–155. [Google Scholar] [CrossRef]
- Yamazaki, S.; Numano, R.; Abe, M.; Hida, A.; Takahashi, R.; Ueda, M.; Block, G.D.; Sakaki, Y.; Menaker, M.; Tei, H. Resetting central and peripheral circadian oscillators in transgenic rats. Science 2000, 288, 682–685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoo, S.H.; Yamazaki, S.; Lowrey, P.L.; Shimomura, K.; Ko, C.H.; Buhr, E.D.; Siepka, S.M.; Hong, H.K.; Oh, W.J.; Yoo, O.J.; et al. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc. Natl. Acad. Sci. USA 2004, 101, 5339–5346. [Google Scholar] [CrossRef] [Green Version]
- Stephan, F.K.; Zucker, I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc. Natl. Acad. Sci. USA 1972, 69, 1583–1586. [Google Scholar] [CrossRef] [Green Version]
- Moore, R.Y.; Eichler, V.B. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res. 1972, 42, 201–206. [Google Scholar] [CrossRef]
- Schmidt, T.M.; Chen, S.K.; Hattar, S. Intrinsically photosensitive retinal ganglion cells: Many subtypes, diverse functions. Trends Neurosci. 2011, 34, 572–580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Provencio, I.; Rodriguez, I.R.; Jiang, G.; Hayes, W.P.; Moreira, E.F.; Rollag, M.D. A novel human opsin in the inner retina. J. Neurosci. 2000, 20, 600–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hattar, S.; Liao, H.W.; Takao, M.; Berson, D.M.; Yau, K.W. Melanopsin-containing retinal ganglion cells: Architecture, projections, and intrinsic photosensitivity. Science 2002, 295, 1065–1070. [Google Scholar] [CrossRef] [Green Version]
- Berson, D.M.; Dunn, F.A.; Takao, M. Phototransduction by retinal ganglion cells that set the circadian clock. Science 2002, 295, 1070–1073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebling, F.J. The role of glutamate in the photic regulation of the suprachiasmatic nucleus. Prog. Neurobiol. 1996, 50, 109–132. [Google Scholar] [CrossRef]
- Pickard, G.E. Bifurcating axons of retinal ganglion cells terminate in the hypothalamic suprachiasmatic nucleus and the intergeniculate leaflet of the thalamus. Neurosci. Lett. 1985, 55, 211–217. [Google Scholar] [CrossRef]
- Hattar, S.; Kumar, M.; Park, A.; Tong, P.; Tung, J.; Yau, K.W.; Berson, D.M. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J. Comp. Neurol. 2006, 497, 326–349. [Google Scholar] [CrossRef] [Green Version]
- McDougal, D.H.; Gamlin, P.D. The influence of intrinsically-photosensitive retinal ganglion cells on the spectral sensitivity and response dynamics of the human pupillary light reflex. Vision Res. 2010, 50, 72–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Provencio, I.; Jiang, G.; De Grip, W.J.; Hayes, W.P.; Rollag, M.D. Melanopsin: An opsin in melanophores, brain, and eye. Proc. Natl. Acad. Sci. USA 1998, 95, 340–345. [Google Scholar] [CrossRef] [Green Version]
- Panda, S.; Provencio, I.; Tu, D.C.; Pires, S.S.; Rollag, M.D.; Castrucci, A.M.; Pletcher, M.T.; Sato, T.K.; Wiltshire, T.; Andahazy, M.; et al. Melanopsin is required for non-image-forming photic responses in blind mice. Science 2003, 301, 525–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucas, R.J.; Douglas, R.H.; Foster, R.G. Characterization of an ocular photopigment capable of driving pupillary constriction in mice. Nat. Neurosci. 2001, 4, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Lucas, R.J.; Foster, R.G. Neither functional rod photoreceptors nor rod or cone outer segments are required for the photic inhibition of pineal melatonin. Endocrinology 1999, 140, 1520–1524. [Google Scholar] [CrossRef] [PubMed]
- Göz, D.; Studholme, K.; Lappi, D.A.; Rollag, M.D.; Provencio, I.; Morin, L.P. Targeted destruction of photosensitive retinal ganglion cells with a saporin conjugate alters the effects of light on mouse circadian rhythms. PLoS ONE 2008, 3, e3153. [Google Scholar] [CrossRef] [PubMed]
- Hatori, M.; Le, H.; Vollmers, C.; Keding, S.R.; Tanaka, N.; Buch, T.; Waisman, A.; Schmedt, C.; Jegla, T.; Panda, S. Inducible ablation of melanopsin-expressing retinal ganglion cells reveals their central role in non-image forming visual responses. PLoS ONE 2008, 3, e2451. [Google Scholar] [CrossRef]
- Güler, A.D.; Ecker, J.L.; Lall, G.S.; Haq, S.; Altimus, C.M.; Liao, H.W.; Barnard, A.R.; Cahill, H.; Badea, T.C.; Zhao, H.; et al. Melanopsin cells are the principal conduits for rod-cone input to non-image-forming vision. Nature 2008, 453, 102–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaidi, F.H.; Hull, J.T.; Peirson, S.N.; Wulff, K.; Aeschbach, D.; Gooley, J.J.; Brainard, G.C.; Gregory-Evans, K.; Rizzo, J.F., 3rd; Czeisler, C.A.; et al. Short-wavelength light sensitivity of circadian, pupillary, and visual awareness in humans lacking an outer retina. Curr. Biol. 2007, 17, 2122–2128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rollag, M.D.; Berson, D.M.; Provencio, I. Melanopsin, ganglion-cell photoreceptors, and mammalian photoentrainment. J. Biol. Rhythms 2003, 18, 227–234. [Google Scholar] [CrossRef]
- Lledo, V.E.; Alkozi, H.A.; Pintor, J. Yellow Filter Effect on Melatonin Secretion in the Eye: Role in IOP Regulation. Curr. Eye Res. 2019, 44, 614–618. [Google Scholar] [CrossRef] [PubMed]
- Bartness, T.J.; Goldman, B.D. Mammalian pineal melatonin: A clock for all seasons. Experientia 1989, 45, 939–945. [Google Scholar] [CrossRef]
- Arendt, J. Melatonin and the pineal gland: Influence on mammalian seasonal and circadian physiology. Rev. Reprod. 1998, 3, 13–22. [Google Scholar] [CrossRef]
- Lerner, A.B.; Case, J.D.; Takahashi, Y. Isolation of melatonin and 5-methoxyindole-3-acetic acid from bovine pineal glands. J. Biol. Chem. 1960, 235, 1992–1997. [Google Scholar] [CrossRef]
- Bubenik, G.A.; Brown, G.M.; Uhlir, I.; Grota, L.J. Immunohistological localization of N-acetylindolealkylamines in pineal gland, retina and cerebellum. Brain Res. 1974, 81, 233–242. [Google Scholar] [CrossRef]
- Aimoto, T.; Rohde, B.H.; Chiou, G.C.; Lauber, J.K. N-acetyltransferase activity and melatonin level in the eyes of glaucomatous chickens. J. Ocul. Pharmacol. 1985, 1, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Quay, W.B. Increases in volume, fluid content, and lens weight of eyes following systemic administration of melatonin. J. Pineal. Res. 1984, 1, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Abe, M.; Itoh, M.T.; Miyata, M.; Shimizu, K.; Sumi, Y. Circadian rhythm of serotonin N -acetyltransferase activity in rat lens. Exp. Eye Res. 2000, 70, 805–808. [Google Scholar] [CrossRef]
- Mhatre, M.C.; van Jaarsveld, A.S.; Reiter, R.J. Melatonin in the lacrimal gland: First demonstration and experimental manipulation. Biochem. Biophys. Res. Commun. 1988, 153, 1186–1192. [Google Scholar] [CrossRef]
- Klein, D.C.; Coon, S.L.; Roseboom, P.H.; Weller, J.L.; Bernard, M.; Gastel, J.A.; Zatz, M.; Iuvone, P.M.; Rodriguez, I.R.; Begay, V.; et al. The melatonin rhythm-generating enzyme: Molecular regulation of serotonin N-acetyltransferase in the pineal gland. Recent Prog. Horm. Res. 1997, 52, 307–357, discussion 357–308. [Google Scholar]
- Pevet, P. The internal time-giver role of melatonin. A key for our health. Rev. Neurol. 2014, 170, 646–652. [Google Scholar] [CrossRef]
- Møller, M.; Baeres, F.M. The anatomy and innervation of the mammalian pineal gland. Cell Tissue Res. 2002, 309, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Reppert, S.M.; Godson, C.; Mahle, C.D.; Weaver, D.R.; Slaugenhaupt, S.A.; Gusella, J.F. Molecular characterization of a second melatonin receptor expressed in human retina and brain: The Mel1b melatonin receptor. Proc. Natl. Acad. Sci. USA 1995, 92, 8734–8738. [Google Scholar] [CrossRef] [Green Version]
- Reppert, S.M.; Weaver, D.R.; Ebisawa, T. Cloning and characterization of a mammalian melatonin receptor that mediates reproductive and circadian responses. Neuron 1994, 13, 1177–1185. [Google Scholar] [CrossRef]
- Duncan, M.J.; Takahashi, J.S.; Dubocovich, M.L. 2-[125I]Iodomelatonin Binding Sites in Hamster Brain Membranes: Pharmacological Characteristics and Regional Distribution*. Endocrinology 1988, 122, 1825–1833. [Google Scholar] [CrossRef] [PubMed]
- Nosjean, O.; Ferro, M.; Coge, F.; Beauverger, P.; Henlin, J.M.; Lefoulon, F.; Fauchere, J.L.; Delagrange, P.; Canet, E.; Boutin, J.A. Identification of the melatonin-binding site MT3 as the quinone reductase 2. J. Biol. Chem. 2000, 275, 31311–31317. [Google Scholar] [CrossRef] [Green Version]
- Dubocovich, M.L.; Markowska, M. Functional MT1 and MT2 melatonin receptors in mammals. Endocrine 2005, 27, 101–110. [Google Scholar] [CrossRef]
- Wiechmann, A.F.; Udin, S.B.; Summers Rada, J.A. Localization of Mel1b melatonin receptor-like immunoreactivity in ocular tissues of Xenopus laevis. Exp. Eye Res. 2004, 79, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Rada, J.A.; Wiechmann, A.F. Melatonin receptors in chick ocular tissues: Implications for a role of melatonin in ocular growth regulation. Investig. Ophthalmol. Vis. Sci. 2006, 47, 25–33. [Google Scholar] [CrossRef] [Green Version]
- Meyer, P.; Pache, M.; Loeffler, K.U.; Brydon, L.; Jockers, R.; Flammer, J.; Wirz-Justice, A.; Savaskan, E. Melatonin MT-1-receptor immunoreactivity in the human eye. Br. J. Ophthalmol. 2002, 86, 1053–1057. [Google Scholar] [CrossRef]
- Pintor, J.J.; Carracedo, G.; Mediero, A.; Guzman–Aranguez, A.; Irazu, M.; Pelaez, T.; Peral, A. Melatonin Increases the Rate of Corneal Re–epithelialisation in New Zealand White Rabbits. Investig. Ophthalmol. Vis. Sci. 2005, 46, 2152. [Google Scholar]
- Crooke, A.; Guzman-Aranguez, A.; Mediero, A.; Alarma-Estrany, P.; Carracedo, G.; Pelaez, T.; Peral, A.; Pintor, J. Effect of melatonin and analogues on corneal wound healing: Involvement of Mt2 melatonin receptor. Curr. Eye Res. 2015, 40, 56–65. [Google Scholar] [CrossRef]
- Osborne, N.N.; Chidlow, G. The presence of functional melatonin receptors in the iris-ciliary processes of the rabbit eye. Exp. Eye Res. 1994, 59, 3–9. [Google Scholar] [CrossRef]
- Roberts, J.E.; Wiechmann, A.F.; Hu, D.N. Melatonin receptors in human uveal melanocytes and melanoma cells. J. Pineal. Res. 2000, 28, 165–171. [Google Scholar] [CrossRef]
- Alkozi, H.; Sánchez-Naves, J.; de Lara, M.J.; Carracedo, G.; Fonseca, B.; Martinez-Aguila, A.; Pintor, J. Elevated intraocular pressure increases melatonin levels in the aqueous humour. Acta Ophthalmol. 2017, 95, e185–e189. [Google Scholar] [CrossRef] [PubMed]
- Martin, X.D.; Malina, H.Z.; Brennan, M.C.; Hendrickson, P.H.; Lichter, P.R. The ciliary body—The third organ found to synthesize indoleamines in humans. Eur. J. Ophthalmol. 1992, 2, 67–72. [Google Scholar] [CrossRef]
- Alkozi, H.A.; Wang, X.; Perez de Lara, M.J.; Pintor, J. Presence of melanopsin in human crystalline lens epithelial cells and its role in melatonin synthesis. Exp. Eye Res. 2017, 154, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Savaskan, E.; Wirz-Justice, A.; Olivieri, G.; Pache, M.; Kräuchi, K.; Brydon, L.; Jockers, R.; Müller-Spahn, F.; Meyer, P. Distribution of melatonin MT1 receptor immunoreactivity in human retina. J. Histochem. Cytochem. 2002, 50, 519–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scher, J.; Wankiewicz, E.; Brown, G.M.; Fujieda, H. MT(1) melatonin receptor in the human retina: Expression and localization. Investig. Ophthalmol. Vis. Sci. 2002, 43, 889–897. [Google Scholar]
- Pandi-Perumal, S.R.; Trakht, I.; Srinivasan, V.; Spence, D.W.; Maestroni, G.J.; Zisapel, N.; Cardinali, D.P. Physiological effects of melatonin: Role of melatonin receptors and signal transduction pathways. Prog. Neurobiol. 2008, 85, 335–353. [Google Scholar] [CrossRef]
- Natesan, A.K.; Cassone, V.M. Melatonin receptor mRNA localization and rhythmicity in the retina of the domestic chick, Gallus domesticus. Vis. Neurosci. 2002, 19, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Dubocovich, M.L. Melatonin is a potent modulator of dopamine release in the retina. Nature 1983, 306, 782–784. [Google Scholar] [CrossRef] [PubMed]
- Pierce, M.E.; Besharse, J.C. Circadian regulation of retinomotor movements. I. Interaction of melatonin and dopamine in the control of cone length. J. Gen. Physiol. 1985, 86, 671–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiechmann, A.F.; Yang, X.-L.; Wu, S.M.; Hollyfield, J.G. Melatonin enhances horizontal cell sensitivity in salamander retina. Brain Res. 1988, 453, 377–380. [Google Scholar] [CrossRef]
- White, M.P.; Fisher, L.J. Effects of exogenous melatonin on circadian disc shedding in the albino rat retina. Vision Res. 1989, 29, 167–179. [Google Scholar] [CrossRef]
- Benloucif, S.; Burgess, H.J.; Klerman, E.B.; Lewy, A.J.; Middleton, B.; Murphy, P.J.; Parry, B.L.; Revell, V.L. Measuring melatonin in humans. J. Clin. Sleep Med. 2008, 4, 66–69. [Google Scholar] [CrossRef]
- Gradisar, M.; Wolfson, A.R.; Harvey, A.G.; Hale, L.; Rosenberg, R.; Czeisler, C.A. The sleep and technology use of Americans: Findings from the National Sleep Foundation’s 2011 Sleep in America poll. J. Clin. Sleep Med. 2013, 9, 1291–1299. [Google Scholar] [CrossRef]
- Thapan, K.; Arendt, J.; Skene, D.J. An action spectrum for melatonin suppression: Evidence for a novel non-rod, non-cone photoreceptor system in humans. J. Physiol. 2001, 535, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Brainard, G.C.; Sliney, D.; Hanifin, J.P.; Glickman, G.; Byrne, B.; Greeson, J.M.; Jasser, S.; Gerner, E.; Rollag, M.D. Sensitivity of the human circadian system to short-wavelength (420-nm) light. J. Biol. Rhythms 2008, 23, 379–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lockley, S.W.; Brainard, G.C.; Czeisler, C.A. High sensitivity of the human circadian melatonin rhythm to resetting by short wavelength light. J. Clin. Endocrinol. Metab. 2003, 88, 4502–4505. [Google Scholar] [CrossRef]
- Lemola, S.; Perkinson-Gloor, N.; Brand, S.; Dewald-Kaufmann, J.F.; Grob, A. Adolescents’ electronic media use at night, sleep disturbance, and depressive symptoms in the smartphone age. J. Youth Adolesc. 2015, 44, 405–418. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X.; Korkmaz, A.; Ma, S. Obesity and metabolic syndrome: Association with chronodisruption, sleep deprivation, and melatonin suppression. Ann. Med. 2012, 44, 564–577. [Google Scholar] [CrossRef]
- Zhao, Q.-F.; Tan, L.; Wang, H.-F.; Jiang, T.; Tan, M.-S.; Tan, L.; Xu, W.; Li, J.-Q.; Wang, J.; Lai, T.-J.; et al. The prevalence of neuropsychiatric symptoms in Alzheimer’s disease: Systematic review and meta-analysis. J. Affect. Disord. 2016, 190, 264–271. [Google Scholar] [CrossRef]
- Weissová, K.; Bartoš, A.; Sládek, M.; Nováková, M.; Sumová, A. Moderate Changes in the Circadian System of Alzheimer’s Disease Patients Detected in Their Home Environment. PLoS ONE 2016, 11, e0146200. [Google Scholar] [CrossRef]
- Breen, D.P.; Vuono, R.; Nawarathna, U.; Fisher, K.; Shneerson, J.M.; Reddy, A.B.; Barker, R.A. Sleep and circadian rhythm regulation in early Parkinson disease. JAMA Neurol. 2014, 71, 589–595. [Google Scholar] [CrossRef]
- Videnovic, A.; Noble, C.; Reid, K.J.; Peng, J.; Turek, F.W.; Marconi, A.; Rademaker, A.W.; Simuni, T.; Zadikoff, C.; Zee, P.C. Circadian Melatonin Rhythm and Excessive Daytime Sleepiness in Parkinson Disease. JAMA Neurol. 2014, 71, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Leng, Y.; Musiek, E.S.; Hu, K.; Cappuccio, F.P.; Yaffe, K. Association between circadian rhythms and neurodegenerative diseases. Lancet Neurol. 2019, 18, 307–318. [Google Scholar] [CrossRef]
- Skene, D.J.; Vivien-Roels, B.; Sparks, D.L.; Hunsaker, J.C.; Pévet, P.; Ravid, D.; Swaab, D.F. Daily variation in the concentration of melatonin and 5-methoxytryptophol in the human pineal gland: Effect of age and Alzheimer’s disease. Brain Res. 1990, 528, 170–174. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.Y.; Zhou, J.N.; van Heerikhuize, J.; Hofman, M.A.; Swaab, D.F. Decreased melatonin levels in postmortem cerebrospinal fluid in relation to aging, Alzheimer’s disease, and apolipoprotein E-epsilon4/4 genotype. J. Clin. Endocrinol. Metab. 1999, 84, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.H.; Swaab, D.F. Disturbance and strategies for reactivation of the circadian rhythm system in aging and Alzheimer’s disease. Sleep. Med. 2007, 8, 623–636. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.; Mirick, D.K.; Stevens, R.G. Night shift work, light at night, and risk of breast cancer. J. Natl. Cancer Inst. 2001, 93, 1557–1562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frank, J.R.; Ovens, H. Shiftwork and emergency medical practice. Can. J. Emerg. Med. 2002, 4, 421–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Books, C.; Coody, L.C.; Kauffman, R.; Abraham, S. Night Shift Work and Its Health Effects on Nurses. Health Care Manag. (Frederick) 2017, 36, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Navarro Gil, F.J.; Huete-Toral, F.; Crooke, A.; Dominguez Godinez, C.O.; Carracedo, G.; Pintor, J. Effect of Melatonin and Its Analogs on Tear Secretion. J. Pharmacol. Exp. Ther. 2019, 371, 186–190. [Google Scholar] [CrossRef]
- Hoyle, C.H.; Peral, A.; Pintor, J. Melatonin potentiates tear secretion induced by diadenosine tetraphosphate in the rabbit. Eur. J. Pharmacol. 2006, 552, 159–161. [Google Scholar] [CrossRef]
- Crespo-Morales, M.; Alkozi, H.A.; López-García, A.; Pintor, J.J.; Diebold, Y. Melatonin receptors are present in the porcine ocular surface and are involved in ex vivo corneal wound healing. Investig. Ophthalmol. Vis. Sci. 2018, 59, 4371. [Google Scholar]
- Wang, F.; Zhou, J.; Lu, Y.; Chu, R. Effects of 530 nm green light on refractive status, melatonin, MT1 receptor, and melanopsin in the guinea pig. Curr. Eye Res. 2011, 36, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Chen, Y.; Zhou, X.; Zhang, X.; Guan, X.; Mao, J. Regulation of Retinal Melanopsin on Lens-induced Myopia in Guinea Pigs. Optom. Vis. Sci. 2020, 97, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Abe, M.; Reiter, R.J.; Orhii, P.B.; Hara, M.; Poeggeler, B. Inhibitory effect of melatonin on cataract formation in newborn rats: Evidence for an antioxidative role for melatonin. J. Pineal. Res. 1994, 17, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.R.; Reiter, R.J.; Fujimori, O.; Oh, C.S.; Duan, Y.P. Cataractogenesis and lipid peroxidation in newborn rats treated with buthionine sulfoximine: Preventive actions of melatonin. J. Pineal. Res. 1997, 22, 117–123. [Google Scholar] [CrossRef]
- Bardak, Y.; Ozertürk, Y.; Ozgüner, F.; Durmuş, M.; Delibaş, N. Effect of melatonin against oxidative stress in ultraviolet-B exposed rat lens. Curr. Eye Res. 2000, 20, 225–230. [Google Scholar] [CrossRef]
- Karslioglu, I.; Ertekin, M.V.; Taysi, S.; Koçer, I.; Sezen, O.; Gepdiremen, A.; Koç, M.; Bakan, N. Radioprotective effects of melatonin on radiation-induced cataract. J. Radiat. Res. 2005, 46, 277–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yağci, R.; Aydin, B.; Erdurmuş, M.; Karadağ, R.; Gürel, A.; Durmuş, M.; Yiğitoğlu, R. Use of melatonin to prevent selenite-induced cataract formation in rat eyes. Curr. Eye Res. 2006, 31, 845–850. [Google Scholar] [CrossRef]
- Taysi, S.; Memisogullari, R.; Koc, M.; Yazici, A.T.; Aslankurt, M.; Gumustekin, K.; Al, B.; Ozabacigil, F.; Yilmaz, A.; Tahsin Ozder, H. Melatonin reduces oxidative stress in the rat lens due to radiation-induced oxidative injury. Int. J. Radiat. Biol. 2008, 84, 803–808. [Google Scholar] [CrossRef]
- Shirazi, A.; Haddadi, G.H.; Asadi-Amoli, F.; Sakhaee, S.; Ghazi-Khansari, M.; Avand, A. Radioprotective effect of melatonin in reducing oxidative stress in rat lenses. Cell J. 2011, 13, 79–82. [Google Scholar] [CrossRef] [Green Version]
- Pintor, J. Light-induced ATP release from the lens. Purinergic Signal 2018, 14, 499–504. [Google Scholar] [CrossRef]
- Liang, F.Q.; Aleman, T.S.; Yang, Z.; Cideciyan, A.V.; Jacobson, S.G.; Bennett, J. Melatonin delays photoreceptor degeneration in the rds/rds mouse. Neuroreport 2001, 12, 1011–1014. [Google Scholar] [CrossRef] [PubMed]
- Kaur, C.; Sivakumar, V.; Yong, Z.; Lu, J.; Foulds, W.S.; Ling, E.A. Blood-retinal barrier disruption and ultrastructural changes in the hypoxic retina in adult rats: The beneficial effect of melatonin administration. J. Pathol. 2007, 212, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Diéguez, H.H.; González Fleitas, M.F.; Aranda, M.L.; Calanni, J.S.; Keller Sarmiento, M.I.; Chianelli, M.S.; Alaimo, A.; Sande, P.H.; Romeo, H.E.; Rosenstein, R.E.; et al. Melatonin protects the retina from experimental nonexudative age-related macular degeneration in mice. J. Pineal. Res. 2020, 68, e12643. [Google Scholar] [CrossRef]
- Yi, C.; Pan, X.; Yan, H.; Guo, M.; Pierpaoli, W. Effects of melatonin in age-related macular degeneration. Ann. N. Y. Acad. Sci. 2005, 1057, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Craig, J.P.; Nichols, K.K.; Akpek, E.K.; Caffery, B.; Dua, H.S.; Joo, C.-K.; Liu, Z.; Nelson, J.D.; Nichols, J.J.; Tsubota, K.; et al. TFOS DEWS II Definition and Classification Report. Ocul. Surf. 2017, 15, 276–283. [Google Scholar] [CrossRef]
- Gomes, J.A.P.; Azar, D.T.; Baudouin, C.; Efron, N.; Hirayama, M.; Horwath-Winter, J.; Kim, T.; Mehta, J.S.; Messmer, E.M.; Pepose, J.S.; et al. TFOS DEWS II iatrogenic report. Ocul. Surf. 2017, 15, 511–538. [Google Scholar] [CrossRef]
- Shen, M.; Wang, J.; Tao, A.; Chen, Q.; Lin, S.; Qu, J.; Lu, F. Diurnal variation of upper and lower tear menisci. Am. J. Ophthalmol. 2008, 145, 801–806. [Google Scholar] [CrossRef]
- Li, M.; Du, C.; Zhu, D.; Shen, M.; Cui, L.; Wang, J. Daytime variations of tear osmolarity and tear meniscus volume. Eye Contact Lens 2012, 38, 282–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayaki, M.; Tachi, N.; Hashimoto, Y.; Kawashima, M.; Tsubota, K.; Negishi, K. Diurnal variation of human tear meniscus volume measured with tear strip meniscometry self-examination. PLoS ONE 2019, 14, e0215922. [Google Scholar] [CrossRef] [PubMed]
- Niimi, J.; Tan, B.; Chang, J.; Zhou, Y.; Ghanekar, A.; Wong, M.; Lee, A.; Lin, M.C. Diurnal Pattern of Tear Osmolarity and Its Relationship to Corneal Thickness and Deswelling. Cornea 2013, 32, 1305–1310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oncel, B.A.; Pinarci, E.; Akova, Y.A. Diurnal variation of the tear osmolarity in normal subjects measured by a new microchip system. Eur. J. Ophthalmol. 2012, 22 (Suppl. 7), S1–S4. [Google Scholar] [CrossRef]
- Ayaki, M.; Tsubota, K.; Kawashima, M.; Kishimoto, T.; Mimura, M.; Negishi, K. Sleep Disorders are a Prevalent and Serious Comorbidity in Dry Eye. Investig. Ophthalmol. Vis. Sci. 2018, 59, Des143–Des150. [Google Scholar] [CrossRef]
- Li, S.; Ning, K.; Zhou, J.; Guo, Y.; Zhang, H.; Zhu, Y.; Zhang, L.; Jia, C.; Chen, Y.; Sol Reinach, P.; et al. Sleep deprivation disrupts the lacrimal system and induces dry eye disease. Exp. Mol. Med. 2018, 50, e451. [Google Scholar] [CrossRef]
- Ayaki, M.; Kawashima, M.; Negishi, K.; Tsubota, K. High prevalence of sleep and mood disorders in dry eye patients: Survey of 1,000 eye clinic visitors. Neuropsychiatr. Dis. Treat. 2015, 11, 889–894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayaki, M.; Kawashima, M.; Negishi, K.; Kishimoto, T.; Mimura, M.; Tsubota, K. Sleep and mood disorders in dry eye disease and allied irritating ocular diseases. Sci. Rep. 2016, 6, 22480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawashima, M.; Uchino, M.; Yokoi, N.; Uchino, Y.; Dogru, M.; Komuro, A.; Sonomura, Y.; Kato, H.; Kinoshita, S.; Tsubota, K. The association of sleep quality with dry eye disease: The Osaka study. Clin. Ophthalmol. 2016, 10, 1015–1021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.B.; Koh, J.W.; Hyon, J.Y.; Wee, W.R.; Kim, J.J.; Shin, Y.J. Sleep deprivation reduces tear secretion and impairs the tear film. Investig. Ophthalmol. Vis. Sci. 2014, 55, 3525–3531. [Google Scholar] [CrossRef]
- Carracedo, G.; Carpena, C.; Concepción, P.; Díaz, V.; García-García, M.; Jemni, N.; Lledó, V.E.; Martín, M.; Pastrana, C.; Pelissier, R.; et al. Presence of melatonin in human tears. J. Optom. 2017, 10, 3–4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ljubimov, A.V.; Saghizadeh, M. Progress in corneal wound healing. Prog. Retin. Eye Res. 2015, 49, 17–45. [Google Scholar] [CrossRef] [Green Version]
- Doughty, M.J. Morphometric analysis of the surface cells of rabbit corneal epithelium by scanning electron microscopy. Am. J. Anat. 1990, 189, 316–328. [Google Scholar] [CrossRef]
- Lavker, R.M.; Dong, G.; Cheng, S.Z.; Kudoh, K.; Cotsarelis, G.; Sun, T.T. Relative proliferative rates of limbal and corneal epithelia. Implications of corneal epithelial migration, circadian rhythm, and suprabasally located DNA-synthesizing keratinocytes. Investig. Ophthalmol. Vis. Sci. 1991, 32, 1864–1875. [Google Scholar]
- Sandvig, K.U.; Haaskjold, E.; Refsum, S.B. Time dependency in the regenerative response to injury of the rat corneal epithelium. Chronobiol. Int. 1994, 11, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Masuda, A.; Oishi, T. Circadian rhythms of corneal mitotic rate, retinal melatonin and immunoreactive visual pigments, and the effects of melatonin on the rhythms in the Japanese quail. J. Comp. Physiol. A 1995, 176, 465–471. [Google Scholar] [CrossRef]
- Xue, Y.; Liu, P.; Wang, H.; Xiao, C.; Lin, C.; Liu, J.; Dong, D.; Fu, T.; Yang, Y.; Wang, Z.; et al. Modulation of Circadian Rhythms Affects Corneal Epithelium Renewal and Repair in Mice. Investig. Ophthalmol. Vis. Sci. 2017, 58, 1865–1874. [Google Scholar] [CrossRef] [PubMed]
- Flitcroft, D.I. The complex interactions of retinal, optical and environmental factors in myopia aetiology. Prog. Retin. Eye Res. 2012, 31, 622–660. [Google Scholar] [CrossRef] [PubMed]
- Wildsoet, C.F.; Chia, A.; Cho, P.; Guggenheim, J.A.; Polling, J.R.; Read, S.; Sankaridurg, P.; Saw, S.M.; Trier, K.; Walline, J.J.; et al. IMI—Interventions Myopia Institute: Interventions for Controlling Myopia Onset and Progression Report. Investig. Ophthalmol. Vis. Sci. 2019, 60, M106–M131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weiss, S.; Schaeffel, F. Diurnal growth rhythms in the chicken eye: Relation to myopia development and retinal dopamine levels. J. Comp. Physiol. A 1993, 172, 263–270. [Google Scholar] [CrossRef]
- Liu, J.H.; Farid, H. Twenty-four-hour change in axial length in the rabbit eye. Investig. Ophthalmol. Vis. Sci. 1998, 39, 2796–2799. [Google Scholar]
- Stone, R.A.; Quinn, G.E.; Francis, E.L.; Ying, G.S.; Flitcroft, D.I.; Parekh, P.; Brown, J.; Orlow, J.; Schmid, G. Diurnal axial length fluctuations in human eyes. Investig. Ophthalmol. Vis. Sci. 2004, 45, 63–70. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, R.; Read, S.A.; Collins, M.J. Diurnal variations in axial length, choroidal thickness, intraocular pressure, and ocular biometrics. Investig. Ophthalmol. Vis. Sci. 2011, 52, 5121–5129. [Google Scholar] [CrossRef] [Green Version]
- Tan, C.S.; Ouyang, Y.; Ruiz, H.; Sadda, S.R. Diurnal variation of choroidal thickness in normal, healthy subjects measured by spectral domain optical coherence tomography. Investig. Ophthalmol. Vis. Sci. 2012, 53, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Rada, J.A.; Achen, V.R.; Perry, C.A.; Fox, P.W. Proteoglycans in the human sclera. Evidence for the presence of aggrecan. Investig. Ophthalmol. Vis. Sci. 1997, 38, 1740–1751. [Google Scholar]
- Devadas, M.; Morgan, I. Light controls scleral precursor synthesis. Neuroreport 1996, 7, 2010–2012. [Google Scholar] [CrossRef]
- Nickla, D.L.; Rada, J.A.; Wallman, J. Isolated chick sclera shows a circadian rhythm in proteoglycan synthesis perhaps associated with the rhythm in ocular elongation. J. Comp. Physiol. A 1999, 185, 81–90. [Google Scholar] [CrossRef]
- Read, S.A.; Alonso-Caneiro, D.; Free, K.A.; Labuc-Spoors, E.; Leigh, J.K.; Quirk, C.J.; Yang, Z.Y.; Vincent, S.J. Diurnal variation of anterior scleral and conjunctival thickness. Ophthalmic Physiol. Opt. 2016, 36, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Schaeffel, F. Melatonin and deprivation myopia in chickens. Neurochem. Int. 1996, 28, 95–107. [Google Scholar] [CrossRef]
- Stone, R.A.; McGlinn, A.M.; Baldwin, D.A.; Tobias, J.W.; Iuvone, P.M.; Khurana, T.S. Image defocus and altered retinal gene expression in chick: Clues to the pathogenesis of ametropia. Investig. Ophthalmol. Vis. Sci. 2011, 52, 5765–5777. [Google Scholar] [CrossRef] [PubMed]
- Stone, R.A.; Cohen, Y.; McGlinn, A.M.; Davison, S.; Casavant, S.; Shaffer, J.; Khurana, T.S.; Pardue, M.T.; Iuvone, P.M. Development of Experimental Myopia in Chicks in a Natural Environment. Investig. Ophthalmol. Vis. Sci. 2016, 57, 4779–4789. [Google Scholar] [CrossRef]
- Rose, K.A.; Morgan, I.G.; Ip, J.; Kifley, A.; Huynh, S.; Smith, W.; Mitchell, P. Outdoor activity reduces the prevalence of myopia in children. Ophthalmology 2008, 115, 1279–1285. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.C.; Tsai, C.L.; Wu, H.L.; Yang, Y.H.; Kuo, H.K. Outdoor activity during class recess reduces myopia onset and progression in school children. Ophthalmology 2013, 120, 1080–1085. [Google Scholar] [CrossRef]
- He, M.; Xiang, F.; Zeng, Y.; Mai, J.; Chen, Q.; Zhang, J.; Smith, W.; Rose, K.; Morgan, I.G. Effect of Time Spent Outdoors at School on the Development of Myopia Among Children in China: A Randomized Clinical Trial. JAMA 2015, 314, 1142–1148. [Google Scholar] [CrossRef] [Green Version]
- Jin, J.X.; Hua, W.J.; Jiang, X.; Wu, X.Y.; Yang, J.W.; Gao, G.P.; Fang, Y.; Pei, C.L.; Wang, S.; Zhang, J.Z.; et al. Effect of outdoor activity on myopia onset and progression in school-aged children in northeast China: The Sujiatun Eye Care Study. BMC Ophthalmol. 2015, 15, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Read, S.A.; Collins, M.J.; Vincent, S.J. Light Exposure and Eye Growth in Childhood. Investig. Ophthalmol. Vis. Sci. 2015, 56, 6779–6787. [Google Scholar] [CrossRef] [PubMed]
- Landis, E.G.; Yang, V.; Brown, D.M.; Pardue, M.T.; Read, S.A. Dim Light Exposure and Myopia in Children. Investig. Ophthalmol. Vis. Sci. 2018, 59, 4804–4811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, P.C.; Chen, C.T.; Lin, K.K.; Sun, C.C.; Kuo, C.N.; Huang, H.M.; Poon, Y.C.; Yang, M.L.; Chen, C.Y.; Huang, J.C.; et al. Myopia Prevention and Outdoor Light Intensity in a School-Based Cluster Randomized Trial. Ophthalmology 2018, 125, 1239–1250. [Google Scholar] [CrossRef] [Green Version]
- Cohen, Y.; Peleg, E.; Belkin, M.; Polat, U.; Solomon, A.S. Ambient illuminance, retinal dopamine release and refractive development in chicks. Exp. Eye Res. 2012, 103, 33–40. [Google Scholar] [CrossRef]
- Zhou, X.; Pardue, M.T.; Iuvone, P.M.; Qu, J. Dopamine signaling and myopia development: What are the key challenges. Prog. Retin. Eye Res. 2017, 61, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Qu, X. The Effects of High Lighting on the Development of Form-Deprivation Myopia in Guinea Pigs. Investig. Ophthalmol. Vis. Sci. 2019, 60, 4319–4327. [Google Scholar] [CrossRef] [Green Version]
- Zisapel, N. Melatonin-dopamine interactions: From basic neurochemistry to a clinical setting. Cell Mol. Neurobiol. 2001, 21, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Tosini, G.; Dirden, J.C. Dopamine inhibits melatonin release in the mammalian retina: In vitro evidence. Neurosci. Lett. 2000, 286, 119–122. [Google Scholar] [CrossRef]
- Kearney, S.; O’Donoghue, L.; Pourshahidi, L.K.; Cobice, D.; Saunders, K.J. Myopes have significantly higher serum melatonin concentrations than non-myopes. Ophthalmic. Physiol. Opt. 2017, 37, 557–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chakraborty, R.; Micic, G.; Thorley, L.; Nissen, T.R.; Lovato, N.; Collins, M.J.; Lack, L.C. Myopia, or near-sightedness, is associated with delayed melatonin circadian timing and lower melatonin output in young adult humans. Sleep 2020. [Google Scholar] [CrossRef]
- Flanagan, S.C.; Cobice, D.; Richardson, P.; Sittlington, J.J.; Saunders, K.J. Elevated Melatonin Levels Found in Young Myopic Adults Are Not Attributable to a Shift in Circadian Phase. Investig. Ophthalmol. Vis. Sci. 2020, 61, 45. [Google Scholar] [CrossRef]
- Seidemann, A.; Schaeffel, F. Effects of longitudinal chromatic aberration on accommodation and emmetropization. Vision Res. 2002, 42, 2409–2417. [Google Scholar] [CrossRef] [Green Version]
- Foulds, W.S.; Barathi, V.A.; Luu, C.D. Progressive myopia or hyperopia can be induced in chicks and reversed by manipulation of the chromaticity of ambient light. Investig. Ophthalmol. Vis. Sci. 2013, 54, 8004–8012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Schaeffel, F.; Jiang, B.; Feldkaemper, M. Effects of Light of Different Spectral Composition on Refractive Development and Retinal Dopamine in Chicks. Investig. Ophthalmol. Vis. Sci. 2018, 59, 4413–4424. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.; Qian, Y.F.; He, J.C.; Hu, M.; Zhou, X.T.; Dai, J.H.; Qu, X.M.; Chu, R.Y. Effects of different monochromatic lights on refractive development and eye growth in guinea pigs. Exp. Eye Res. 2011, 92, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.F.; Dai, J.H.; Liu, R.; Chen, M.J.; Zhou, X.T.; Chu, R.Y. Effects of the chromatic defocus caused by interchange of two monochromatic lights on refraction and ocular dimension in guinea pigs. PLoS ONE 2013, 8, e63229. [Google Scholar] [CrossRef] [Green Version]
- Zou, L.; Zhu, X.; Liu, R.; Ma, F.; Yu, M.; Liu, H.; Dai, J. Effect of Altered Retinal Cones/Opsins on Refractive Development under Monochromatic Lights in Guinea Pigs. J. Ophthalmol. 2018, 2018, 9197631. [Google Scholar] [CrossRef]
- Shiels, A.; Hejtmancik, J.F. Biology of Inherited Cataracts and Opportunities for Treatment. Annu. Rev. Vis. Sci. 2019, 5, 123–149. [Google Scholar] [CrossRef]
- Beebe, D.C.; Holekamp, N.M.; Shui, Y.B. Oxidative damage and the prevention of age-related cataracts. Ophthalmic Res. 2010, 44, 155–165. [Google Scholar] [CrossRef] [Green Version]
- Von Sallmann, L.; Grimes, P. Effect of age on cell division, 3H-thymidine incorporation, and diurnal rhythm in the lens epithelium of rats. Investig. Ophthalmol. 1966, 5, 560–567. [Google Scholar]
- Brewitt, B.; Talian, J.C.; Zelenka, P.S. Cell cycle synchrony in the developing chicken lens epithelium. Dev. Biol. 1992, 152, 315–322. [Google Scholar] [CrossRef]
- Erichsen, J.H.; Brøndsted, A.E.; Kessel, L. Effect of cataract surgery on regulation of circadian rhythms. J. Cataract Refract. Surg. 2015, 41, 1997–2009. [Google Scholar] [CrossRef] [PubMed]
- Green, C.B.; Besharse, J.C. Retinal circadian clocks and control of retinal physiology. J. Biol. Rhythms 2004, 19, 91–102. [Google Scholar] [CrossRef]
- Wiechmann, A.F.; Smith, A.R. Melatonin receptor RNA is expressed in photoreceptors and displays a diurnal rhythm in Xenopus retina. Brain Res. Mol. Brain Res. 2001, 91, 104–111. [Google Scholar] [CrossRef]
- Klettner, A.; Kampers, M.; Töbelmann, D.; Roider, J.; Dittmar, M. The Influence of Melatonin and Light on VEGF Secretion in Primary RPE Cells. Biomolecules 2021, 11, 114. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, T.; Sieving, P.A.; Iuvone, P.M.; Bush, R.A. The melatonin antagonist luzindole protects retinal photoreceptors from light damage in the rat. Investig. Ophthalmol. Vis. Sci. 1998, 39, 2458–2465. [Google Scholar]
- Liang, F.Q.; Green, L.; Wang, C.; Alssadi, R.; Godley, B.F. Melatonin protects human retinal pigment epithelial (RPE) cells against oxidative stress. Exp. Eye Res. 2004, 78, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Rosen, R.B.; Hu, D.N.; Chen, M.; McCormick, S.A.; Walsh, J.; Roberts, J.E. Effects of melatonin and its receptor antagonist on retinal pigment epithelial cells against hydrogen peroxide damage. Mol. Vis. 2012, 18, 1640–1648. [Google Scholar] [PubMed]
- Chang, C.C.; Huang, T.Y.; Chen, H.Y.; Huang, T.C.; Lin, L.C.; Chang, Y.J.; Hsia, S.M. Protective Effect of Melatonin against Oxidative Stress-Induced Apoptosis and Enhanced Autophagy in Human Retinal Pigment Epithelium Cells. Oxid. Med. Cell Longev. 2018, 2018, 9015765. [Google Scholar] [CrossRef]
- Do Carmo Buonfiglio, D.; Peliciari-Garcia, R.A.; do Amaral, F.G.; Peres, R.; Nogueira, T.C.; Afeche, S.C.; Cipolla-Neto, J. Early-stage retinal melatonin synthesis impairment in streptozotocin-induced diabetic wistar rats. Investig. Ophthalmol. Vis. Sci. 2011, 52, 7416–7422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hikichi, T.; Tateda, N.; Miura, T. Alteration of melatonin secretion in patients with type 2 diabetes and proliferative diabetic retinopathy. Clin. Ophthalmol. 2011, 5, 655–660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozdemir, G.; Ergün, Y.; Bakariş, S.; Kılınç, M.; Durdu, H.; Ganiyusufoğlu, E. Melatonin prevents retinal oxidative stress and vascular changes in diabetic rats. Eye 2014, 28, 1020–1027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, T.; Chang, Q.; Cai, J.; Fan, J.; Zhang, X.; Xu, G. Protective Effects of Melatonin on Retinal Inflammation and Oxidative Stress in Experimental Diabetic Retinopathy. Oxid. Med. Cell Longev. 2016, 2016, 3528274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergua, A.; Mardin, C.Y.; Horn, F.K. Tele-transmission of stereoscopic images of the optic nerve head in glaucoma via internet. Telemed. e-Health 2009, 15, 439–444. [Google Scholar] [CrossRef]
- Tham, Y.C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef] [PubMed]
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The pathophysiology and treatment of glaucoma: A review. JAMA 2014, 311, 1901–1911. [Google Scholar] [CrossRef] [Green Version]
- Friedman, D.S.; Wilson, M.R.; Liebmann, J.M.; Fechtner, R.D.; Weinreb, R.N. An evidence-based assessment of risk factors for the progression of ocular hypertension and glaucoma. Am. J. Ophthalmol. 2004, 138, S19–S31. [Google Scholar] [CrossRef] [PubMed]
- Topouzis, F.; Harris, A.; Wilson, M.R.; Koskosas, A.; Founti, P.; Yu, F.; Anastasopoulos, E.; Pappas, T.; Coleman, A.L. Increased likelihood of glaucoma at the same screening intraocular pressure in subjects with pseudoexfoliation: The Thessaloniki Eye Study. Am. J. Ophthalmol. 2009, 148, 606–613.e601. [Google Scholar] [CrossRef]
- Mwanza, J.C.; Tulenko, S.E.; Barton, K.; Herndon, L.W.; Mathenge, E.; Hall, A.; Kim, H.Y.; Hay-Smith, G.; Budenz, D.L. Eight-Year Incidence of Open-Angle Glaucoma in the Tema Eye Survey. Ophthalmology 2019, 126, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K.; Niranjan, D.G.; Agrawal, S.S.; Srivastava, S.; Saxena, R. Recent advances in pharmacotherapy of glaucoma. Indian J. Pharmacol. 2008, 40, 197–208. [Google Scholar] [CrossRef]
- Boland, M.V.; Ervin, A.M.; Friedman, D.S.; Jampel, H.D.; Hawkins, B.S.; Vollenweider, D.; Chelladurai, Y.; Ward, D.; Suarez-Cuervo, C.; Robinson, K.A. Comparative effectiveness of treatments for open-angle glaucoma: A systematic review for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2013, 158, 271–279. [Google Scholar] [CrossRef]
- Davson, H. Aqueous humour and the intraocular pressure. In Physiology of the Eye, 4th ed.; Davson, H., Ed.; Academic Press: Cambridge, MA, USA, 1980; pp. 9–81. [Google Scholar]
- Quaranta, L.; Katsanos, A.; Russo, A.; Riva, I. 24-h intraocular pressure and ocular perfusion pressure in glaucoma. Surv. Ophthalmol. 2013, 58, 26–41. [Google Scholar] [CrossRef] [PubMed]
- McCannel, C.; Koskela, T.; Brubaker, R.F. Topical flurbiprofen pretreatment does not block apraclonidine’s effect on aqueous flow in humans. Arch. Ophthalmol. 1991, 109, 810–811. [Google Scholar] [CrossRef]
- Gabelt, B.T.; Kaufman, P.L. Changes in aqueous humor dynamics with age and glaucoma. Prog. Retin. Eye Res. 2005, 24, 612–637. [Google Scholar] [CrossRef]
- Larsson, L.I.; Rettig, E.S.; Brubaker, R.F. Aqueous flow in open-angle glaucoma. Arch. Ophthalmol. 1995, 113, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Renard, E.; Palombi, K.; Gronfier, C.; Pepin, J.L.; Noel, C.; Chiquet, C.; Romanet, J.P. Twenty-four hour (Nyctohemeral) rhythm of intraocular pressure and ocular perfusion pressure in normal-tension glaucoma. Investig. Ophthalmol. Vis. Sci. 2010, 51, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Forrester, J.V.; Dick, A.D.; McMenamin, P.G.; Roberts, F.; Pearlman, E. Chapter 1—Anatomy of the eye and orbit. In The Eye, 4th ed.; Forrester, J.V., Dick, A.D., McMenamin, P.G., Roberts, F., Pearlman, E., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2016; pp. 1–102. [Google Scholar]
- Liu, H.; Fan, S.; Gulati, V.; Camras, L.J.; Zhan, G.; Ghate, D.; Camras, C.B.; Toris, C.B. Aqueous humor dynamics during the day and night in healthy mature volunteers. Arch. Ophthalmol. 2011, 129, 269–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becker, B. The decline in aqueous secretion and outflow facility with age. Am. J. Ophthalmol. 1958, 46, 731–736. [Google Scholar] [CrossRef]
- Toris, C.B.; Yablonski, M.E.; Wang, Y.L.; Camras, C.B. Aqueous humor dynamics in the aging human eye. Am. J. Ophthalmol. 1999, 127, 407–412. [Google Scholar] [CrossRef]
- Brubaker, R.F. Flow of aqueous humor in humans [The Friedenwald Lecture]. Investig. Ophthalmol. Vis. Sci. 1991, 32, 3145–3166. [Google Scholar]
- Alkozi, H.A.; Navarro, G.; Franco, R.; Pintor, J. Melatonin and the control of intraocular pressure. Prog. Retin. Eye Res. 2020, 75, 100798. [Google Scholar] [CrossRef]
- Waldhauser, F.; Weiszenbacher, G.; Tatzer, E.; Gisinger, B.; Waldhauser, M.; Schemper, M.; Frisch, H. Alterations in nocturnal serum melatonin levels in humans with growth and aging. J. Clin. Endocrinol. Metab. 1988, 66, 648–652. [Google Scholar] [CrossRef] [PubMed]
- Grippo, T.M.; Liu, J.H.; Zebardast, N.; Arnold, T.B.; Moore, G.H.; Weinreb, R.N. Twenty-four-hour pattern of intraocular pressure in untreated patients with ocular hypertension. Investig. Ophthalmol. Vis. Sci. 2013, 54, 512–517. [Google Scholar] [CrossRef] [Green Version]
- O’Brien, E.; Murphy, J.; Tyndall, A.; Atkins, N.; Mee, F.; McCarthy, G.; Staessen, J.; Cox, J.; O’Malley, K. Twenty-four-hour ambulatory blood pressure in men and women aged 17 to 80 years: The Allied Irish Bank Study. J. Hypertens. 1991, 9, 355–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.H.; Zhang, X.; Kripke, D.F.; Weinreb, R.N. Twenty-four-hour intraocular pressure pattern associated with early glaucomatous changes. Investig. Ophthalmol. Vis. Sci. 2003, 44, 1586–1590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Staessen, J.A.; Fagard, R.H.; Lijnen, P.J.; Thijs, L.; Van Hoof, R.; Amery, A.K. Mean and range of the ambulatory pressure in normotensive subjects from a meta-analysis of 23 studies. Am. J. Cardiol. 1991, 67, 723–727. [Google Scholar] [CrossRef]
- Millar-Craig, M.W.; Bishop, C.N.; Raftery, E.B. Circadian variation of blood-pressure. Lancet 1978, 1, 795–797. [Google Scholar] [CrossRef]
- Graham, S.L.; Drance, S.M. Nocturnal hypotension: Role in glaucoma progression. Surv. Ophthalmol. 1999, 43 (Suppl. 1), S10–S16. [Google Scholar] [CrossRef]
- Leske, M.C.; Heijl, A.; Hyman, L.; Bengtsson, B. Early Manifest Glaucoma Trial: Design and baseline data. Ophthalmology 1999, 106, 2144–2153. [Google Scholar] [CrossRef]
- Wong, T.Y.; Mitchell, P. The eye in hypertension. Lancet 2007, 369, 425–435. [Google Scholar] [CrossRef]
- Hayreh, S.S. Role of nocturnal arterial hypotension in the development of ocular manifestations of systemic arterial hypertension. Curr. Opin. Ophthalmol. 1999, 10, 474–482. [Google Scholar] [CrossRef]
- Sehi, M.; Flanagan, J.G.; Zeng, L.; Cook, R.J.; Trope, G.E. Relative change in diurnal mean ocular perfusion pressure: A risk factor for the diagnosis of primary open-angle glaucoma. Investig. Ophthalmol. Vis. Sci. 2005, 46, 561–567. [Google Scholar] [CrossRef]
- Liu, J.H.; Gokhale, P.A.; Loving, R.T.; Kripke, D.F.; Weinreb, R.N. Laboratory assessment of diurnal and nocturnal ocular perfusion pressures in humans. J. Ocul. Pharmacol. Ther. 2003, 19, 291–297. [Google Scholar] [CrossRef]
- Choi, J.; Jeong, J.; Cho, H.S.; Kook, M.S. Effect of nocturnal blood pressure reduction on circadian fluctuation of mean ocular perfusion pressure: A risk factor for normal tension glaucoma. Investig. Ophthalmol. Vis. Sci. 2006, 47, 831–836. [Google Scholar] [CrossRef] [PubMed]
- Sehi, M.; Flanagan, J.G.; Zeng, L.; Cook, R.J.; Trope, G.E. Anterior optic nerve capillary blood flow response to diurnal variation of mean ocular perfusion pressure in early untreated primary open-angle glaucoma. Investig. Ophthalmol. Vis. Sci. 2005, 46, 4581–4587. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Kim, K.H.; Jeong, J.; Cho, H.S.; Lee, C.H.; Kook, M.S. Circadian fluctuation of mean ocular perfusion pressure is a consistent risk factor for normal-tension glaucoma. Investig. Ophthalmol. Vis. Sci. 2007, 48, 104–111. [Google Scholar] [CrossRef]
- Sung, K.R.; Lee, S.; Park, S.B.; Choi, J.; Kim, S.T.; Yun, S.C.; Kang, S.Y.; Cho, J.W.; Kook, M.S. Twenty-four hour ocular perfusion pressure fluctuation and risk of normal-tension glaucoma progression. Investig. Ophthalmol. Vis. Sci. 2009, 50, 5266–5274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wurtman, R.J.; Axelrod, J.; Phillips, L.S. Melatonin Synthesis in the Pineal Gland: Control by Light. Science 1963, 142, 1071–1073. [Google Scholar] [CrossRef]
- Ma, X.P.; Shen, M.Y.; Shen, G.L.; Qi, Q.R.; Sun, X.H. Melatonin concentrations in serum of primary glaucoma patients. Int. J. Ophthalmol. 2018, 11, 1337–1341. [Google Scholar] [CrossRef] [PubMed]
- Pintor, J.; Martin, L.; Pelaez, T.; Hoyle, C.H.; Peral, A. Involvement of melatonin MT(3) receptors in the regulation of intraocular pressure in rabbits. Eur. J. Pharmacol. 2001, 416, 251–254. [Google Scholar] [CrossRef]
- Belforte, N.A.; Moreno, M.C.; de Zavalia, N.; Sande, P.H.; Chianelli, M.S.; Keller Sarmiento, M.I.; Rosenstein, R.E. Melatonin: A novel neuroprotectant for the treatment of glaucoma. J. Pineal. Res. 2010, 48, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Aguila, A.; Fonseca, B.; Bergua, A.; Pintor, J. Melatonin analogue agomelatine reduces rabbit’s intraocular pressure in normotensive and hypertensive conditions. Eur. J. Pharmacol. 2013, 701, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Pescosolido, N.; Gatto, V.; Stefanucci, A.; Rusciano, D. Oral treatment with the melatonin agonist agomelatine lowers the intraocular pressure of glaucoma patients. Ophthalmic Physiol. Opt. 2015, 35, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Ismail, S.A.; Mowafi, H.A. Melatonin provides anxiolysis, enhances analgesia, decreases intraocular pressure, and promotes better operating conditions during cataract surgery under topical anesthesia. Anesth. Analg. 2009, 108, 1146–1151. [Google Scholar] [CrossRef] [PubMed]
- Carracedo-Rodríguez, G.; Martínez-Águila, A.; Rodriguez-Pomar, C.; Bodas-Romero, J.; Sanchez-Naves, J.; Pintor, J. Effect of nutritional supplement based on melatonin on the intraocular pressure in normotensive subjects. Int. Ophthalmol. 2020, 40, 419–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blumenthal, E.Z.; Weinreb, R.N. Assessment of the retinal nerve fiber layer in clinical trials of glaucoma neuroprotection. Surv. Ophthalmol. 2001, 45 (Suppl. 3), S305–S312. [Google Scholar] [CrossRef]
- Drouyer, E.; Dkhissi-Benyahya, O.; Chiquet, C.; WoldeMussie, E.; Ruiz, G.; Wheeler, L.A.; Denis, P.; Cooper, H.M. Glaucoma alters the circadian timing system. PLoS ONE 2008, 3, e3931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mojon, D.S.; Hess, C.W.; Goldblum, D.; Bohnke, M.; Korner, F.; Mathis, J. Primary open-angle glaucoma is associated with sleep apnea syndrome. Ophthalmologica 2000, 214, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Jean-Louis, G.; Zizi, F.; Lazzaro, D.R.; Wolintz, A.H. Circadian rhythm dysfunction in glaucoma: A hypothesis. J. Circadian Rhythms 2008, 6, 1. [Google Scholar] [CrossRef] [Green Version]
- Gubin, D.G.; Malishevskaya capital Te, C.; Astakhov, Y.S.; Astakhov, S.Y.; Cornelissen, G.; Kuznetsov, V.A.; Weinert, D. Progressive retinal ganglion cell loss in primary open-angle glaucoma is associated with temperature circadian rhythm phase delay and compromised sleep. Chronobiol. Int. 2019, 36, 564–577. [Google Scholar] [CrossRef]
- Mabuchi, F.; Yoshimura, K.; Kashiwagi, K.; Shioe, K.; Yamagata, Z.; Kanba, S.; Iijima, H.; Tsukahara, S. High prevalence of anxiety and depression in patients with primary open-angle glaucoma. J. Glaucoma 2008, 17, 552–557. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Y.; Ding, J.; Wang, N. Changes in the circadian rhythm in patients with primary glaucoma. PLoS ONE 2013, 8, e62841. [Google Scholar] [CrossRef] [Green Version]
- Guo, Z.Z.; Jiang, S.M.; Zeng, L.P.; Tang, L.; Li, N.; Xu, Z.P.; Wei, X. ipRGCs: Possible causation accounts for the higher prevalence of sleep disorders in glaucoma patients. Int. J. Ophthalmol. 2017, 10, 1163–1167. [Google Scholar] [CrossRef] [PubMed]
- Agorastos, A.; Skevas, C.; Matthaei, M.; Otte, C.; Klemm, M.; Richard, G.; Huber, C.G. Depression, anxiety, and disturbed sleep in glaucoma. J. Neuropsychiatry Clin. Neurosci. 2013, 25, 205–213. [Google Scholar] [CrossRef]
- Skalicky, S.; Goldberg, I. Depression and quality of life in patients with glaucoma: A cross-sectional analysis using the Geriatric Depression Scale-15, assessment of function related to vision, and the Glaucoma Quality of Life-15. J. Glaucoma 2008, 17, 546–551. [Google Scholar] [CrossRef]
- Hasler, B.P.; Buysse, D.J.; Kupfer, D.J.; Germain, A. Phase relationships between core body temperature, melatonin, and sleep are associated with depression severity: Further evidence for circadian misalignment in non-seasonal depression. Psychiatry Res. 2010, 178, 205–207. [Google Scholar] [CrossRef] [Green Version]
- Kellner, M.; Yassouridis, A.; Manz, B.; Steiger, A.; Holsboer, F.; Wiedemann, K. Corticotropin-releasing hormone inhibits melatonin secretion in healthy volunteers—A potential link to low-melatonin syndrome in depression? Neuroendocrinology 1997, 65, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.A.; Marcu, S.; Kayumov, L.; Shapiro, C.M. Altered sleep architecture and higher incidence of subsyndromal depression in low endogenous melatonin secretors. Eur. Arch. Psychiatry. Clin. Neurosci. 2010, 260, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Torres-Farfan, C.; Richter, H.G.; Rojas-Garcia, P.; Vergara, M.; Forcelledo, M.L.; Valladares, L.E.; Torrealba, F.; Valenzuela, G.J.; Seron-Ferre, M. mt1 Melatonin receptor in the primate adrenal gland: Inhibition of adrenocorticotropin-stimulated cortisol production by melatonin. J. Clin. Endocrinol. Metab. 2003, 88, 450–458. [Google Scholar] [CrossRef] [Green Version]
- Gillespie, C.F.; Nemeroff, C.B. Hypercortisolemia and depression. Psychosom. Med. 2005, 67 (Suppl. 1), S26–S28. [Google Scholar] [CrossRef]
- Chiou, G.C.; McLaughlin, M.A. Studies on the involvement of melatonergic mechanism in intraocular pressure regulation. Ophthalmic Res. 1984, 16, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Huete-Toral, F.; Crooke, A.; Martínez-Águila, A.; Pintor, J. Melatonin receptors trigger cAMP production and inhibit chloride movements in nonpigmented ciliary epithelial cells. J. Pharmacol. Exp. Ther. 2015, 352, 119–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pintor, J.; Peláez, T.; Hoyle, C.H.; Peral, A. Ocular hypotensive effects of melatonin receptor agonists in the rabbit: Further evidence for an MT 3 receptor. Br. J. Pharmacol. 2003, 138, 831–836. [Google Scholar] [CrossRef] [Green Version]
- Crooke, A.; Huete-Toral, F.; Martínez-Águila, A.; Martín-Gil, A.; Pintor, J. Involvement of carbonic anhydrases in the ocular hypotensive effect of melatonin analogue 5-MCA-NAT. J. Pineal Res. 2012, 52. [Google Scholar] [CrossRef] [PubMed]
- Conway, S.; Canning, S.J.; Howell, H.E.; Mowat, E.S.; Barrett, P.; Drew, J.E.; Delagrange, P.; Lesieur, D.; Morgan, P.J. Characterisation of human melatonin mt1 and MT2 receptors by CRE-luciferase reporter assay. Eur. J. Pharmacol. 2000, 390, 15–24. [Google Scholar] [CrossRef]
- Millan, M.J.; Gobert, A.; Lejeune, F.; Dekeyne, A.; Newman-Tancredi, A.; Pasteau, V.; Rivet, J.M.; Cussac, D. The novel melatonin agonist agomelatine (S20098) is an antagonist at 5-hydroxytryptamine2C receptors, blockade of which enhances the activity of frontocortical dopaminergic and adrenergic pathways. J. Pharmacol. Exp. Ther. 2003, 306, 954–964. [Google Scholar] [CrossRef] [Green Version]
- De Berardis, D.; Di Iorio, G.; Acciavatti, T.; Conti, C.; Serroni, N.; Olivieri, L.; Cavuto, M.; Martinotti, G.; Janiri, L.; Saverio Moschetta, F.; et al. The Emerging Role of Melatonin Agonists in the Treatment of Major Depression: Focus on Agomelatine. CNS Neurol. Disord. Drug Targets 2011, 10, 119–132. [Google Scholar] [CrossRef]
- Meyer, W. Trendelenburg position. Arch. Klin. Chir. 1885, 31, 495–525. [Google Scholar]
- Anderson, M.G.; Smith, R.S.; Hawes, N.L.; Zabaleta, A.; Chang, B.; Wiggs, J.L.; John, S.W. Mutations in genes encoding melanosomal proteins cause pigmentary glaucoma in DBA/2J mice. Nat. Genet. 2002, 30, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Águila, A.; Fonseca, B.; Pérez de Lara, M.J.; Pintor, J. Assessment of inner retina dysfunction and progressive ganglion cell loss in a mouse model of glaucoma. Exp. Eye Res. 2014, 122, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Águila, A.; Fonseca, B.; Pérez de Lara, M.J.; Pintor, J. Effect of Melatonin and 5-Methoxycarbonylamino-N-Acetyltryptamine on the Intraocular Pressure of Normal and Glaucomatous Mice. J. Pharmacol. Exp. Ther. 2016, 357, 293–299. [Google Scholar] [CrossRef] [Green Version]
- Samples, J.R.; Krause, G.; Lewy, A.J. Effect of melatonin on intraocular pressure. Curr. Eye Res. 1988, 7, 649–653. [Google Scholar] [CrossRef] [PubMed]
- Sakanaka, K.; Kawazu, K.; Tomonari, M.; Kitahara, T.; Nakashima, M.; Nishida, K.; Nakamura, J.; Sasaki, H.; Higuchi, S. Ocular pharmacokinetic/pharmacodynamic modeling for multiple anti-glaucoma drugs. Biol. Pharm. Bull. 2008, 31, 1590–1595. [Google Scholar] [CrossRef] [Green Version]
- Yu, Z.; Yanxia, H.; Limin, G.; Yun, Z.; Mingxuan, Z.; Fuyao, X.; Cheng, T.; Jufang, H.; Dan, C. Melatonin alleviates pyroptosis of retinal neurons following acute intraocular hypertension. CNS Neurol. Disord. Drug Targets 2020. [Google Scholar] [CrossRef]
- Park, S.W.; Lee, H.S.; Sung, M.S.; Kim, S.J. The effect of melatonin on retinal ganglion cell survival in ischemic retina. Chonnam Med. J. 2012, 48, 116–122. [Google Scholar] [CrossRef] [Green Version]
- Ayoub, M.A.; Couturier, C.; Lucas-Meunier, E.; Angers, S.; Fossier, P.; Bouvier, M.; Jockers, R. Monitoring of ligand-independent dimerization and ligand-induced conformational changes of melatonin receptors in living cells by bioluminescence resonance energy transfer. J. Biol. Chem. 2002, 277, 21522–21528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayoub, M.A.; Levoye, A.; Delagrange, P.; Jockers, R. Preferential formation of MT1/MT2 melatonin receptor heterodimers with distinct ligand interaction properties compared with MT 2 homodimers. Mol. Pharmacol. 2004, 66, 312–321. [Google Scholar] [CrossRef]
- Kamal, M.; Gbahou, F.; Guillaume, J.L.; Daulat, A.M.; Benleulmi-Chaachoua, A.; Luka, M.; Chen, P.; Anaraki, D.K.; Baroncini, M.; la Cour, C.M.; et al. Convergence of melatonin and serotonin (5-HT) signaling at MT2/5-HT2C receptor heteromers. J. Biol. Chem. 2015, 290, 11537–11546. [Google Scholar] [CrossRef] [Green Version]
- Alkozi, H.A.; Navarro, G.; Aguinaga, D.; Reyes-Resina, I.; Sanchez-Naves, J.; Pérez de Lara, M.J.; Franco, R.; Pintor, J. Adreno-melatonin receptor complexes control ion homeostasis and intraocular pressure - their disruption contributes to hypertensive glaucoma. Br. J. Pharmacol. 2020, 177, 2090–2105. [Google Scholar] [CrossRef]
- Crooke, A.; Huete-Toral, F.; Martínez-Águila, A.; Martín-Gil, A.; Pintor, J. Melatonin and its analog 5-methoxycarbonylamino-N-acetyltryptamine potentiate adrenergic receptor-mediated ocular hypotensive effects in rabbits: Significance for combination therapy in glaucoma. J. Pharmacol. Exp. Ther. 2013, 346, 138–145. [Google Scholar] [CrossRef] [Green Version]
- Serle, J.B.; Wang, R.F.; Peterson, W.M.; Plourde, R.; Yerxa, B.R. Effect of 5-MCA-NAT, a putative melatonin MT3 receptor agonist, on intraocular pressure in glaucomatous monkey eyes. J. Glaucoma 2004, 13, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Grzybowski, A.; Kanclerz, P.; Tsubota, K.; Lanca, C.; Saw, S.M. A review on the epidemiology of myopia in school children worldwide. BMC Ophthalmol. 2020, 20, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Troilo, D.; Smith, E.L.; Nickla, D.L.; Ashby, R.; Tkatchenko, A.V.; Ostrin, L.A.; Gawne, T.J.; Pardue, M.T.; Summers, J.A.; Kee, C.S.; et al. IMI—Report on Experimental Models of Emmetropization and Myopia. Investig. Ophthalmol. Vis. Sci. 2019, 60, M31–M88. [Google Scholar] [CrossRef] [Green Version]
- Mao, J.; Liu, S.; Qin, W.; Li, F.; Wu, X.; Tan, Q. Levodopa inhibits the development of form-deprivation myopia in guinea pigs. Optom. Vis. Sci. 2010, 87, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, M.; Coon, S.L.; Klein, D.C. RGS2 is a feedback inhibitor of melatonin production in the pineal gland. FEBS Lett. 2013, 587, 1392–1398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, L.; Liu, R.; Zhang, X.; Chu, R.; Dai, J.; Zhou, H.; Liu, H. Upregulation of regulator of G-protein signaling 2 in the sclera of a form deprivation myopic animal model. Mol. Vis. 2014, 20, 977–987. [Google Scholar] [PubMed]
- Kim, Y.W.; Kim, J.S.; Lee, S.Y.; Ha, A.; Lee, J.; Park, Y.J.; Kim, Y.K.; Jeoung, J.W.; Park, K.H. Twenty-four-Hour Intraocular Pressure-Related Patterns from Contact Lens Sensors in Normal-Tension Glaucoma and Healthy Eyes: The Exploring Nyctohemeral Intraocular pressure related pattern for Glaucoma Management (ENIGMA) Study. Ophthalmology 2020, 127, 1487–1497. [Google Scholar] [CrossRef]
| Ocular Pathology | Reference | Compound | Target | Results |
|---|---|---|---|---|
| Dry eye | Navarro Gil et al. [79] | Melatonin and analogs (Agomelatine, IIK7, and 5-MCA-NAT) | Rabbits | The topical instillation of analogs of melatonin increased tears secretion: 39% with agomelatine (MT2 receptor), 28% with IIK7 (MT2 receptor), and 20% with 5-MCA-NAT (unknown receptors). Conversely, there were no changes with the melatonin. |
| Hoyle et al. [80] | Ap4A + Melatonin | Rabbits | The topical instillation of Ap4A increased tear secretion by 10%, while melatonin showed no effect. The synergic effect of both molecules increased tear secretion by 34%. | |
| Corneal wound healing | Crooke et al. [47] | Melatonin and analogs (IIK7 and 5-MCA-NAT) | Rabbits | The topical instillation of melatonin and IIK7 improved rate of corneal healing by around 47%, while 5-MCA-NAT showed no effect. This effect is mediated by the MT2 receptor, which is present in corneal epithelial cells. |
| Crespo-Moral et al. [81] | Melatonin | Ex vivo Porcine eyeball | The topical exposition of melatonin in an ex vivo corneal-wound model accelerated the healing process. Notably, 60 µg/mL melatonin accelerated the healing rate during 48 h and 90 µg/mL melatonin during 72 h. Unexpectedly, 120 µg/mL melatonin decreased the healing rate after 96 h. | |
| Myopia | Wang et al. [82] | Blue light | Guinea pigs | The retinal stimulation with blue light (480 nm) reduced eye growth, which was related to stimulate synthesis of melanopsin in retina and sclera, and to reduce both synthesis of MT1 receptor and production of melatonin in pineal gland. |
| Zheng et al. [83] | AA92593 (melanopsin antagonist) | Guinea pigs | The retinal melanopsin inhibition by intravitreal injection of an antagonist AA92593 increased both the eye growth and melatonin levels in the retina, these variables being directly correlated (r = 0.74). | |
| Cataracts | Different authors [84,85,86,87,88,89,90] | Melatonin | Rats | The use of melatonin in rat models reduced lipid peroxidation and promoted both the synthesis of glutathione and antioxidative activity of different enzymes, leading to a reduction in cataract formation. |
| Pintor et al. [91] | Yellow light and AA92593 | Rabbits | The inhibition of melanopsin present in the lens epithelium with a yellow filter (absorbance between 465-480 nm) and its antagonist AA92593 reduced the concentration of ATP in the aqueous humour. | |
| Retinal damage | Liang et al. [92] | Melatonin | Mice | The muscular injection of melatonin reduced damage of photoreceptors and their apoptosis in a retinal degeneration model. |
| Kaur et al. [93] | Melatonin | Rats | The intraperitoneal injection of melatonin reduced levels of VEGF, nitrite, and melatonin in the retina of hypoxic rats, also reducing retinal vascular permeability. | |
| Age-related macular degeneration | Dieguez et al. [94] | Melatonin | Mice | The antioxidant effect of subcutaneous implantation of a pellet of melatonin helped to preserve visual function and retinal structures in a non-exudative AMD model. |
| Yi et al. [95] | Melatonin | Humans | In this case series study, the daily oral administration of 3 mg melatonin for 3 months improved the signs of AMD in terms of retinal blood and retinal exudates in more than 90% of patients. |
| Compound | Animal Model/ Patients | Results | Reference |
|---|---|---|---|
| Melatonin | New Zealand white rabbits (NZWR) | 22.0% reduction | Pintor et al. [228] |
| Hypertensive NZWR | 43.6% reduction | Martinez-Águila et al. [207] | |
| Control (C57BL/6J) and glaucomatous mice (DBA/2J) | 19.4 normotensive vs 32.6% glaucomatous mice | Martinez-Águila et al. [236] | |
| Normotensive patients | 17% reduction with 0.5 mg; From 17.9 to 13.8 mmHg (23%) with 10 mg; From 15.7 to 14.7 mmHg (7%) with 1 mg. | Samples et al. [237]; Ismail et al. [209] and Carracedo et al. [210] | |
| Agomelatine | New Zealand white rabbits (NZWR) | 20.8% reduction | Martinez-Águila et al. [207] |
| Hypertensive NZWR | 68.8% reduction | Martinez-Águila et al. [207] | |
| Glaucomatous patients | From 23.4 to 14.3 mmHg (34%) with 25 mg | Pescosolido et al. [208] | |
| 5-MCA-NAT | New Zealand white rabbits (NZWR) | 42.5% reduction | Pintor et al. [228] |
| Hypertensive NZWR | 85.6% reduction | Martinez-Águila et al. [207] | |
| Control (C57BL/6J) and glaucomatous mice (DBA/2J) | 20.7% normotensive vs 29.3% glaucomatous mice. 13% reduction with 3 months treatment vs placebo | Martinez-Águila et al. [236] | |
| Glaucomatous monkeys (Macaca fascicularis) | 7.0 mmHg (19%) reduction | Serle et al. [246] | |
| Other melatonin analogs | New Zealand white rabbits (NZWR) | N-Acetyltryptamine 6.0%, 6-chloromelatonin 9.0%, 1-Iodomelatonin 9.0% and 2-phenylmelatonin 8.7% reduction | Pintor et al. [228] |
| Melatonin + Prazosin | Glaucomatous mice (DBA/2J) | From 16.6 to 9.0 mmHg (46%) reduction | Hanan et al. [244] |
| 5-MCA-NAT + Anhydrases | New Zealand white rabbits (NZWR) | 5-MCA-NAT increase dorzolamide effect by 45% vs 32% dorzolamide only for 3 days. | Crooke et al. [229] |
| Melatonin + adrenergic compounds | New Zealand white rabbits (NZWR) | Melatonin increase brimonidine effect by 29.3% vs 40.6% brimonidine only and 5-MCA-NAT 39.1%, for 4 days. Melatonin increase timolol effect by 39.8% vs 25.8% timolol only and 5-MCA-NAT 42.6% only one day. | Crooke et al. [245] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Águila, A.; Martín-Gil, A.; Carpena-Torres, C.; Pastrana, C.; Carracedo, G. Influence of Circadian Rhythm in the Eye: Significance of Melatonin in Glaucoma. Biomolecules 2021, 11, 340. https://doi.org/10.3390/biom11030340
Martínez-Águila A, Martín-Gil A, Carpena-Torres C, Pastrana C, Carracedo G. Influence of Circadian Rhythm in the Eye: Significance of Melatonin in Glaucoma. Biomolecules. 2021; 11(3):340. https://doi.org/10.3390/biom11030340
Chicago/Turabian StyleMartínez-Águila, Alejandro, Alba Martín-Gil, Carlos Carpena-Torres, Cristina Pastrana, and Gonzalo Carracedo. 2021. "Influence of Circadian Rhythm in the Eye: Significance of Melatonin in Glaucoma" Biomolecules 11, no. 3: 340. https://doi.org/10.3390/biom11030340
APA StyleMartínez-Águila, A., Martín-Gil, A., Carpena-Torres, C., Pastrana, C., & Carracedo, G. (2021). Influence of Circadian Rhythm in the Eye: Significance of Melatonin in Glaucoma. Biomolecules, 11(3), 340. https://doi.org/10.3390/biom11030340









