Chitosan as a Valuable Biomolecule from Seafood Industry Waste in the Design of Green Food Packaging
Abstract
:1. Introduction
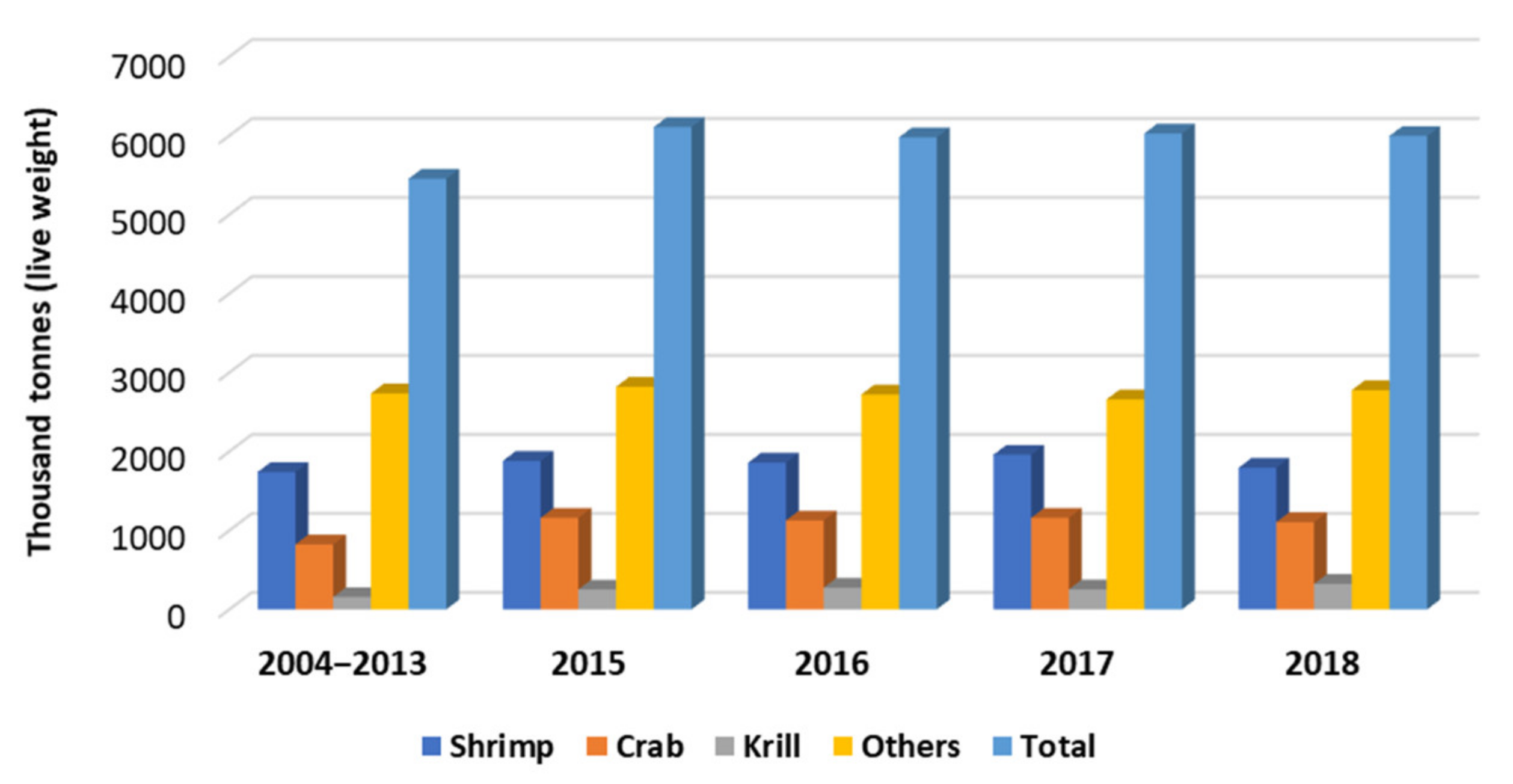
2. Seafood Industry Waste
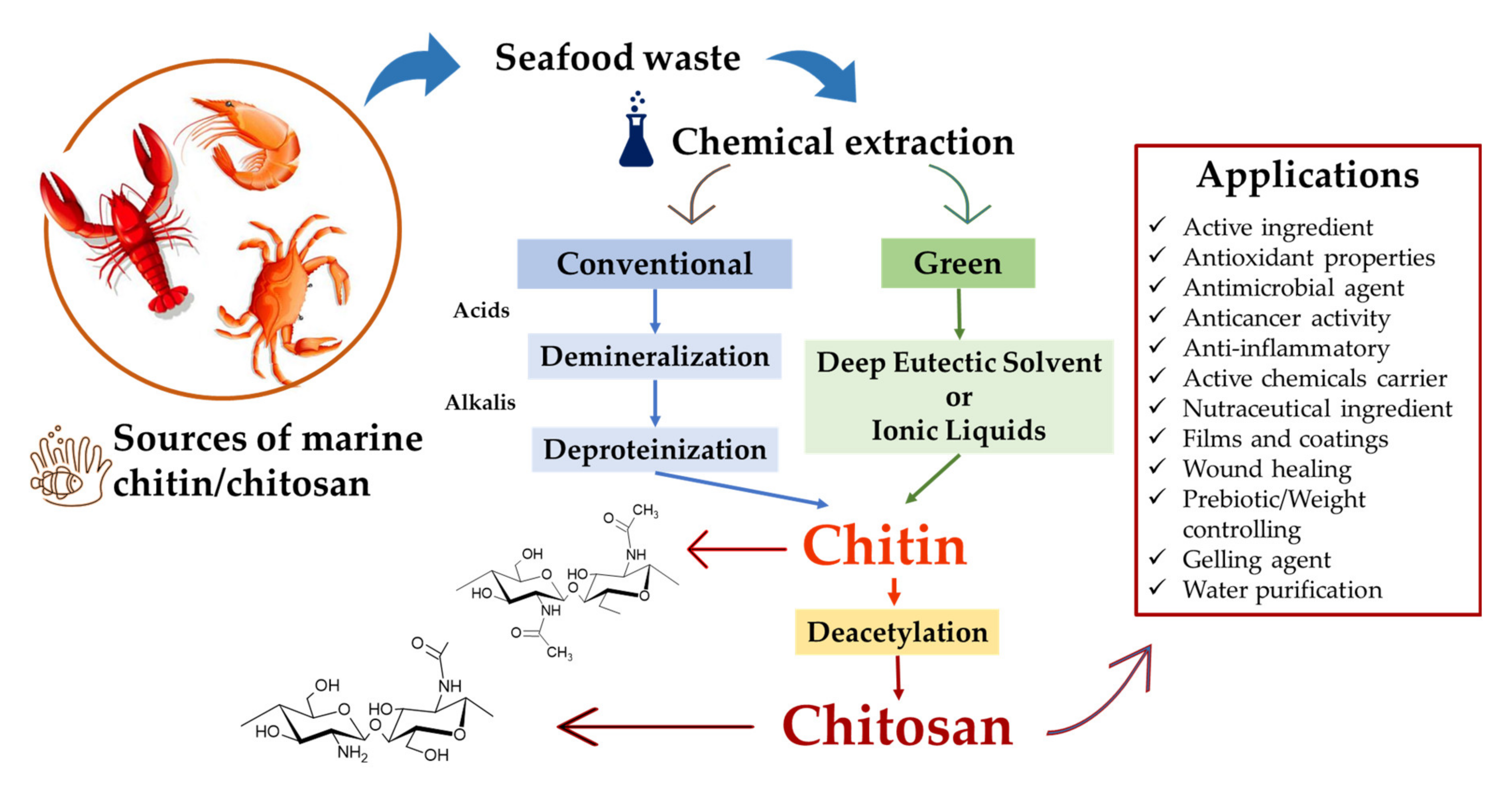
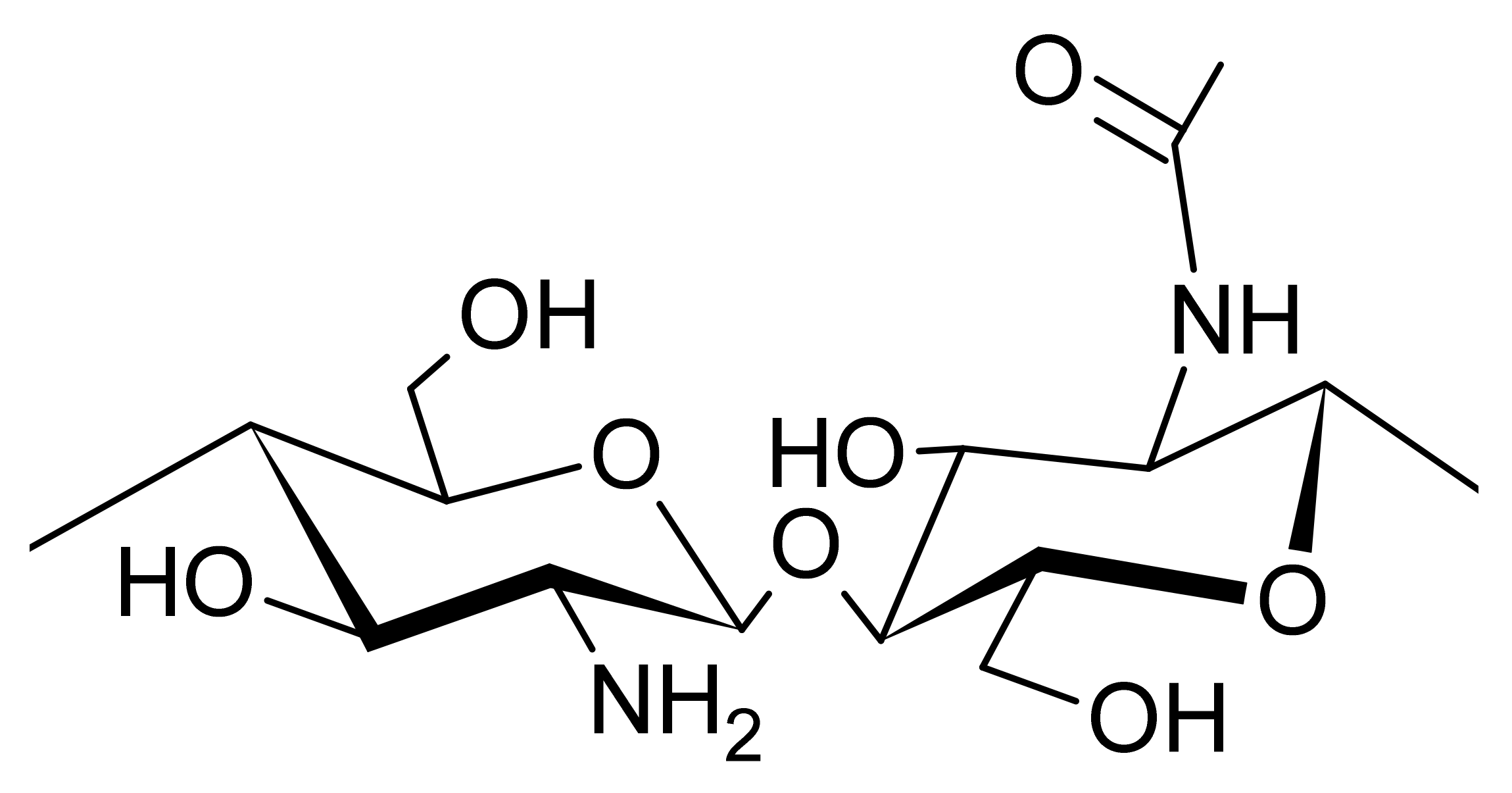
3. Chitin and Chitosan
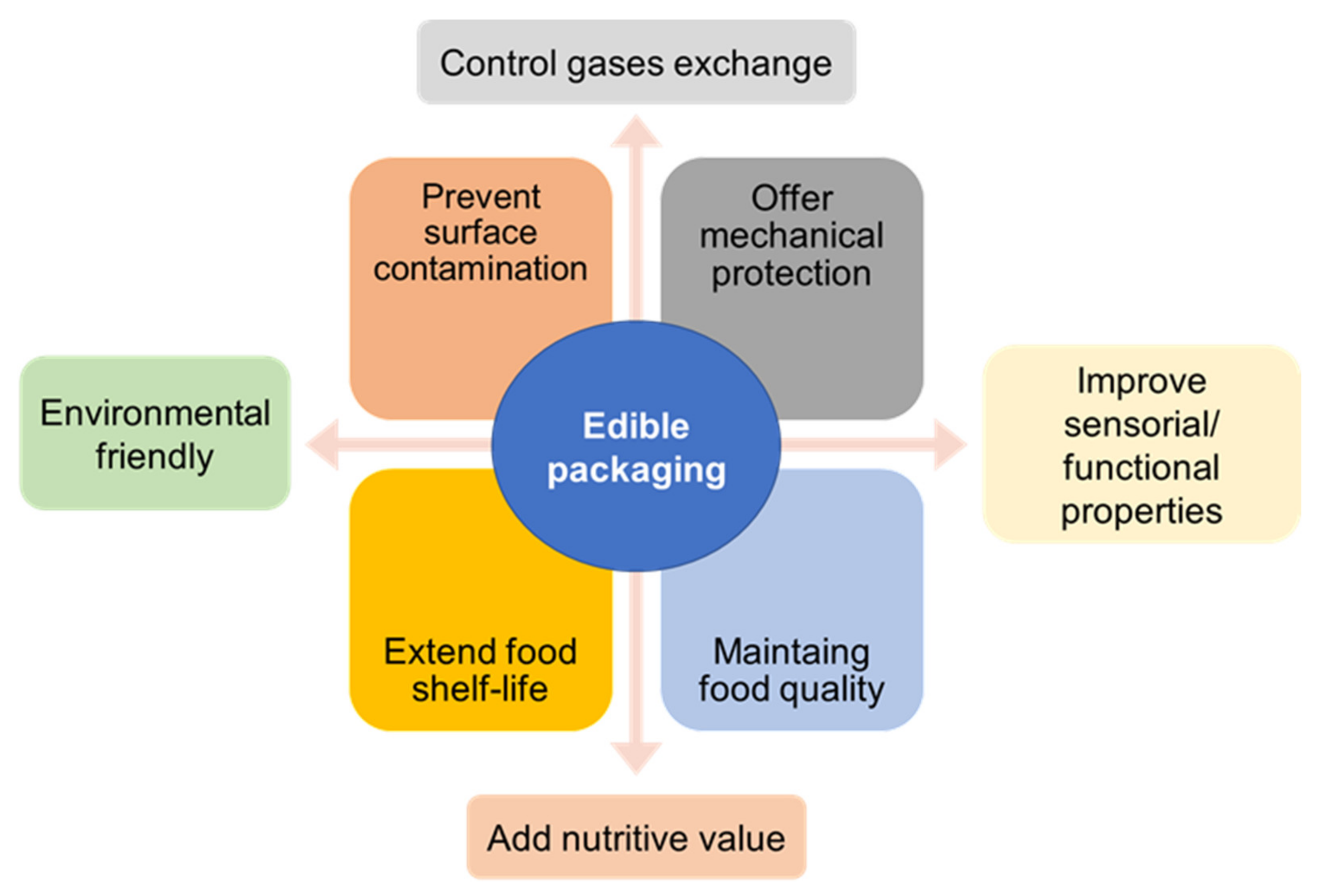
3.1. Food Packaging
3.1.1. Chitosan-Based Films to Food Packaging
3.1.2. Plasticizing Biodegradable Films with Green Solvents
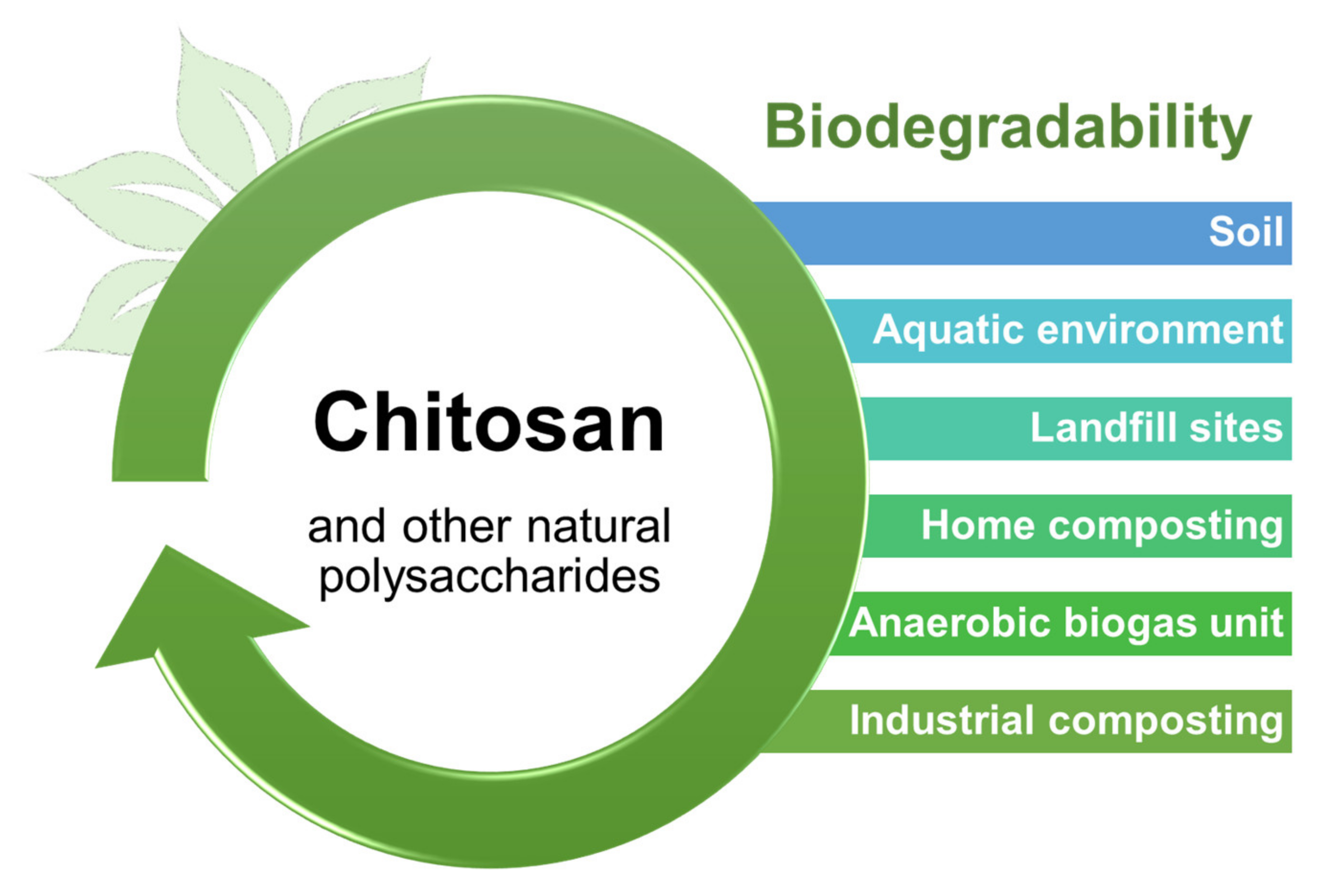
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ozogul, F.; Cagalj, M.; Šimat, V.; Ozogul, Y.; Tkaczewska, J.; Hassoun, A.; Kaddour, A.A.; Kuley, E.; Rathod, N.B.; Phadke, G.G. Recent developments in valorisation of bioactive ingredients in discard/seafood processing by-products. Trends Food Sci. Technol. 2021, 116, 559–582. [Google Scholar] [CrossRef]
- Schmitz, C.; González Auza, L.; Koberidze, D.; Rasche, S.; Fischer, R.; Bortesi, B. Conversion of Chitin to Defined Chitosan Oligomers: Current Status and Future Prospects. Mar. Drugs 2019, 17, 452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khayrova, A.; Lopatin, S.; Varlamov, V. Obtaining chitin, chitosan and their melanin complexes from insects. Int. J. Biol. Macromol. 2021, 167, 1319–1328. [Google Scholar] [CrossRef] [PubMed]
- Mujtaba, M.; Morsi, R.E.; Kerch, G.; Elsabee, M.Z.; Kaya, M.; Labidi, J.; Khawar, K.M. Current advancements in chitosan-based film production for food technology; A review. Int. J. Biol. Macromol. 2019, 121, 889–904. [Google Scholar] [CrossRef] [PubMed]
- Tavassoli-Kafrani, E.; Shekarchizadeh, H.; Masoudpour-Behabadi, M. Development of edible films and coatings from alginates and carrageenans. Carbohydr. Polym. 2016, 137, 360–374. [Google Scholar] [CrossRef] [PubMed]
- Quirós-Sauceda, A.E.; Ayala-Zavala, J.F.; Olivas, G.I.; González-Aguilar, G.A. Edible coatings as encapsulating matrices for bioactive compounds: A review. J. Food Sci. Technol. 2014, 51, 1674–1685. [Google Scholar] [CrossRef] [Green Version]
- Rivero, S.; Damonte, L.; Garcia, M.A.; Pinotti, A. An Insight into the Role of Glycerol in Chitosan Films. Food Biophys. 2016, 11, 117–127. [Google Scholar] [CrossRef]
- Almeida, C.M.R.; Magalhães, J.M.C.S.; Souza, H.K.S.; Gonçalves, M.P. The role of choline chloride-based deep eutectic solvent and curcumin on chitosan films properties. Food Hydrocoll. 2018, 81, 456–466. [Google Scholar] [CrossRef]
- Pereira, P.F.; Andrade, C.T. Optimized pH-responsive film based on a eutectic mixture-plasticized chitosan. Carbohydr. Polym. 2017, 165, 238–246. [Google Scholar] [CrossRef]
- Sousa, A.M.M.; Souza, H.K.S.; Latona, N.; Liu, C.-K.; Gonçalves, M.P.; Liu, L. Choline chloride based ionic liquid analogues as tool for the fabrication of agar films with improved mechanical properties. Carbohydr. Polym. 2014, 111, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Abbott, A.P.; Harris, R.C.; Ryder, K.S.; D’Agostino, C.; Gladden, L.F.; Mantle, M.D. Glycerol eutectics as sustainable solvent systems. Green Chem. 2011, 13, 82–90. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef] [Green Version]
- Zdanowicz, M.; Wilpiszewska, K.; Spychaj, T. Deep eutectic solvents for polysaccharides processing. A review. Carbohydr. Polym. 2018, 200, 361–380. [Google Scholar] [CrossRef]
- Mathew, G.M.; Sukumaran, R.K.; Sindhu, R.; Binod, P.; Pandey, A. Green remediation of the potential hazardous shellfish wastes generated from the processing industries and their bioprospecting. Environ. Technol. Innov. 2021, 24, 101979. [Google Scholar] [CrossRef]
- Kruijssen, F.; Tedesco, I.; Ward, A.; Pincus, L.; Love, D.; Thorne-Lyman, A.L. Loss and waste in fish value chains: A review of the evidence from low and middle-income countries. Glob. Food Sec. 2020, 26, 100434. [Google Scholar] [CrossRef]
- Jouffray, J.-B.; Crona, B.; Wassénius, E.; Bebbington, J.; Scholtens, B. Leverage points in the financial sector for seafood sustainability. Sci. Adv. 2019, 5, eaax3324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shamshak, G.L.; Anderson, J.L.; Asche, F.; Garlock, T.; Love, D.C. U.S. seafood consumption. J. World Aquac. Soc. 2019, 50, 715–727. [Google Scholar] [CrossRef]
- Food and Agriculture Organization (FAO). Statistics Division. Commodity Balances—Livestock and Fish Primary Equivalent; FAO: Rome, Italy, 2021; Available online: http://www.fao.org/faostat/en/#home (accessed on 26 April 2021).
- Food and Agriculture Organization (FAO). The State of World Fisheries and Aquaculture 2020—Sustainability in Action; FAO: Rome, Italy, 2020. Available online: https://www.fao.org/documents/card/en/c/ca9229en (accessed on 18 October 2021).
- Maulu, S.; Hasimuna, O.J.; Monde, C.; Mweemba, M. An assessment of post-harvest fish losses and preservation practices in Siavonga district, Southern Zambia. Fish. Aquat. Sci. 2020, 23, 25. [Google Scholar] [CrossRef]
- Tacias-Pascacio, V.G.; Castañeda-Valbuena, D.; Morellon-Sterling, R.; Tavano, O.; Berenguer-Murcia, Á.; Vela-Gutiérrez, G.; Rather, I.A.; Fernandez-Lafuente, R. Bioactive peptides from fisheries residues: A review of use of papain in proteolysis reactions. Int. J. Biol. Macromol. 2021, 184, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Haghighi, H.; Licciardello, F.; Fava, P.; Siesler, H.W.; Pulvirenti, A. Recent advances on chitosan-based films for sustainable food packaging applications. Food Packag. Shelf Life 2020, 26, 100551–100567. [Google Scholar] [CrossRef]
- Sanchez-Salvador, J.L.; Balea, A.; Monte, M.C.; Negro, C.; Blanco, A. Chitosan grafted/cross-linked with biodegradable polymers: A review. Int. J. Biol. Macromol. 2021, 178, 325–343. [Google Scholar] [CrossRef] [PubMed]
- Crini, G. Historical review on chitin and chitosan biopolymers. Environ. Chem. Lett. 2019, 17, 1623–1643. [Google Scholar] [CrossRef]
- Van den Broek, L.A.M.; Knoop, R.J.I.; Kappen, F.H.J.; Boeriu, C.G. Chitosan films and blends for packaging material. Carbohydr. Polym. 2015, 116, 237–242. [Google Scholar] [CrossRef]
- Hoell, I.A.; Vaaje-Kolstad, G.; Eijsink, V.G.H. Structure and function of enzymes acting on chitin and chitosan. Biotechnol. Genet. Eng. Rev. 2010, 27, 331–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdel-Rahman, R.M.; Hrdina, R.; Abdel-Mohsen, A.M.; Fouda, M.M.G.; Soliman, A.Y.; Mohamed, F.K.; Mohsin, K.; Pinto, T.D. Chitin and chitosan from Brazilian Atlantic Coast: Isolation, characterization and antibacterial activity. Int. J. Biol. Macromol. 2015, 80, 107–120. [Google Scholar] [CrossRef]
- Bastos, D.S.; Barreto, B.N.; Souza, H.K.S.; Bastos, M.; Rocha-Leão, M.H.M.; Andrade, C.T.; Gonçalves, M.P. Characterization of a chitosan sample extracted from Brazilian shrimps and its application to obtain insoluble complexes with a commercial whey protein isolate. Food Hydrocoll. 2010, 24, 709–718. [Google Scholar] [CrossRef]
- Morin-Crini, N.; Lichtfouse, E.; Torri, G.; Crini, G. Applications of chitosan in food, pharmaceuticals, medicine, cosmetics, agriculture, textiles, pulp and paper, biotechnology, and environmental chemistry. Environ. Chem. Lett. 2019, 17, 1667–1692. [Google Scholar] [CrossRef] [Green Version]
- Markets and Markets. Chitosan Market. 2018. Available online: https://www.marketsandmarkets.com/Market-Reports/chitosan-market-202177578.html (accessed on 19 October 2021).
- Pandit, A.; Indurkar, A.; Deshpande, C.; Jain, R.; Dandekar, P. A systematic review of physical techniques for chitosan degradation. Carbohydr. Polym. Technol. Appl. 2021, 2, 100033. [Google Scholar]
- Muñoz, I.; Rodríguez, C.; Gillet, D.; Moerschbacher, B.M. Life cycle assessment of chitosan production in India and Europe. Int. J. Life Cycle Assess. 2018, 23, 1151–1160. [Google Scholar] [CrossRef] [Green Version]
- Muxika, A.; Etxabide, A.; Uranga, J.; Guerrero, P.; de la Caba, K. Chitosan as a bioactive polymer: Processing, properties and applications. Int. J. Biol. Macromol. 2017, 105, 1358–1368. [Google Scholar] [CrossRef]
- Philibert, T.; Lee, B.H.; Fabien, N. Current Status and New Perspectives on Chitin and Chitosan as Functional Biopolymers. Appl. Biochem. Biotechnol. 2017, 181, 1314–1337. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Huang, W.-C.; Guo, N.; Zhang, S.; Xue, C.; Mao, X. Two-step separation of chitin from shrimp shells using citric acid and deep eutectic solvents with the assistance of microwave. Polymers 2019, 11, 409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Setoguchi, T.; Kato, T.; Yamamoto, K.; Kadokawa, J. Facile production of chitin from crab shells using ionic liquid and citric acid. Int. J. Biol. Macromol. 2012, 50, 861–864. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Lu, X.; Sun, N.; Rogers, R.D. Dissolution or extraction of crustacean shells using ionic liquids to obtain high molecular weight purified chitin and direct production of chitin films and fibers. Green Chem. 2010, 12, 968. [Google Scholar] [CrossRef]
- Younes, I.; Rinaudo, M. Chitin and Chitosan Preparation from Marine Sources. Structure, Properties and Applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef] [Green Version]
- González-Espinosa, Y.; Sabagh, B.; Moldenhauer, E.; Clarke, P.; Goycoolea, F.M. Characterisation of chitosan molecular weight distribution by multi-detection asymmetric flow-field flow fractionation (AF4) and SEC. Int. J. Biol. Macromol. 2019, 136, 911–919. [Google Scholar] [CrossRef]
- Kasaai, M. A review of several reported procedures to determine the degree of N-acetylation for chitin and chitosan using infrared spectroscopy. Carbohydr. Polym. 2008, 71, 497–508. [Google Scholar] [CrossRef]
- Brugnerotto, J.; Desbrières, J.; Roberts, G.; Rinaudo, M. Characterization of chitosan by steric exclusion chromatography. Polymer 2001, 42, 09921–09927. [Google Scholar] [CrossRef]
- Kasaai, M.R. Calculation of Mark-Houwink-Sakurada (MHS) equation viscometric constants for chitosan in any solvent-temperature system using experimental reported viscometric constants data. Carbohydr. Polym. 2007, 68, 477–488. [Google Scholar] [CrossRef]
- Annu Raja, A.N. Recent development in chitosan-based electrochemical sensors and its sensing application. Int. J. Biol. Macromol. 2020, 164, 4231–4244. [Google Scholar] [CrossRef]
- Oliveira, H.C.; Gomes, B.C.R.; Pelegrino, M.T.; Seabra, A.B. Nitric oxide-releasing chitosan nanoparticles alleviate the effects of salt stress in maize plants. Nitric Oxide 2016, 61, 10–19. [Google Scholar] [CrossRef]
- EPA—Environmental Protection Agency of United States. Safer Chemical Ingredients List. Available online: https://www.epa.gov/saferchoice/safer-ingredients#pop9012764 (accessed on 10 May 2021).
- ANVISA—Agência Nacional de Vigilância Sanitária. Alegações de Propriedade Funcional Aprovadas pela ANVISA. Available online: https://www.gov.br/agricultura/pt-br/assuntos/inspecao/produtos-vegetal/legislacao-1/biblioteca-de-normas-vinhos-e-bebidas/alegacoes-de-propriedade-funcional-aprovadas_anvisa.pdf/view (accessed on 10 May 2021).
- Lopez-Santamarina, A.; Mondragon, A.D.C.; Lamas, A.; Miranda, J.M.; Franco, C.M.; Cepeda, A. Animal-origin prebiotics based on chitin: An alternative for the Future? A critical review. Foods 2020, 9, 782. [Google Scholar] [CrossRef]
- Trivedi, V.; Satia, M.; Deschamps, A.; Maquet, V.; Shah, R.; Zinzuwadia, P.; Trivedi, J. Single-blind, placebo controlled randomised clinical study of chitosan for body weight reduction. Nutr. J. 2016, 15, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Liao, D.; Zou, Y.; Chi, H. The effects of chitosan supplementation on body weight and body composition: A systematic review and meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2020, 60, 1815–1825. [Google Scholar] [CrossRef]
- Preuss, H.; Kaats, G. Chitosan as a Dietary Supplement for Weight Loss: A Review. Curr. Nutr. Food Sci. 2006, 2, 297–311. [Google Scholar] [CrossRef]
- Friedman, M.; Juneja, V.K. Review of antimicrobial and antioxidative activities of chitosans in food. J. Food Prot. 2010, 73, 1737–1761. [Google Scholar] [CrossRef]
- Ma, Z.; Garrido-Maestu, A.; Jeong, K.C. Application, mode of action, and in vivo activity of chitosan and its micro- and nanoparticles as antimicrobial agents: A review. Carbohydr. Polym. 2017, 176, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Sahariah, P.; Másson, M. Antimicrobial chitosan and chitosan derivatives: A review of the structure-activity relationship. Biomacromolecules 2017, 18, 3846–3868. [Google Scholar] [CrossRef] [PubMed]
- Verlee, A.; Mincke, S.; Stevens, C.V. Recent developments in antibacterial and antifungal chitosan and its derivatives. Carbohydr. Polym. 2017, 164, 268–283. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Bonilla, A.; Cerrada, M.L.; Fernández-García, M. Antimicrobial activity of chitosan in food, agriculture and biomedicine. In Polymeric Materials with Antimicrobial Activity: From Synthesis to Applications; Muñoz-Bonilla, A., Cerrada, M.L., Fernández-García, M., Eds.; Royal Society of Chemistry: Cambridge, UK, 2014; pp. 22–53. [Google Scholar] [CrossRef]
- Cazón, P.; Velazquez, G.; Ramírez, J.A.; Vázquez, M. Polysaccharide-based films and coatings for food packaging: A review. Food Hydrocoll. 2017, 68, 136–148. [Google Scholar] [CrossRef]
- Duan, C.; Meng, X.; Meng, J.; Khan, M.I.H.; Dai, L.; Khan, A.; An, X.; Zhang, J.; Huq, T.; Ni, Y. Chitosan as a preservative for fruits and vegetables: A review on chemistry and antimicrobial properties. J. Bioresour. Bioprod. 2019, 4, 11–21. [Google Scholar] [CrossRef]
- Teixeira-Costa, B.E.; Silva Pereira, B.C.; Lopes, G.K.; Andrade, C.T. Encapsulation and antioxidant activity of assai pulp oil (Euterpe oleracea) in chitosan/alginate polyelectrolyte complexes. Food Hydrocoll. 2020, 109, 106097. [Google Scholar] [CrossRef]
- Klinmalai, P.; Hagiwara, T.; Sakiyama, T.; Ratanasumawong, S. Chitosan effects on physical properties, texture, and microstructure of flat rice noodles. LWT Food Sci. Technol. 2017, 76, 117–123. [Google Scholar] [CrossRef]
- Han, M.; Clausen, M.P.; Christensen, M.; Vossen, E.; van Hecke, T.; Bertram, H.C. Enhancing the health potential of processed meat: The effect of chitosan or carboxymethyl cellulose enrichment on inherent microstructure, water mobility and oxidation in a meat-based food matrix. Food Funct. 2018, 9, 4017–4027. [Google Scholar] [CrossRef]
- Pereda, M.; Amica, G.; Marcovich, N.E. Development and characterization of edible chitosan/olive oil emulsion films. Carbohydr. Polym. 2012, 87, 1318–1325. [Google Scholar] [CrossRef]
- Morelli, S.; Holdich, R.G.; Dragosavac, M.M. Chitosan and Poly (Vinyl Alcohol) microparticles produced by membrane emulsification for encapsulation and pH controlled release. Chem. Eng. J. 2016, 288, 451–460. [Google Scholar] [CrossRef] [Green Version]
- Rachtanapun, C.; Tantala, J.; Klinmalai, P.; Ratanasumawong, S. Effect of chitosan on Bacillus cereus inhibition and quality of cooked rice during storage. Int. J. Food Sci. Technol. 2015, 50, 2419–2426. [Google Scholar] [CrossRef]
- Bonilla, J.; Poloni, T.; Lourenço, R.V.; Sobral, P.J.A. Antioxidant potential of eugenol and ginger essential oils with gelatin/chitosan films. Food Biosci. 2018, 23, 107–114. [Google Scholar] [CrossRef]
- Menconi, A.; Hernandez-Velasco, X.; Latorre, J.D.; Kallapura, G.; Pumford, N.R.; Morgan, M.J.; Hargis, B.M.; Tellez, G. Effect of chitosan as a biological sanitizer for Salmonella typhimurium and aerobic gram negative spoilage bacteria present on chicken skin. Int. J. Poult. Sci. 2013, 12, 318–321. [Google Scholar] [CrossRef] [Green Version]
- Rajaei, A.; Hadian, M.; Mohsenifar, A.; Rahmani-Cherati, T.; Tabatabaei, M. A coating based on clove essential oils encapsulated by chitosan-myristic acid nanogel efficiently enhanced the shelf-life of beef cutlets. Food Packag. Shelf Life 2017, 14, 137–145. [Google Scholar] [CrossRef]
- Zhou, W.; He, Y.; Liu, F.; Liao, L.; Huang, X.; Li, R.; Zou, Y.; Zhou, L.; Zou, L.; Liu, Y.; et al. Carboxymethyl chitosan-pullulan edible films enriched with galangal essential oil: Characterization and application in mango preservation. Carbohydr. Polym. 2021, 256, 117579. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, C.; Wu, X.; Long, Y.; Su, Y. Chitosan augments tetramycin against soft rot in kiwifruit and enhances its improvement for kiwifruit growth, quality and aroma. Biomolecules 2021, 11, 1257. [Google Scholar] [CrossRef]
- Hai, L.V.; Zhai, L.; Kim, H.C.; Panicker, P.S.; Pham, D.H.; Kim, J. Chitosan Nanofiber and Cellulose Nanofiber Blended Composite Applicable for Active Food Packaging. Nanomaterials 2020, 10, 1752. [Google Scholar] [CrossRef]
- Wu, T.; Huang, J.; Jiang, Y.; Hu, Y.; Ye, X.; Liu, D.; Chen, J. Formation of hydrogels based on chitosan/alginate for the delivery of lysozyme and their antibacterial activity. Food Chem. 2018, 240, 361–369. [Google Scholar] [CrossRef]
- Yaroslavov, A.A.; Efimova, A.A.; Krasnikov, E.A.; Trosheva, K.S.; Popov, A.S.; Melik-Nubarov, N.S.; Krivtsov, G.G. Chitosan-based multi-liposomal complexes: Synthesis, biodegradability and cytotoxicity. Int. J. Biol. Macromol. 2021, 177, 455–462. [Google Scholar] [CrossRef]
- Muşat, V.; Anghel, E.; Zaharia, A.; Atkinson, I.; Mocioiu, O.; Buşilă, M.; Alexandru, P. A chitosan-agarose polysaccharide-based hydrogel for biomimetic remineralization of dental enamel. Biomolecules 2021, 11, 1137. [Google Scholar] [CrossRef]
- Sousa, C.F.V.; Saraiva, C.A.; Correia, T.R.; Pesqueira, T.; Patrício, S.G.; Rial-Hermida, M.I.; Borges, J.; Mano, J.F. Bioinstructive layer-by-layer-coated customizable 3D printed perfusable microchannels embedded in photocrosslinkable hydrogels for vascular tissue engineering. Biomolecules 2021, 11, 863. [Google Scholar] [CrossRef] [PubMed]
- Pop, N.L.; Nan, A.; Urda-Cimpean, A.E.; Florea, A.; Toma, V.A.; Moldovan, R.; Decea, N.; Mitrea, D.R.; Orasan, R. Chitosan functionalized magnetic nanoparticles to provide neural regeneration and recovery after experimental model induced peripheral nerve injury. Biomolecules 2021, 11, 676. [Google Scholar] [CrossRef]
- Ladiè, R.; Cosentino, C.; Tagliaro, I.; Antonini, C.; Bianchini, G.; Bertini, S. Supramolecular structuring of hyaluronan-lactose-modified chitosan matrix: Towards high-performance biopolymers with excellent biodegradation. Biomolecules 2021, 11, 389. [Google Scholar] [CrossRef]
- Marinho, S.; Illanes, M.; Ávila-Román, J.; Motilva, V.; Talero, E. Anti-inflammatory effects of rosmarinic acid-loaded nanovesicles in acute colitis through modulation of NLRP3 inflammasome. Biomolecules 2021, 11, 162. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro-Santos, R.; Andrade, M.; Melo, N.R.; de Sanches-Silva, A. Use of essential oils in active food packaging: Recent advances and future trends. Trends Food Sci. Technol. 2017, 61, 132–140. [Google Scholar] [CrossRef]
- Sohail, M.; Sun, D.-W.; Zhu, Z. Recent developments in intelligent packaging for enhancing food quality and safety. Crit. Rev. Food Sci. Nutr. 2018, 58, 2650–2662. [Google Scholar] [CrossRef]
- Kamdem, D.P.; Shen, Z.; Nabinejad, O. Development of biodegradable composite chitosan-based films incorporated with xylan and carvacrol for food packaging application. Food Packag. Shelf Life 2019, 21, 100344–100366. [Google Scholar] [CrossRef]
- Carina, D.; Sharma, S.; Jaiswal, A.K.; Jaiswal, S. Seaweeds polysaccharides in active food packaging: A review of recent progress. Trends Food Sci. Technol. 2021, 110, 559–572. [Google Scholar] [CrossRef]
- Groh, K.J.; Backhaus, T.; Carney-Almroth, B.; Geueke, B.; Inostroza, P.A.; Lennquist, A.; Leslie, H.A.; Maffini, M.; Slunge, D.; Trasande, L.; et al. Overview of known plastic packaging-associated chemicals and their hazards. Sci. Total Environ. 2019, 651, 3253–3268. [Google Scholar] [CrossRef]
- Kleine Jäger, J.; Piscicelli, L. Collaborations for circular food packaging: The set-up and partner selection process. Sustain. Prod. Consum. 2021, 26, 733–740. [Google Scholar] [CrossRef]
- Soares, J.; Miguel, I.; Venâncio, C.; Lopes, I.; Oliveira, M. Public views on plastic pollution: Knowledge, perceived impacts, and pro-environmental behaviours. J. Hazard. Mater. 2021, 412, 125227–125235. [Google Scholar] [CrossRef]
- Rai, P.; Mehrotra, S.; Priya, S.; Gnansounou, E.; Sharma, S.K. Recent advances in the sustainable design and applications of biodegradable polymers. Bioresour. Technol. 2021, 325, 124739–124751. [Google Scholar] [CrossRef] [PubMed]
- Flury, M.; Narayan, R. Biodegradable plastic as an integral part of the solution to plastic waste pollution of the environment. Curr. Opin. Green Sustain. Chem. 2021, 30, 100490. [Google Scholar] [CrossRef]
- Otoni, C.G.; Avena-Bustillos, R.J.; Azeredo, H.M.C.; Lorevice, M.V.; Moura, M.R.; Mattoso, L.H.C.; McHugh, T.H. Recent Advances on Edible Films Based on Fruits and Vegetables-A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1151–1169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petkoska, A.T.; Daniloski, D.; D’Cunha, N.M.; Naumovski, N.; Broach, A.T. Edible packaging: Sustainable solutions and novel trends in food packaging. Food Res. Int. 2021, 140, 109981. [Google Scholar] [CrossRef]
- Mkandawire, M.; Aryee, A.N. Resurfacing and modernization of edible packaging material technology. Curr. Opin. Food Sci. 2018, 19, 104–112. [Google Scholar] [CrossRef]
- Liang, J.; Yan, H.; Zhang, J.; Dai, W.; Gao, X.; Zhou, Y.; Wan, X.; Puligundla, P. Preparation and characterization of antioxidant edible chitosan films incorporated with epigallocatechin gallate nanocapsules. Carbohydr. Polym. 2017, 171, 300–306. [Google Scholar] [CrossRef]
- Leceta, I.; Guerrero, P.; Cabezudo, S.; de la Caba, K. Environmental assessment of chitosan-based films. J. Clean. Prod. 2013, 41, 312–318. [Google Scholar] [CrossRef]
- Oberlintner, A.; Bajić, M.; Kalčíková, G.; Likozar, B.; Novak, U. Biodegradability study of active chitosan biopolymer films enriched with Quercus polyphenol extract in different soil types. Environ. Technol. Innov. 2021, 21, 101318. [Google Scholar] [CrossRef]
- Liu, M.; Zhou, Y.; Zhang, Y.; Yu, C.; Cao, S. Preparation and structural analysis of chitosan films with and without sorbitol. Food Hydrocoll. 2013, 33, 186–191. [Google Scholar] [CrossRef]
- Zakaria, S.; Chia, C.H.; Wan Ahmad, W.H.; Kaco, H.; Chook, S.W.; Chan, C.H. Mechanical and Antibacterial Properties of Paper Coated with Chitosan. Sains Malays. 2015, 44, 905–911. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, Z.; Zhang, L.; Wang, X.; Li, L. Effects of plasticizer type and concentration on rheological, physico-mechanical and structural properties of chitosan/zein film. Int. J. Biol. Macromol. 2020, 143, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Kerch, G.; Korkhov, V. Effect of storage time and temperature on structure, mechanical and barrier properties of chitosan-based films. Eur. Food Res. Technol. 2011, 232, 17–22. [Google Scholar] [CrossRef]
- Zhong, Y.; Li, Y. Effects of storage conditions and acid solvent types on structural, mechanical and physical properties of kudzu starch (Pueraria lobata)-chitosan composite films. Starch Stärke 2011, 63, 579–586. [Google Scholar] [CrossRef]
- Wang, H.; Ding, F.; Ma, L.; Zhang, Y. Edible films from chitosan-gelatin: Physical properties and food packaging application. Food Biosci. 2021, 40, 100871. [Google Scholar] [CrossRef]
- Abugoch, L.E.; Tapia, C.; Villamán, M.C.; Yazdani-Pedram, M.; Díaz-Dosque, M. Characterization of quinoa protein-chitosan blend edible films. Food Hydrocoll. 2011, 25, 879–886. [Google Scholar] [CrossRef]
- Madian, N.G.; Mohamed, N. Enhancement of the dynamic mechanical properties of chitosan thin films by crosslinking with greenly synthesized silver nanoparticles. J. Mater. Res. Technol. 2020, 9, 12970–12975. [Google Scholar] [CrossRef]
- Pavinatto, A.; de Almeida Mattos, A.V.; Malpass, A.C.G.; Okura, M.H.; Balogh, D.T.; Sanfelice, R.C. Coating with chitosan-based edible films for mechanical/biological protection of strawberries. Int. J. Biol. Macromol. 2020, 151, 1004–1011. [Google Scholar] [CrossRef]
- Da Costa, J.C.M.; Miki, K.S.L.; da Ramos, A.S.; Teixeira-Costa, B.E. Development of biodegradable films based on purple yam starch/chitosan for food application. Heliyon 2020, 6, 1–10. [Google Scholar] [CrossRef]
- Riaz, A.; Lei, S.; Akhtar, H.M.S.; Wan, P.; Chen, D.; Jabbar, S.; Abid, M.; Hashim, M.M.; Zeng, X. Preparation and characterization of chitosan-based antimicrobial active food packaging film incorporated with apple peel polyphenols. Int. J. Biol. Macromol. 2018, 114, 547–555. [Google Scholar] [CrossRef]
- Giannakas, A.; Patsaoura, A.; Barkoula, N.-M.; Ladavos, A. A novel solution blending method for using olive oil and corn oil as plasticizers in chitosan based organoclay nanocomposites. Carbohydr. Polym. 2017, 157, 550–557. [Google Scholar] [CrossRef]
- Shahbazi, Y. The properties of chitosan and gelatin films incorporated with ethanolic red grape seed extract and Ziziphora clinopodioides essential oil as biodegradable materials for active food packaging. Int. J. Biol. Macromol. 2017, 99, 746–753. [Google Scholar] [CrossRef]
- Ren, L.; Yan, X.; Zhou, J.; Tong, J.; Su, X. Influence of chitosan concentration on mechanical and barrier properties of corn starch/chitosan films. Int. J. Biol. Macromol. 2017, 105, 1636–1643. [Google Scholar] [CrossRef] [PubMed]
- Siripatrawan, U.; Vitchayakitti, W. Improving functional properties of chitosan films as active food packaging by incorporating with propolis. Food Hydrocoll. 2016, 61, 695–702. [Google Scholar] [CrossRef]
- Lekjing, S. A chitosan-based coating with or without clove oil extends the shelf life of cooked pork sausages in refrigerated storage. Meat Sci. 2016, 111, 192–197. [Google Scholar] [CrossRef]
- Cardoso, G.P.; Dutra, M.P.; Fontes, P.R.; Ramos, A.D.L.S.; Gomide, L.A.D.M.; Ramos, E.M. Selection of a chitosan gelatin-based edible coating for color preservation of beef in retail display. Meat Sci. 2016, 114, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Sady, S.; Błaszczyk, A.; Kozak, W.; Boryło, P.; Szindler, M. Quality assessment of innovative chitosan-based biopolymers for edible food packaging applications. Food Packag. Shelf Life 2021, 30, 100756. [Google Scholar] [CrossRef]
- Kalia, A.; Kaur, M.; Shami, A.; Jawandha, S.K.; Alghuthaymi, M.A.; Thakur, A.; Abd-Elsalam, K.A. Nettle-Leaf Extract Derived ZnO/CuO Nanoparticle-Biopolymer-Based Antioxidant and Antimicrobial Nanocomposite Packaging Films and Their Impact on Extending the Post-Harvest Shelf Life of Guava Fruit. Biomolecules 2021, 11, 224. [Google Scholar] [CrossRef] [PubMed]
- Gubanova, G.N.; Petrova, V.A.; Kononova, S.V.; Popova, E.N.; Smirnova, V.E.; Bugrov, A.N.; Klechkovskaya, V.V.; Skorik, Y.A. Thermal properties and structural features of multilayer films based on chitosan and anionic polysaccharides. Biomolecules 2021, 11, 762. [Google Scholar] [CrossRef]
- Liu, J.; Liu, S.; Wu, Q.; Gu, Y.; Kan, J.; Jin, C. Effect of protocatechuic acid incorporation on the physical, mechanical, structural and antioxidant properties of chitosan film. Food Hydrocoll. 2017, 73, 90–100. [Google Scholar] [CrossRef]
- Abbott, A.P.; Boothby, D.; Capper, G.; Davies, D.L.; Rasheed, R. Deep Eutectic Solvents Formed Between Choline Chloride and Carboxylic Acids. J. Am. Chem. Soc. 2004, 126, 9142. [Google Scholar] [CrossRef] [PubMed]
- Mbous, Y.P.; Hayyan, M.; Wong, W.F.; Looi, C.Y.; Hashim, M.A. Unraveling the cytotoxicity and metabolic pathways of binary natural deep eutectic solvent systems. Sci. Rep. 2017, 7, 41257. [Google Scholar] [CrossRef] [PubMed]

| Application | Purpose | Product | Reference |
|---|---|---|---|
| Nutraceutical/functional ingredient | Body weight reduction | Oral capsules | [50] |
| Food packaging | Assai polyelectrolyte complexes | [58] | |
| Additive in food products | Texture controlling agent | Rice noodles; meat product | [59,60] |
| Emulsifying/gelling agent | Films; microparticles | [61,62] | |
| Pastes | [33] | ||
| Biological, antimicrobial or antioxidant activity | Preservation agent in powder | Rice | [63] |
| Film/coatings | Fish fillet | [64] | |
| Chicken fillet | [65] | ||
| Meat cutlets | [66] | ||
| Fruits | [57,67,68] | ||
| Nanofibers | Active food packaging | [69] | |
| Hydrogels | Delivery of enzymes | [70] | |
| Liposomes | Delivery of bioactive substances | [71] | |
| Dentistry application | Hydrogel | Remineralization of enamel surface | [72] |
| Biomedical application | Layer-by-layer coating | 3D multilayered microchannels in hydrogels | [73] |
| Nanoparticles | Chitosan functionalized magnetic nanoparticles | [74] | |
| Self-assembly system | Polyelectrolyte complexes | [75] | |
| Nanovesicles | Chitosan/nutriose coated niosomes | [76] |
| Films Matrix | Composite Ingredients | Applications | Main Results | References |
|---|---|---|---|---|
| Chitosan/Zein | Glycerol, PEG-400, and sorbitol | Potential food application | Permeability increased with higher plasticizer concentration. PEG-400 promoted a better barrier property. | [94] |
| Chitosan | Aloe vera gel and silver nanoparticles (SNPs) | Potential biomedical applications | SNPs decrease the crystallinity of the films. SNPs affected the structure and viscoelastic behavior of chitosan films. | [99] |
| Chitosan | Glycerol | Strawberries | Protection of the fruit against fungi. Maintenance of flavor, appearance, texture, and aroma. | [100] |
| Chitosan/purple yam starch | Glycerol | Coating of apples | The films preserved the quality of apples for four weeks of storage. Weight loss from the coated apples was less significant than the uncoated. | [101] |
| Chitosan | Apple peel polyphenols (APP) | Potential bio-composite food packaging material | APP significantly increased thickness, density, solubility, opacity, and swelling ratio of films. APP decreased the moisture content, water vapor permeability, tensile strength and elongation at break of the films. | [102] |
| Chitosan/organoclay nanocomposites (OrgMMT) | Olive oil and corn oil as plasticizers | Potential food packaging applications | OrgMMT significantly reduced the elongation at break of all oil containing samples, acting as stress concentrator upon deformation. Corn oil was a less effective as plasticizer than olive oil. | [103] |
| Chitosan/gelatin | Red grape seed extract and Ziziphora clinopodioides essential oil | Potential food packaging applications | The addition of red grape seed extract and Z. clinopodioides essential oil improved total phenolic content, antibacterial and antioxidant activities, thickness, and water vapor barrier property. | [104] |
| Chitosan/corn starch | Glycerol as plasticizer | Potential food/pharmaceutical applications | The water vapor permeability and moisture content of films increased with an increase in chitosan concentration. | [105] |
| Chitosan hydrochloride (CHC) | Glycerol as plasticizer and epigallocatechin gallate (EGCG) nanocapsules (NCs) | Potential food packaging applications | The incorporation of nanocapsules into the CHC films increased their tensile strength and percentage of elongation at break. The produced films could prevent lipids oxidation of fatty food products | [89] |
| Chitosan | Propolis extract | Potential food packaging applications | All chitosan/Propolis films inhibited bacteria on contact surface underneath the film. Mechanical properties, oxygen and moisture barrier, antioxidant and antimicrobial properties were improved by the addition of Propolis extract into the films. | [106] |
| Chitosan | Clove oil (Syzygium aromaticum) | Cooked pork sausages | The shelf life of cooked pork sausages increased from 14 to 20 days with the chitosan/clove oil films. | [107] |
| Chitosan/gelatin | Glycerol as plasticizer | Beef steaks | Myoglobin oxidation during retail display was reduced and the percentage of deoxymyoglobin increased with gelatin content in films. | [108] |
| Chitosan | Glycerine, chokeberry pomace extract | Potential food packaging applications | Enhanced water vapor permeability and reduced oxygen permeability by addition of chokeberry extracts. The films showed significant antioxidant properties. | [109] |
| Chitosan | Urtica dioica leaf extract derived copper oxide (CuO) and zinc oxide (ZnO) nanoparticles | Food film packaging and shelf life of guava fruit | Low antioxidant activity and greater antimicrobial properties. Decreased moisture content, water holding capacity, and solubility. The nanocomposite chitosan films improved the quality and shelf-life attributes of guava by one week when compared to unpackaged fruits. | [110] |
| Multilayer chitosan composites films | Sodium sulfoethyl cellulose (SEC), sodium alginate (ALG), and sodium hyaluronate (HA) | Potential biomedical and food applications | Effect of the polyelectrolyte complex layer on the properties of the poly-layer composites decreases in the order CS/SEC > CS/HA > CS/ALG. | [111] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teixeira-Costa, B.E.; Andrade, C.T. Chitosan as a Valuable Biomolecule from Seafood Industry Waste in the Design of Green Food Packaging. Biomolecules 2021, 11, 1599. https://doi.org/10.3390/biom11111599
Teixeira-Costa BE, Andrade CT. Chitosan as a Valuable Biomolecule from Seafood Industry Waste in the Design of Green Food Packaging. Biomolecules. 2021; 11(11):1599. https://doi.org/10.3390/biom11111599
Chicago/Turabian StyleTeixeira-Costa, Barbara E., and Cristina T. Andrade. 2021. "Chitosan as a Valuable Biomolecule from Seafood Industry Waste in the Design of Green Food Packaging" Biomolecules 11, no. 11: 1599. https://doi.org/10.3390/biom11111599
APA StyleTeixeira-Costa, B. E., & Andrade, C. T. (2021). Chitosan as a Valuable Biomolecule from Seafood Industry Waste in the Design of Green Food Packaging. Biomolecules, 11(11), 1599. https://doi.org/10.3390/biom11111599








