Nanoscale Sub-Compartmentalization of the Dendritic Spine Compartment
Abstract
:1. Introduction
2. Dendritic Spines, Discrete Subcellular Compartment of the Neuronal Membrane
3. Cannabinoids and Cannabinoid Receptors
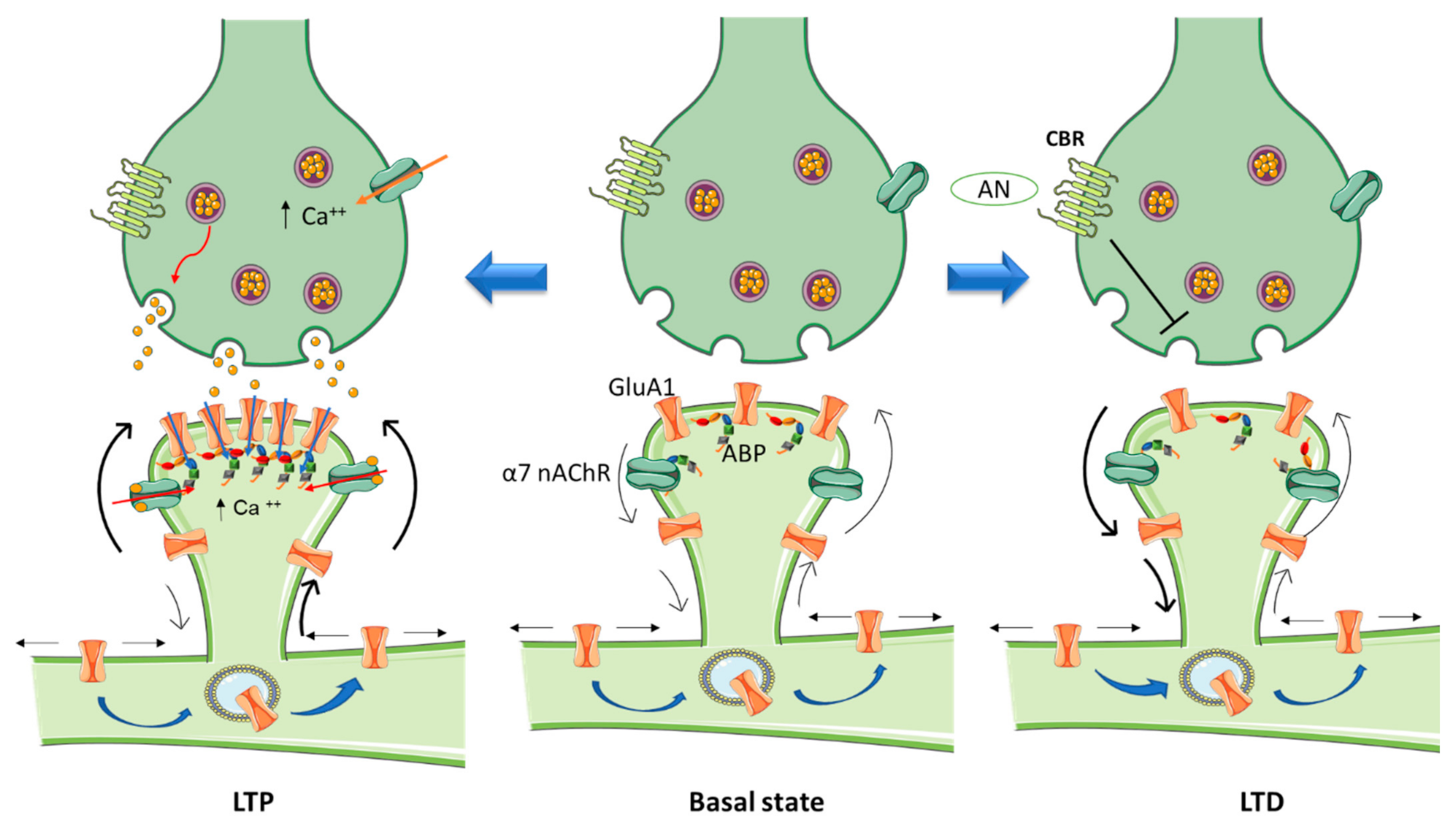
4. Cannabinoid Regulation of Synaptic Function and Compartmentalization
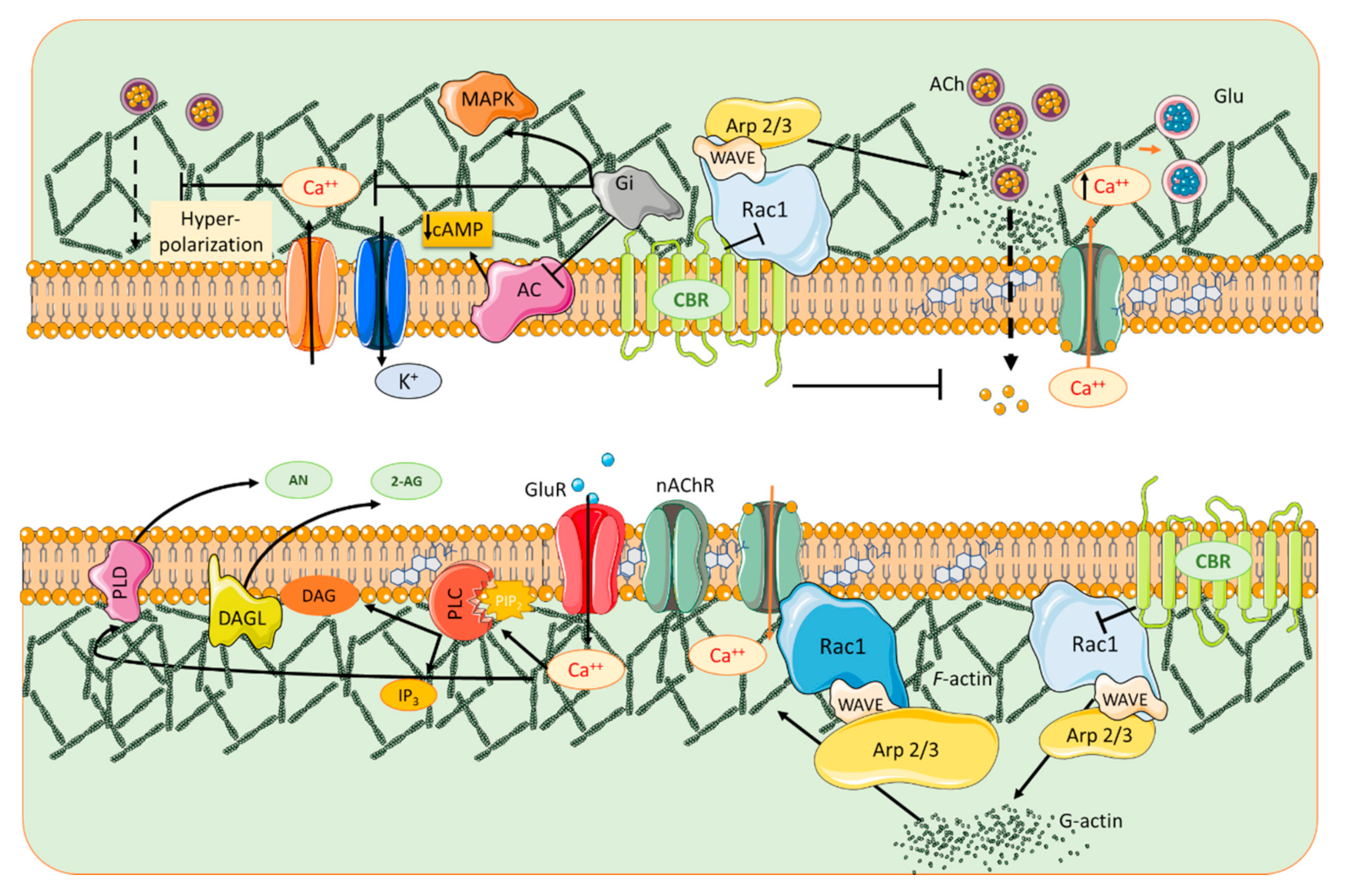
5. Cholinergic Signaling Contribution to Glutamatergic Receptor Compartmentalization
6. Cannabinoids and Nicotinic Receptors
7. Importance of Lipids in Dendritic Spine Compartmentalization
Impact of the Lipid Microenvironment on nAChRs and CBRs
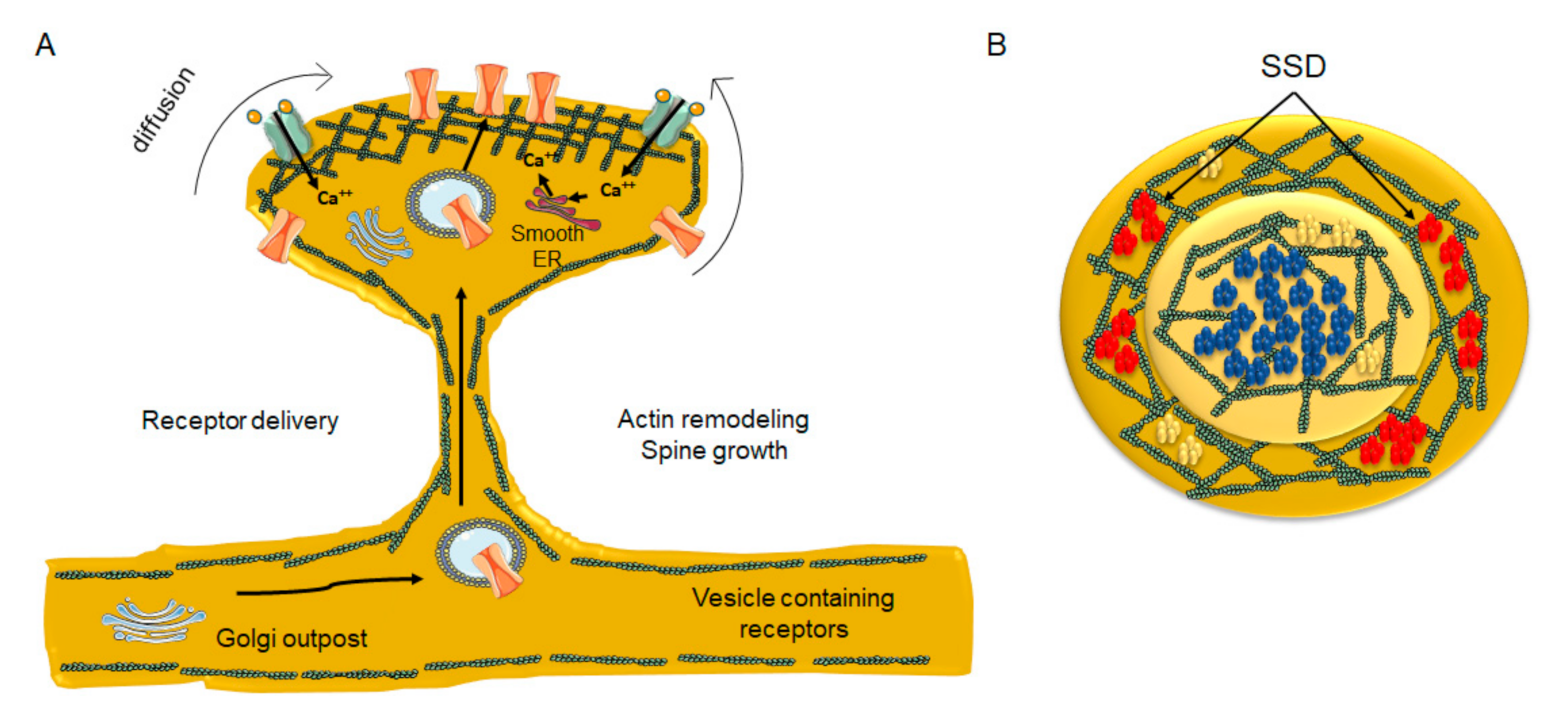
8. Contribution of Actin Dynamics to the Compartmentalization of the Dendritic Spine
9. Nanodomain Cluster Organization Emerges as a Common Organizing Principle at CNS Synapses
10. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Kusumi, A.; Shirai, Y.M.; Koyama-Honda, I.; Suzuki, K.G.N.; Fujiwara, T.K. Hierarchical organization of the plasma membrane: Investigations by single-molecule tracking vs. fluorescence correlation spectroscopy. FEBS Lett. 2010, 584, 1814–1823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kusumi, A.; Fujiwara, T.K.; Tsunoyama, T.A.; Kasai, R.S.; Liu, A.-A.; Hirosawa, K.M.; Kinoshita, M.; Matsumori, N.; Komura, N.; Ando, H.; et al. Defining raft domains in the plasma membrane. Traffic 2020, 21, 106–137. [Google Scholar] [CrossRef]
- Jacobson, K.; Liu, P. Complexity Revealed: A Hierarchy of Clustered Membrane Proteins. Biophys. J. 2016, 111, 1–2. [Google Scholar] [CrossRef] [Green Version]
- Simons, K.; Ikonen, E. Functional rafts in cell membranes. Nature 1997, 387, 569–572. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, K.; Dietrich, C. Looking at lipid rafts? Trends Cell Biol. 1999, 9, 87–91. [Google Scholar] [CrossRef]
- Simons, K.; Toomre, D. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 2000, 1, 31–39. [Google Scholar] [CrossRef]
- Brusés, J.L.; Chauvet, N.; Rutishauser, U. Membrane lipid rafts are necessary for the maintenance of the (alpha)7 nicotinic acetylcholine receptor in somatic spines of ciliary neurons. J. Neurosci. 2001, 21, 504–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayashi, T.; Su, T.-P. Sigma-1 receptors at galactosylceramide-enriched lipid microdomains regulate oligodendrocyte differentiation. Proc. Natl. Acad. Sci. USA 2004, 101, 14949–14954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, J.A.; Halverson-Tamboli, R.A.; Rasenick, M.M. Lipid raft microdomains and neurotransmitter signalling. Nat. Rev. Neurosci. 2007, 8, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Egawa, J.; Pearn, M.L.; Lemkuil, B.P.; Patel, P.M.; Head, B.P. Membrane lipid rafts and neurobiology: Age-related changes in membrane lipids and loss of neuronal function. J. Physiol. 2016, 594, 4565–4579. [Google Scholar] [CrossRef] [Green Version]
- Nagappan, G.; Lu, B. Activity-dependent modulation of the BDNF receptor TrkB: Mechanisms and implications. Trends Neurosci. 2005, 28, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.C.; Berg, D.K.; Gómez-Varela, D. Lateral mobility of nicotinic acetylcholine receptors on neurons is determined by receptor composition, local domain, and cell type. J. Neurosci. 2010, 30, 8841–8851. [Google Scholar] [CrossRef] [Green Version]
- Dunaevsky, A.; Tashiro, A.; Majewska, A.; Mason, C.; Yuste, R. Developmental regulation of spine motility in the mammalian central nervous system. Proc. Natl. Acad. Sci. USA 1999, 96, 13438–13443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heine, M.; Holcman, D. Asymmetry between Pre- and Postsynaptic Transient Nanodomains Shapes Neuronal Communication. Trends Neurosci. 2020, 43, 182–196. [Google Scholar] [CrossRef] [PubMed]
- Hering, H.; Sheng, M. Dentritic spines: Structure, dynamics and regulation. Nat. Rev. Neurosci. 2001, 2, 880–888. [Google Scholar] [CrossRef]
- Trommald, M.; Hulleberg, G. Dimensions and density of dendritic spines from rat dentate granule cells based on reconstructions from serial electron micrographs. J. Comp. Neurol. 1997, 377, 15–28. [Google Scholar] [CrossRef]
- Arellano, J.I.; Benavides-Piccione, R.; Defelipe, J.; Yuste, R. Ultrastructure of dendritic spines: Correlation between synaptic and spine morphologies. Front. Neurosci. 2007, 1, 131–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, K.M.; Stevens, J.K. Dendritic spines of CA 1 pyramidal cells in the rat hippocampus: Serial electron microscopy with reference to their biophysical characteristics. J. Neurosci. 1989, 9, 2982–2997. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, J.; Nagaoka, A.; Watanabe, S.; Ellis-Davies, G.C.R.; Kitamura, K.; Kano, M.; Matsuzaki, M.; Kasai, H. In vivo two-photon uncaging of glutamate revealing the structure-function relationships of dendritic spines in the neocortex of adult mice. J. Physiol. 2011, 589, 2447–2457. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, M.; Ellis-Davies, G.C.R.; Nemoto, T.; Miyashita, Y.; Iino, M.; Kasai, H. Dendritic spine geometry is critical for AMPA receptor expression in hippocampal CA1 pyramidal neurons. Nat. Neurosci. 2001, 4, 1086–1092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Citri, A.; Malenka, R.C. Synaptic Plasticity: Multiple Forms, Functions, and Mechanisms. Neuropsychopharmacology 2008, 33, 18–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bliss, T.V.P.; Cooke, S.F. Long-term potentiation and long-term depression: A clinical perspective. Clinics 2011, 66 (Suppl. 1), 3–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuzaki, M.; Honkura, N.; Ellis-Davies, G.C.R.; Kasai, H. Structural basis of long-term potentiation in single dendritic spines. Nature 2004, 429, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Lang, C.; Barco, A.; Zablow, L.; Kandel, E.R.; Siegelbaum, S.A.; Zakharenko, S.S. Transient expansion of synaptically connected dendritic spines upon induction of hippocampal long-term potentiation. Proc. Natl. Acad. Sci. USA 2004, 101, 16665–16670. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Q.; Homma, K.J.; Poo, M. Shrinkage of dendritic spines associated with long-term depression of hippocampal synapses. Neuron 2004, 44, 749–757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nägerl, U.V.; Eberhorn, N.; Cambridge, S.B.; Bonhoeffer, T. Bidirectional activity-dependent morphological plasticity in hippocampal neurons. Neuron 2004, 44, 759–767. [Google Scholar] [CrossRef] [Green Version]
- Tønnesen, J.; Katona, G.; Rózsa, B.; Nägerl, U.V. Spine neck plasticity regulates compartmentalization of synapses. Nat. Neurosci. 2014, 17, 678–685. [Google Scholar] [CrossRef] [Green Version]
- Vanderklish, P.W.; Edelman, G.M. Dendritic spines elongate after stimulation of group 1 metabotropic glutamate receptors in cultured hippocampal neurons. Proc. Natl. Acad. Sci. USA 2002, 99, 1639–1644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tønnesen, J.; Nägerl, U.V. Dendritic Spines as Tunable Regulators of Synaptic Signals. Front. Psychiatry 2016, 7, 101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, W.; Connor, J.A. Dendritic spines as individual neuronal compartments for synaptic Ca2+ responses. Nature 1991, 354, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Guthrie, P.B.; Segal, M.; Kater, S.B. Independent regulation of calcium revealed by imaging dendritic spines. Nature 1991, 354, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Honigmann, A.; Pralle, A. Compartmentalization of the Cell Membrane. J. Mol. Biol. 2016, 428, 4739–4748. [Google Scholar] [CrossRef] [PubMed]
- Correa, F.; Wolfson, M.L.; Valchi, P.; Aisemberg, J.; Franchi, A.M. Endocannabinoid system and pregnancy. Reproduction 2016, 152, R191–R200. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, V. The endocannabinoid system: Its general strategy of action, tools for its pharmacological manipulation and potential therapeutic exploitation. Pharmacol. Res. 2009, 60, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Devane, W.A.; Hanus, L.; Breuer, A.; Pertwee, R.G.; Stevenson, L.A.; Griffin, G.; Gibson, D.; Mandelbaum, A.; Etinger, A.; Mechoulam, R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 1992, 258, 1946–1949. [Google Scholar] [CrossRef]
- Rankin, L.; Fowler, C.J. The Basal Pharmacology of Palmitoylethanolamide. Int. J. Mol. Sci. 2020, 21, 7942. [Google Scholar] [CrossRef] [PubMed]
- Hebert-Chatelain, E.; Desprez, T.; Serrat, R.; Bellocchio, L.; Soria-Gomez, E.; Busquets-Garcia, A.; Pagano Zottola, A.C.; Delamarre, A.; Cannich, A.; Vincent, P.; et al. A cannabinoid link between mitochondria and memory. Nature 2016, 539, 555–559. [Google Scholar] [CrossRef]
- Matsuda, L.A.; Lolait, S.J.; Brownstein, M.J.; Young, A.C.; Bonner, T.I. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 1990, 346, 561–564. [Google Scholar] [CrossRef]
- Munro, S.; Thomas, K.L.; Abu-Shaar, M. Molecular characterization of a peripheral receptor for cannabinoids. Nature 1993, 365, 61–65. [Google Scholar] [CrossRef]
- Herkenham, M.; Lynn, A.B.; Little, M.D.; Johnson, M.R.; Melvin, L.S.; de Costa, B.R.; Rice, K.C. Cannabinoid receptor localization in brain. Proc. Natl. Acad. Sci. USA 1990, 87, 1932–1936. [Google Scholar] [CrossRef] [Green Version]
- Núñez, E.; Benito, C.; Pazos, M.R.; Barbachano, A.; Fajardo, O.; González, S.; Tolón, R.M.; Romero, J. Cannabinoid CB2 receptors are expressed by perivascular microglial cells in the human brain: An immunohistochemical study. Synapse 2004, 53, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Van Sickle, M.D.; Duncan, M.; Kingsley, P.J.; Mouihate, A.; Urbani, P.; Mackie, K.; Stella, N.; Makriyannis, A.; Piomelli, D.; Davison, J.S.; et al. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science 2005, 310, 329–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ong, W.Y.; Mackie, K. A light and electron microscopic study of the CB1 cannabinoid receptor in the primate spinal cord. J. Neurocytol. 1999, 28, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Kano, M.; Ohno-Shosaku, T.; Hashimotodani, Y.; Uchigashima, M.; Watanabe, M. Endocannabinoid-mediated control of synaptic transmission. Physiol. Rev. 2009, 89, 309–380. [Google Scholar] [CrossRef]
- Deadwyler, S.A.; Hampson, R.E.; Mu, J.; Whyte, A.; Childers, S. Cannabinoids modulate voltage sensitive potassium A-current in hippocampal neurons via a cAMP-dependent process. J. Pharmacol. Exp. Ther. 1995, 273, 734–743. [Google Scholar] [PubMed]
- Vásquez, C.; Navarro-Polanco, R.A.; Huerta, M.; Trujillo, X.; Andrade, F.; Trujillo-Hernández, B.; Hernández, L. Effects of cannabinoids on endogenous K+ and Ca2+ currents in HEK293 cells. Can. J. Physiol. Pharmacol. 2003, 81, 436–442. [Google Scholar] [CrossRef]
- Howlett, A.C.; Breivogel, C.S.; Childers, S.R.; Deadwyler, S.A.; Hampson, R.E.; Porrino, L.J. Cannabinoid physiology and pharmacology: 30 years of progress. Neuropharmacology 2004, 47 (Suppl. 1), 345–358. [Google Scholar] [CrossRef]
- Hampson, R.E.; Evans, G.J.; Mu, J.; Zhuang, S.Y.; King, V.C.; Childers, S.R.; Deadwyler, S.A. Role of cyclic AMP dependent protein kinase in cannabinoid receptor modulation of potassium “A-current” in cultured rat hippocampal neurons. Life Sci. 1995, 56, 2081–2088. [Google Scholar] [CrossRef]
- Howlett, A.C.; Barth, F.; Bonner, T.I.; Cabral, G.; Casellas, P.; Devane, W.A.; Felder, C.C.; Herkenham, M.; Mackie, K.; Martin, B.R.; et al. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol. Rev. 2002, 54, 161–202. [Google Scholar] [CrossRef]
- Bouaboula, M.; Perrachon, S.; Milligan, L.; Canat, X.; Rinaldi-Carmona, M.; Portier, M.; Barth, F.; Calandra, B.; Pecceu, F.; Lupker, J.; et al. A selective inverse agonist for central cannabinoid receptor inhibits mitogen-activated protein kinase activation stimulated by insulin or insulin-like growth factor 1. Evidence for a new model of receptor/ligand interactions. J. Biol. Chem. 1997, 272, 22330–22339. [Google Scholar] [CrossRef] [Green Version]
- Bouaboula, M.; Poinot-Chazel, C.; Bourrié, B.; Canat, X.; Calandra, B.; Rinaldi-Carmona, M.; Le Fur, G.; Casellas, P. Activation of mitogen-activated protein kinases by stimulation of the central cannabinoid receptor CB1. Biochem. J. 1995, 312 Pt 2, 637–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Derkinderen, P.; Ledent, C.; Parmentier, M.; Girault, J.A. Cannabinoids activate p38 mitogen-activated protein kinases through CB1 receptors in hippocampus. J. Neurochem. 2001, 77, 957–960. [Google Scholar] [CrossRef] [PubMed]
- Muccioli, G.G. Endocannabinoid biosynthesis and inactivation, from simple to complex. Drug Discov. Today 2010, 15, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Alger, B.E. Endocannabinoid signaling in neural plasticity. Curr. Top. Behav. Neurosci. 2009, 1, 141–172. [Google Scholar] [CrossRef] [PubMed]
- Edwards, D.A.; Zhang, L.; Alger, B.E. Metaplastic control of the endocannabinoid system at inhibitory synapses in hippocampus. Proc. Natl. Acad. Sci. USA 2008, 105, 8142–8147. [Google Scholar] [CrossRef] [Green Version]
- Melis, M.; Greco, B.; Tonini, R. Interplay between synaptic endocannabinoid signaling and metaplasticity in neuronal circuit function and dysfunction. Eur. J. Neurosci. 2014, 39, 1189–1201. [Google Scholar] [CrossRef]
- Iremonger, K.J.; Wamsteeker Cusulin, J.I.; Bains, J.S. Changing the tune: Plasticity and adaptation of retrograde signals. Trends Neurosci. 2013, 36, 471–479. [Google Scholar] [CrossRef]
- Hohmann, T.; Feese, K.; Ghadban, C.; Dehghani, F.; Grabiec, U. On the influence of cannabinoids on cell morphology and motility of glioblastoma cells. PLoS ONE 2019, 14, e0212037. [Google Scholar] [CrossRef] [PubMed]
- Njoo, C.; Agarwal, N.; Lutz, B.; Kuner, R. The Cannabinoid Receptor CB1 Interacts with the WAVE1 Complex and Plays a Role in Actin Dynamics and Structural Plasticity in Neurons. PLOS Biol. 2015, 13, e1002286. [Google Scholar] [CrossRef] [Green Version]
- Ladarre, D.; Roland, A.B.; Biedzinski, S.; Ricobaraza, A.; Lenkei, Z. Polarized cellular patterns of endocannabinoid production and detection shape cannabinoid signaling in neurons. Front. Cell. Neurosci. 2014, 8, 426. [Google Scholar] [CrossRef] [Green Version]
- Leterrier, C.; Lainé, J.; Darmon, M.; Boudin, H.; Rossier, J.; Lenkei, Z. Constitutive activation drives compartment-selective endocytosis and axonal targeting of type 1 cannabinoid receptors. J. Neurosci. 2006, 26, 3141–3153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nimchinsky, E.A.; Sabatini, B.L.; Svoboda, K. Structure and function of dendritic spines. Annu. Rev. Physiol. 2002, 64, 313–353. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, A.F.; Reyes, B.A.S.; Ramalhosa, F.; Sousa, N.; Van Bockstaele, E.J. Repeated administration of a synthetic cannabinoid receptor agonist differentially affects cortical and accumbal neuronal morphology in adolescent and adult rats. Brain Struct. Funct. 2016, 221, 407–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spiga, S.; Lintas, A.; Diana, M. Altered Mesolimbic Dopamine System in THC Dependence. Curr. Neuropharmacol. 2011, 9, 200–204. [Google Scholar] [CrossRef] [Green Version]
- Spiga, S.; Lintas, A.; Migliore, M.; Diana, M. Altered architecture and functional consequences of the mesolimbic dopamine system in cannabis dependence. Addict. Biol. 2010, 15, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Monday, H.R.; Bourdenx, M.; Jordan, B.A.; Castillo, P.E. Cb1-receptor-mediated inhibitory ltd triggers presynaptic remodeling via protein synthesis and ubiquitination. Elife 2020, 9, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Incontro, S.; Sammari, M.; Azzaz, F.; Inglebert, Y.; Ankri, N.; Russier, M.; Fantini, J.; Debanne, D. Endocannabinoids tune intrinsic excitability in O-LM interneurons by direct modulation of post-synaptic Kv7 channels. J. Neurosci. 2021. [Google Scholar] [CrossRef]
- Okamura, K.; Tanaka, H.; Yagita, Y.; Saeki, Y.; Taguchi, A.; Hiraoka, Y.; Zeng, L.H.; Colman, D.R.; Miki, N. Cadherin activity is required for activity-induced spine remodeling. J. Cell Biol. 2004, 167, 961–972. [Google Scholar] [CrossRef]
- McKinney, R.A. Excitatory amino acid involvement in dendritic spine formation, maintenance and remodelling. J. Physiol. 2010, 588, 107–116. [Google Scholar] [CrossRef]
- Dani, J.A.; Bertrand, D. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 699–729. [Google Scholar] [CrossRef]
- Taly, A.; Corringer, P.-J.; Guedin, D.; Lestage, P.; Changeux, J.-P. Nicotinic receptors: Allosteric transitions and therapeutic targets in the nervous system. Nat. Rev. Drug Discov. 2009, 8, 733–750. [Google Scholar] [CrossRef] [PubMed]
- Livingstone, P.D.; Wonnacott, S. Nicotinic acetylcholine receptors and the ascending dopamine pathways. Biochem. Pharmacol. 2009, 78, 744–755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albuquerque, E.X.; Pereira, E.F.R.; Alkondon, M.; Rogers, S.W. Mammalian nicotinic acetylcholine receptors: From structure to function. Physiol. Rev. 2009, 89, 73–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gotti, C.; Clementi, F.; Fornari, A.; Gaimarri, A.; Guiducci, S.; Manfredi, I.; Moretti, M.; Pedrazzi, P.; Pucci, L.; Zoli, M. Structural and functional diversity of native brain neuronal nicotinic receptors. Biochem. Pharmacol. 2009, 78, 703–711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McQuiston, A.R. Acetylcholine release and inhibitory interneuron activity in hippocampal CA1. Front. Synaptic Neurosci. 2014, 6, 20. [Google Scholar] [CrossRef]
- Marchi, M.; Risso, F.; Viola, C.; Cavazzani, P.; Raiteri, M. Direct evidence that release-stimulating alpha7* nicotinic cholinergic receptors are localized on human and rat brain glutamatergic axon terminals. J. Neurochem. 2002, 80, 1071–1078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lozada, A.F.; Wang, X.; Gounko, N.V.; Massey, K.A.; Duan, J.; Liu, Z.; Berg, D.K. Induction of dendritic spines by β2-containing nicotinic receptors. J. Neurosci. 2012, 32, 8391–8400. [Google Scholar] [CrossRef] [PubMed]
- Lozada, A.F.; Wang, X.; Gounko, N.V.; Massey, K.A.; Duan, J.; Liu, Z.; Berg, D.K. Glutamatergic synapse formation is promoted by α7-containing nicotinic acetylcholine receptors. J. Neurosci. 2012, 32, 7651–7661. [Google Scholar] [CrossRef] [Green Version]
- Halff, A.W.; Gómez-Varela, D.; John, D.; Berg, D.K. A Novel Mechanism for Nicotinic Potentiation of Glutamatergic Synapses. J. Neurosci. 2014, 34, 2051–2064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balfour, D.J.K. The role of mesoaccumbens dopamine in nicotine dependence. Curr. Top. Behav. Neurosci. 2015, 24, 55–98. [Google Scholar] [CrossRef] [PubMed]
- Pidoplichko, V.I.; DeBiasi, M.; Williams, J.T.; Dani, J.A. Nicotine activates and desensitizes midbrain dopamine neurons. Nature 1997, 390, 401–404. [Google Scholar] [CrossRef]
- Corrigall, W.A.; Franklin, K.B.; Coen, K.M.; Clarke, P.B. The mesolimbic dopaminergic system is implicated in the reinforcing effects of nicotine. Psychopharmacology 1992, 107, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Di Chiara, G. Role of dopamine in the behavioural actions of nicotine related to addiction. Eur. J. Pharmacol. 2000, 393, 295–314. [Google Scholar] [CrossRef]
- Wise, R.A. Dopamine, learning and motivation. Nat. Rev. Neurosci. 2004, 5, 483–494. [Google Scholar] [CrossRef]
- Watkins, S.S.; Epping-Jordan, M.P.; Koob, G.F.; Markou, A. Blockade of nicotine self-administration with nicotinic antagonists in rats. Pharmacol. Biochem. Behav. 1999, 62, 743–751. [Google Scholar] [CrossRef]
- Cohen, C.; Bergis, O.E.; Galli, F.; Lochead, A.W.; Jegham, S.; Biton, B.; Leonardon, J.; Avenet, P.; Sgard, F.; Besnard, F.; et al. SSR591813, a novel selective and partial alpha4beta2 nicotinic receptor agonist with potential as an aid to smoking cessation. J. Pharmacol. Exp. Ther. 2003, 306, 407–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Picciotto, M.R.; Zoli, M.; Rimondini, R.; Léna, C.; Marubio, L.M.; Pich, E.M.; Fuxe, K.; Changeux, J.P. Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature 1998, 391, 173–177. [Google Scholar] [CrossRef]
- Marubio, L.M.; Gardier, A.M.; Durier, S.; David, D.; Klink, R.; Arroyo-Jimenez, M.M.; McIntosh, J.M.; Rossi, F.; Champtiaux, N.; Zoli, M.; et al. Effects of nicotine in the dopaminergic system of mice lacking the alpha4 subunit of neuronal nicotinic acetylcholine receptors. Eur. J. Neurosci. 2003, 17, 1329–1337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xi, Z.; Spiller, K.; Gardner, E.L. Mechanism-based medication development for the treatment of nicotine dependence. Acta Pharmacol. Sin. 2009, 30, 723–739. [Google Scholar] [CrossRef] [Green Version]
- Scott, L.; Zelenin, S.; Malmersjö, S.; Kowalewski, J.M.; Markus, E.Z.; Nairn, A.C.; Greengard, P.; Brismar, H.; Aperia, A. Allosteric changes of the NMDA receptor trap diffusible dopamine 1 receptors in spines. Proc. Natl. Acad. Sci. USA 2006, 103, 762–767. [Google Scholar] [CrossRef] [Green Version]
- Cepeda, C.; Levine, M.S. Where do you think you are going? The NMDA-D1 receptor trap. Sci. STKE 2006, 2006, pe20. [Google Scholar] [CrossRef]
- Calabresi, P.; Gubellini, P.; Centonze, D.; Picconi, B.; Bernardi, G.; Chergui, K.; Svenningsson, P.; Fienberg, A.A.; Greengard, P. Dopamine and cAMP-regulated phosphoprotein 32 kDa controls both striatal long-term depression and long-term potentiation, opposing forms of synaptic plasticity. J. Neurosci. 2000, 20, 8443–8451. [Google Scholar] [CrossRef]
- Kerr, J.N.; Wickens, J.R. Dopamine D-1/D-5 receptor activation is required for long-term potentiation in the rat neostriatum in vitro. J. Neurophysiol. 2001, 85, 117–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sawaguchi, T.; Goldman-Rakic, P.S. The role of D1-dopamine receptor in working memory: Local injections of dopamine antagonists into the prefrontal cortex of rhesus monkeys performing an oculomotor delayed-response task. J. Neurophysiol. 1994, 71, 515–528. [Google Scholar] [CrossRef] [PubMed]
- Fiorentini, C.; Gardoni, F.; Spano, P.; Di Luca, M.; Missale, C. Regulation of dopamine D1 receptor trafficking and desensitization by oligomerization with glutamate N-methyl-D-aspartate receptors. J. Biol. Chem. 2003, 278, 20196–20202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maurer, S.V.; Williams, C.L. The Cholinergic System Modulates Memory and Hippocampal Plasticity via Its Interactions with Non-Neuronal Cells. Front. Immunol. 2017, 8, 1489. [Google Scholar] [CrossRef] [Green Version]
- Prado, V.F.; Janickova, H.; Al-Onaizi, M.A.; Prado, M.A.M. Cholinergic circuits in cognitive flexibility. Neuroscience 2017, 345, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Almeida, L.E.F.; Spornick, N.A.; Kenyon, N.; Kamimura, S.; Khaibullina, A.; Nouraie, M.; Quezado, Z.M.N. Modulation of social deficits and repetitive behaviors in a mouse model of autism: The role of the nicotinic cholinergic system. Psychopharmacology 2015, 232, 4303–4316. [Google Scholar] [CrossRef] [PubMed]
- Oz, M. Receptor-independent actions of cannabinoids on cell membranes: Focus on endocannabinoids. Pharmacol. Ther. 2006, 111, 114–144. [Google Scholar] [CrossRef] [PubMed]
- Pertwee, R.G. Receptors and channels targeted by synthetic cannabinoid receptor agonists and antagonists. Curr. Med. Chem. 2010, 17, 1360–1381. [Google Scholar] [CrossRef] [Green Version]
- Scherma, M.; Muntoni, A.; Melis, M.; Fattore, L.; Fadda, P.; Fratta, W.; Pistis, M. Interactions between the endocannabinoid and nicotinic cholinergic systems: Preclinical evidence and therapeutic perspectives. Psychopharmacology 2016, 233, 1765–1777. [Google Scholar] [CrossRef]
- Jackson, K.J.; Marks, M.J.; Vann, R.E.; Chen, X.; Gamage, T.F.; Warner, J.A.; Damaj, M.I. Role of alpha5 nicotinic acetylcholine receptors in pharmacological and behavioral effects of nicotine in mice. J. Pharmacol. Exp. Ther. 2010, 334, 137–146. [Google Scholar] [CrossRef] [Green Version]
- Howlett, A.C.; Bidaut-Russell, M.; Devane, W.A.; Melvin, L.S.; Johnson, M.R.; Herkenham, M. The cannabinoid receptor: Biochemical, anatomical and behavioral characterization. Trends Neurosci. 1990, 13, 420–423. [Google Scholar] [CrossRef]
- Lichtman, A.H.; Cook, S.A.; Martin, B.R. Investigation of brain sites mediating cannabinoid-induced antinociception in rats: Evidence supporting periaqueductal gray involvement. J. Pharmacol. Exp. Ther. 1996, 276, 585–593. [Google Scholar] [PubMed]
- Sañudo-Peña, M.C.; Romero, J.; Seale, G.E.; Fernandez-Ruiz, J.J.; Walker, J.M. Activational role of cannabinoids on movement. Eur. J. Pharmacol. 2000, 391, 269–274. [Google Scholar] [CrossRef]
- Justinova, Z.; Goldberg, S.R.; Heishman, S.J.; Tanda, G. Self-administration of cannabinoids by experimental animals and human marijuana smokers. Pharmacol. Biochem. Behav. 2005, 81, 285–299. [Google Scholar] [CrossRef] [Green Version]
- Ahsan, H.M.; de la Peña, J.B.I.; Botanas, C.J.; Kim, H.J.; Yu, G.Y.; Cheong, J.H. Conditioned place preference and self-administration induced by nicotine in adolescent and adult rats. Biomol. Ther. 2014, 22, 460–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scherma, M.; Justinová, Z.; Zanettini, C.; Panlilio, L.V.; Mascia, P.; Fadda, P.; Fratta, W.; Makriyannis, A.; Vadivel, S.K.; Gamaleddin, I.; et al. The anandamide transport inhibitor AM404 reduces the rewarding effects of nicotine and nicotine-induced dopamine elevations in the nucleus accumbens shell in rats. Br. J. Pharmacol. 2012, 165, 2539–2548. [Google Scholar] [CrossRef] [Green Version]
- Balerio, G.N.; Aso, E.; Berrendero, F.; Murtra, P.; Maldonado, R. Delta9-tetrahydrocannabinol decreases somatic and motivational manifestations of nicotine withdrawal in mice. Eur. J. Neurosci. 2004, 20, 2737–2748. [Google Scholar] [CrossRef]
- Balerio, G.N.; Aso, E.; Maldonado, R. Role of the cannabinoid system in the effects induced by nicotine on anxiety-like behaviour in mice. Psychopharmacology 2006, 184, 504–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valjent, E.; Mitchell, J.M.; Besson, M.-J.; Caboche, J.; Maldonado, R. Behavioural and biochemical evidence for interactions between Delta 9-tetrahydrocannabinol and nicotine. Br. J. Pharmacol. 2002, 135, 564–578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acquas, E.; Pisanu, A.; Marrocu, P.; Goldberg, S.R.; Di Chiara, G. Δ9-tetrahydrocannabinol enhances cortical and hippocampal acetylcholine release in vivo: A microdialysis study. Eur. J. Pharmacol. 2001, 419, 155–161. [Google Scholar] [CrossRef]
- Pisanu, A.; Acquas, E.; Fenu, S.; Di Chiara, G. Modulation of Δ9-THC-induced increase of cortical and hippocampal acetylcholine release by μ opioid and D1 dopamine receptors. Neuropharmacology 2006, 50, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Tzavara, E.T.; Wade, M.; Nomikos, G.G. Biphasic effects of cannabinoids on acetylcholine release in the hippocampus: Site and mechanism of action. J. Neurosci. 2003, 23, 9374–9384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gamaleddin, I.H.; Trigo, J.M.; Gueye, A.B.; Zvonok, A.; Makriyannis, A.; Goldberg, S.R.; Le Foll, B. Role of the endogenous cannabinoid system in nicotine addiction: Novel insights. Front. Psychiatry 2015, 6, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solinas, M.; Scherma, M.; Tanda, G.; Wertheim, C.E.; Fratta, W.; Goldberg, S.R. Nicotinic facilitation of delta9-tetrahydrocannabinol discrimination involves endogenous anandamide. J. Pharmacol. Exp. Ther. 2007, 321, 1127–1134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oz, M.; Ravindran, A.; Diaz-Ruiz, O.; Zhang, L.; Morales, M. The endogenous cannabinoid anandamide inhibits alpha7 nicotinic acetylcholine receptor-mediated responses in Xenopus oocytes. J. Pharmacol. Exp. Ther. 2003, 306, 1003–1010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oz, M.; Zhang, L.; Ravindran, A.; Morales, M.; Lupica, C.R. Differential effects of endogenous and synthetic cannabinoids on alpha7-nicotinic acetylcholine receptor-mediated responses in Xenopus Oocytes. J. Pharmacol. Exp. Ther. 2004, 310, 1152–1160. [Google Scholar] [CrossRef] [Green Version]
- Lagalwar, S.; Bordayo, E.Z.; Hoffmann, K.L.; Fawcett, J.R.; Frey, W.H. 2nd Anandamides inhibit binding to the muscarinic acetylcholine receptor. J. Mol. Neurosci. 1999, 13, 55–61. [Google Scholar] [CrossRef]
- Spivak, C.E.; Lupica, C.R.; Oz, M. The Endocannabinoid Anandamide Inhibits the Function of α4β2 Nicotinic Acetylcholine Receptors. Mol. Pharmacol. 2007, 72, 1024–1032. [Google Scholar] [CrossRef] [Green Version]
- Oz, M. Receptor-independent effects of endocannabinoids on ion channels. Curr. Pharm. Des. 2006, 12, 227–239. [Google Scholar] [CrossRef]
- Oz, M.; Jackson, S.N.; Woods, A.S.; Morales, M.; Zhang, L. Additive effects of endogenous cannabinoid anandamide and ethanol on alpha7-nicotinic acetylcholine receptor-mediated responses in Xenopus Oocytes. J. Pharmacol. Exp. Ther. 2005, 313, 1272–1280. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.N.; Singhal, S.K.; Woods, A.S.; Morales, M.; Shippenberg, T.; Zhang, L.; Oz, M. Volatile anesthetics and endogenous cannabinoid anandamide have additive and independent inhibitory effects on alpha(7)-nicotinic acetylcholine receptor-mediated responses in Xenopus oocytes. Eur. J. Pharmacol. 2008, 582, 42–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabatini, B.L.; Oertner, T.G.; Svoboda, K. The life cycle of Ca(2+) ions in dendritic spines. Neuron 2002, 33, 439–452. [Google Scholar] [CrossRef] [Green Version]
- Fujii, H.; Inoue, M.; Okuno, H.; Sano, Y.; Takemoto-Kimura, S.; Kitamura, K.; Kano, M.; Bito, H. Nonlinear decoding and asymmetric representation of neuronal input information by CaMKIIα and calcineurin. Cell Rep. 2013, 3, 978–987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, J.-Y.; Parra-Bueno, P.; Laviv, T.; Szatmari, E.M.; Lee, S.-J.R.; Yasuda, R. CaMKII Autophosphorylation Is Necessary for Optimal Integration of Ca(2+) Signals during LTP Induction, but Not Maintenance. Neuron 2017, 94, 800–808. [Google Scholar] [CrossRef] [Green Version]
- Murakoshi, H.; Wang, H.; Yasuda, R. Local, persistent activation of Rho GTPases during plasticity of single dendritic spines. Nature 2011, 472, 100. [Google Scholar] [CrossRef] [PubMed]
- Borovac, J.; Bosch, M.; Okamoto, K. Regulation of actin dynamics during structural plasticity of dendritic spines: Signaling messengers and actin-binding proteins. Mol. Cell. Neurosci. 2018, 91, 122–130. [Google Scholar] [CrossRef]
- Costa, J.F.; Dines, M.; Lamprecht, R. The Role of Rac GTPase in Dendritic Spine Morphogenesis and Memory. Front. Synaptic Neurosci. 2020, 12, 12. [Google Scholar] [CrossRef]
- Chernomordik, L.V.; Kozlov, M.M. Protein-lipid interplay in fusion and fission of biological membranes. Annu. Rev. Biochem. 2003, 72, 175–207. [Google Scholar] [CrossRef]
- Puchkov, D.; Haucke, V. Greasing the synaptic vesicle cycle by membrane lipids. Trends Cell Biol. 2013, 23, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Dieterich, D.C.; Kreutz, M.R. Proteomics of the Synapse—A Quantitative Approach to Neuronal Plasticity *. Mol. Cell. Proteomics 2016, 15, 368–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jurado, S.; Benoist, M.; Lario, A.; Knafo, S.; Petrok, C.N.; Esteban, J.A. PTEN is recruited to the postsynaptic terminal for NMDA receptor-dependent long-term depression. EMBO J. 2010, 29, 2827–2840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, M.G.; Ahmed, T.; Korovaichuk, A.; Venero, C.; Menchón, S.A.; Salas, I.; Munck, S.; Herreras, O.; Balschun, D.; Dotti, C.G. Constitutive hippocampal cholesterol loss underlies poor cognition in old rodents. EMBO Mol. Med. 2014, 6, 902–917. [Google Scholar] [CrossRef]
- Tulodziecka, K.; Diaz-Rohrer, B.B.; Farley, M.M.; Chan, R.B.; Di Paolo, G.; Levental, K.R.; Waxham, M.N.; Levental, I. Remodeling of the postsynaptic plasma membrane during neural development. Mol. Biol. Cell 2016, 27, 3480–3489. [Google Scholar] [CrossRef]
- Merrill, A.H.J. Sphingolipid and glycosphingolipid metabolic pathways in the era of sphingolipidomics. Chem. Rev. 2011, 111, 6387–6422. [Google Scholar] [CrossRef]
- Borgmeyer, M.; Coman, C.; Has, C.; Schött, H.-F.; Li, T.; Westhoff, P.; Cheung, Y.F.H.; Hoffmann, N.; Yuanxiang, P.; Behnisch, T.; et al. Multiomics of synaptic junctions reveals altered lipid metabolism and signaling following environmental enrichment. Cell Rep. 2021, 37, 109797. [Google Scholar] [CrossRef]
- van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane lipids: Where they are and how they behave. Nat. Rev. Mol. Cell Biol. 2008, 9, 112–124. [Google Scholar] [CrossRef]
- Casares, D.; Escribá, P.V.; Rosselló, C.A. Membrane Lipid Composition: Effect on Membrane and Organelle Structure, Function and Compartmentalization and Therapeutic Avenues. Int. J. Mol. Sci. 2019, 20, 2167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goñi, F.M.; Sot, J.; Alonso, A. Biophysical properties of sphingosine, ceramides and other simple sphingolipids. Biochem. Soc. Trans. 2014, 42, 1401–1408. [Google Scholar] [CrossRef]
- Sohn, J.; Lin, H.; Fritch, M.R.; Tuan, R.S. Influence of cholesterol/caveolin-1/caveolae homeostasis on membrane properties and substrate adhesion characteristics of adult human mesenchymal stem cells. Stem Cell Res. Ther. 2018, 9, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, E.S.; Oh, Y.; Sung, B.J. Interdomain exchange and the flip-flop of cholesterol in ternary component lipid membranes and their effects on heterogeneous cholesterol diffusion. Phys. Rev. E 2021, 104, 44402. [Google Scholar] [CrossRef]
- Baier, C.J.; Fantini, J.; Barrantes, F.J. Disclosure of cholesterol recognition motifs in transmembrane domains of the human nicotinic acetylcholine receptor. Sci. Rep. 2011, 1, 69. [Google Scholar] [CrossRef] [Green Version]
- Fantini, J.; Di Scala, C.; Evans, L.S.; Williamson, P.T.F.; Barrantes, F.J. A mirror code for protein-cholesterol interactions in the two leaflets of biological membranes. Sci. Rep. 2016, 6, 21907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oddi, S.; Dainese, E.; Fezza, F.; Lanuti, M.; Barcaroli, D.; De Laurenzi, V.; Centonze, D.; Maccarrone, M. Functional characterization of putative cholesterol binding sequence (CRAC) in human type-1 cannabinoid receptor. J. Neurochem. 2011, 116, 858–865. [Google Scholar] [CrossRef]
- Hua, T.; Vemuri, K.; Pu, M.; Qu, L.; Han, G.W.; Wu, Y.; Zhao, S.; Shui, W.; Li, S.; Korde, A.; et al. Crystal Structure of the Human Cannabinoid Receptor CB(1). Cell 2016, 167, 750–762. [Google Scholar] [CrossRef] [Green Version]
- Krishna Kumar, K.; Shalev-Benami, M.; Robertson, M.J.; Hu, H.; Banister, S.D.; Hollingsworth, S.A.; Latorraca, N.R.; Kato, H.E.; Hilger, D.; Maeda, S.; et al. Structure of a Signaling Cannabinoid Receptor 1-G Protein Complex. Cell 2019, 176, 448–458. [Google Scholar] [CrossRef] [Green Version]
- Allende, M.L.; Zhu, H.; Kono, M.; Hoachlander-Hobby, L.E.; Huso, V.L.; Proia, R.L. Genetic defects in the sphingolipid degradation pathway and their effects on microglia in neurodegenerative disease. Cell. Signal. 2021, 78, 109879. [Google Scholar] [CrossRef]
- Hering, H.; Lin, C.C.; Sheng, M. Lipid rafts in the maintenance of synapses, dendritic spines, and surface AMPA receptor stability. J. Neurosci. 2003, 23, 3262–3271. [Google Scholar] [CrossRef] [Green Version]
- Carrasco, P.; Sahún, I.; McDonald, J.; Ramírez, S.; Jacas, J.; Gratacós, E.; Sierra, A.Y.; Serra, D.; Herrero, L.; Acker-Palmer, A.; et al. Ceramide levels regulated by carnitine palmitoyltransferase 1C control dendritic spine maturation and cognition. J. Biol. Chem. 2012, 287, 21224–21232. [Google Scholar] [CrossRef] [Green Version]
- Hammond, G.R.V.; Schiavo, G. Polyphosphoinositol lipids: Under-PPInning synaptic function in health and disease. Dev. Neurobiol. 2007, 67, 1232–1247. [Google Scholar] [CrossRef] [PubMed]
- Richards, D.A.; Mateos, J.M.; Hugel, S.; De Paola, V.; Caroni, P.; Gähwiler, B.H.; McKinney, R.A. Glutamate induces the rapid formation of spine head protrusions in hippocampal slice cultures. Proc. Natl. Acad. Sci. USA 2005, 102, 6166–6171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ueda, Y.; Hayashi, Y. PIP3 regulates Spinule formation in dendritic spines during structural long-term potentiation. J. Neurosci. 2013, 33, 11040–11047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, H.L.; Janmey, P.A. Phosphoinositide Regulation of the Actin Cytoskeleton. Annu. Rev. Physiol. 2003, 65, 761–789. [Google Scholar] [CrossRef]
- Kumar, V.; Zhang, M.X.; Swank, M.W.; Kunz, J.; Wu, G.Y. Regulation of dendritic morphogenesis by Ras-PI3K-Akt-mTOR and Ras-MAPK signaling pathways. J. Neurosci. 2005, 25, 11288–11299. [Google Scholar] [CrossRef] [Green Version]
- Kelleher, R.J., 3rd; Govindarajan, A.; Tonegawa, S. Translational regulatory mechanisms in persistent forms of synaptic plasticity. Neuron 2004, 44, 59–73. [Google Scholar] [CrossRef] [Green Version]
- McLaughlin, S.; Murray, D. Plasma membrane phosphoinositide organization by protein electrostatics. Nature 2005, 438, 605–611. [Google Scholar] [CrossRef]
- Calabrese, B.; Halpain, S. Essential role for the PKC target MARCKS in maintaining dendritic spine morphology. Neuron 2005, 48, 77–90. [Google Scholar] [CrossRef] [Green Version]
- Horne, E.A.; Dell’Acqua, M.L. Phospholipase C is required for changes in postsynaptic structure and function associated with NMDA receptor-dependent long-term depression. J. Neurosci. 2007, 27, 3523–3534. [Google Scholar] [CrossRef]
- Arroyo, A.I.; Camoletto, P.G.; Morando, L.; Sassoe-Pognetto, M.; Giustetto, M.; Van Veldhoven, P.P.; Schuchman, E.H.; Ledesma, M.D. Pharmacological reversion of sphingomyelin-induced dendritic spine anomalies in a Niemann Pick disease type A mouse model. EMBO Mol. Med. 2014, 6, 398–413. [Google Scholar] [CrossRef] [Green Version]
- Stoffel, W. Functional analysis of acid and neutral sphingomyelinases in vitro and in vivo. Chem. Phys. Lipids 1999, 102, 107–121. [Google Scholar] [CrossRef]
- Franco-Villanueva, A.; Fernández-López, E.; Gabandé-Rodríguez, E.; Bañón-Rodríguez, I.; Esteban, J.A.; Antón, I.M.; Ledesma, M.D. WIP modulates dendritic spine actin cytoskeleton by transcriptional control of lipid metabolic enzymes. Hum. Mol. Genet. 2014, 23, 4383–4395. [Google Scholar] [CrossRef] [Green Version]
- Barrantes, F.J. Structural basis for lipid modulation of nicotinic acetylcholine receptor function. Brain Res. Brain Res. Rev. 2004, 47, 71–95. [Google Scholar] [CrossRef]
- Barrantes, F.J. Cholesterol effects on nicotinic acetylcholine receptor. J. Neurochem. 2007, 103, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Baier, C.J.; Gallegos, C.E.; Levi, V.; Barrantes, F.J. Cholesterol modulation of nicotinic acetylcholine receptor surface mobility. Eur. Biophys. J. 2010, 39, 213–227. [Google Scholar] [CrossRef]
- Mosqueira, A.; Camino, P.A.; Barrantes, F.J. Antibody-induced crosslinking and cholesterol-sensitive, anomalous diffusion of nicotinic acetylcholine receptors. J. Neurochem. 2020, 152, 663–674. [Google Scholar] [CrossRef]
- Mosqueira, A.; Camino, P.A.; Barrantes, F.J. Cholesterol modulates acetylcholine receptor diffusion by tuning confinement sojourns and nanocluster stability. Sci. Rep. 2018, 8, 11974. [Google Scholar] [CrossRef]
- Pediconi, M.F.; Gallegos, C.E.; De Los Santos, E.B.; Barrantes, F.J. Metabolic cholesterol depletion hinders cell-surface trafficking of the nicotinic acetylcholine receptor. Neuroscience 2004, 128, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Borroni, V.; Kamerbeek, C.; Pediconi, M.F.; Barrantes, F.J. Lovastatin Differentially Regulates α7 and α4 Neuronal Nicotinic Acetylcholine Receptor Levels in Rat Hippocampal Neurons. Molecules 2020, 25, 4838. [Google Scholar] [CrossRef] [PubMed]
- Baier, C.J.; Barrantes, F.J. Sphingolipids are necessary for nicotinic acetylcholine receptor export in the early secretory pathway. J. Neurochem. 2007, 101, 1072–1084. [Google Scholar] [CrossRef]
- Yeliseev, A.; Iyer, M.R.; Joseph, T.T.; Coffey, N.J.; Cinar, R.; Zoubak, L.; Kunos, G.; Gawrisch, K. Cholesterol as a modulator of cannabinoid receptor CB2 signaling. Sci. Reports 2021 111 2021, 11, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Bari, M.; Spagnuolo, P.; Fezza, F.; Oddi, S.; Pasquariello, N.; Finazzi-Agrò, A.; Maccarrone, M. Effect of lipid rafts on Cb2 receptor signaling and 2-arachidonoyl-glycerol metabolism in human immune cells. J. Immunol. 2006, 177, 4971–4980. [Google Scholar] [CrossRef] [Green Version]
- Di Scala, C.; Fantini, J.; Yahi, N.; Barrantes, F.J.; Chahinian, H. Anandamide Revisited: How Cholesterol and Ceramides Control Receptor-Dependent and Receptor-Independent Signal Transmission Pathways of a Lipid Neurotransmitter. Biomolecules 2018, 8, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kapus, A.; Janmey, P. Plasma membrane--cortical cytoskeleton interactions: A cell biology approach with biophysical considerations. Compr. Physiol. 2013, 3, 1231–1281. [Google Scholar] [CrossRef]
- Kusumi, A.; Nakada, C.; Ritchie, K.; Murase, K.; Suzuki, K.; Murakoshi, H.; Kasai, R.S.; Kondo, J.; Fujiwara, T. Paradigm shift of the plasma membrane concept from the two-dimensional continuum fluid to the partitioned fluid: High-speed single-molecule tracking of membrane molecules. Annu. Rev. Biophys. Biomol. Struct. 2005, 34, 351–378. [Google Scholar] [CrossRef] [Green Version]
- Andrade, D.M.; Clausen, M.P.; Keller, J.; Mueller, V.; Wu, C.; Bear, J.E.; Hell, S.W.; Lagerholm, B.C.; Eggeling, C. Cortical actin networks induce spatio-temporal confinement of phospholipids in the plasma membrane--a minimally invasive investigation by STED-FCS. Sci. Rep. 2015, 5, 11454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujiwara, T.; Ritchie, K.; Murakoshi, H.; Jacobson, K.; Kusumi, A. Phospholipids undergo hop diffusion in compartmentalized cell membrane. J. Cell Biol. 2002, 157, 1071–1081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Auth, T.; Gov, N.S. Diffusion in a fluid membrane with a flexible cortical cytoskeleton. Biophys. J. 2009, 96, 818–830. [Google Scholar] [CrossRef] [Green Version]
- Hotulainen, P.; Hoogenraad, C.C. Actin in dendritic spines: Connecting dynamics to function. J. Cell Biol. 2010, 189, 619–629. [Google Scholar] [CrossRef] [Green Version]
- Kwik, J.; Boyle, S.; Fooksman, D.; Margolis, L.; Sheetz, M.P.; Edidin, M. Membrane cholesterol, lateral mobility, and the phosphatidylinositol 4,5-bisphosphate-dependent organization of cell actin. Proc. Natl. Acad. Sci. USA 2003, 100, 13964–13969. [Google Scholar] [CrossRef] [Green Version]
- Cingolani, L.A.; Goda, Y. Actin in action: The interplay between the actin cytoskeleton and synaptic efficacy. Nat. Rev. Neurosci. 2008, 9, 344–356. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.C.; Stavoe, A.K.H.; Colón-Ramos, D.A. The actin cytoskeleton in presynaptic assembly. Cell Adh. Migr. 2013, 7, 379–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michel, K.; Müller, J.A.; Oprişoreanu, A.-M.; Schoch, S. The presynaptic active zone: A dynamic scaffold that regulates synaptic efficacy. Exp. Cell Res. 2015, 335, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Rust, M.B.; Maritzen, T. Relevance of presynaptic actin dynamics for synapse function and mouse behavior. Exp. Cell Res. 2015, 335, 165–171. [Google Scholar] [CrossRef]
- Bartol, T.M.; Bromer, C.; Kinney, J.; Chirillo, M.A.; Bourne, J.N.; Harris, K.M.; Sejnowski, T.J. Nanoconnectomic upper bound on the variability of synaptic plasticity. Elife 2015, 4, e10778. [Google Scholar] [CrossRef] [Green Version]
- Bourne, J.N.; Chirillo, M.A.; Harris, K.M. Presynaptic ultrastructural plasticity along CA3→CA1 axons during long-term potentiation in mature hippocampus. J. Comp. Neurol. 2013, 521, 3898–3912. [Google Scholar] [CrossRef] [Green Version]
- Meyer, D.; Bonhoeffer, T.; Scheuss, V. Balance and stability of synaptic structures during synaptic plasticity. Neuron 2014, 82, 430–443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gundelfinger, E.D.; Fejtova, A. Molecular organization and plasticity of the cytomatrix at the active zone. Curr. Opin. Neurobiol. 2012, 22, 423–430. [Google Scholar] [CrossRef]
- Matz, J.; Gilyan, A.; Kolar, A.; McCarvill, T.; Krueger, S.R. Rapid structural alterations of the active zone lead to sustained changes in neurotransmitter release. Proc. Natl. Acad. Sci. USA 2010, 107, 8836–8841. [Google Scholar] [CrossRef] [Green Version]
- Monday, H.R.; Castillo, P.E. Closing the gap: Long-term presynaptic plasticity in brain function and disease. Curr. Opin. Neurobiol. 2017, 45, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Runge, K.; Cardoso, C.; de Chevigny, A. Dendritic Spine Plasticity: Function and Mechanisms. Front. Synaptic Neurosci. 2020, 12, 36. [Google Scholar] [CrossRef] [PubMed]
- Nakahata, Y.; Yasuda, R. Plasticity of Spine Structure: Local Signaling, Translation and Cytoskeletal Reorganization. Front. Synaptic Neurosci. 2018, 10, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holcman, D.; Triller, A. Modeling synaptic dynamics driven by receptor lateral diffusion. Biophys. J. 2006, 91, 2405–2415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Specht, C.G. Subsynaptic Domains in Super-Resolution Microscopy: The Treachery of Images. Front. Mol. Neurosci. 2019, 12, 161. [Google Scholar] [CrossRef]
- Hruska, M.; Henderson, N.; Le Marchand, S.J.; Jafri, H.; Dalva, M.B. Synaptic nanomodules underlie the organization and plasticity of spine synapses. Nat. Neurosci. 2018, 21, 671–682. [Google Scholar] [CrossRef]
- Wegner, W.; Mott, A.C.; Grant, S.G.N.; Steffens, H.; Willig, K.I. In vivo STED microscopy visualizes PSD95 sub-structures and morphological changes over several hours in the mouse visual cortex. Sci. Rep. 2018, 8, 219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moretto, E.; Longatti, A.; Murru, L.; Chamma, I.; Sessa, A.; Zapata, J.; Hosy, E.; Sainlos, M.; Saint-Pol, J.; Rubinstein, E.; et al. TSPAN5 Enriched Microdomains Provide a Platform for Dendritic Spine Maturation through Neuroligin-1 Clustering. Cell Rep. 2019, 29, 1130–1146. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Annaert, W. The Nanoscopic Organization of Synapse Structures: A Common Basis for Cell Communication. Membranes 2021, 11, 248. [Google Scholar] [CrossRef]
- Triller, A.; Choquet, D. Surface trafficking of receptors between synaptic and extrasynaptic membranes: And yet they do move! Trends Neurosci. 2005, 28, 133–139. [Google Scholar] [CrossRef]
- Song, I.; Huganir, R.L. Regulation of AMPA receptors during synaptic plasticity. Trends Neurosci. 2002, 25, 578–588. [Google Scholar] [CrossRef]
- Choquet, D.; Triller, A. The role of receptor diffusion in the organization of the postsynaptic membrane. Nat. Rev. Neurosci. 2003, 4, 251–265. [Google Scholar] [CrossRef]
- Triller, A.; Choquet, D. New concepts in synaptic biology derived from single-molecule imaging. Neuron 2008, 59, 359–374. [Google Scholar] [CrossRef] [Green Version]
- Blanpied, T.A.; Scott, D.B.; Ehlers, M.D. Dynamics and regulation of clathrin coats at specialized endocytic zones of dendrites and spines. Neuron 2002, 36, 435–449. [Google Scholar] [CrossRef] [Green Version]
- Rácz, B.; Blanpied, T.A.; Ehlers, M.D.; Weinberg, R.J. Lateral organization of endocytic machinery in dendritic spines. Nat. Neurosci. 2004, 7, 917–918. [Google Scholar] [CrossRef]
- Lu, J.; Helton, T.D.; Blanpied, T.A.; Rácz, B.; Newpher, T.M.; Weinberg, R.J.; Ehlers, M.D. Postsynaptic positioning of endocytic zones and AMPA receptor cycling by physical coupling of dynamin-3 to Homer. Neuron 2007, 55, 874–889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Groc, L.; Choquet, D. Measurement and characteristics of neurotransmitter receptor surface trafficking (Review). Mol. Membr. Biol. 2008, 25, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Kusters, R.; van der Heijden, T.; Kaoui, B.; Harting, J.; Storm, C. Forced transport of deformable containers through narrow constrictions. Phys. Rev. E. Stat. Nonlin. Soft Matter Phys. 2014, 90, 33006. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Fourriere, L.; Gleeson, P.A. Local Secretory Trafficking Pathways in Neurons and the Role of Dendritic Golgi Outposts in Different Cell Models. Front. Mol. Neurosci. 2020, 13, 597391. [Google Scholar] [CrossRef]
- Frotscher, M.; Studer, D.; Graber, W.; Chai, X.; Nestel, S.; Zhao, S. Fine structure of synapses on dendritic spines. Front. Neuroanat. 2014, 8, 94. [Google Scholar] [CrossRef] [Green Version]
- Renner, M.; Specht, C.G.; Triller, A. Molecular dynamics of postsynaptic receptors and scaffold proteins. Curr. Opin. Neurobiol. 2008, 18, 532–540. [Google Scholar] [CrossRef]
- Newpher, T.M.; Ehlers, M.D. Glutamate receptor dynamics in dendritic microdomains. Neuron 2008, 58, 472–497. [Google Scholar] [CrossRef] [Green Version]
- Bürli, T.; Baer, K.; Ewers, H.; Sidler, C.; Fuhrer, C.; Fritschy, J.-M. Single Particle Tracking of α7 Nicotinic AChR in Hippocampal Neurons Reveals Regulated Confinement at Glutamatergic and GABAergic Perisynaptic Sites. PLoS ONE 2010, 5, e11507. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Földy, C.; Soltesz, I. Distinct endocannabinoid control of GABA release at perisomatic and dendritic synapses in the hippocampus. J. Neurosci. 2010, 30, 7993–8000. [Google Scholar] [CrossRef]
- Dudok, B.; Barna, L.; Ledri, M.; Szabó, S.I.; Szabadits, E.; Pintér, B.; Woodhams, S.G.; Henstridge, C.M.; Balla, G.Y.; Nyilas, R.; et al. Cell-specific STORM superresolution imaging reveals nanoscale organization of cannabinoid signaling. Nat. Neurosci. 2015, 18, 75. [Google Scholar] [CrossRef]
- Goncalves, J.; Bartol, T.M.; Camus, C.; Levet, F.; Menegolla, A.P.; Sejnowski, T.J.; Sibarita, J.-B.; Vivaudou, M.; Choquet, D.; Hosy, E. Nanoscale co-organization and coactivation of AMPAR, NMDAR, and mGluR at excitatory synapses. Proc. Natl. Acad. Sci. USA 2020, 117, 14503–14511. [Google Scholar] [CrossRef]
- Cognet, L.; Groc, L.; Lounis, B.; Choquet, D. Multiple routes for glutamate receptor trafficking: Surface diffusion and membrane traffic cooperate to bring receptors to synapses. Sci. STKE 2006, 2006, pe13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martini, L.; Waldhoer, M.; Pusch, M.; Kharazia, V.; Fong, J.; Lee, J.H.; Freissmuth, C.; Whistler, J.L. Ligand-induced down-regulation of the cannabinoid 1 receptor is mediated by the G-protein-coupled receptor-associated sorting protein GASP1. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2007, 21, 802–811. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Brown, S.; Roche, J.P.; Hsieh, C.; Celver, J.P.; Kovoor, A.; Chavkin, C.; Mackie, K. Distinct domains of the CB1 cannabinoid receptor mediate desensitization and internalization. J. Neurosci. 1999, 19, 3773–3780. [Google Scholar] [CrossRef] [Green Version]
- Delgado-Peraza, F.; Ahn, K.H.; Nogueras-Ortiz, C.; Mungrue, I.N.; Mackie, K.; Kendall, D.A.; Yudowski, G.A. Mechanisms of Biased β-Arrestin-Mediated Signaling Downstream from the Cannabinoid 1 Receptor. Mol. Pharmacol. 2016, 89, 618–629. [Google Scholar] [CrossRef] [Green Version]
- Roche, J.P.; Bounds, S.; Brown, S.; Mackie, K. A mutation in the second transmembrane region of the CB1 receptor selectively disrupts G protein signaling and prevents receptor internalization. Mol. Pharmacol. 1999, 56, 611–618. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vallés, A.S.; Barrantes, F.J. Nanoscale Sub-Compartmentalization of the Dendritic Spine Compartment. Biomolecules 2021, 11, 1697. https://doi.org/10.3390/biom11111697
Vallés AS, Barrantes FJ. Nanoscale Sub-Compartmentalization of the Dendritic Spine Compartment. Biomolecules. 2021; 11(11):1697. https://doi.org/10.3390/biom11111697
Chicago/Turabian StyleVallés, Ana Sofía, and Francisco J. Barrantes. 2021. "Nanoscale Sub-Compartmentalization of the Dendritic Spine Compartment" Biomolecules 11, no. 11: 1697. https://doi.org/10.3390/biom11111697
APA StyleVallés, A. S., & Barrantes, F. J. (2021). Nanoscale Sub-Compartmentalization of the Dendritic Spine Compartment. Biomolecules, 11(11), 1697. https://doi.org/10.3390/biom11111697





