Sgt1 Regulates α-Synuclein Subcellular Localization and Expression of Parkinson’s Disease Related Genes, PINK1 and PARK9
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plasmids
2.2. Cell Culture and Transfection
2.3. Preparation of Protein Lysates
2.4. Western Blot Analysis
2.5. Immunofluorescence Staining and Confocal Microscopy
2.6. Fluorescence-Based Quantification of Nuclear-Cytoplasmic Localization
2.7. Real Time/Quantitative PCR (RT-qPCR)
2.8. Cell Viability Assay
2.9. Statistical Analysis
3. Results
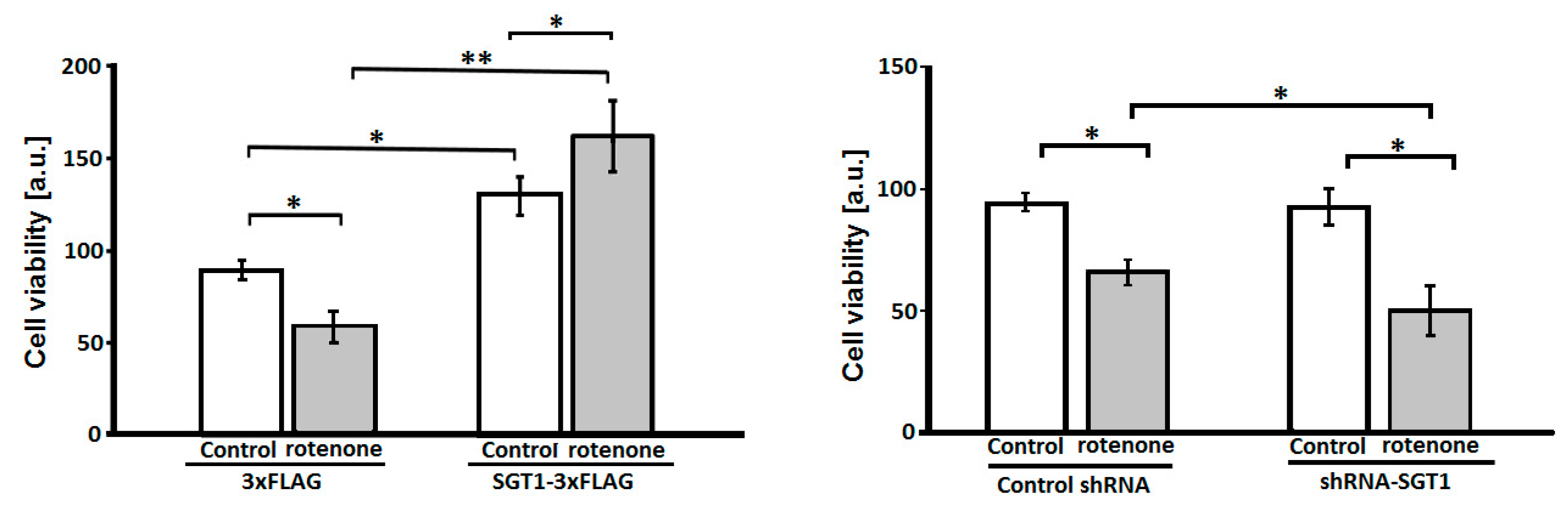
3.1. SGT1 Protects HEK293 Cells from Rotenone-Induced Toxicity
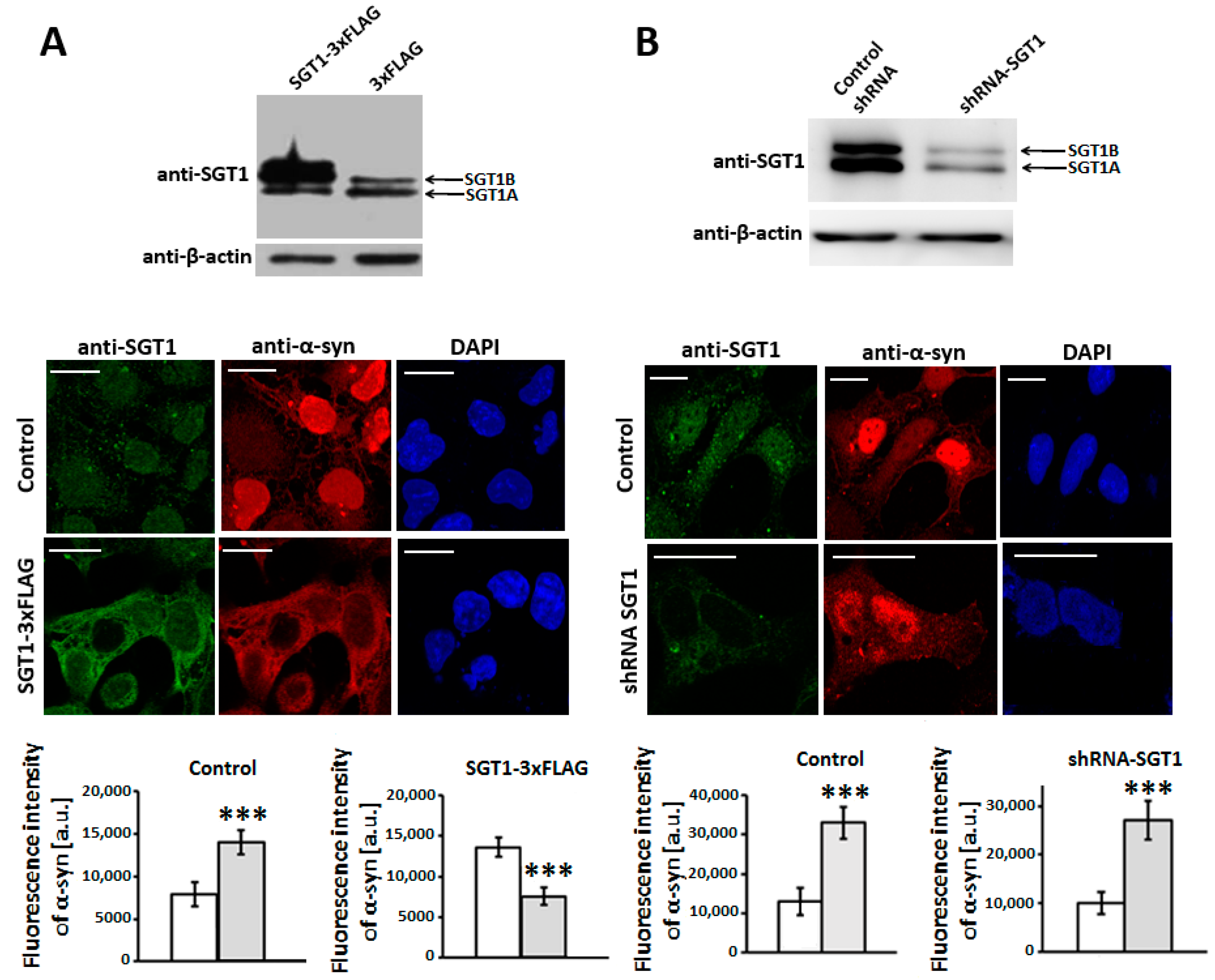
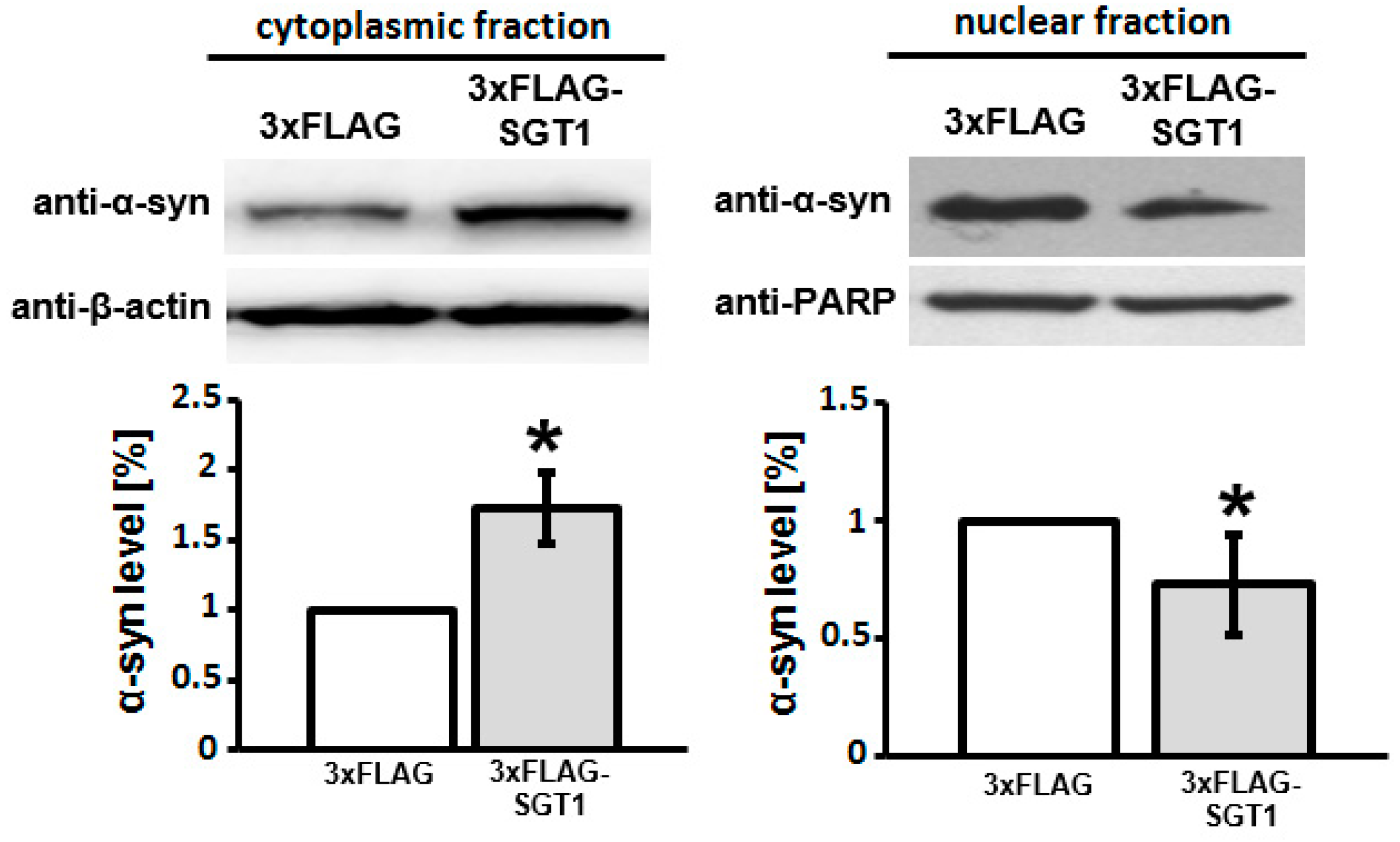
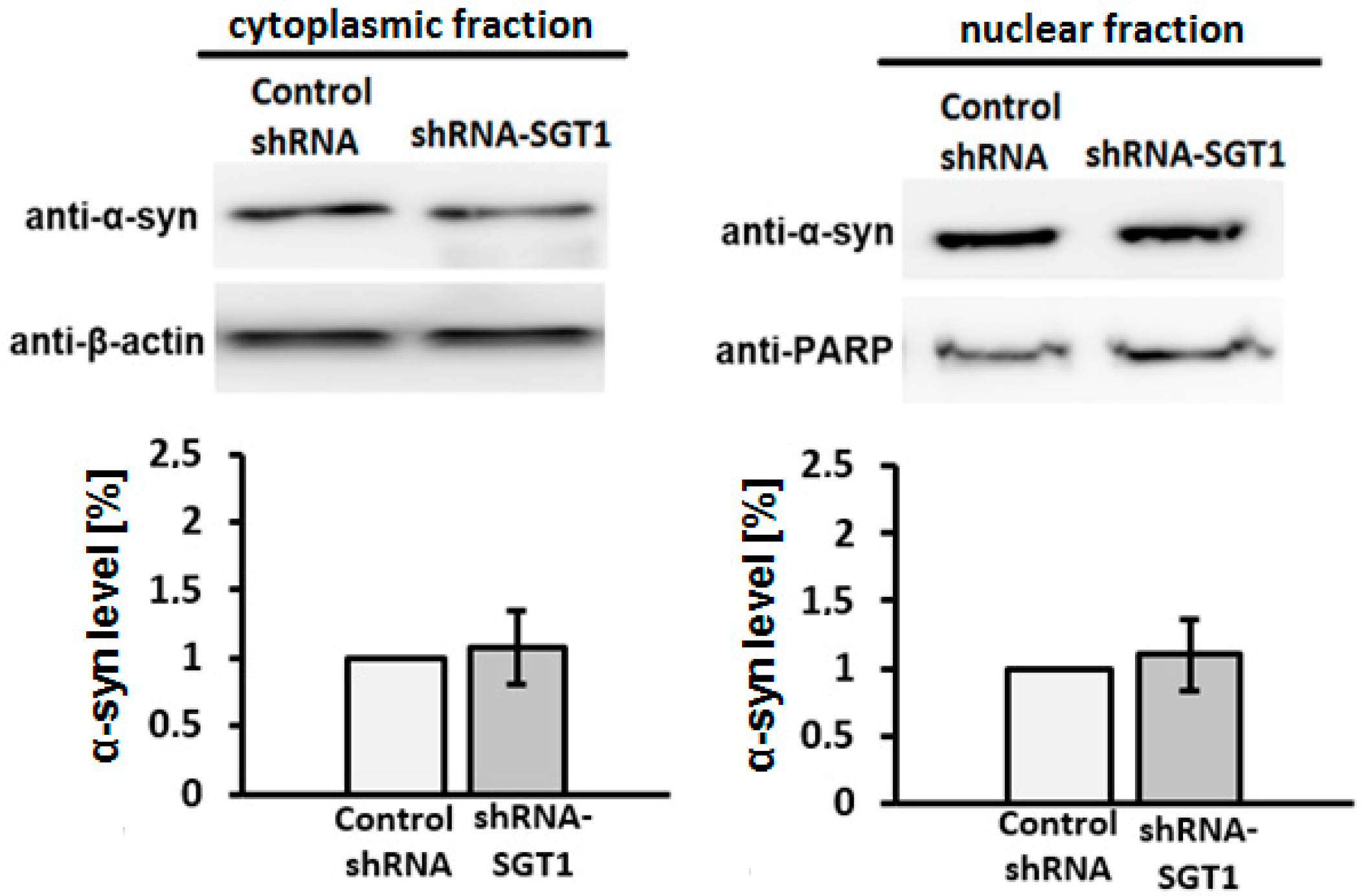
3.2. SGT1 Affects α-Synuclein Subcellular Localization in HEK293 Cells
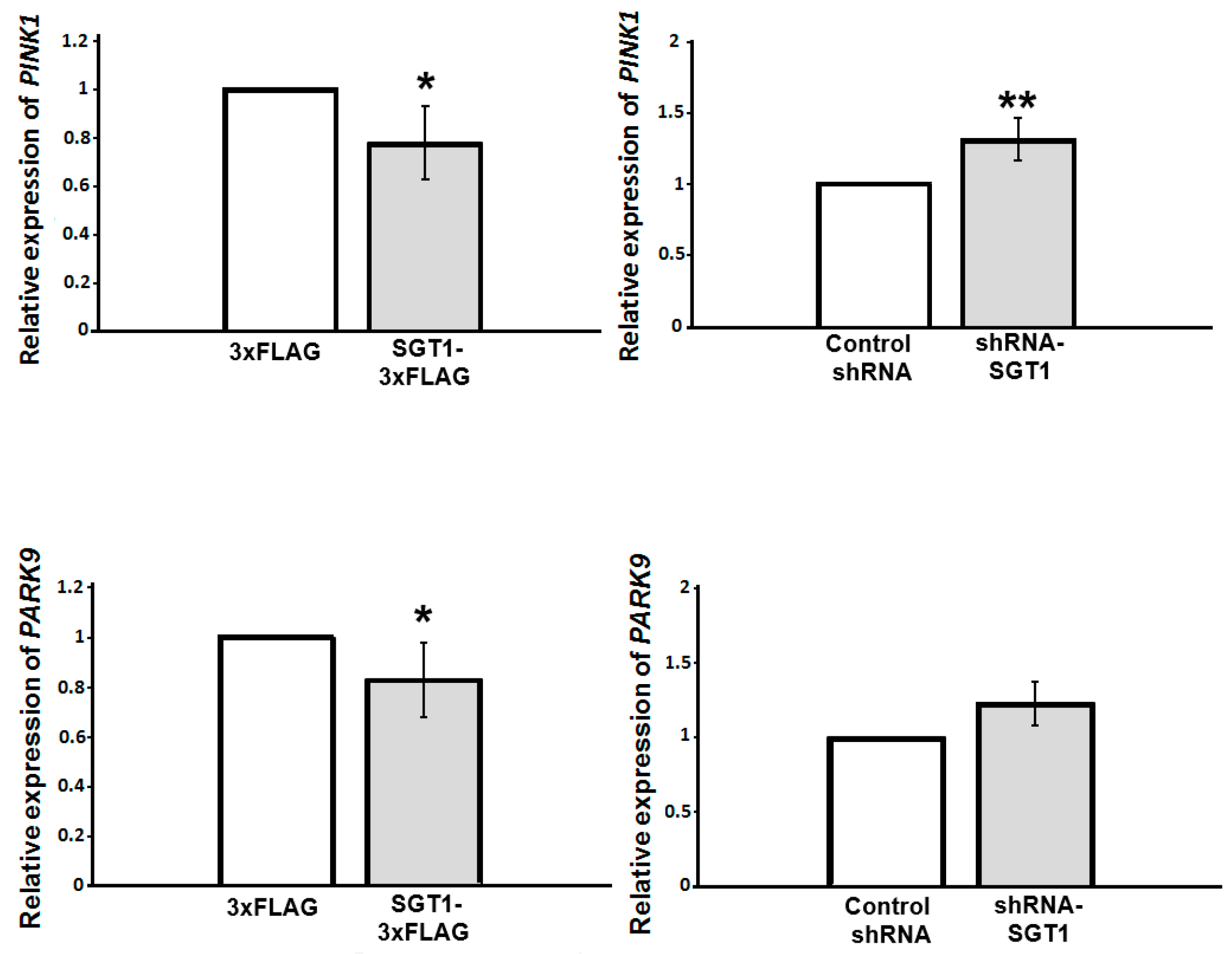
3.3. Altered SGT1 Level Triggers Changes in PINK1 and PARK9 Gene Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Compliance with Ethical Standards
Abbreviations
References
- Kouli, A.; Torsney, K.M.; Kuan, W.L. Parkinson’s Disease: Etiology, Neuropathology, and Pathogenesis; Codon Publications: Brisbane, Australia, 2018; Chapter 1. [Google Scholar]
- Emamzadeh, F.N. Alpha-synuclein structure, functions, and interactions. J. Res. Med. Sci. 2016, 21, 29. [Google Scholar] [CrossRef] [PubMed]
- Somayaji, M.; Lanseur, Z.; Choi, S.J.; Sulzer, D.; Mosharov, E.V. Roles for α-Synuclein in Gene Expression. Genes 2021, 12, 1166. [Google Scholar] [CrossRef]
- Kontopoulos, E.; Parvin, J.D.; Feany, M.B. Alpha-synuclein acts in the nucleus to inhibit histone acetylation and promote neurotoxicity. Hum. Mol. Genet. 2006, 15, 3012–3023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Z.; Xu, Z.; Wu, Y.; Zhou, Y. Determining nuclear localization of alpha-synuclein in mouse brains. Neuroscience 2011, 199, 318–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davidi, D.; Schechter, M.; Elhadi, S.A.; Matatov, A.; Nathanson, L.; Sharon, R. α-Synuclein Translocates to the Nucleus to Activate Retinoic-Acid-Dependent Gene Transcription. iScience 2020, 23, 100910. [Google Scholar] [CrossRef] [Green Version]
- Pinho, R.; Paiva, I.; Jercic, K.G.; Fonseca-Ornelas, L.; Gerhardt, E.; Fahlbusch, C.; Garcia-Esparcia, P.; Kerimoglu, C.; Pavlou, M.A.S.; Villar-Piqué, A.; et al. Nuclear localization and phosphorylation modulate pathological effects of alpha-synuclein. Hum. Mol. Genet. 2019, 28, 31–50. [Google Scholar] [CrossRef]
- Goers, J.; Manning-Bog, A.B.; McCormack, A.L.; Millett, I.S.; Doniach, S.; Di Monte, D.A.; Uversky, V.N.; Fink, A.L. Nuclear localization of alpha-synuclein and its interaction with histones. Biochemistry 2003, 42, 8465–8471. [Google Scholar] [CrossRef]
- Vasquez, V.; Mitra, J.; Hegde, P.M.; Pandey, A.; Sengupta, S.; Mitra, S.; Rao, K.S.; Hegde, M.L. Chromatin-Bound Oxidized α-Synuclein Causes Strand Breaks in Neuronal Genomes in in vitro Models of Parkinson’s Disease. J. Alzheimer’s Dis. 2017, 60, S133–S150. [Google Scholar] [CrossRef]
- Decressac, M.; Kadkhodaei, B.; Mattsson, B.; Laguna, A.; Perlmann, T.; Björklund, A. α-Synuclein-induced down-regulation of Nurr1 disrupts GDNF signaling in nigral dopamine neurons. Sci. Transl. Med. 2012, 4, 163ra156. [Google Scholar] [CrossRef]
- Kitagawa, K.; Skowyra, D.; Elledge, S.J.; Harper, J.W.; Hieter, P. SGT1 encodes an essential component of the yeast kinetochore assembly pathway and a novel subunit of the SCF ubiquitin ligase complex. Mol. Cell 1999, 21–33. [Google Scholar] [CrossRef]
- Lee, Y.T.; Jacob, J.; Michowski, W.; Nowotny, M.; Kuznicki, J.; Chazin, W.J. Human Sgt1 binds HSP90 through the CHORD-Sgt1 domain and not the tetratricopeptide repeat domain. J. Biol. Chem. 2004, 279, 16511–16517. [Google Scholar] [CrossRef] [Green Version]
- Correia, D.S.J.; Miranda, Y.; Leonard, N.; Ulevitch, R. SGT1 is essential for Nod1 activation. Proc. Natl. Acad. Sci. USA 2007, 104, 6764–6769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spiechowicz, M.; Bernstein, H.G.; Dobrowolny, H.; Leśniak, W.; Mawrin, C.; Bogerts, B.; Kuźnicki, J.; Filipek, A. Density of Sgt1-immunopositive neurons is decreased in the cerebral cortex of Alzheimer’s disease brain. Neurochem. Int. 2006, 49, 487–493. [Google Scholar] [CrossRef]
- Bohush, A.; Niewiadomska, G.; Weis, S.; Filipek, A. HSP90 and Its Novel Co-Chaperones, SGT1 and CHP-1, in Brain of Patients with Parkinson’s Disease and Dementia with Lewy Bodies. J. Parkinson’s Dis. 2019, 9, 97–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bohush, A.; Bieganowski, P.; Filipek, A. Hsp90 and Its Co-Chaperones in Neurodegenerative Diseases. Int. J. Mol. Sci. 2019, 20, 4976. [Google Scholar] [CrossRef] [Green Version]
- Bohush, A.; Filipek, A. HSP90 Co-Chaperone, CacyBP/SIP, Protects α-Synuclein from Aggregation. Cells 2020, 9, 2254. [Google Scholar] [CrossRef] [PubMed]
- Filipek, A.; Leśniak, W. Current view on cellular function of S100A6 and its ligands, CacyBP/SIP and Sgt1. Post. Biochem. 2018, 64, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Miyagishi, M.; Matsumoto, S.; Taira, K. Generation of an shRNAi expression library against the whole human transcripts. Virus Res. 2004, 102, 117–124. [Google Scholar] [CrossRef]
- Prus, W.; Zabka, M.; Bieganowski, P.; Filipek, A. Nuclear translocation of Sgt1 depends on its phosphorylation state. Int. J. Biochem. Cell Biol. 2011, 43, 1747–1753. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Góral, A.; Bartkowska, K.; Djavadian, R.L.; Filipek, A. CacyBP/SIP, a Hsp90 binding chaperone, in cellular stress response. Int. J. Biochem. Cell Biol. 2018, 99, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Kelley, J.B.; Paschal, B.M. Fluorescence-based quantification of nucleocytoplasmic transport. Methods 2019, 157, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Sherer, T.B.; Betarbet, R.; Testa, C.M.; Seo, B.B.; Richardson, J.R.; Kim, J.H.; Miller, G.W.; Yagi, T.; Matsuno-Yagi, A.; Greenamyre, J.T. Mechanism of toxicity in rotenone models of Parkinson’s disease. J. Neurosci. 2003, 23, 10756–10764. [Google Scholar] [CrossRef] [PubMed]
- Baptista, M.J.; O’Farrell, C.; Daya, S.; Ahmad, R.; Miller, D.W.; Hardy, J.; Farrer, M.J.; Cookson, M.R. Co-ordinate transcriptional regulation of dopamine synthesis genes by alpha-synuclein in human neuroblastoma cell lines. J. Neurochem. 2003, 85, 957–968. [Google Scholar] [CrossRef] [PubMed]
- Zambon, F.; Cherubini, M.; Fernandes, H.J.R.; Lang, C.; Ryan, B.J.; Volpato, V.; Bengoa-Vergniory, N.; Vingill, S.; Attar, M.; Booth, H.D.E.; et al. Cellular α-synuclein pathology is associated with bioenergetic dysfunction in Parkinson’s iPSC-derived dopamine neurons. Hum. Mol. Genet. 2019, 28, 2001–2013. [Google Scholar] [CrossRef]
- Morais, V.A.; Verstreken, P.; Roethig, A.; Smet, J.; Snellinx, A.; Vanbrabant, M.; Haddad, D.; Frezza, C.; Mandemakers, W.; Vogt-Weisenhorn, D.; et al. Parkinson’s disease mutations in PINK1 result in decreased Complex I activity and deficient synaptic function. EMBO Mol. Med. 2009, 99–111. [Google Scholar] [CrossRef]
- Murphy, K.E.; Cottle, L.; Gysbers, A.M.; Cooper, A.A.; Halliday, G.M. ATP13A2 (PARK9) protein levels are reduced in brain tissue of cases with Lewy bodies. Acta Neuropathol. Commun. 2013, 1, 11. [Google Scholar] [CrossRef] [Green Version]
| Plasmid | Description | Source/Reference |
|---|---|---|
| pcDNA4-α-synuclein-3×FLAG | plasmid for α-synuclein expression in eukaryotic cells with 3×FLAG tag | [17] |
| p3×FLAG-CMV-10 | plasmid for protein expression in eukaryotic cells encoding 3×FLAG tag | Sigma-Aldrich |
| p3×FLAG-CMV-10-SGT1A | plasmid for SGT1A expression in eukaryotic cells with 3×FLAG tag | [20] |
| p3×FLAG-CMV-10-SGT1B | plasmid for SGT1B expression in eukaryotic cells with 3×FLAG tag | Present work |
| pPURhU6-Luc4 | plasmid encoding control shRNA against Renilla Luciferase mRNA | Present work |
| pPURhU6-SGT1 | plasmid encoding shRNA against SGT1 mRNA | Present work |
| Gene | Forward | Reverse |
|---|---|---|
| 18S | 5′-CGCCGCTAGAGGTGAAATTC-3′ | 5′-TTGGCAAATGCTTTCGCTC-3′ |
| PINK1 | 5′-TATGGAGCAGTCACTTACAGAAAATCC-3′ | 5′-GGTGAAGGCGCGGAGAA-3′ |
| PARK9 | 5′-ATGATGGCTGGGATCCCTTT-3′ | 5′-AACTATCCTCTTTGTCTCTTATTTCGATAAC-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bohush, A.; Góral, A.; Sierant, M.; Nawrot, B.; Leśniak, W.; Filipek, A. Sgt1 Regulates α-Synuclein Subcellular Localization and Expression of Parkinson’s Disease Related Genes, PINK1 and PARK9. Biomolecules 2021, 11, 1675. https://doi.org/10.3390/biom11111675
Bohush A, Góral A, Sierant M, Nawrot B, Leśniak W, Filipek A. Sgt1 Regulates α-Synuclein Subcellular Localization and Expression of Parkinson’s Disease Related Genes, PINK1 and PARK9. Biomolecules. 2021; 11(11):1675. https://doi.org/10.3390/biom11111675
Chicago/Turabian StyleBohush, Anastasiia, Agnieszka Góral, Małgorzata Sierant, Barbara Nawrot, Wiesława Leśniak, and Anna Filipek. 2021. "Sgt1 Regulates α-Synuclein Subcellular Localization and Expression of Parkinson’s Disease Related Genes, PINK1 and PARK9" Biomolecules 11, no. 11: 1675. https://doi.org/10.3390/biom11111675
APA StyleBohush, A., Góral, A., Sierant, M., Nawrot, B., Leśniak, W., & Filipek, A. (2021). Sgt1 Regulates α-Synuclein Subcellular Localization and Expression of Parkinson’s Disease Related Genes, PINK1 and PARK9. Biomolecules, 11(11), 1675. https://doi.org/10.3390/biom11111675






