Neuropathological Changes in the Brains of Suicide Killers
Abstract
:1. Introduction
2. Materials and Methods
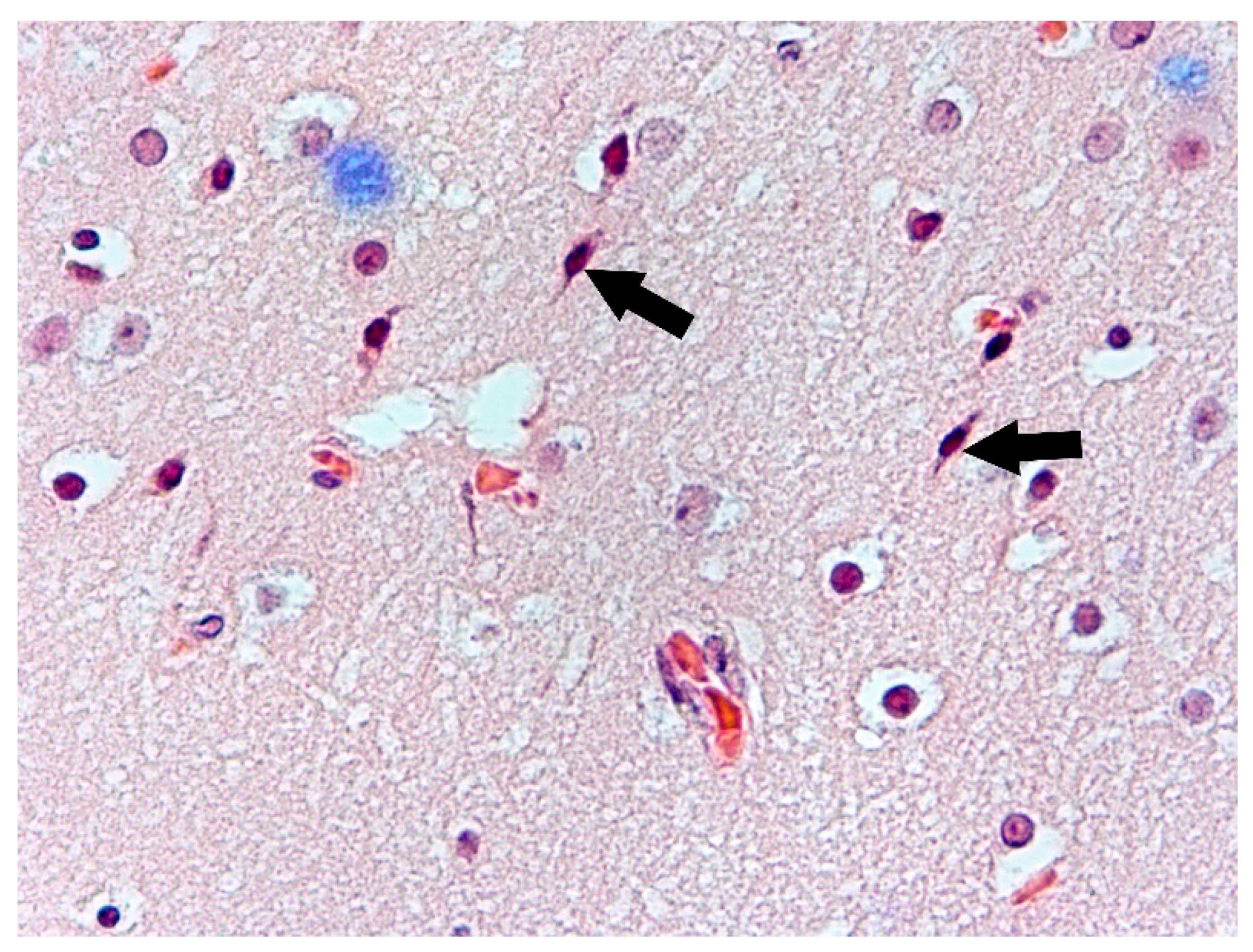
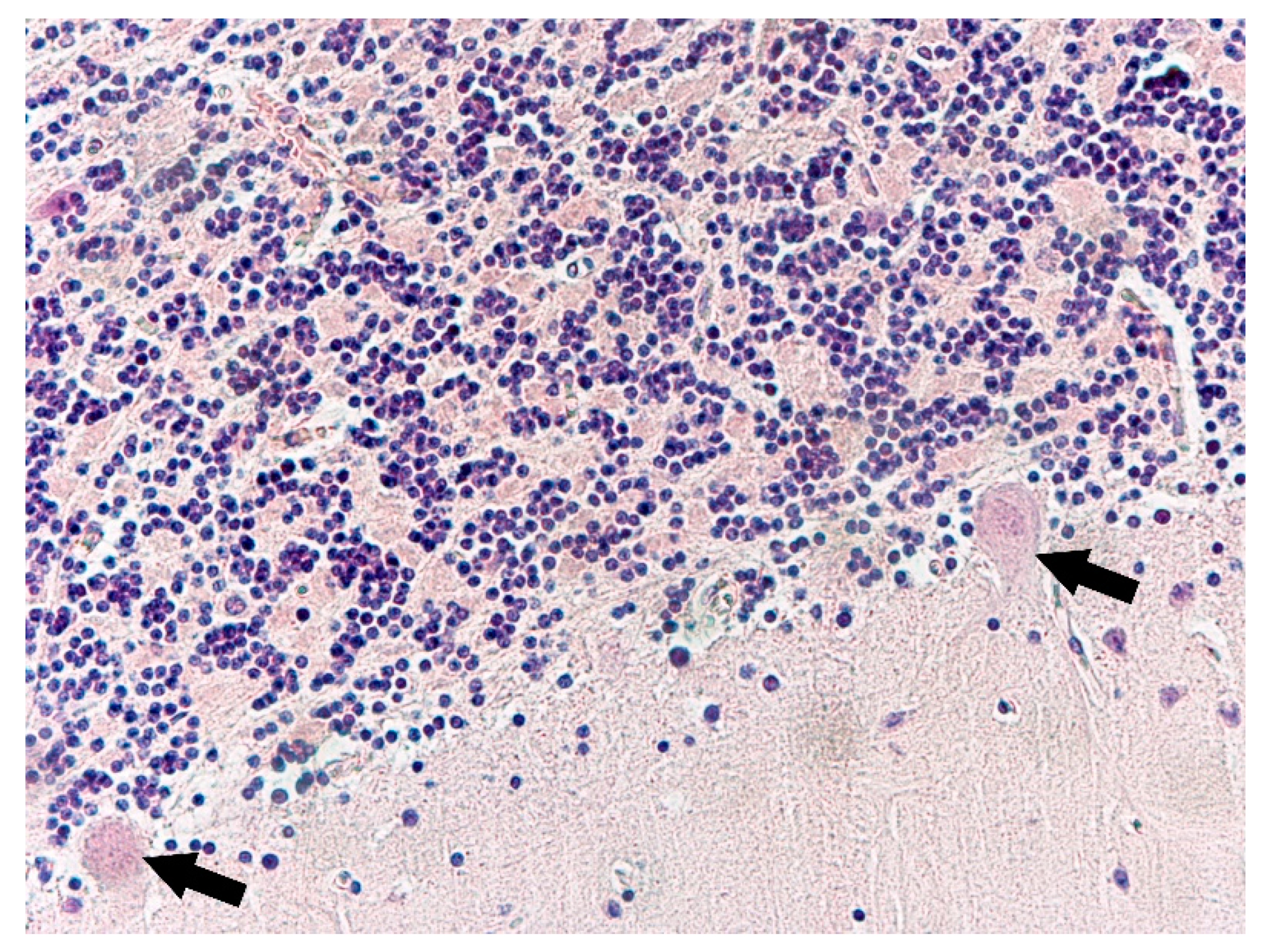
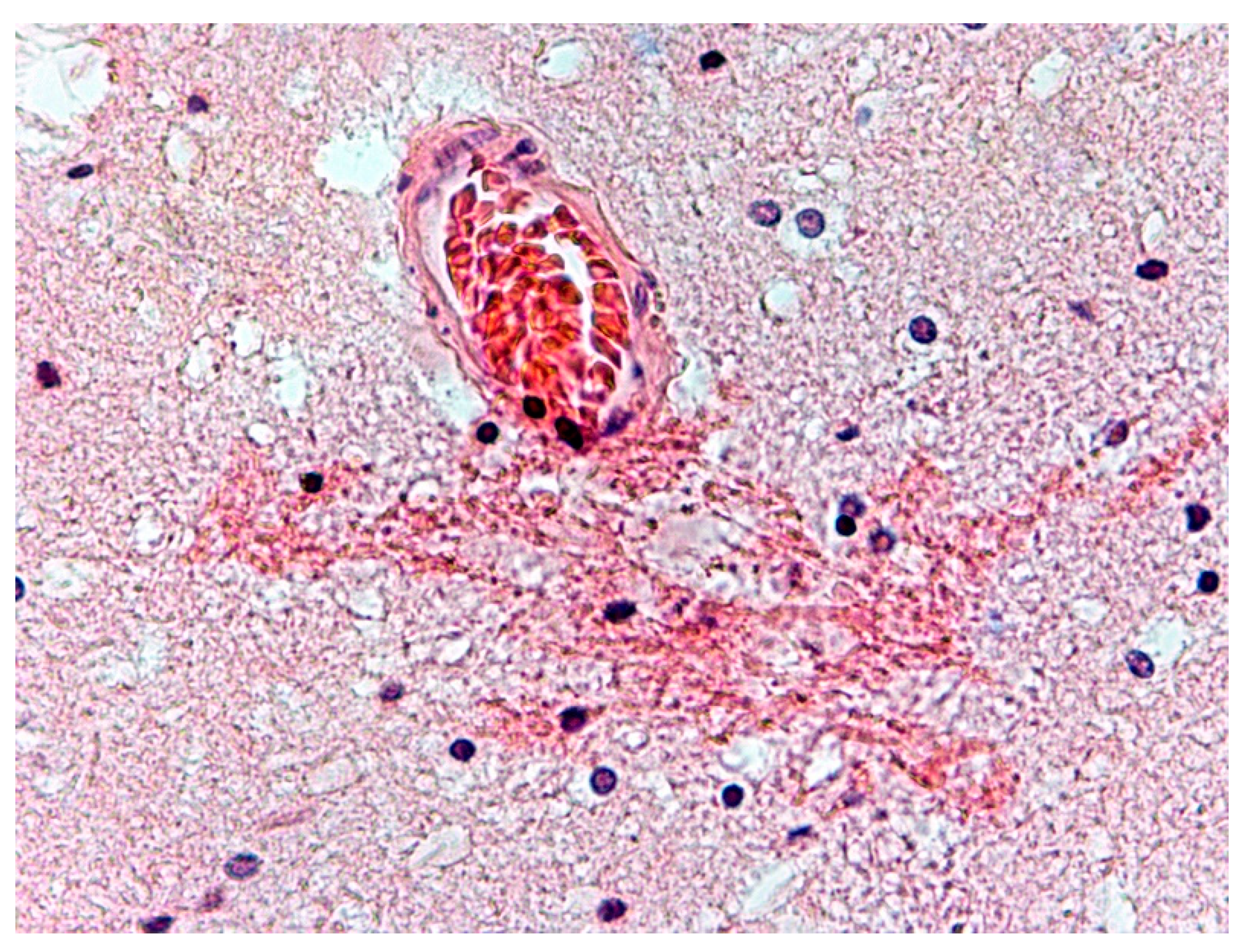
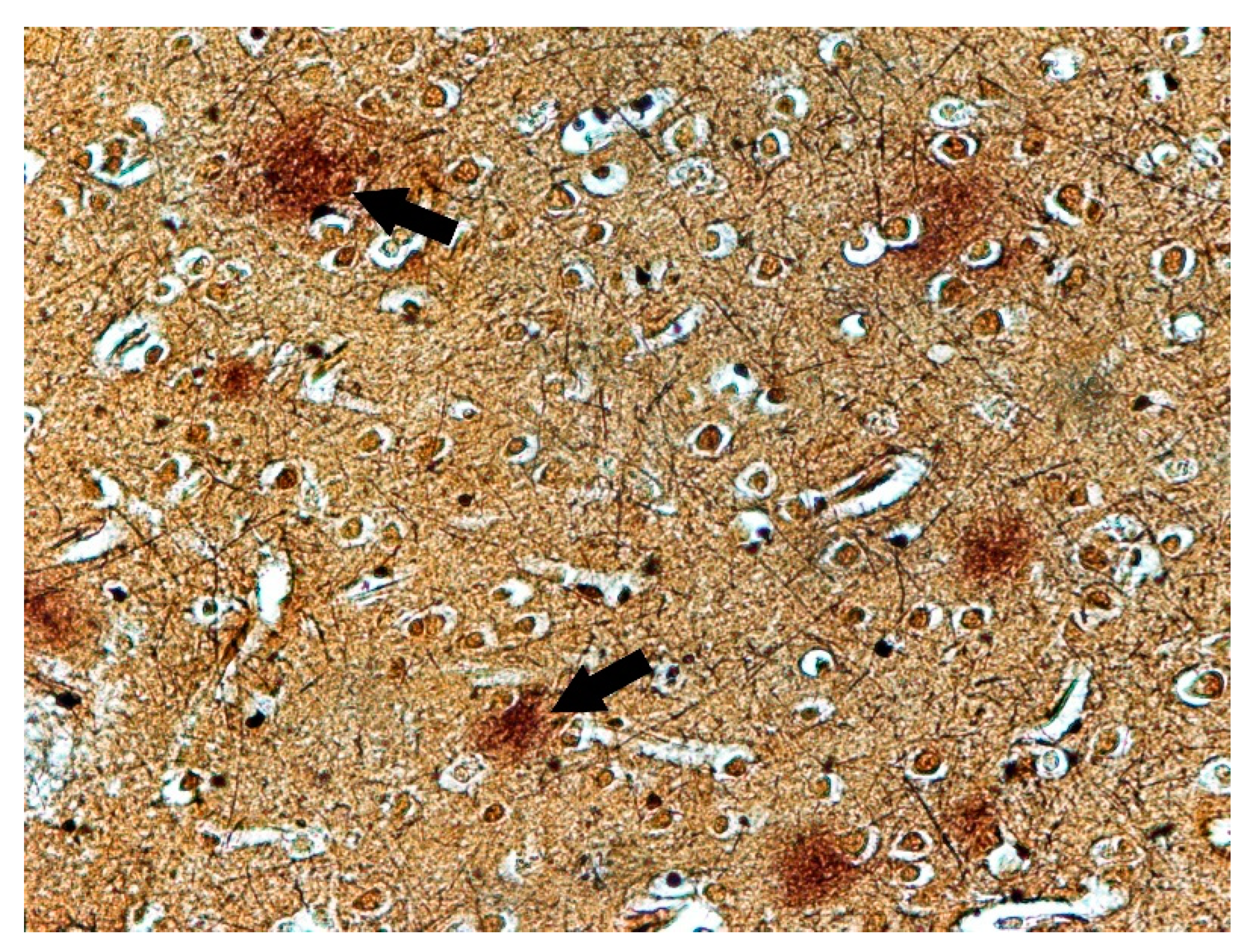
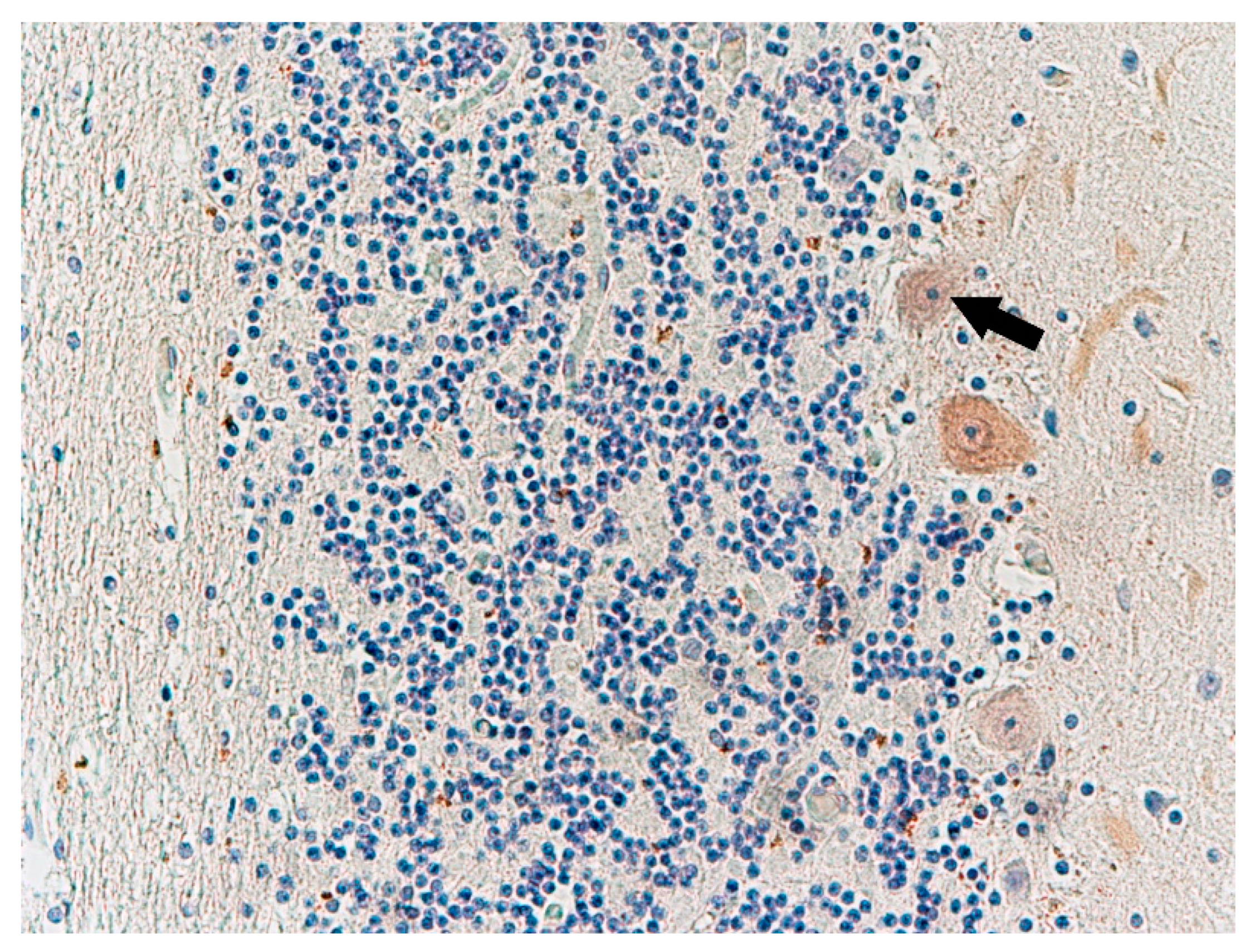
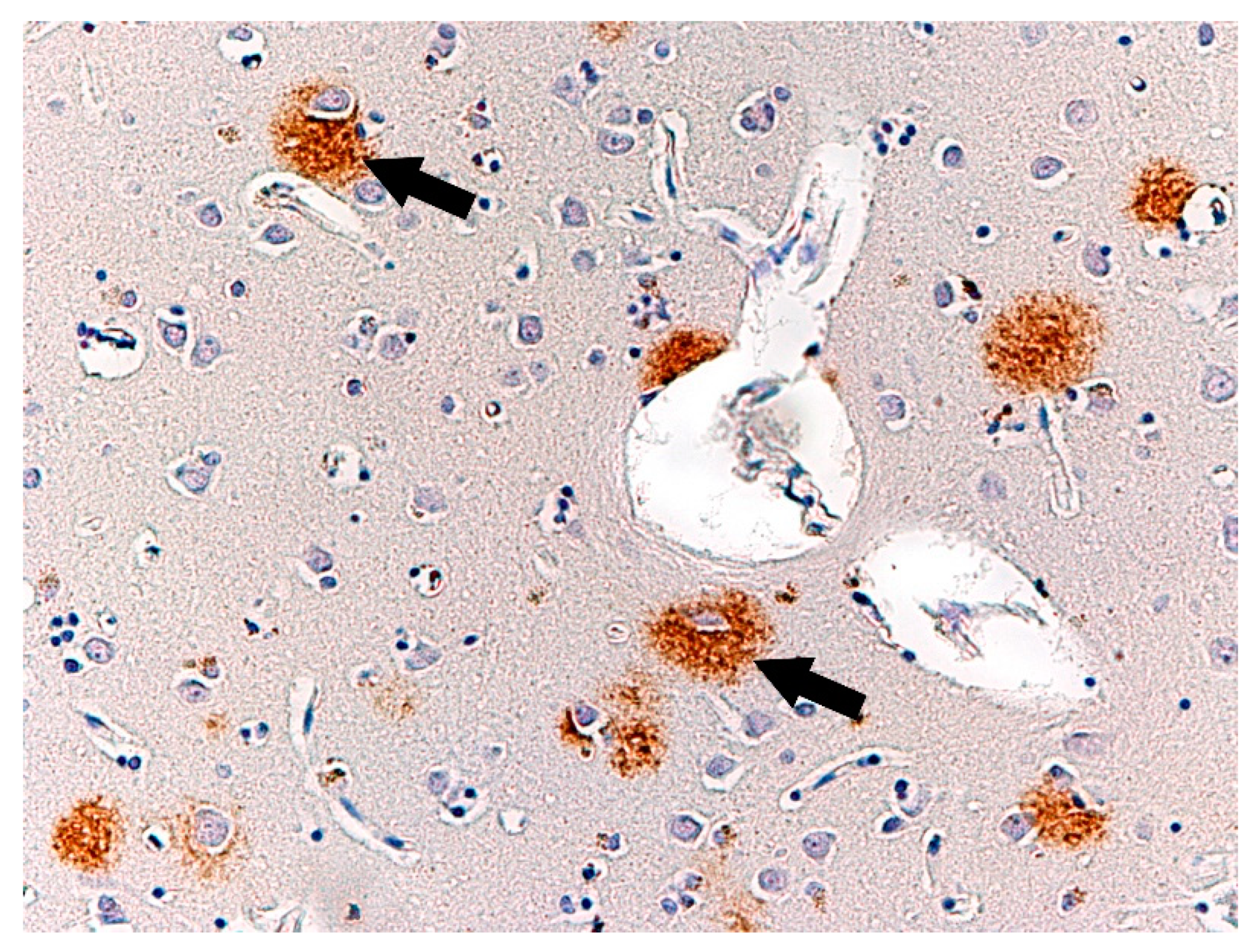
3. Results
Histochemical Methods
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marzuk, P.M.; Tardiff, K.; Hirsch, C.S. The epidemiology of murder-suicide. JAMA 1992, 267, 3179–3183. [Google Scholar] [CrossRef]
- Zimmermann, M.; Tsokos, M. Typology of murder-suicides in Berlin according to a longitudinal study based on autopsy files. Forensic Sci. Med. Pathol. 2021, 17, 247–253. [Google Scholar] [CrossRef]
- Eliason, S. Murder-suicide: A review of the recent literature. J. Am. Acad. Psychiatry Law 2009, 37, 371–376. [Google Scholar]
- Milroy, C.M.; Drastas, M.; Ranson, D. Homicide–suicide in Victoria, Australia. Am. J. Forensic Med. Pathol. 1997, 18, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Liem, M.; Roberts, D.W. Intimate Partner Homicide by Presence or Absence of a Self-Destructive Act. Homicide Stud. 2009, 13, 339–354. [Google Scholar] [CrossRef]
- Belfrage, H.; Rying, M. Characteristics of spousal homicide perpetrators: A study of all cases of spousal homicide in Sweden 1990–1999. Crim. Behav. Ment. Health 2004, 14, 121–133. [Google Scholar] [CrossRef]
- Heitzman, J. Stres w Etiologii Przestępstw Agresywnych. [Stress in the Aetiology of Violent Crime]; Wydawnictwo Uniwersytetu Jagielońskiego: Kraków, Poland, 2002. [Google Scholar]
- Tanaka, M.; Tóth, F.; Polyák, H.; Szabó, Á.; Mándi, Y.; Vécsei, L. Immune Influencers in Action: Metabolites and Enzymes of the Tryptophan-Kynurenine Metabolic Pathway. Biomedicines 2021, 9, 734. [Google Scholar] [CrossRef]
- Roma, P.; Pazzelli, F.; Pompili, M.; Lester, D.; Girardi, P.; Ferracuti, S. Mental illness in homicide-suicide: A review. J. Am. Acad. Psychiatry Law 2012, 40, 462–468. [Google Scholar]
- Flynn, S.; Gask, L.; Appleby, L.; Shaw, J. Homicide-suicide and the role of mental disorder: A national consecutive case series. Soc. Psychiatry Psychiatr. Epidemiol. 2016, 51, 877–884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hillbrand, M. The Oxford Handbook of Behavioral Emergencies and Crises; Kleespies, P.M., Ed.; Oxford University Press: New York, NY, USA, 2017; Volume XIX, pp. 215–226. [Google Scholar]
- Flynn, S.; Gask, L.; Shaw, J. Newspaper reporting of homicide-suicide and mental illness. BJPsych Bull. 2015, 39, 268–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coccaro, E.F.; Kavoussi, R.J.; Hauger, R.L. Physiological responses to d-fenfluramine and ipsapirone challenge correlate with indices of aggression in males with personality disorder. Int. Clin. Psychopharmacol. 1995, 10, 177–179. [Google Scholar] [CrossRef] [PubMed]
- Barkataki, I.; Kumari, V.; Das, M.; Taylor, P.; Sharma, T. Volumetric structural brain abnormalities in men with schizophre-nia or antisocial personality disorder. Behav. Brain Res. 2006, 169, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Laakso, E.; Hakko, H.; Räsänen, P.; Riala, K. Suicidality and unhealthy weight control behaviors among female underaged psychiatric inpatients. Compr. Psychiatry 2013, 54, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Mighdoll, M.I.; Tao, R.; Kleinman, J.E.; Hyde, T.M. Myelin, myelin-related disorders, and psychosis. Schizophr. Res. 2015, 161, 85–93. [Google Scholar] [CrossRef]
- Kaufman, K.R.; Newman, N.B.; Dawood, A. Capgras delusion with violent behavior in Alzheimer dementia: Case analysis with literature review. Ann. Clin. Psychiatry 2014, 26, 187–191. [Google Scholar] [PubMed]
- Mega, M.S.; Cummings, J.L.; Fiorello, T.; Gornbein, J. The spectrum of behavioral changes in Alzheimer’s disease. Neurology 1996, 46, 130–135. [Google Scholar] [CrossRef]
- Wragg, R.E.; Jeste, D.V. Overview of depression and psychosis in Alzheimer’s disease. Am. J. Psychiatry 1989, 146, 577–587. [Google Scholar]
- Bartoszewska, M. Molecular mechanisms of Alzheimer disease. Postępy Biologii Komórki 2008, 3, 333–350. [Google Scholar]
- Gill, S.; Wang, M.; Mouches, P.; Rajashekar, D.; Sajobi, T.; MacMaster, F.P.; Smith, E.E.; Forkert, N.D.; Ismail, Z. Alzheimer’s Disease Neuroimaging Initiative. Neural correlates of the impulse dyscontrol domain of mild behavioral impairment. Int. J. Geriatr. Psychiatry 2021, 36, 1398–1406. [Google Scholar] [CrossRef]
- Trzepacz, P.T.; Yu, P.; Bhamidipati, P.K.; Willis, B.; Forrester, T.; Tabas, L.; Schwarz, A.J.; Saykin, A.J. Alzheimer’s Disease Neuroimaging Initiative. Frontolimbic atrophy is associated with agitation and aggression in mild cognitive impairment and Alzheimer’s disease. Alzheimer’s Dement. 2013, 9 (Suppl. 5), S95–S104. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Meiberth, D.; Newport, B.; Jessen, F. Anatomical correlates of the neuropsychiatric symptoms in Alzheimer’s disease. Curr. Alzheimer Res. 2015, 12, 266–277. [Google Scholar] [CrossRef]
- Plog, B.A.; Nedergaard, M. The Glymphatic System in Central Nervous System Health and Disease: Past, Present, and Future. Annu. Rev. Pathol. 2018, 13, 379–394. [Google Scholar] [CrossRef] [Green Version]
- Nagelhus, E.A.; Ottersen, O.P. Physiological Roles of Aquaporin-4 in Brain. Physiol. Rev. 2013, 93, 1543–1562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heizmann, C.W.; Braun, K. Changes in Ca2+-binding proteins in human neurodegenerative disorders. Trends Neurosci. 1992, 15, 259–264. [Google Scholar] [CrossRef]
- Lledo, P.M.; Somasundaram, B.; Morton, A.J.; Emson, P.C.; Mason, W.T. Stable transfection of calbindin-D28k into the GH3 cell line alters calcium currents and intracellular calcium homeostasis. Neuron 1992, 9, 943–954. [Google Scholar] [CrossRef]
- Røttingen, J.; Iversen, J.G. Ruled by waves? Intracellular and intercellular calcium signalling. Acta Physiol. Scand. 2000, 169, 203–219. [Google Scholar] [CrossRef]
- Laure-Kamionowska, M.; Maślińska, D. Calbindin positive Purkinje cells in the pathology of human cerebellum occurring at the time of its development. Folia Neuropathol. 2009, 47, 305. [Google Scholar]
- Parent, A.; Fortin, M.; Côté, P.Y.; Cicchetti, F. Calcium-binding proteins in primate basal ganglia. Neurosci. Res. 1996, 25, 309–334. [Google Scholar] [CrossRef]
- Ganel, R.; Rothstein, J.D. Glutamate transporter dysfunction and neuronal death. In Ionotropic Glutamate Receptors in the CNS; Monyer, H., Gabriel, A., Eds.; Springer: Berlin, Germany, 1999; pp. 472–493. [Google Scholar]
- Barton, D.A.; Esler, M.D.; Dawood, T.; Lambert, E.A.; Haikerwal, D.; Brenchley, C.; Socratous, F.; Hastings, J.; Guo, L.; Wiesner, G.; et al. Elevated brain serotonin turnover in patients with de-pression: Effect of genotype and therapy. Arch. Gen. Psychiatry 2008, 65, 38–46. [Google Scholar] [CrossRef] [Green Version]
- Paul, E.D.; Johnson, P.L.; Shekhar, A.; Lowry, C.A. The Deakin/Graeff hypothesis: Focus on serotonergic inhibition of panic. Neurosci. Biobehav. Rev. 2014, 46 Pt 3, 379–396. [Google Scholar] [CrossRef] [Green Version]
- Gosek, P.; Heitzman, J.; Stefanowski, B.; Antosik-Wójcińska, A.Z.; Parnowski, T. Symptomatic differences and symptoms stability in unipolar and bipolar depression. Medical charts review in 99 inpatients. Psychiatr. Polska 2019, 53, 655–672. [Google Scholar] [CrossRef]
- Völlm, B.A.; Clarke, M.; Herrando, V.T.; Seppänen, A.O.; Gosek, P.; Heitzman, J.; Bulten, E. European Psychiatric Association (EPA) guidance on forensic psychiatry: Evidence based assessment and treatment of mentally disordered offenders. Eur. Psychiatry 2018, 51, 58–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heitzman, J.; Gosek, P.; Luks, M.; Pilszyk, A.; Kotowska, J.; Pacholski, M. Implementation of the European Psychiatric Association (EPA) guidance on forensic psychiatry in Poland. Current state and required measures. Psychiatr. Polska 2020, 54, 553–570. [Google Scholar] [CrossRef] [PubMed]
- Swatt, M.; He, N. Exploring the Difference Between Male and Female Intimate Partner Homicides: Revisiting the Concept of Situated Transactions. Homicide Stud. 2006, 10, 279–292. [Google Scholar] [CrossRef]
- Harper, D.W.; Voigt, L. Homicide Followed by Suicide: An Integrated Theoretical Perspective. Homicide Stud. 2007, 11, 295–318. [Google Scholar] [CrossRef]
- McCarthy, K.J.; Mehta, R.; Haberland, N.A. Gender, power, and violence: A systematic review of measures and their association with male perpetration of IPV. PLoS ONE 2018, 13, e0207091. [Google Scholar] [CrossRef] [PubMed]
- Mojahed, A.; Alaidarous, N.; Kopp, M.; Pogarell, A.; Thiel, F.; Garthus-Niegel, S. Prevalence of Intimate Partner Violence among Intimate Partners During the Perinatal Period: A Narrative Literature Review. Front. Psychiatry 2021, 12, 601236. [Google Scholar] [CrossRef]
- Liem, M.; Barber, C.; Markwalder, N.; Killias, M.; Nieuwbeerta, P. Homicide–suicide and other violent deaths: An international comparison. Forensic Sci. Int. 2011, 207, 70–76. [Google Scholar] [CrossRef]
- Milroy, C.M. The epidemiology of homicide-suicide (dyadic death). Forensic Sci. Int. 1995, 71, 117–122. [Google Scholar] [CrossRef]
- Gillespie, M.; Hearn, V.; Silverman, R.A. Suicide Following Homicide in Canada. Homicide Stud. 1998, 2, 46–63. [Google Scholar] [CrossRef]
- LeComte, D.; Fornes, P. Homicide followed by suicide: Paris and its suburbs, 1991–1996. J. Forensic Sci. 1998, 43, 760–764. [Google Scholar] [CrossRef] [PubMed]
- Milroy, C.M. Reasons for homicide and suicide in episodes of dyadic death in Yorkshire and Humbershire. Med. Sci. Law 1995, 35, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.; Barraclough, B.M. Suicide preceded by murder: The epidemiology of homicide–suicide in England and Wales 1988–92. Psychol. Med. 2002, 32, 577–584. [Google Scholar]
- Haines, J.; Williams, C.H.L.; Lester, D. Murder-suicide: A reaction to interpersonal crisis. Forensic Sci. Int. 2010, 202, 93–96. [Google Scholar] [CrossRef]
- Holland, M.; Brown, S.V.; Hall, J.E.; Logan, J.E. Circumstances preceding homicide-suicides involving child victims: A qual-itative analysis. J. Interpers. Violence 2018, 33, 379–401. [Google Scholar] [CrossRef] [PubMed]
- Coid, J. The epidemiology of abnormal homicide and murder followed by suicide. Psychol. Med. 1983, 13, 855–860. [Google Scholar] [CrossRef]
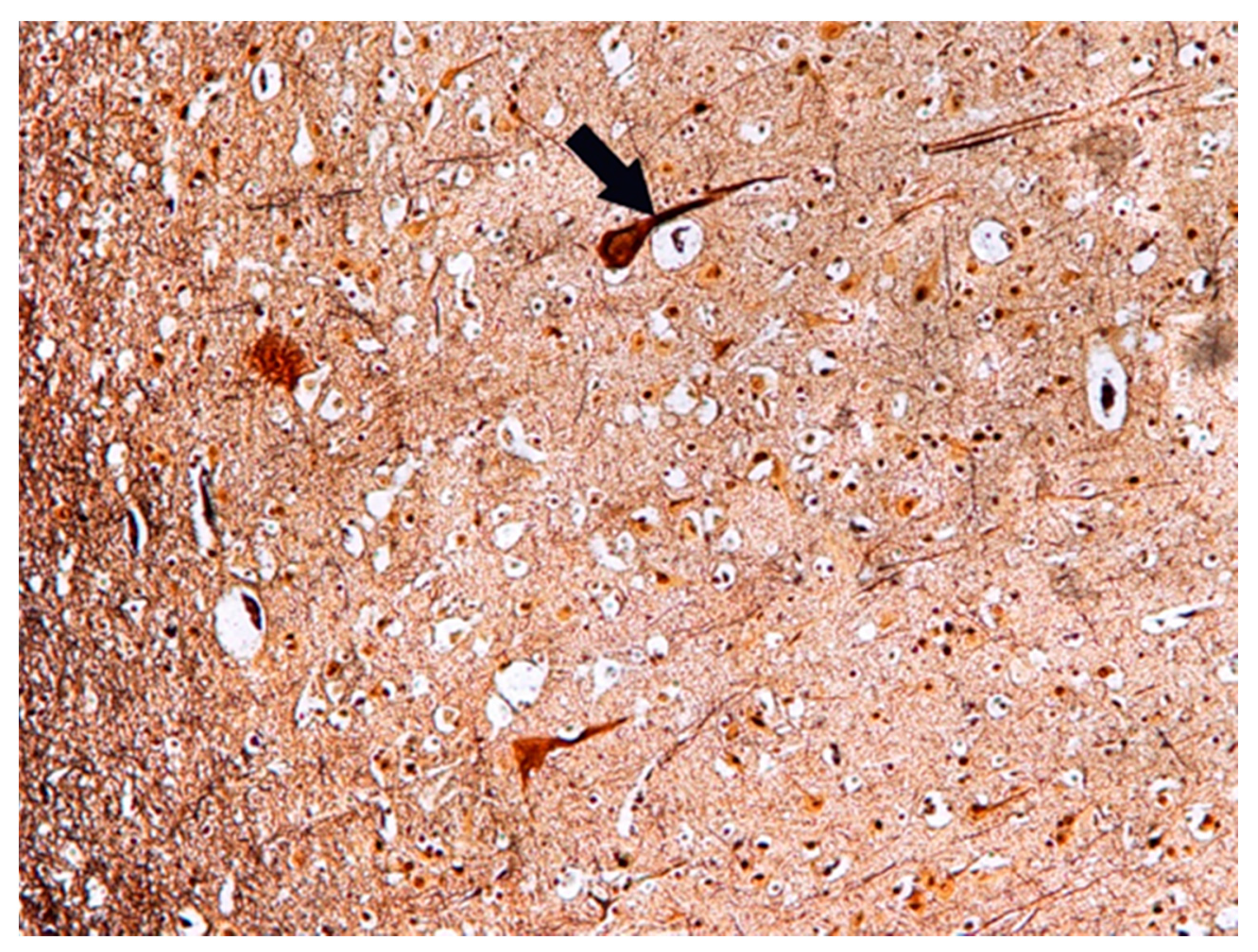
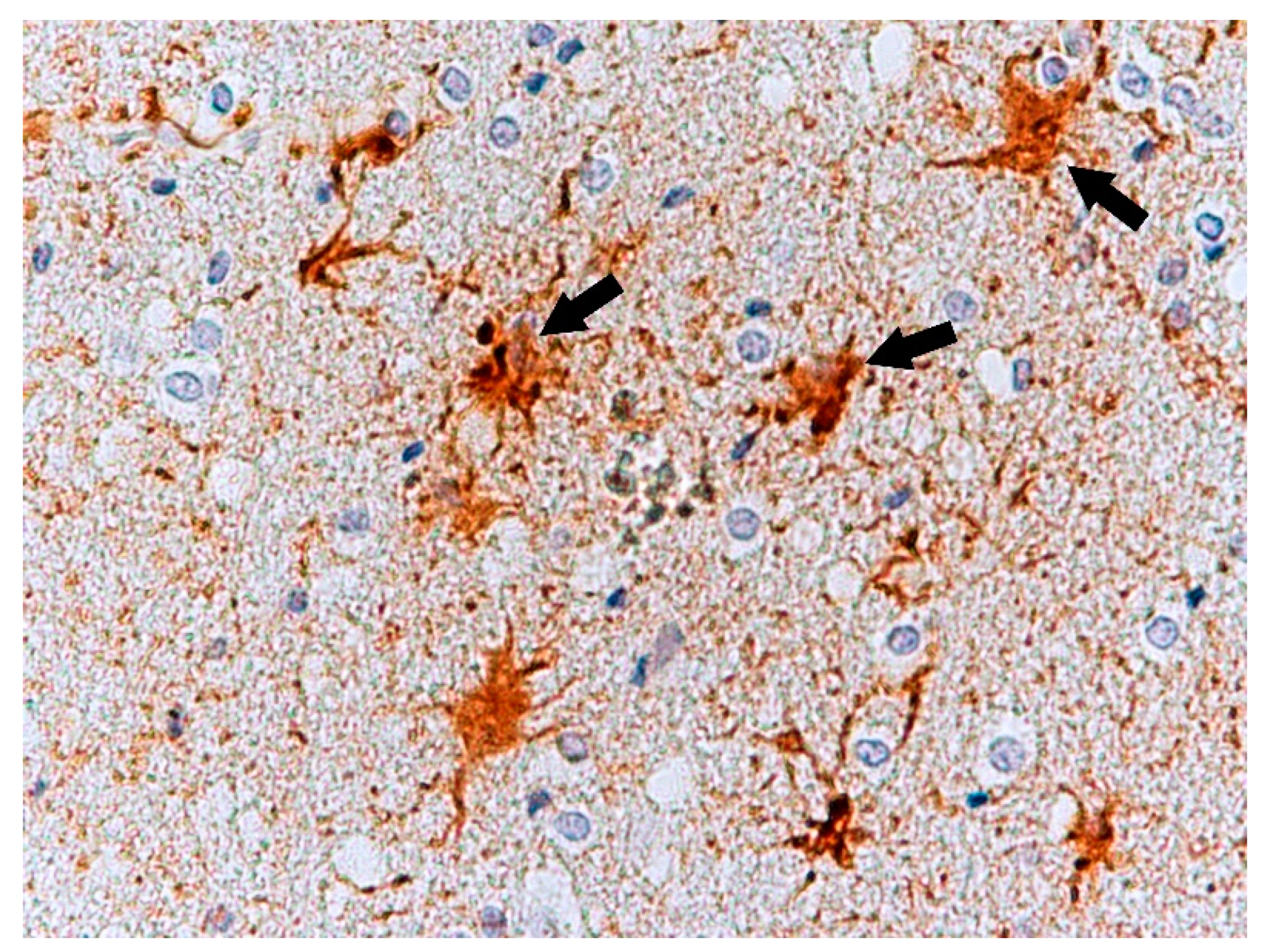

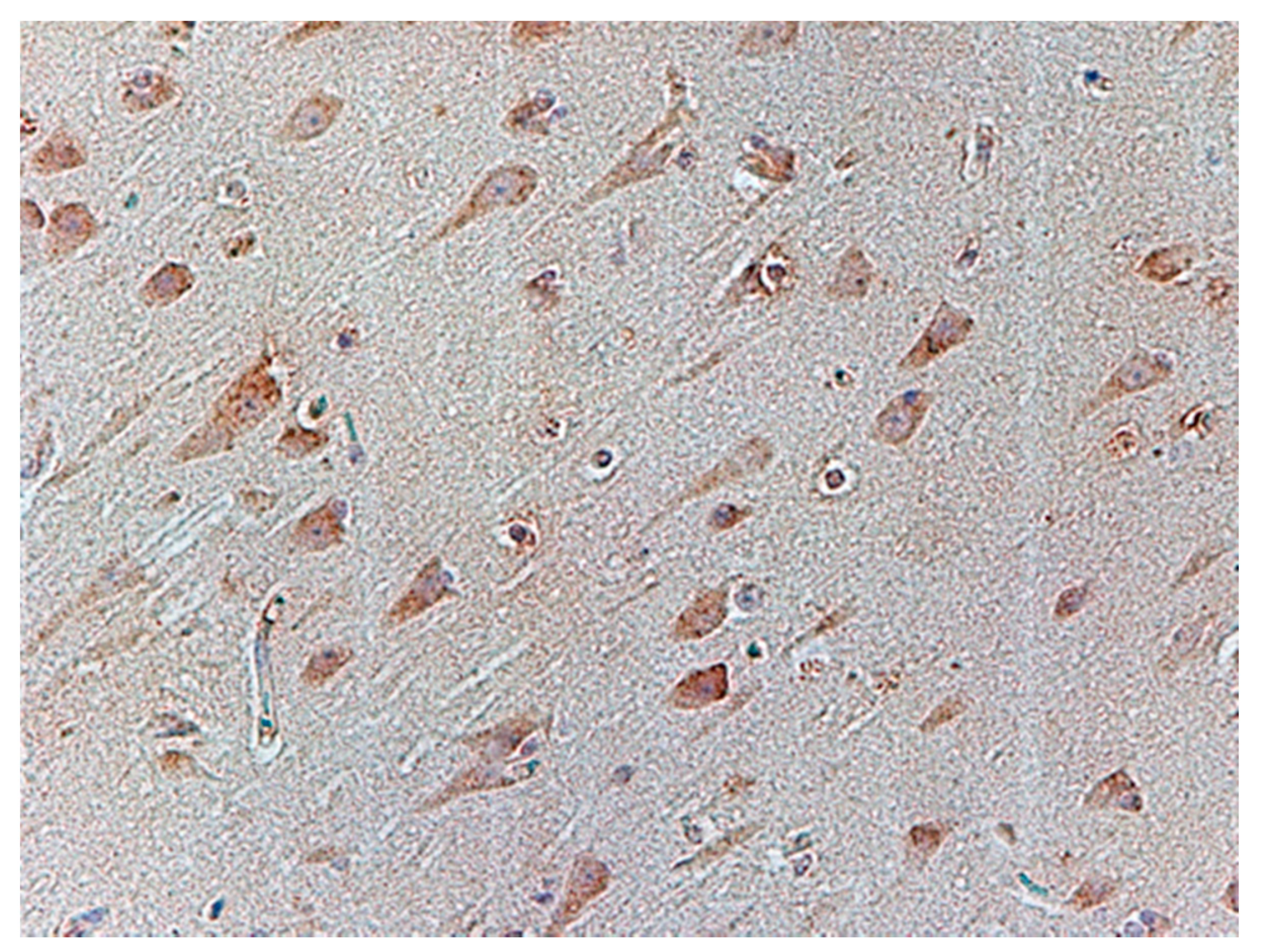
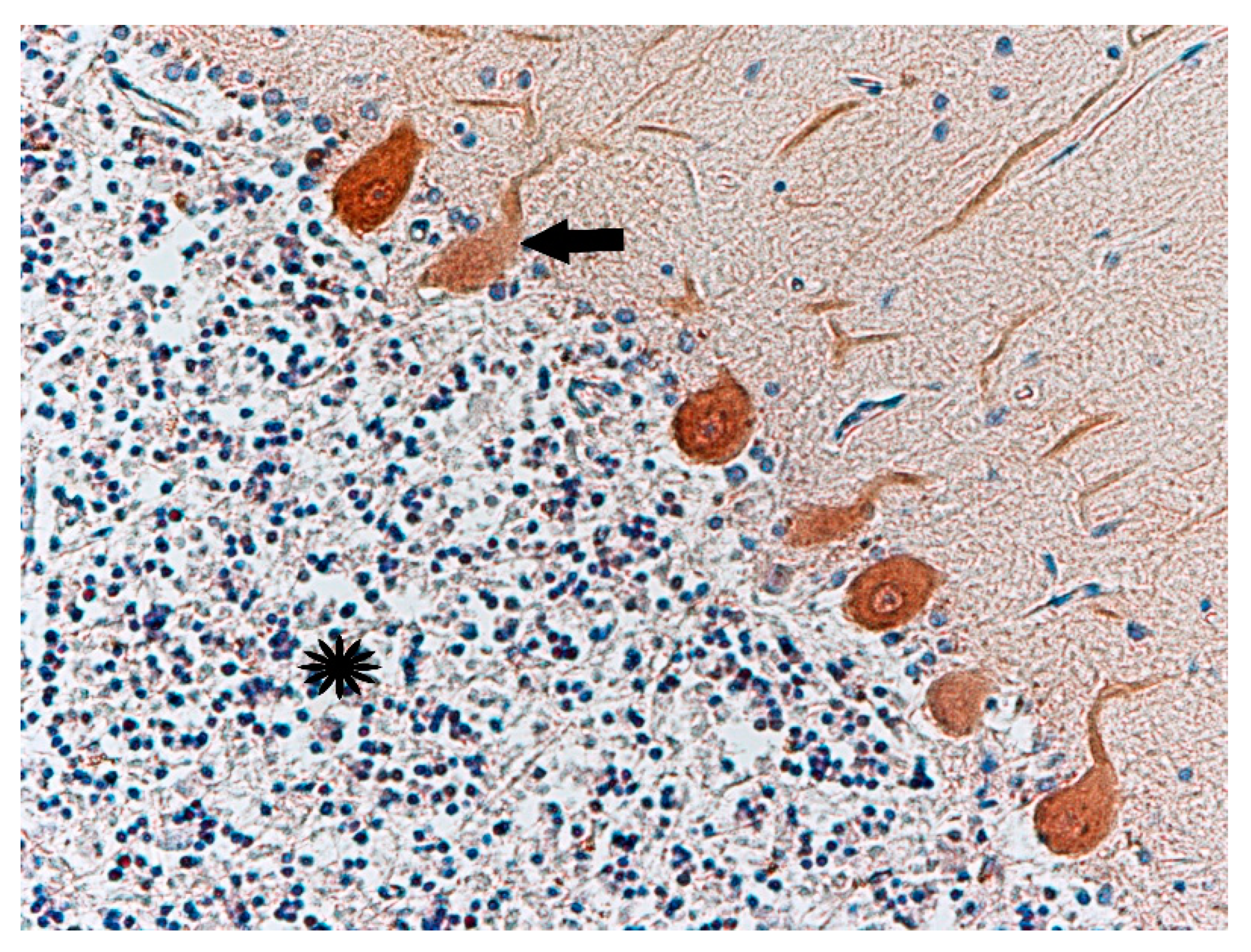
| No. | Age | Sex | Tested Structure | Time of Autopsy after Death | Cause of Death |
|---|---|---|---|---|---|
| 1 | 34 | Male | Frontal lobe, cerebellum, basal ganglia | 24 h | Hanging |
| 2 | 58 | Male | Frontal, occipital, and temporal lobes, basal ganglia, cerebellum | 72 h | Exsanguination |
| 3 | 54 | Male | Frontal and temporal lobes with the Brodmann area 28 | 72 h | Fall from height |
| 4 | 19 | Male | Frontal, occipital, and temporal lobes, mammillary bodies | 72 h | Hanging |
| 5 | 60 | Male | Frontal and temporal lobes with the Brodmann area 28, cerebellum | 72 h | Hanging |
| 6 | 34 | Male | Frontal and temporal lobes, basal ganglia | 24 h | Fall from height |
| 7 | 33 | Male | Frontal, occipital, and temporal lobes, thalamus | 24 h | Exsanguination |
| 8 | 37 | Male | Frontal, occipital, and temporal lobes, basal ganglia, midbrain | 72 h | Hanging |
| No. | Age | Sex | Tested Structure | Time of Autopsy after Death | Cause of Death |
|---|---|---|---|---|---|
| 1 | 55 | Male | Frontal, occipital, and temporal lobes, basal ganglia, cerebellum | 96 h | Traffic accident |
| 2 | 27 | Male | Frontal, occipital, and temporal lobes, basal ganglia, cerebellum, cerebellum | 96h | Traffic accident |
| 3 | 39 | Male | Frontal, occipital, and temporal lobes, basal ganglia, cerebellum | 96 h | Traffic accident |
| 4 | 21 | Male | Frontal, occipital, and temporal lobes, basal ganglia, mammillary bodies, cerebellum | 72 h | Traffic accident |
| 5 | 29 | Male | Frontal, occipital, and temporal lobes, basal ganglia, cerebellum | 72 h | Traffic accident |
| 6 | 42 | Male | Frontal, occipital, and temporal lobes, basal ganglia, cerebellum | 96 h | Traffic accident |
| 7 | 80 | Male | Frontal, occipital, and temporal lobes, basal ganglia, midbrain, cerebellum | 72 h | Traffic accident |
| 8 | 43 | Male | Frontal, occipital, and temporal lobes, basal ganglia, midbrain, cerebellum | 72 h | Traffic accident |
| No. | Age | Bielschowsky’s Stain | βA(1–40) | βA(1–42) | GFAP | Serotonin 5-HT2A | GLT1 | GLAST | Calbindin-D28k |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 34 | Senile plaques in the frontal lobe | − | + | + | + | + | + | + |
| 2 | 58 | Senile plaques in the occipital lobe | + | + | + | − | + | + | + |
| 3 | 54 | − | − | − | + | + | + | + | × |
| 4 | 19 | − | − | − | + | + | + | + | × |
| 5 | 60 | Senile plaques in the occipital lobe | − | + | + | + | + | + | + |
| 6 | 34 | − | − | − | + | + | − | − | × |
| 7 | 33 | Senile plaques in the frontal lobe | + | − | + | + | − | − | × |
| 8 | 37 | − | − | − | + | + | + | + | × |
| No. | Age | Bielschowsky’s Stain | βA(1–40) | βA(1–42) | GFAP | Serotonin 5-HT2A | GLT1 | GLAST | Calbindin-D28k |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 55 | − | − | − | + | + | + | + | + |
| 2 | 27 | − | − | − | + | + | + | + | + |
| 3 | 39 | − | − | − | + | + | + | + | + |
| 4 | 21 | − | − | − | + | + | + | + | + |
| 5 | 29 | − | − | − | + | + | + | + | + |
| 6 | 42 | − | − | − | + | + | + | + | + |
| 7 | 80 | − | − | − | + | + | + | + | + |
| 8 | 43 | − | − | − | + | + | + | + | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stępień, T.; Heitzman, J.; Wierzba-Bobrowicz, T.; Gosek, P.; Krajewski, P.; Chrzczonowicz-Stępień, A.; Berent, J.; Jurek, T.; Bolechała, F. Neuropathological Changes in the Brains of Suicide Killers. Biomolecules 2021, 11, 1674. https://doi.org/10.3390/biom11111674
Stępień T, Heitzman J, Wierzba-Bobrowicz T, Gosek P, Krajewski P, Chrzczonowicz-Stępień A, Berent J, Jurek T, Bolechała F. Neuropathological Changes in the Brains of Suicide Killers. Biomolecules. 2021; 11(11):1674. https://doi.org/10.3390/biom11111674
Chicago/Turabian StyleStępień, Tomasz, Janusz Heitzman, Teresa Wierzba-Bobrowicz, Paweł Gosek, Paweł Krajewski, Agnieszka Chrzczonowicz-Stępień, Jarosław Berent, Tomasz Jurek, and Filip Bolechała. 2021. "Neuropathological Changes in the Brains of Suicide Killers" Biomolecules 11, no. 11: 1674. https://doi.org/10.3390/biom11111674
APA StyleStępień, T., Heitzman, J., Wierzba-Bobrowicz, T., Gosek, P., Krajewski, P., Chrzczonowicz-Stępień, A., Berent, J., Jurek, T., & Bolechała, F. (2021). Neuropathological Changes in the Brains of Suicide Killers. Biomolecules, 11(11), 1674. https://doi.org/10.3390/biom11111674







