A Time-Kill Assay Study on the Synergistic Bactericidal Activity of Pomegranate Rind Extract and Zn (II) against Methicillin-Resistant Staphylococcus aureus (MRSA), Staphylococcus epidermidis, Escherichia coli, and Pseudomonas aeruginosa
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. PRE Preparation and Evaluation
2.3. Microbiological Evaluations
2.4. Statistical Analysis
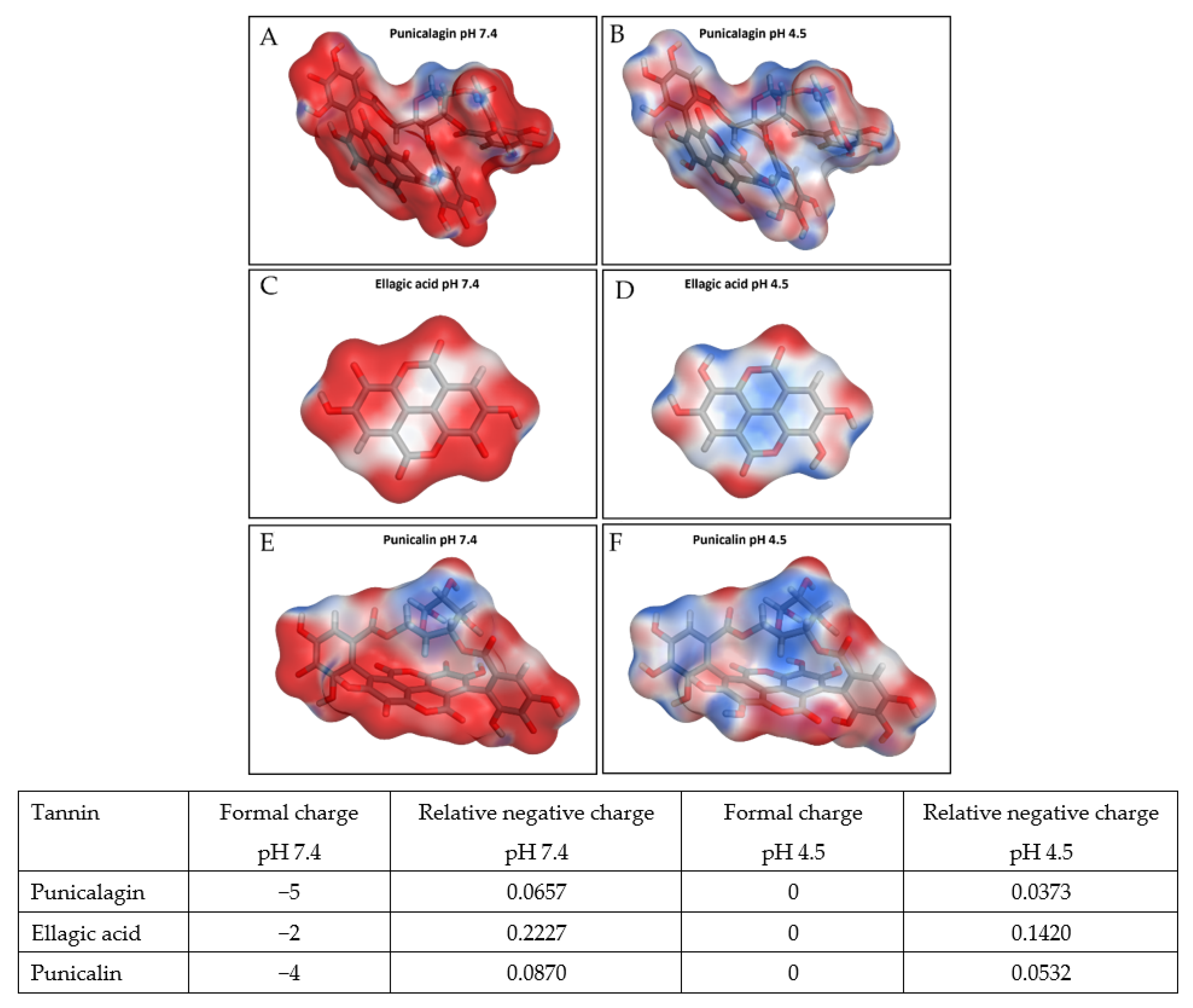
2.5. Computational Surface Charge Analysis
3. Results
3.1. PRE Evaluation
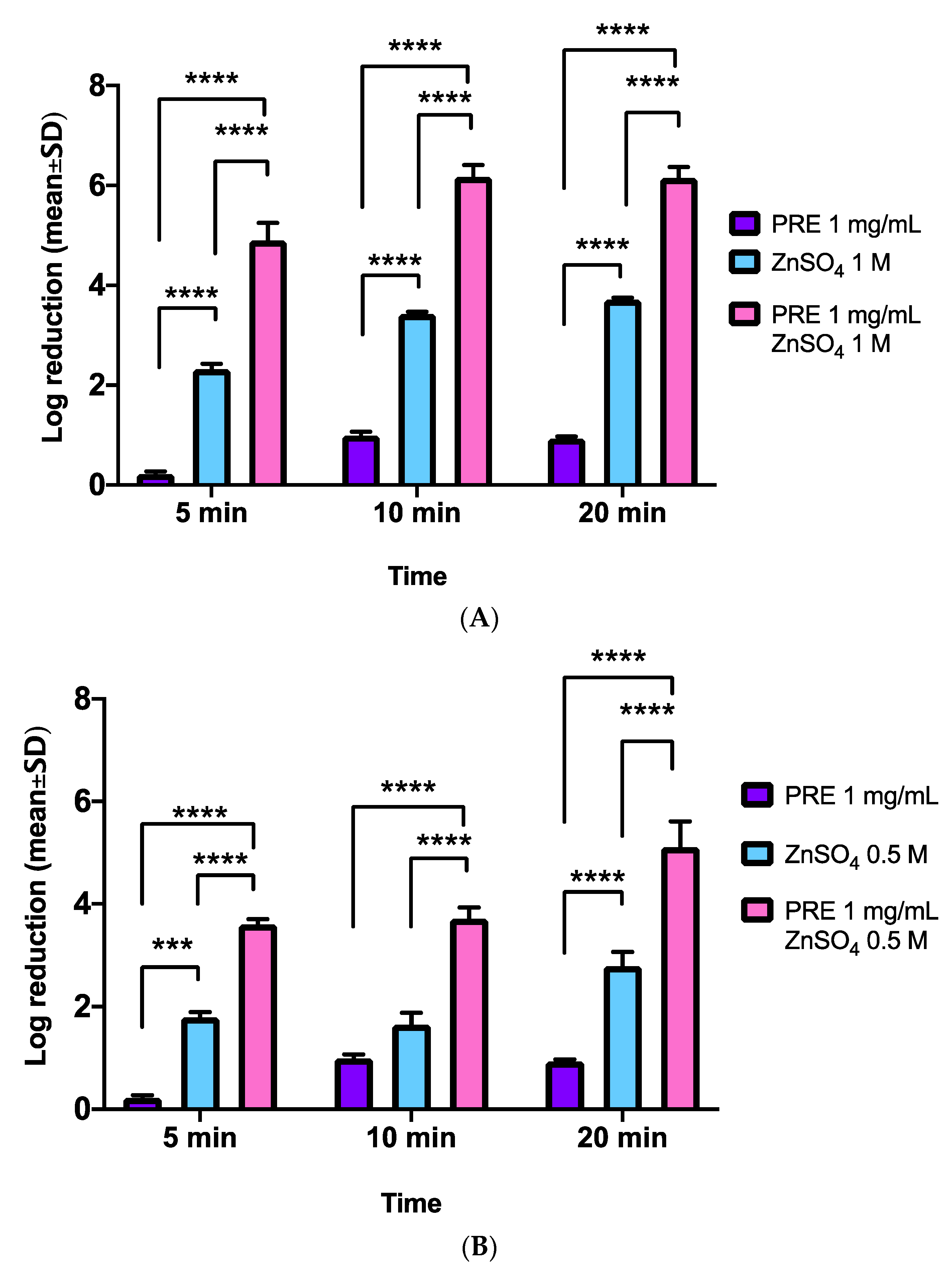
3.2. MRSA
3.3. S. epidermidis
3.4. E. coli
3.5. P. aeruginosa
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ventola, C.L. The antibiotic resistance crisis: Part 1: Causes and threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- WHO. Antimicrobial Resistance. 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 12 December 2021).
- Vilar-Compte, D.; Camacho-Ortiz, A.; Ponce-de-León, S. Infection control in limited resources countries: Challenges and priorities. Curr. Infect. Dis. Rep. 2017, 19, 20. [Google Scholar] [CrossRef]
- Celiksoy, V.; Heard, C.M. The antimicrobial potential of pomegranate. In Pomegranate; InfoTechOpen: London, UK, 2021; Available online: https://cdn.intechopen.com/pdfs/75059.pdf (accessed on 13 December 2021).
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic compounds as beneficial phytochemicals in pomegranate (Punica granatum L.) peel: A review. Food Chem. 2018, 261, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Duman, A.D.; Ozgen, M.; Dayisoylu, K.S.; Erbil, N.; Durgac, C. Antimicrobial activity of six pomegranate (Punica granatum L.) varieties and their relation to some of their pomological and phytonutrient characteristics. Molecules 2009, 14, 1808–1817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Zoreky, N.S. Antimicrobial activity of pomegranate (Punica granatum L.) fruit peels. Int. J. Food Microbiol. 2002, 134, 244–248. [Google Scholar] [CrossRef]
- Nozohour, Y.; Golmohammadi, R.; Mirnejad, R.; Fartashvand, M. Antibacterial activity of pomegranate (Punica granatum l.) seed and peel alcoholic extracts on Staphylococcus aureus and Pseudomonas aeruginosa isolated from health centers. J. Appl. Biotechnol. Rep. 2018, 5, 32–36. [Google Scholar] [CrossRef]
- Li, G.; Xu, Y.; Pan, L.; Xia, X. Punicalagin damages the membrane of Salmonella typhimurium. J. Food Prot. 2020, 83, 2102–2106. [Google Scholar] [CrossRef]
- Mousavi, S.; Weschka, D.; Bereswill, S.; Heimesaat, M.M. Preclinical evaluation of oral urolithin-a for the treatment of acute campylobacteriosis in campylobacter jejuni infected microbiota-depleted IL-10−/− mice. Pathogens 2020, 10, 7. [Google Scholar] [CrossRef]
- Fischbach, M.A. Combination therapies for combating antimicrobial resistance. Curr. Opin. Microbiol. 2011, 14, 519–523. [Google Scholar] [CrossRef] [Green Version]
- Schmid, A.; Wolfensberger, A.; Nemeth, J.; Schreiber, P.W.; Sax, H.; Kuster, S.P. Monotherapy versus combination therapy for multidrug-resistant Gram-negative infections: Systematic review and meta-analysis. Sci. Rep. 2019, 9, 15290. [Google Scholar] [CrossRef]
- Lee, Y.; Puumala, E.; Robbins, N.; Cowen, L.E. Antifungal drug resistance: Molecular mechanisms in Candida albicans and beyond. Chem. Rev. 2021, 121, 3390–3411. [Google Scholar] [CrossRef]
- Khameneh, B.; Diab, R.; Ghazvini, K.; Bazzaz, B.S.F. Breakthroughs in bacterial resistance mechanisms and the potential ways to combat them. Microb. Pathog. 2016, 95, 32–42. [Google Scholar] [CrossRef]
- Woerther, P.-L.; Burdet, C.; Chachaty, E.; Andremont, A. Trends in human fecal carriage of extended-spectrum β-lactamases in the community: Toward the globalization of CTX-M. Clin. Microbiol. Rev. 2013, 26, 744–758. [Google Scholar] [CrossRef] [Green Version]
- Khameneh, B.; Iranshahy, M.; Soheili, V.; Bazzaz, B.S.F. Review on plant antimicrobials: A mechanistic viewpoint. Antimicrob. Resist. Infect. Control 2019, 8, 118. [Google Scholar] [CrossRef] [Green Version]
- Stewart, G.S.; Jassim, S.A.; Denyer, S.P.; Newby, P.; Linley, K.; Dhir, V.K. The specific and sensitive detection of bacterial pathogens within 4 h using bacteriophage amplification. J. Appl. Microbiol. 1998, 84, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Gould, S.W.J.; Fielder, M.D.; Kelly, A.F.; Naughton, D.P. Anti-microbial activities of pomegranate rind extracts: Enhancement by cupric sulphate against clinical isolates of S. aureus, MRSA and PVL positive CA-MSSA. BMC Complement. Altern. Med. 2009, 9, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Houston, D.M.; Bugert, J.J.; Denyer, S.P.; Heard, C.M. Potentiated virucidal activity of pomegranate rind extract (PRE) and punicalagin against Herpes simplex virus (HSV) when co-administered with zinc (II) ions, and antiviral activity of PRE against HSV and aciclovir-resistant HSV. PLoS ONE 2017, 12, e0188609. [Google Scholar]
- Celiksoy, V.; Moses, R.L.; Sloan, A.J.; Moseley, R.; Heard, C.M. Synergistic in vitro antibacterial activity of pomegranate rind extract and zinc (II) against Micrococcus luteus under planktonic and biofilm conditions. Pharmaceutics 2021, 13, 851. [Google Scholar] [CrossRef]
- Miles, A.A.; Misra, S.S. The estimation of the bactericidal power of the blood. J. Hyg. 1938, 38, 732–749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hostynek, J.J.; Maibach, H.I. Skin irritation potential of copper compounds. Toxicol. Mech. Methods 2004, 14, 205–213. [Google Scholar] [CrossRef]
- Li, H. Selected biomarkers revealed potential skin toxicity caused by certain copper compounds. Sci. Rep. 2016, 6, 37664. [Google Scholar] [CrossRef] [PubMed]
- Houston, D.M.J. Towards a Nanomedicine-Based Virucidal System. Ph.D. Thesis, Cardiff University, Cardiff, UK, 2011. [Google Scholar]
- Gordon, R.J.; Lowy, F.D. Pathogenesis of Methicillin-Resistant Staphylococcus aureus Infection. Clin. Infect. Dis. 2008, 46, S350–S359. [Google Scholar] [CrossRef] [Green Version]
- Otto, M. Staphylococcus epidermidis—The ‘accidental’ pathogen. Nat. Rev. Microbiol. 2009, 7, 555–567. [Google Scholar] [CrossRef] [Green Version]
- Smith, L.; Fratamico, P.M. Escherichia coli as a Pathogen. In Foodborne Diseases, 3rd ed.; Dodd, C.E.R., Aldsworth, T., Stein, R.A., Cliver, D.O., Riemann, H.P., Eds.; Academic Press: Cambridge, MA, USA, 2017; Chapter 7; pp. 189–208. ISBN 9780123850072. [Google Scholar]
- Azam, M.W.; Khan, A.U. Updates on the pathogenicity status of Pseudomonas aeruginosa. Drug Discov. Today 2019, 24, 350–359. [Google Scholar] [CrossRef]
- McCarrell, E.M.; Gould, S.W.J.; Fielder, M.D.; Kelly, A.F.; El Sankary, W.; Naughton, D.P. Antimicrobial activities of pomegranate rind extracts: Enhancement by addition of metal salts and vitamin C. BMC Complement. Altern. Med. 2008, 8, 64. [Google Scholar] [CrossRef] [Green Version]
- David, R. Why zinc is bad for bacteria. Nat. Rev. Genet. 2011, 10, 4. [Google Scholar] [CrossRef]
- Dörr, T.; Moynihan, P.J.; Mayer, C. Editorial: Bacterial Cell Wall Structure and Dynamics. Front. Microbiol. 2019, 10, 2051. [Google Scholar] [CrossRef] [Green Version]
- Brown, D.G.; May-Dracka, T.L.; Gagnon, M.M.; Tommasi, R. Trends and exceptions of physical properties on antibacterial activity for Gram-positive and Gram-negative pathogens. J. Med. Chem. 2014, 57, 10144–10161. [Google Scholar] [CrossRef] [PubMed]
- Wafa, B.A.; Makni, M.; Ammar, S.; Khannous, L.; Hassana, A.B.; Bouaziz, M.; Gdoura, R. Antimicrobial effect of the Tunisian Nana variety Punica granatum L. extracts against Salmonella enterica (serovars Kentucky and Enteritidis) isolated from chicken meat and phenolic composition of its peel extract. Int. J. Food Microbiol. 2017, 241, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Wilson, W.W.; Wade, M.M.; Holman, S.C.; Champlin, F.R. Status of methods for assessing bacterial cell surface charge properties based on Zeta potential measurements. J. Microbiol. Methods 2001, 43, 153–164. [Google Scholar] [CrossRef]
- Houston, D.M.; Bugert, J.J.; Denyer, S.P.; Heard, C.M. Anti-inflammatory activity of Punica granatum L. (pomegranate) rind extracts applied topically to ex vivo skin. Eur. J. Pharm. Biopharm. 2017, 112, 30–37. [Google Scholar] [CrossRef]
- Celiksoy, V.; Moses, R.L.; Sloan, A.J.; Moseley, R.; Heard, C.M. Evaluation of the in vitro oral wound healing effects of pomegranate (Punica granatum) rind extract and punicalagin, in combination with Zn (II). Biomolecules 2020, 10, 1234. [Google Scholar] [CrossRef]
- Sharquie, K.E.; Al-Mashhadani, S.A.; Salman, H.A. Topical 10% zinc sulfate solution for treatment of melasma. Dermatol. Surg. 2008, 34, 1346–1349. [Google Scholar] [CrossRef] [PubMed]
- Sharquie, K.E.; Al-Mashhadani, S.A.; Noaimi, A.A.; Hasan, A.A. Topical zinc sulphate (25%) solution: A new therapy for actinic keratosis. J. Cutan. Aesthet. Surg. 2012, 5, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Mahajan, V.K.; Mehta, K.S.; Chauhan, P.S. Zinc therapy in dermatology: A review. Dermatol. Res. Pract. 2014, 2014, 709152. [Google Scholar] [CrossRef] [PubMed]
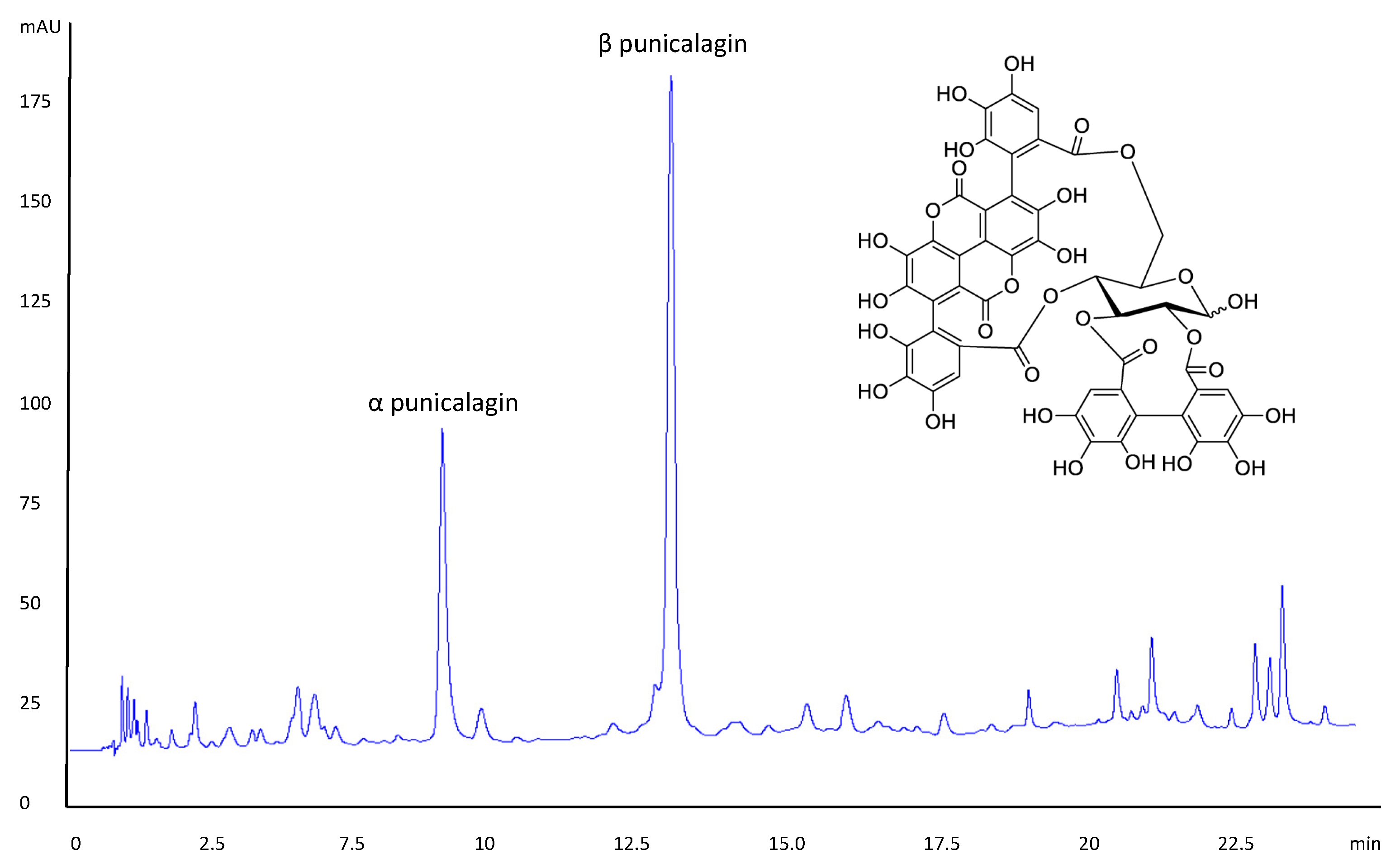



| Time (min) | A. % MeOH + 0.1% TFA | B. % H2O + 0.1% TFA |
|---|---|---|
| 0 | 5 | 95 |
| 7 | 10 | 90 |
| 15 | 20 | 80 |
| 20 | 40 | 60 |
| 25 | 60 | 40 |
| 30 | 5 | 95 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alrashidi, A.; Jafar, M.; Higgins, N.; Mulligan, C.; Varricchio, C.; Moseley, R.; Celiksoy, V.; Houston, D.M.J.; Heard, C.M. A Time-Kill Assay Study on the Synergistic Bactericidal Activity of Pomegranate Rind Extract and Zn (II) against Methicillin-Resistant Staphylococcus aureus (MRSA), Staphylococcus epidermidis, Escherichia coli, and Pseudomonas aeruginosa. Biomolecules 2021, 11, 1889. https://doi.org/10.3390/biom11121889
Alrashidi A, Jafar M, Higgins N, Mulligan C, Varricchio C, Moseley R, Celiksoy V, Houston DMJ, Heard CM. A Time-Kill Assay Study on the Synergistic Bactericidal Activity of Pomegranate Rind Extract and Zn (II) against Methicillin-Resistant Staphylococcus aureus (MRSA), Staphylococcus epidermidis, Escherichia coli, and Pseudomonas aeruginosa. Biomolecules. 2021; 11(12):1889. https://doi.org/10.3390/biom11121889
Chicago/Turabian StyleAlrashidi, Amal, Mohammed Jafar, Niamh Higgins, Ciara Mulligan, Carmine Varricchio, Ryan Moseley, Vildan Celiksoy, David M. J. Houston, and Charles M. Heard. 2021. "A Time-Kill Assay Study on the Synergistic Bactericidal Activity of Pomegranate Rind Extract and Zn (II) against Methicillin-Resistant Staphylococcus aureus (MRSA), Staphylococcus epidermidis, Escherichia coli, and Pseudomonas aeruginosa" Biomolecules 11, no. 12: 1889. https://doi.org/10.3390/biom11121889
APA StyleAlrashidi, A., Jafar, M., Higgins, N., Mulligan, C., Varricchio, C., Moseley, R., Celiksoy, V., Houston, D. M. J., & Heard, C. M. (2021). A Time-Kill Assay Study on the Synergistic Bactericidal Activity of Pomegranate Rind Extract and Zn (II) against Methicillin-Resistant Staphylococcus aureus (MRSA), Staphylococcus epidermidis, Escherichia coli, and Pseudomonas aeruginosa. Biomolecules, 11(12), 1889. https://doi.org/10.3390/biom11121889








