The Vasoactive Effect of Perivascular Adipose Tissue and Hydrogen Sulfide in Thoracic Aortas of Normotensive and Spontaneously Hypertensive Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Guide for the Use and Care of Laboratory Animals
2.2. Experimental Animals and Basic Parameters
2.3. Functional Study
2.4. Measurement of Superoxide Production in Selected Tissues
2.5. Western Blotting
2.6. Statistical Analysis
2.7. Drugs
3. Results
3.1. General Characteristics of Experimental Animals
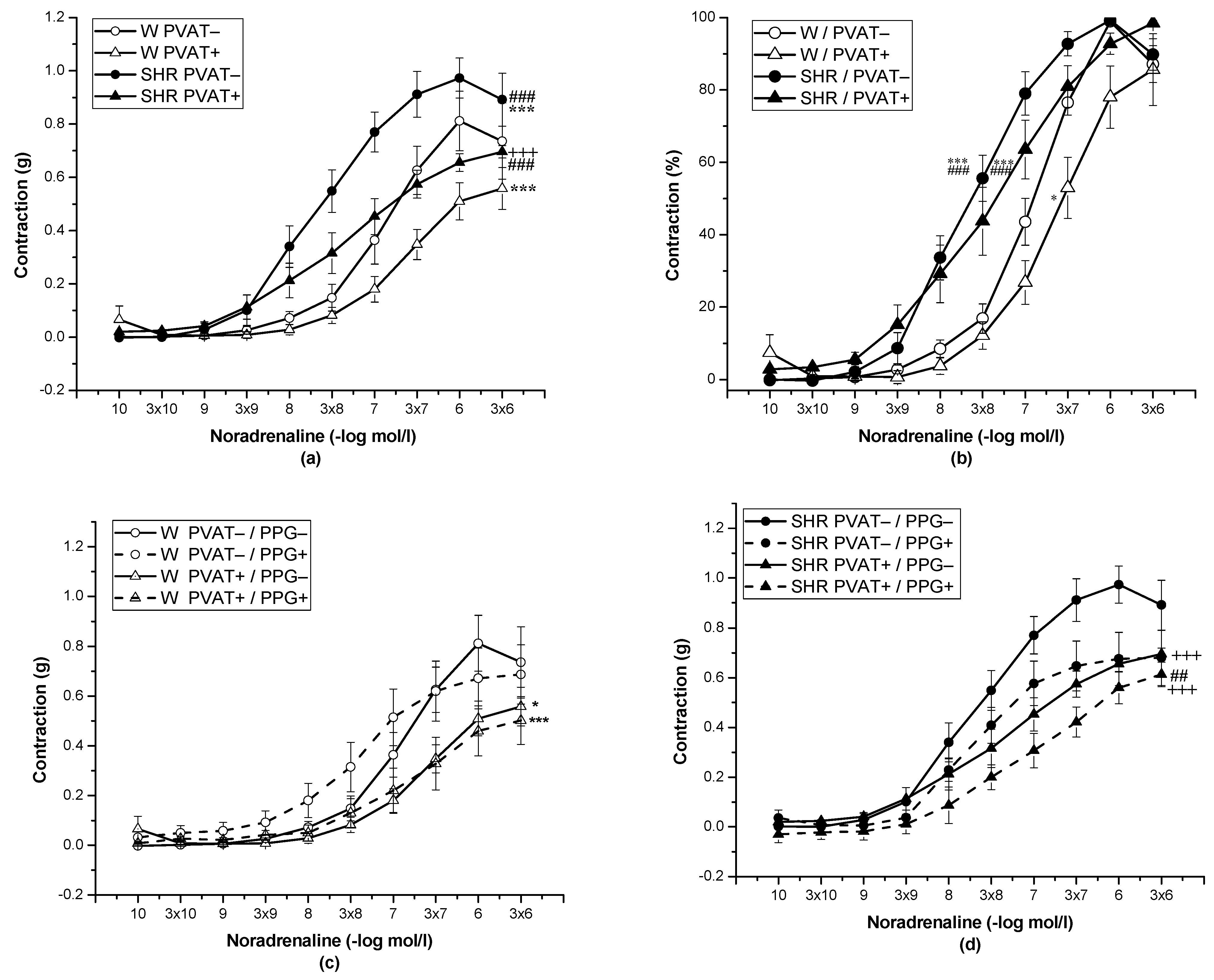
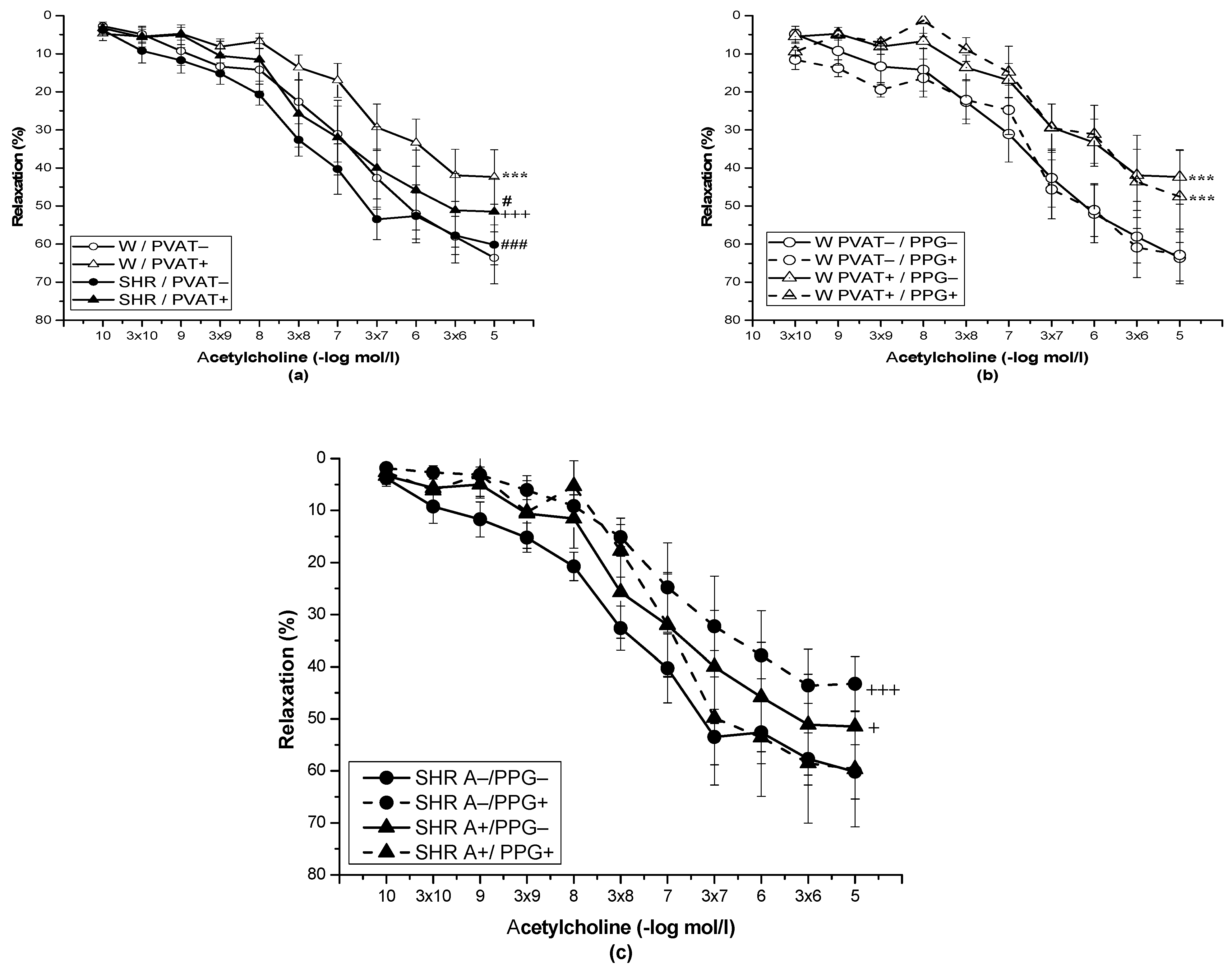
3.2. Role of Perivascular Adipose Tissue and Vasoactive Effect of H2S
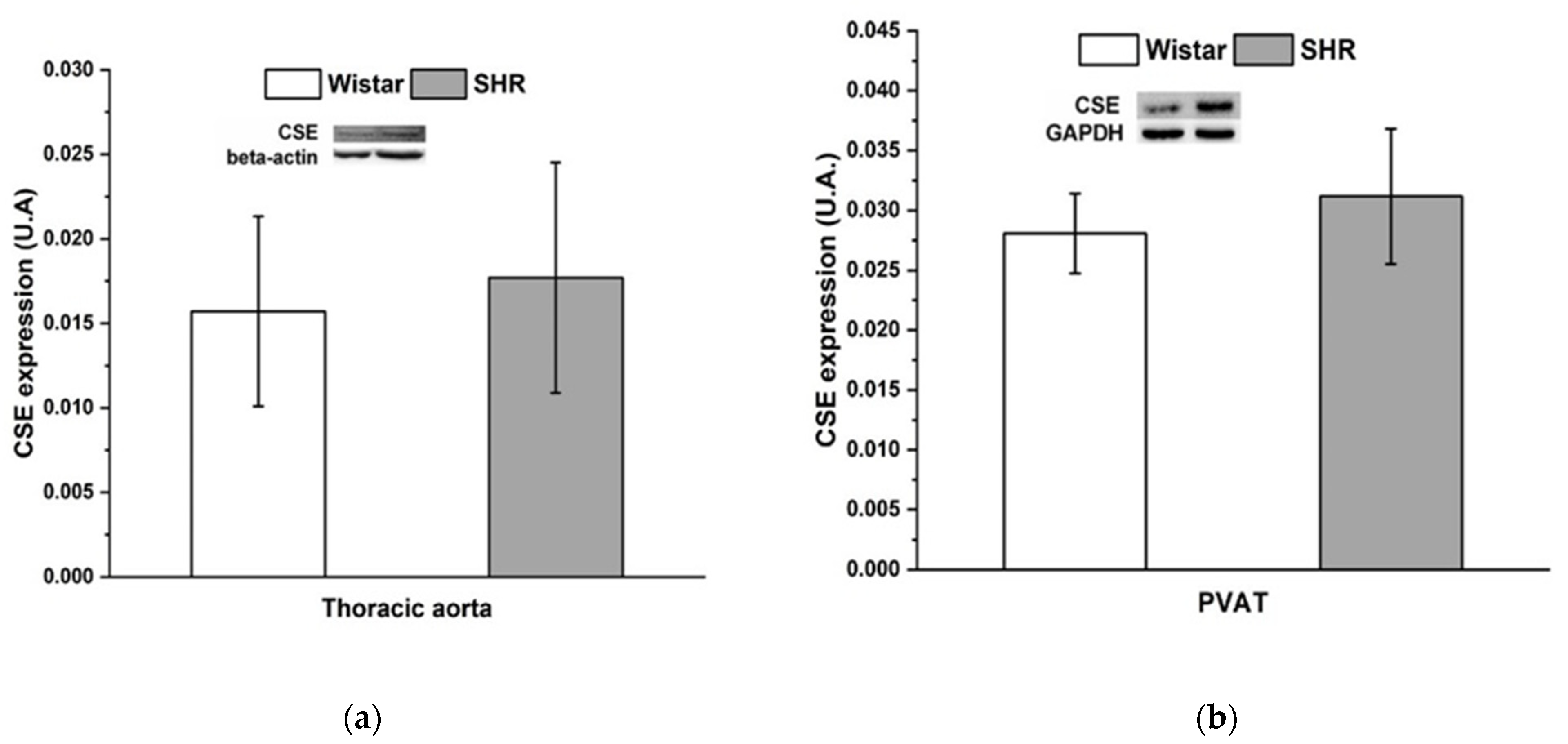
3.3. Measurement of Superoxide Production in Selected Tissues
3.4. Protein Expression of Cystathionine γ-Lyase
4. Discussion
4.1. PVAT-Related Modulation of Vasoactive Responses
4.2. PVAT—Hydrogen Sulfide Interaction
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oriowo, M.A. Perivascular Adipose Tissue, Vascular Reactivity and Hypertension. Med. Princ. Pract. 2015, 24 (Suppl. 1), 29–37. [Google Scholar] [CrossRef]
- Huang Cao, Z.F.; Stoffel, E.; Cohen, P. Role of Perivascular Adipose Tissue in Vascular Physiology and Pathology. Hypertension 2017, 69, 770–777. [Google Scholar] [CrossRef]
- Cacanyiova, S.; Majzunova, M.; Golas, S.; Berenyiova, A. The Role of Perivascular Adipose Tissue and Endogenous Hydrogen Sulfide in Vasoactive Responses of Isolated Mesenteric Arteries in Normotensive and Spontaneously Hypertensive Rats. J. Physiol. Pharmacol. 2019, 70, 295–306. [Google Scholar] [CrossRef]
- Gil-Ortega, M.; Somoza, B.; Huang, Y.; Gollasch, M.; Fernandez-Alfonso, M.S. Regional Differences in Perivascular Adipose Tissue Impacting Vascular Homeostasis. Trends Endocrinol. Metab. 2015, 26, 367–375. [Google Scholar] [CrossRef]
- Löhn, M.; Dubrovska, G.; Lauterbach, B.; Luft, F.C.; Gollasch, M.; Sharma, A.M. Periadventitial Fat Releases a Vascular Relaxing Factor. FASEB J. 2002, 16, 1057–1063. [Google Scholar] [CrossRef]
- Schleifenbaum, J.; Kohn, C.; Voblova, N.; Dubrovska, G.; Zavarirskaya, O.; Gloe, T.; Crean, C.S.; Luft, F.C.; Huang, Y.; Schubert, R.; et al. Systemic Peripheral Artery Relaxation by KCNQ Channel Openers and Hydrogen Sulfide. J. Hypertens. 2010, 28, 1875–1882. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, J.; Lu, Y.; Wang, R. The Vasorelaxant Effect of H2S as a Novel Endogenous Gaseous K(ATP) Channel Opener. EMBO J. 2001, 20, 6008–6016. [Google Scholar] [CrossRef] [Green Version]
- Mustafa, A.K.; Sikka, G.; Gazi, S.K.; Steppan, J.; Jung, S.M.; Bhunia, A.K.; Barodka, V.M.; Gazi, F.K.; Barrow, R.K.; Wang, R.; et al. Hydrogen Sulfide as Endothelium-Derived Hyperpolarizing Factor Sulfhydrates Potassium Channels. Circ. Res. 2011, 109, 1259–1268. [Google Scholar] [CrossRef]
- Liu, Y.-H.; Lu, M.; Hu, L.-F.; Wong, P.T.-H.; Webb, G.D.; Bian, J.-S. Hydrogen Sulfide in the Mammalian Cardiovascular System. Antioxid. Redox Signal. 2012, 17, 141–185. [Google Scholar] [CrossRef]
- Cacanyiova, S.; Berenyiova, A.; Kristek, F.; Drobna, M.; Ondrias, K.; Grman, M. The Adaptive Role of Nitric Oxide and Hydrogen Sulphide in Vasoactive Responses of Thoracic Aorta Is Triggered Already in Young Spontaneously Hypertensive Rats. J. Physiol. Pharmacol. 2016, 67, 501–512. [Google Scholar]
- Ondrias, K.; Stasko, A.; Cacanyiova, S.; Sulova, Z.; Krizanova, O.; Kristek, F.; Malekova, L.; Knezl, V.; Breier, A. H2S and HS− Donor NaHS Releases Nitric Oxide from Nitrosothiols, Metal Nitrosyl Complex, Brain Homogenate and Murine L1210 Leukaemia Cells. Pflugers Arch. 2008, 457, 271–279. [Google Scholar] [CrossRef]
- Yong, Q.C.; Choo, C.H.; Tan, B.H.; Low, C.-M.; Bian, J.-S. Effect of Hydrogen Sulfide on Intracellular Calcium Homeostasis in Neuronal Cells. Neurochem. Int. 2010, 56, 508–515. [Google Scholar] [CrossRef]
- Xie, Z.-Z.; Liu, Y.; Bian, J.-S. Hydrogen Sulfide and Cellular Redox Homeostasis. Oxid. Med. Cell Longev. 2016, 2016, 6043038. [Google Scholar] [CrossRef] [Green Version]
- Lu, C.; Su, L.-Y.; Lee, R.M.K.W.; Gao, Y.-J. Alterations in Perivascular Adipose Tissue Structure and Function in Hypertension. Eur. J. Pharmacol. 2011, 656, 68–73. [Google Scholar] [CrossRef]
- Youngstrom, T.G.; Bartness, T.J. White Adipose Tissue Sympathetic Nervous System Denervation Increases Fat Pad Mass and Fat Cell Number. Am. J. Physiol. 1998, 275, R1488–R1493. [Google Scholar] [CrossRef]
- Bartness, T.J.; Liu, Y.; Shrestha, Y.B.; Ryu, V. Neural Innervation of White Adipose Tissue and the Control of Lipolysis. Front. Neuroendocrinol. 2014, 35, 473–493. [Google Scholar] [CrossRef] [Green Version]
- Zemancikova, A.; Torok, J. Effect of Perivascular Adipose Tissue on Arterial Adrenergic Contractions in Normotensive and Hypertensive Rats with High Fructose Intake. Physiol. Res. 2017, 66, S537–S544. [Google Scholar] [CrossRef]
- Jain, S.K.; Micinski, D.; Lieblong, B.J.; Stapleton, T. Relationship between Hydrogen Sulfide Levels and HDL-Cholesterol, Adiponectin, and Potassium Levels in the Blood of Healthy Subjects. Atherosclerosis 2012, 225, 242–245. [Google Scholar] [CrossRef] [Green Version]
- Yan, H.; Du, J.; Tang, C. The Possible Role of Hydrogen Sulfide on the Pathogenesis of Spontaneous Hypertension in Rats. Biochem. Biophys. Res. Commun. 2004, 313, 22–27. [Google Scholar] [CrossRef]
- Zong, W.; Liu, R.; Sun, F.; Wang, M.; Zhang, P.; Liu, Y.; Tian, Y. Cyclic Voltammetry: A New Strategy for the Evaluation of Oxidative Damage to Bovine Insulin. Protein. Sci. 2010, 19, 263–268. [Google Scholar] [CrossRef] [Green Version]
- Cacanyiova, S.; Golas, S.; Zemancikova, A.; Majzunova, M.; Cebova, M.; Malinska, H.; Huttl, M.; Markova, I.; Berenyiova, A. The Vasoactive Role of Perivascular Adipose Tissue and the Sulfide Signaling Pathway in a Nonobese Model of Metabolic Syndrome. Biomolecules 2021, 11, 108. [Google Scholar] [CrossRef]
- Berenyiova, A.; Drobna, M.; Cebova, M.; Kristek, F.; Cacanyiova, S. Changes in the Vasoactive Effects of Nitric Oxide, Hydrogen Sulfide and the Structure of the Rat Thoracic Aorta: The Role of Age and Essential Hypertension. J. Physiol. Pharmacol. 2018, 69, 1–12. [Google Scholar] [CrossRef]
- Torok, J.; Zemancikova, A.; Kocianova, Z. Interaction of Perivascular Adipose Tissue and Sympathetic Nerves in Arteries from Normotensive and Hypertensive Rats. Physiol. Res. 2016, 65, S391–S399. [Google Scholar] [CrossRef]
- Szasz, T.; Bomfim, G.F.; Webb, R.C. The Influence of Perivascular Adipose Tissue on Vascular Homeostasis. Vasc. Health Risk Manag. 2013, 9, 105–116. [Google Scholar] [CrossRef] [Green Version]
- Padilla, J.; Jenkins, N.T.; Vieira-Potter, V.J.; Laughlin, M.H. Divergent Phenotype of Rat Thoracic and Abdominal Perivascular Adipose Tissues. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 304, R543–R552. [Google Scholar] [CrossRef] [Green Version]
- Farmer, S.R. Molecular Determinants of Brown Adipocyte Formation and Function. Genes Dev. 2008, 22, 1269–1275. [Google Scholar] [CrossRef] [Green Version]
- Tang, E.H.C.; Leung, F.P.; Huang, Y.; Feletou, M.; So, K.-F.; Man, R.Y.K.; Vanhoutte, P.M. Calcium and Reactive Oxygen Species Increase in Endothelial Cells in Response to Releasers of Endothelium-Derived Contracting Factor. Br. J. Pharmacol. 2007, 151, 15–23. [Google Scholar] [CrossRef] [Green Version]
- Boulanger, C.M.; Heymes, C.; Benessiano, J.; Geske, R.S.; Levy, B.I.; Vanhoutte, P.M. Neuronal Nitric Oxide Synthase Is Expressed in Rat Vascular Smooth Muscle Cells: Activation by Angiotensin II in Hypertension. Circ. Res. 1998, 83, 1271–1278. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Vanhoutte, P.M.; Leung, S.W.S. Endothelial Nitric Oxide Synthase-Independent Release of Nitric Oxide in the Aorta of the Spontaneously Hypertensive Rat. J. Pharmacol. Exp. Ther. 2013, 344, 15–22. [Google Scholar] [CrossRef]
- Zhong, G.; Chen, F.; Cheng, Y.; Tang, C.; Du, J. The Role of Hydrogen Sulfide Generation in the Pathogenesis of Hypertension in Rats Induced by Inhibition of Nitric Oxide Synthase. J. Hypertens. 2003, 21, 1879–1885. [Google Scholar] [CrossRef]
- Payne, G.A.; Bohlen, H.G.; Dincer, U.D.; Borbouse, L.; Tune, J.D. Periadventitial Adipose Tissue Impairs Coronary Endothelial Function via PKC-Beta-Dependent Phosphorylation of Nitric Oxide Synthase. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H460–H465. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.H.-H.; Chen, S.-J.; Tsao, C.-M.; Wu, C.-C. Perivascular Adipose Tissue Inhibits Endothelial Function of Rat Aortas via Caveolin-1. PLoS ONE 2014, 9, e99947. [Google Scholar] [CrossRef] [Green Version]
- Wu, R.; Millette, E.; Wu, L.; de Champlain, J. Enhanced Superoxide Anion Formation in Vascular Tissues from Spontaneously Hypertensive and Desoxycorticosterone Acetate-Salt Hypertensive Rats. J. Hypertens. 2001, 19, 741–748. [Google Scholar] [CrossRef]
- Melrose, H. The Effect of Ageing on Perivascular Adipose Tissue Function. Ph.D. Thesis, The University of Manchester, Manchester, UK, August 2016. [Google Scholar]
- Kohn, C.; Schleifenbaum, J.; Szijarto, I.A.; Marko, L.; Dubrovska, G.; Huang, Y.; Gollasch, M. Differential Effects of Cystathionine-Gamma-Lyase-Dependent Vasodilatory H2S in Periadventitial Vasoregulation of Rat and Mouse Aortas. PLoS ONE 2012, 7, e41951. [Google Scholar] [CrossRef]
- Aalbaek, F.; Bonde, L.; Kim, S.; Boedtkjer, E. Perivascular Tissue Inhibits Rho-Kinase-Dependent Smooth Muscle Ca2+ Sensitivity and Endothelium-Dependent H2S Signalling in Rat Coronary Arteries. J. Physiol. 2015, 593, 4747–4764. [Google Scholar] [CrossRef] [Green Version]
- Al-Magableh, M.R.; Hart, J.L. Mechanism of Vasorelaxation and Role of Endogenous Hydrogen Sulfide Production in Mouse Aorta. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2011, 383, 403–413. [Google Scholar] [CrossRef]
- Berg, T.; Walaas, S.I.; Roberg, B.A.; Huynh, T.T.; Jensen, J. Plasma Norepinephrine in Hypertensive Rats Reflects Alpha(2)-Adrenoceptor Release Control Only When Re-Uptake Is Inhibited. Front. Neurol. 2012, 3, 160. [Google Scholar] [CrossRef] [Green Version]
- Stassen, F.R.; Maas, R.G.; Schiffers, P.M.; Janssen, G.M.; De Mey, J.G. A Positive and Reversible Relationship between Adrenergic Nerves and Alpha-1A Adrenoceptors in Rat Arteries. J. Pharmacol. Exp. Ther. 1998, 284, 399–405. [Google Scholar]
- Fang, L.; Zhao, J.; Chen, Y.; Ma, T.; Xu, G.; Tang, C.; Liu, X.; Geng, B. Hydrogen Sulfide Derived from Periadventitial Adipose Tissue Is a Vasodilator. J. Hypertens. 2009, 27, 2174–2185. [Google Scholar] [CrossRef]
- Caliendo, G.; Cirino, G.; Santagada, V.; Wallace, J.L. Synthesis and Biological Effects of Hydrogen Sulfide (H2S): Development of H2S-Releasing Drugs as Pharmaceuticals. J. Med. Chem. 2010, 53, 6275–6286. [Google Scholar] [CrossRef]
- Coletta, C.; Papapetropoulos, A.; Erdelyi, K.; Olah, G.; Modis, K.; Panopoulos, P.; Asimakopoulou, A.; Gero, D.; Sharina, I.; Martin, E.; et al. Hydrogen Sulfide and Nitric Oxide Are Mutually Dependent in the Regulation of Angiogenesis and Endothelium-Dependent Vasorelaxation. Proc. Natl. Acad. Sci. USA 2012, 109, 9161–9166. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.-C.; Chang, H.-H.; Chiang, C.-L.; Liu, C.-H.; Yeh, J.-I.; Chen, M.-F.; Chen, P.-Y.; Kuo, J.-S.; Lee, T.J.F. Role of Perivascular Adipose Tissue-Derived Methyl Palmitate in Vascular Tone Regulation and Pathogenesis of Hypertension. Circulation 2011, 124, 1160–1171. [Google Scholar] [CrossRef] [Green Version]
- Rainbow, R.D.; Norman, R.I.; Everitt, D.E.; Brignell, J.L.; Davies, N.W.; Standen, N.B. Endothelin-I and Angiotensin II Inhibit Arterial Voltage-Gated K+ Channels through Different Protein Kinase C Isoenzymes. Cardiovasc. Res. 2009, 83, 493–500. [Google Scholar] [CrossRef] [Green Version]
- Ketonen, J.; Shi, J.; Martonen, E.; Mervaala, E. Periadventitial Adipose Tissue Promotes Endothelial Dysfunction via Oxidative Stress in Diet-Induced Obese C57Bl/6 Mice. Circ. J. 2010, 74, 1479–1487. [Google Scholar] [CrossRef] [Green Version]


| Parameter | Wistar Rats | Spontaneously Hypertensive Rats |
|---|---|---|
| SBP (mmHg) | 128.14 ± 2.82 | 172.68 ± 2.22 ** |
| BW (g) | 424.5 ± 10.77 | 335.5 ± 0.73 *** |
| HW (mg) | 1240 ± 28.81 | 1275 ± 26.47 |
| TL (mm) | 39.48 ± 0.51 | 37.16 ± 0.47 |
| HW/BW (mg/g) | 2.92 ± 0.05 | 3.80 ± 0.04 *** |
| HW/TL (mg/mm) | 31.42 ± 0.66 | 34.17 ± 0.88 * |
| RFW (mg) | 3320.38 ± 547.13 | 3060.25 ± 132.91 |
| RFW/TL (mg/mm) | 83.68 ± 12.98 | 81.29 ± 3.95 |
| Chol (mmol/L) | 2.05 ± 0.18 | 2.19 ± 0.10 |
| HDL (mg/dL) | 56.92 ± 5.44 | 64.98 ± 2.69 |
| LDL (mg/dL) | 10.33 ± 1.42 | 13.1 ± 0.56 |
| TAG (mmol/L) | 2.23 ± 0.13 | 1.39 ± 0.18 ** |
| GLU (mmol/L) | 7.48 ± 0.36 | 7.36 ± 0.41 |
| Wistar Rats | Spontaneously Hypertensive Rats | |||||||
| PVAT− | PVAT+ | PVAT− | PVAT+ | |||||
| PPG− | PPG+ | PPG− | PPG+ | PPG− | PPG+ | PPG− | PPG+ | |
| EC50 | 7.12 ± 0.09 | 7.68 ± 0.08 ** | 6.83 ± 0.06 | 7.0 ± 0.18 | 7.81 ± 0.15 ** | 7.49 ± 0.31 | 7.63 ± 0.24 * | 7.12 ± 0.17 |
| DL-propargylglycine (g) | Wistar Rats | Spontaneously Hypertensive Rats | ||
| PVAT− | PVAT+ | PVAT− | PVAT+ | |
| 0 ± 0.00 | −0.08 ± 0.02 | 0.14 ± 0.06 * | −0.02 ± 0.03 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golas, S.; Berenyiova, A.; Majzunova, M.; Drobna, M.; Tuorkey, M.J.; Cacanyiova, S. The Vasoactive Effect of Perivascular Adipose Tissue and Hydrogen Sulfide in Thoracic Aortas of Normotensive and Spontaneously Hypertensive Rats. Biomolecules 2022, 12, 457. https://doi.org/10.3390/biom12030457
Golas S, Berenyiova A, Majzunova M, Drobna M, Tuorkey MJ, Cacanyiova S. The Vasoactive Effect of Perivascular Adipose Tissue and Hydrogen Sulfide in Thoracic Aortas of Normotensive and Spontaneously Hypertensive Rats. Biomolecules. 2022; 12(3):457. https://doi.org/10.3390/biom12030457
Chicago/Turabian StyleGolas, Samuel, Andrea Berenyiova, Miroslava Majzunova, Magdalena Drobna, Muobarak J. Tuorkey, and Sona Cacanyiova. 2022. "The Vasoactive Effect of Perivascular Adipose Tissue and Hydrogen Sulfide in Thoracic Aortas of Normotensive and Spontaneously Hypertensive Rats" Biomolecules 12, no. 3: 457. https://doi.org/10.3390/biom12030457
APA StyleGolas, S., Berenyiova, A., Majzunova, M., Drobna, M., Tuorkey, M. J., & Cacanyiova, S. (2022). The Vasoactive Effect of Perivascular Adipose Tissue and Hydrogen Sulfide in Thoracic Aortas of Normotensive and Spontaneously Hypertensive Rats. Biomolecules, 12(3), 457. https://doi.org/10.3390/biom12030457







