Cardiovascular “Patterns” of H2S and SSNO−-Mix Evaluated from 35 Rat Hemodynamic Parameters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Ethical Approval
2.3. Animals, APW Measurement and Data Evaluation
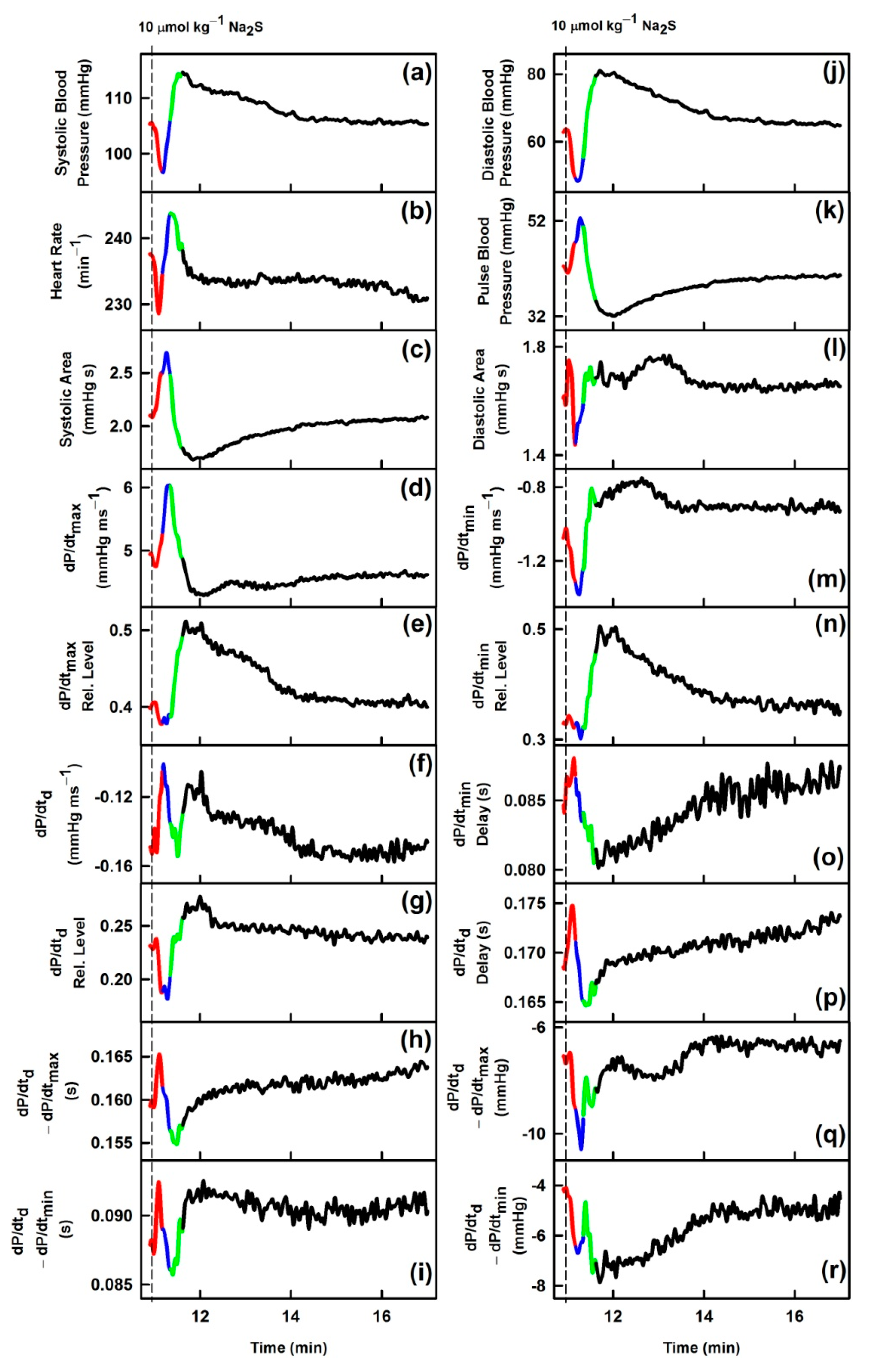
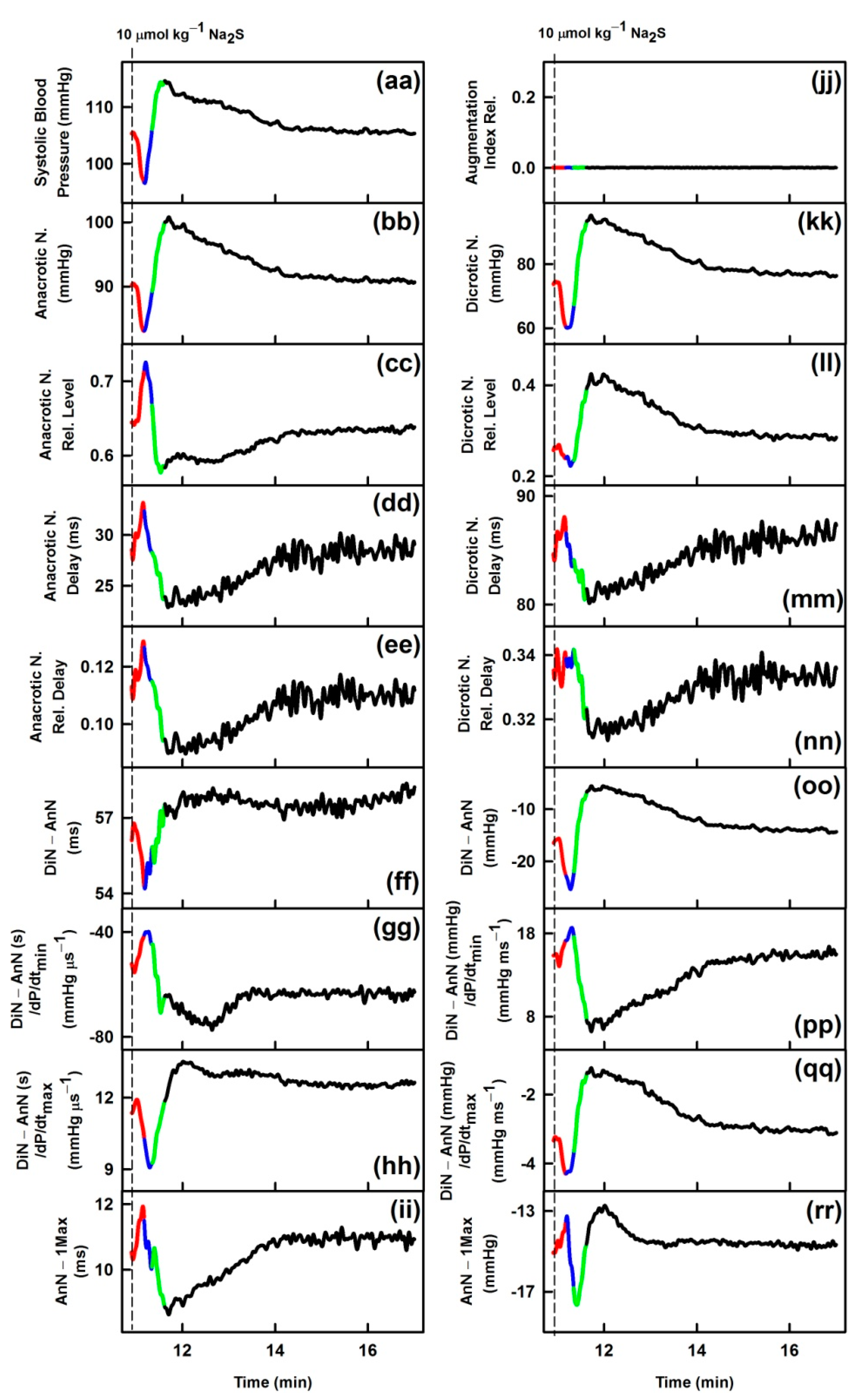
3. Results
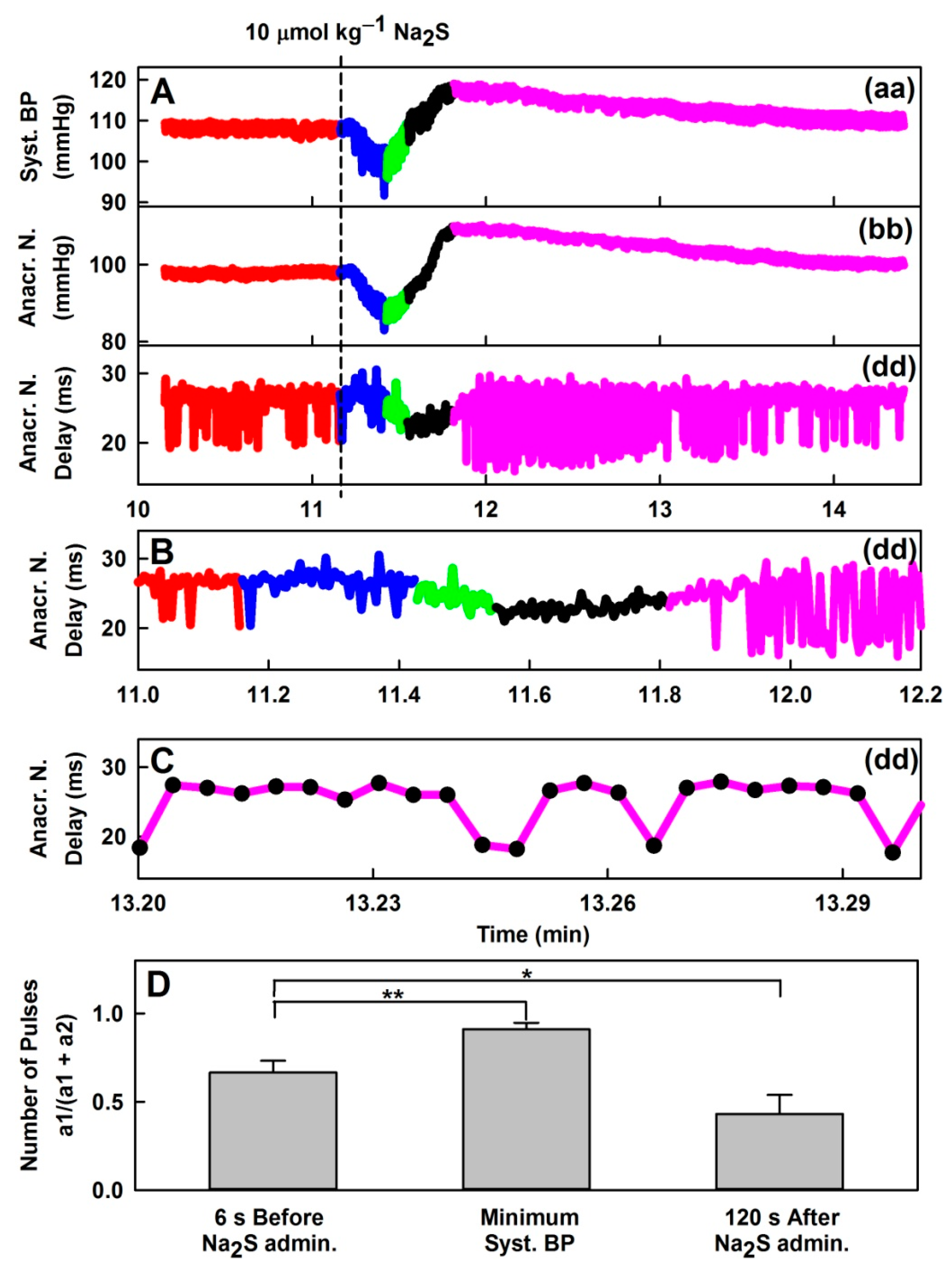
3.1. Effects of Na2S on Time-Dependent Changes of 35 HPs
3.2. Comparison of Transient Effects of Na2S with Published Data of GSNO on 35 HPs
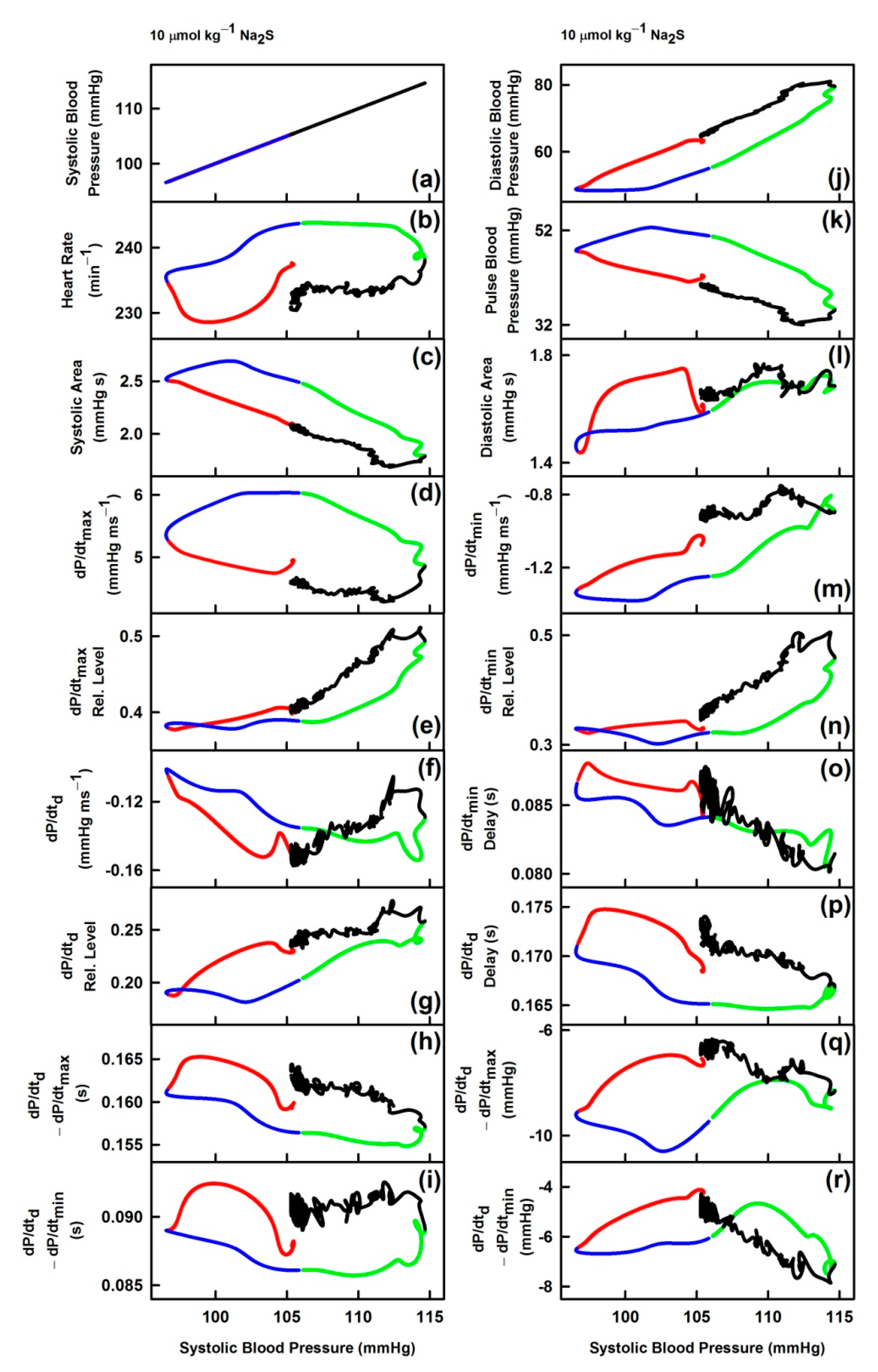
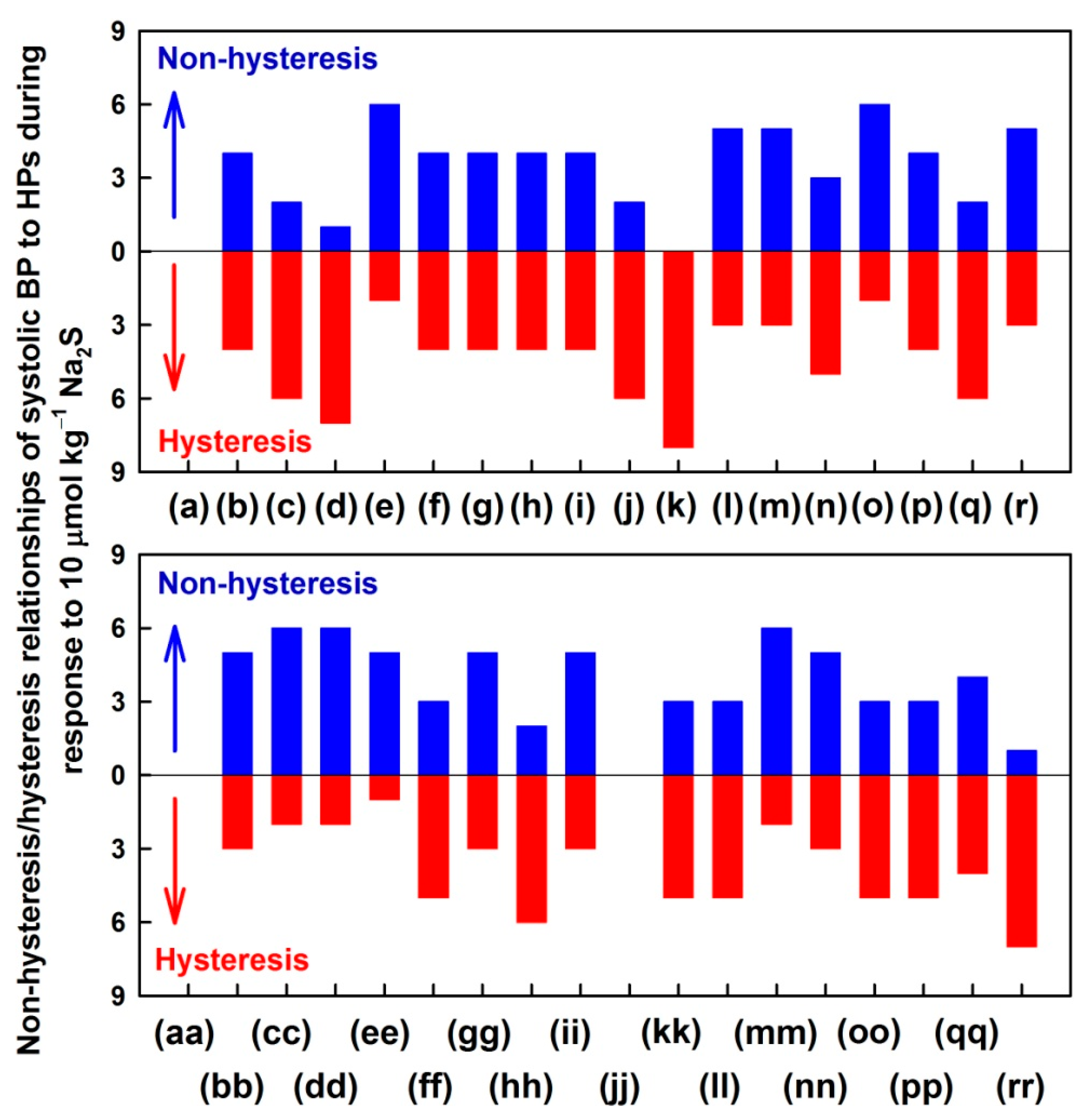
3.3. Non-Hysteresis/Hysteresis Relationships between HPs to Systolic BP after Na2S Administration
3.4. Comparison of Non-Hysteresis/Hysteresis Relationships of Na2S with Published Relationships of GSNO
3.5. Effect of H2S on Distinct Fluctuation of Diastolic BP
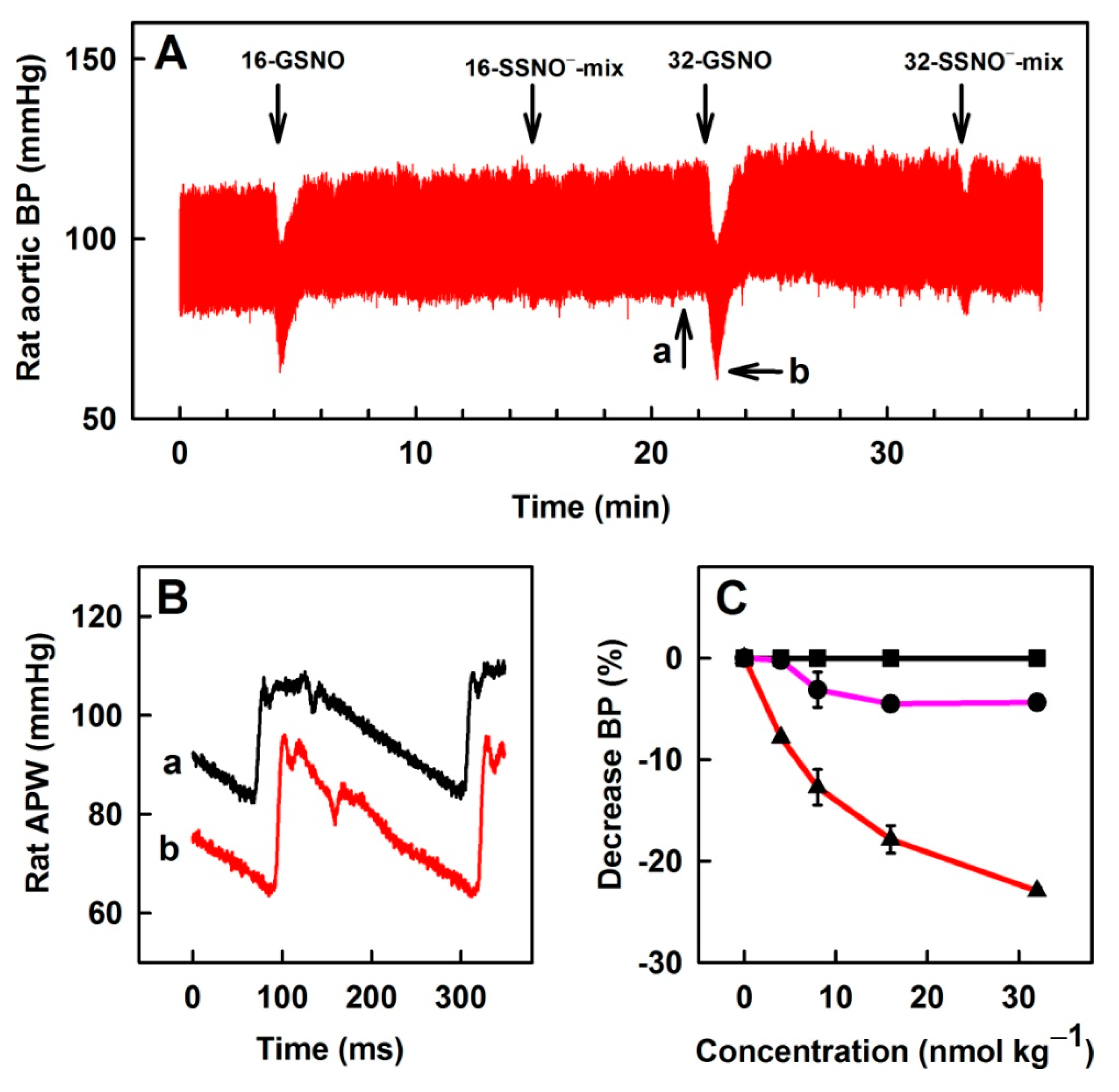
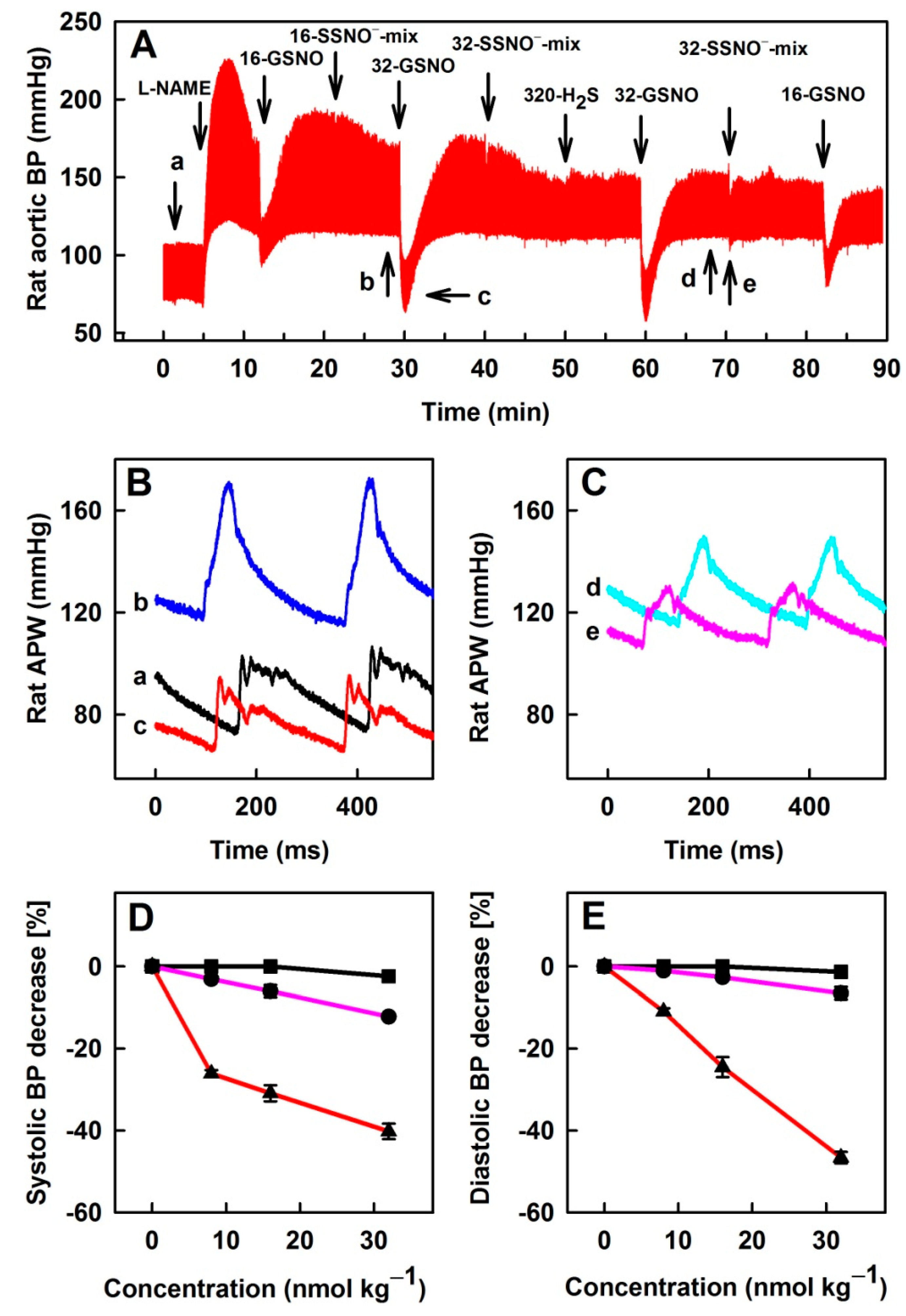
3.6. Comparison of Na2S, GSNO and SSNO−-Mix Effects on Rat BP
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- O’Rourke, M.F.; Pauca, A.; Jiang, X.-J. Pulse wave analysis. Br. J. Clin. Pharmacol. 2001, 51, 507–522. [Google Scholar] [CrossRef]
- Žikić, D. A mathematical model of pressure and flow waveforms in the aortic root. Eur. Biophys. J. 2017, 46, 41–48. [Google Scholar] [CrossRef]
- Marais, L.; Pernot, M.; Khettab, H.; Tanter, M.; Messas, E.; Zidi, M.; Laurent, S.; Boutouyrie, P. Arterial Stiffness Assessment by Shear Wave Elastography and Ultrafast Pulse Wave Imaging: Comparison with Reference Techniques in Normotensives and Hypertensives. Ultrasound Med. Bio. 2019, 45, 758–772. [Google Scholar] [CrossRef]
- Misak, A.; Kurakova, L.; Berenyiova, A.; Tomasova, L.; Grman, M.; Cacanyiova, S.; Ondrias, K. Patterns and Direct/Indirect Signaling Pathways in Cardiovascular System in the Condition of Transient Increase of NO. Biomed. Res. Int. 2020, 2020, 6578213. [Google Scholar] [CrossRef]
- Tomasova, L.; Misak, A.; Kurakova, L.; Grman, M.; Ondrias, K. Characterization of Rat Cardiovascular System by Anacrotic/Dicrotic Notches in the Condition of Increase/Decrease of NO Bioavailability. Int. J. Mol. Sci. 2020, 21, 6685. [Google Scholar] [CrossRef] [PubMed]
- Kurakova, L.; Misak, A.; Tomasova, L.; Cacanyiova, S.; Berenyiova, A.; Ondriasova, E.; Balis, P.; Grman, M.; Ondrias, K. Mathematical relationships of patterns of 35 rat haemodynamic parameters for conditions of hypertension resulting from decreased nitric oxide bioavailability. Exp. Physiol. 2020, 105, 312–334. [Google Scholar] [CrossRef] [PubMed]
- Wang, R. Physiological implications of hydrogen sulfide: A whiff exploration that blossomed. Physiol. Rev. 2012, 92, 791–896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drobná, M.; Misak, A.; Holland, T.; Kristek, F.; Grman, M.; Tomasova, L.; Berenyiova, A.; Cacanyiova, S.; Ondrias, K. Captopril partially decreases the effect of H2S on rat blood pressure and inhibits H2S-induced nitric oxide release from S-nitrosoglutathione. Physiol. Res. 2015, 64, 479–486. [Google Scholar] [CrossRef]
- Cacanyiova, S.; Berenyiova, A.; Balis, P.; Kristek, F.; Grman, M.; Ondrias, K.; Breza, J.; Breza, J., Jr. Nitroso-sulfide coupled signaling triggers specific vasoactive effects in the intrarenal arteries of patients with arterial hypertension. J. Physiol. Pharmacol. 2017, 68, 527–538. [Google Scholar]
- Zhao, W.; Zhang, J.; Lu, Y.; Wang, R. The vasorelaxant effect of H2S as a novel endogenous gaseous KATP channel opener. EMBO J. 2001, 20, 6008–6016. [Google Scholar] [CrossRef] [Green Version]
- Geng, B.; Yang, J.; Qi, Y.; Zhao, J.; Pang, Y.; Du, J.; Tang, C. H2S generated by heart in rat and its effects on cardiac function. Biochem. Biophys. Res. Commun. 2004, 313, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Szijártó, I.A.; Markó, L.; Filipovic, M.R.; Miljkovic, J.L.; Tabeling, C.; Tsvetkov, D.; Wang, N.; Rabelo, L.A.; Witzenrath, M.; Diedrich, A.; et al. Cystathionine γ-Lyase-Produced Hydrogen Sulfide Controls Endothelial NO Bioavailability and Blood Pressure. Hypertension 2018, 71, 1210–1217. [Google Scholar] [CrossRef]
- Yang, G.; Wu, L.; Jiang, B.; Yang, W.; Qi, J.; Cao, K.; Meng, Q.; Mustafa, A.K.; Mu, W.; Zhang, S.; et al. H2S as a physiologic vasorelaxant: Hypertension in mice with deletion of cystathionine gamma-lyase. Science 2008, 322, 587–590. [Google Scholar] [CrossRef] [Green Version]
- Cortese-Krott, M.M.; Kuhnle, G.G.; Dyson, A.; Fernandez, B.O.; Grman, M.; DuMond, J.F.; Barrow, M.P.; McLeod, G.; Nakagawa, H.; Ondrias, K.; et al. Key bioactive reaction products of the NO/H2S interaction are S/N-hybrid species, polysulfides, and nitroxyl. Proc. Natl. Acad. Sci. USA 2015, 112, 4651–4660. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.Y.; Ping, C.Y.; Mok, Y.Y.; Ling, L.; Whiteman, M.; Bhatia, M.; Moore, P.K. Regulation of vascular nitric oxide in vitro and in vivo; a new role for endogenous hydrogen sulphide? Br. J. Pharmacol. 2006, 149, 625–634. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Whiteman, M.; Guan, Y.Y.; Neo, K.L.; Cheng, Y.; Lee, S.W.; Zhao, Y.; Baskar, R.; Tan, C.H.; Moore, P.K. Characterization of a novel, water-soluble hydrogen sulfide-releasing molecule (GYY4137): New insights into the biology of hydrogen sulfide. Circulation 2008, 117, 2351–2360. [Google Scholar] [CrossRef] [Green Version]
- Cacanyiova, S.; Krskova, K.; Zorad, S.; Frimmel, K.; Drobna, M.; Valaskova, Z.; Misak, A.; Golas, S.; Breza, J.; Breza, J., Jr.; et al. Arterial Hypertension and Plasma Glucose Modulate the Vasoactive Effects of Nitroso-Sulfide Coupled Signaling in Human Intrarenal Arteries. Molecules 2020, 25, 2886. [Google Scholar] [CrossRef] [PubMed]
- Cortese-Krott, M.M.; Fernandez, B.O.; Santos, J.L.; Mergia, E.; Grman, M.; Nagy, P.; Kelm, M.; Butler, A.; Feelisch, M. Nitrosopersulfide (SSNO−) accounts for sustained NO bioactivity of S-nitrosothiols following reaction with sulfide. Redox Bio. 2014, 2, 234–244. [Google Scholar] [CrossRef] [Green Version]
- Bogdándi, V.; Ditrói, T.; Bátai, I.Z.; Sándor, Z.; Minnion, M.; Vasas, A.; Galambos, K.; Buglyó, P.; Pintér, E.; Feelisch, M.; et al. Nitrosopersulfide (SSNO−) Is a Unique Cysteine Polysulfidating Agent with Reduction-Resistant Bioactivity. Antioxid. Redox Signal. 2020. [Google Scholar] [CrossRef]
- Grundy, D. Principles and standards for reporting animal experiments in The Journal of Physiology and Experimental Physiology. J. Physiol. 2015, 593, 2547–2549. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.R.; Nilius, B.; Han, J. Gaseous Signaling Molecules in Cardiovascular Function: From Mechanisms to Clinical Translation. Rev. Physiol. Biochem. Pharmacol. 2018, 174, 81–156. [Google Scholar] [CrossRef]
- Bianco, C.L.; Akaike, T.; Ida, T.; Nagy, P.; Bogdandi, V.; Toscano, J.P.; Kumagai, Y.; Henderson, C.F.; Goddu, R.N.; Lin, J.; et al. The reaction of hydrogen sulfide with disulfides: Formation of a stable trisulfide and implications for biological systems. Br. J. Pharmacol. 2019, 176, 671–683. [Google Scholar] [CrossRef]
- Gheibi, S.; Jeddi, S.; Kashfi, K.; Ghasemi, A. Regulation of vascular tone homeostasis by NO and H2S: Implications in hypertension. Biochem. Pharmacol. 2018, 149, 42–59. [Google Scholar] [CrossRef]
- Iring, A.; Jin, Y.J.; Albarrán-Juárez, J.; Siragusa, M.; Wang, S.; Dancs, P.T.; Nakayama, A.; Tonack, S.; Chen, M.; Künne, C.; et al. Shear stress-induced endothelial adrenomedullin signaling regulates vascular tone and blood pressure. J. Clin. Investig. 2019, 129, 2775–2791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigo, G.C.; Herbert, K.E. Regulation of vascular function and blood pressure by circadian variation in redox signalling. Free Radic. Biol. Med. 2018, 119, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Vanhoutte, P.M.; Leung, S.W. Vascular nitric oxide: Beyond eNOS. J. Pharmacol. Sci. 2015, 129, 83–94. [Google Scholar] [CrossRef] [Green Version]
- Puzserova, A.; Bernatova, I. Blood pressure regulation in stress: Focus on nitric oxide-dependent mechanisms. Physiol. Res. 2016, 65, S309–s342. [Google Scholar] [CrossRef]
- Szabo, C. Hydrogen sulfide, an enhancer of vascular nitric oxide signaling: Mechanisms and implications. Am. J. Physiol. Cell Physiol. 2017, 312, C3–c15. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Wu, L.; Wang, R. Interaction of hydrogen sulfide with ion channels. Clin. Exp. Pharmacol. Physiol. 2010, 37, 753–763. [Google Scholar] [CrossRef]
- Cao, X.; Wu, Z.; Xiong, S.; Cao, L.; Sethi, G.; Bian, J.S. The role of hydrogen sulfide in cyclic nucleotide signaling. Biochem. Pharmacol. 2018, 149, 20–28. [Google Scholar] [CrossRef]
- Stubbert, D.; Prysyazhna, O.; Rudyk, O.; Scotcher, J.; Burgoyne, J.R.; Eaton, P. Protein kinase G Iα oxidation paradoxically underlies blood pressure lowering by the reductant hydrogen sulfide. Hypertension 2014, 64, 1344–1351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nier, B.A.; Harrington, L.S.; Carrier, M.J.; Weinberg, P.D. Evidence for a specific influence of the nitrergic pathway on the peripheral pulse waveform in rabbits. Exp. Physiol. 2008, 93, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Wu, Y.M.; Li, Q.; Wang, X.; He, R.R. Electrophysiological effects of hydrogen sulfide on pacemaker cells in sinoatrial nodes of rabbits. Sheng Li Xue Bao 2008, 60, 175–180. [Google Scholar] [PubMed]
- Yong, Q.C.; Hu, L.F.; Wang, S.; Huang, D.; Bian, J.S. Hydrogen sulfide interacts with nitric oxide in the heart: Possible involvement of nitroxyl. Cardiovasc. Res. 2010, 88, 482–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunner, F.; Andrew, P.; Wölkart, G.; Zechner, R.; Mayer, B. Myocardial Contractile Function and Heart Rate in Mice With Myocyte-Specific Overexpression of Endothelial Nitric Oxide Synthase. Circulation 2001, 104, 3097–3102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Layland, J.; Li, J.M.; Shah, A.M. Role of cyclic GMP-dependent protein kinase in the contractile response to exogenous nitric oxide in rat cardiac myocytes. J. Physiol. 2002, 540, 457–467. [Google Scholar] [CrossRef]
- Berenyiova, A.; Grman, M.; Mijuskovic, A.; Stasko, A.; Misak, A.; Nagy, P.; Ondriasova, E.; Cacanyiova, S.; Brezova, V.; Feelisch, M.; et al. The reaction products of sulfide and S-nitrosoglutathione are potent vasorelaxants. Nitric Oxide 2015, 46, 123–130. [Google Scholar] [CrossRef] [Green Version]
- Gladwin, M.T.; Crawford, J.H.; Patel, R.P. The biochemistry of nitric oxide, nitrite, and hemoglobin: Role in blood flow regulation. Free Radic. Biol. Med. 2004, 36, 707–717. [Google Scholar] [CrossRef]
- Farmer, P.J.; Sulc, F. Coordination chemistry of the HNO ligand with hemes and synthetic coordination complexes. J. Inorg. Biochem. 2005, 99, 166–184. [Google Scholar] [CrossRef]
- Vaughn, M.W.; Huang, K.T.; Kuo, L.; Liao, J.C. Erythrocytes possess an intrinsic barrier to nitric oxide consumption. J. Biol. Chem. 2000, 275, 2342–2348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deonikar, P.; Kavdia, M. Contribution of membrane permeability and unstirred layer diffusion to nitric oxide–red blood cell interaction. J. Theor. Bio. 2013, 317, 321–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]

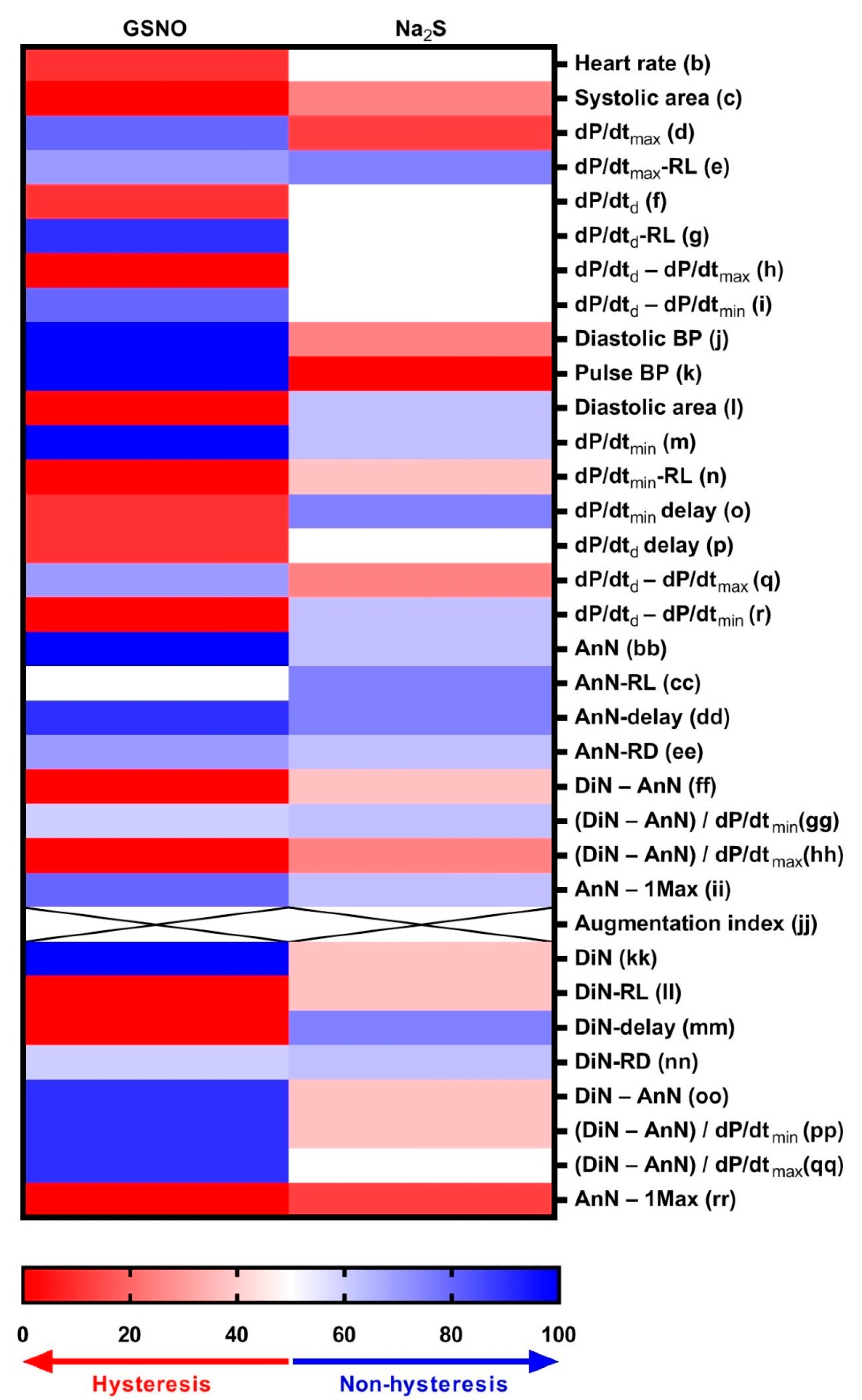
| Description | GSNO | Na2S | |
|---|---|---|---|
| (a) | Systolic blood pressure; in mmHg | ↓ | ↓ |
| (b) | Heart rate; in min−1 | ~ | ↓ |
| (c) | Systolic area; in mmHg s | ↑ | ↑ |
| (d) | dP/dtmax; in mmHg ms−1 | ↑ | ~ |
| (e) | dP/dtmax relative level | ↓ | ↓ |
| (f) | dP/dtd; in mmHg ms−1 | ↓↑ | ~ |
| (g) | dP/dtd relative level | ↓ | ↓ |
| (h) | dP/dtd − dP/dtmax; in s | ↑ | ↑ |
| (i) | dP/dtd − dP/dtmin; in s | ↑↓ | ↑ |
| (j) | Diastolic blood pressure; in mmHg | ↓ | ↓ |
| (k) | Pulse blood pressure; in mmHg | ↑ | ↑ |
| (l) | Diastolic area; in mmHg s | ↓ | ↑ |
| (m) | dP/dtmin; in mmHg ms−1 | ↓ | ↓ |
| (n) | dP/dtmin relative level | ↓ | ~ |
| (o) | dP/dtmin delay; in s | ↑ | ↑ |
| (p) | dP/dtd delay; in s | ↑ | ↑ |
| (q) | dP/dtd − dP/dtmax; in mmHg | ↓ | ↓ |
| (r) | dP/dtd − dP/dtmin; in mmHg | ↑ | ~ |
| (aa) | Systolic blood pressure; in mmHg | ↓ | ↓ |
| (bb) | Anacrotic notch; in mmHg | ↓ | ↓ |
| (cc) | Anacrotic notch relative level | ↑ | ↑ |
| (dd) | Anacrotic notch delay; in ms | ↑ | ↑ |
| (ee) | Anacrotic notch relative Delay | ↑ | ~ |
| (ff) | Dicrotic notch (DiN) − Anacrotic notch (AnN); in s | ↑ | ↑ |
| (gg) | (DiN − AnN)/dP/dtmin; in s/mmHg µs−1 | ↑ | ↑ |
| (hh) | (DiN − AnN)/dP/dtmax; in s/mmHg µs−1 | ↓ | ~ |
| (ii) | AnN − 1Max; in ms | ↑ | ↑ |
| (jj) | Augmentation index relative | ~ | ~ |
| (kk) | Dicrotic notch; in mmHg | ↓ | ↓ |
| (ll) | Dicrotic notch relative level | ↓ | ↓ |
| (mm) | Dicrotic notch delay; in ms | ↑ | ↑ |
| (nn) | Dicrotic notch relative delay | ↑ | ~ |
| (oo) | DiN − AnN; in mmHg | ↓ | ↓ |
| (pp) | (DiN − AnN)/dP/dtmin; in mmHg/mmHg ms−1 | ↑ | ↑ |
| (qq) | (DiN − AnN)/dP/dtmax; in mmHg/mmHg ms−1 | ↓ | ↓ |
| (rr) | AnN − 1Max; in mmHg | ~ | ~ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomasova, L.; Grman, M.; Misak, A.; Kurakova, L.; Ondriasova, E.; Ondrias, K. Cardiovascular “Patterns” of H2S and SSNO−-Mix Evaluated from 35 Rat Hemodynamic Parameters. Biomolecules 2021, 11, 293. https://doi.org/10.3390/biom11020293
Tomasova L, Grman M, Misak A, Kurakova L, Ondriasova E, Ondrias K. Cardiovascular “Patterns” of H2S and SSNO−-Mix Evaluated from 35 Rat Hemodynamic Parameters. Biomolecules. 2021; 11(2):293. https://doi.org/10.3390/biom11020293
Chicago/Turabian StyleTomasova, Lenka, Marian Grman, Anton Misak, Lucia Kurakova, Elena Ondriasova, and Karol Ondrias. 2021. "Cardiovascular “Patterns” of H2S and SSNO−-Mix Evaluated from 35 Rat Hemodynamic Parameters" Biomolecules 11, no. 2: 293. https://doi.org/10.3390/biom11020293
APA StyleTomasova, L., Grman, M., Misak, A., Kurakova, L., Ondriasova, E., & Ondrias, K. (2021). Cardiovascular “Patterns” of H2S and SSNO−-Mix Evaluated from 35 Rat Hemodynamic Parameters. Biomolecules, 11(2), 293. https://doi.org/10.3390/biom11020293






