Bioprinting Au Natural: The Biologics of Bioinks
Abstract
:1. Introduction
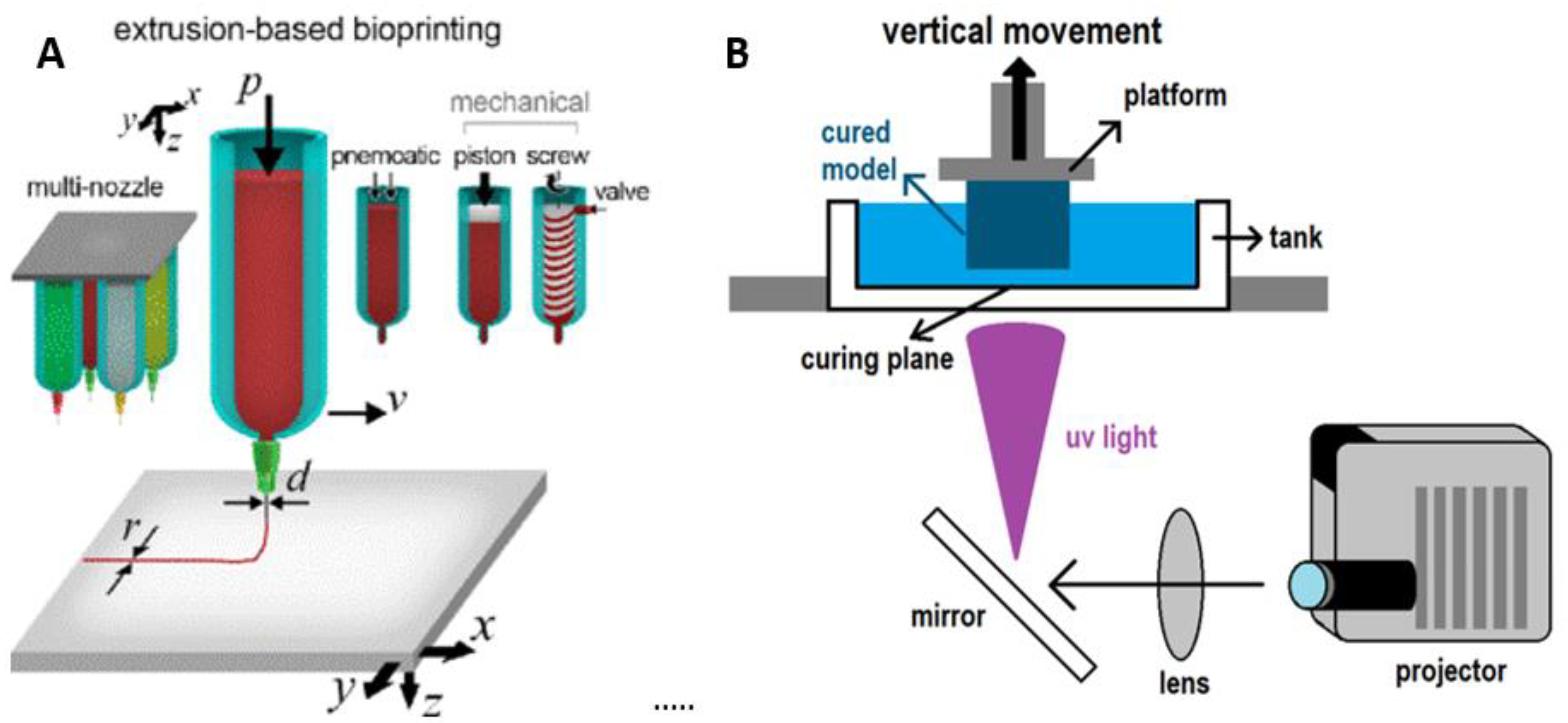
2. Types of Bioprinters
3. Biological Bioinks
3.1. Agarose
3.2. Alginate
3.3. Chitosan
3.4. Collagen
3.5. Extracellular Matrix
3.6. Fibrin
3.7. Gelatin
3.8. Hyaluronic Acid
3.9. Scaffold-Free
3.10. Silk
4. Bioinks Today and Tomorrow
Author Contributions
Funding
Conflicts of Interest
References
- Thevenot, P.; Nair, A.; Dey, J.; Yang, J.; Tang, L. Method to analyze three-dimensional cell distribution and infiltration in degradable scaffolds. Tissue Eng. Part C Methods 2008, 14, 319–331. [Google Scholar] [CrossRef] [Green Version]
- Silva, M.M.; Cyster, L.A.; Barry, J.J.; Yang, X.B.; Oreffo, R.O.; Grant, D.M.; Scotchford, C.A.; Howdle, S.M.; Shakesheff, K.M.; Rose, F.R. The effect of anisotropic architecture on cell and tissue infiltration into tissue engineering scaffolds. Biomaterials 2006, 27, 5909–5917. [Google Scholar] [CrossRef] [PubMed]
- Eltom, A.; Zhong, G. Muhammad, Scaffold Techniques and Designs in Tissue Engineering Functions and Purposes: A Review. Adv. Mater. Sci. Eng. 2019, 2019, 3429527. [Google Scholar] [CrossRef] [Green Version]
- Solchaga, L.A.; Tognana, E.; Penick, K.; Baskaran, H.; Goldberg, V.M.; Caplan, A.I.; Welter, J.F. A rapid seeding technique for the assembly of large cell/scaffold composite constructs. Tissue Eng. 2006, 12, 1851–1863. [Google Scholar] [CrossRef] [PubMed]
- Khalil, S.; Nam, J.; Sun, W. Multi-nozzle deposition for construction of 3D biopolymer tissue scaffolds. Rapid Prototyp. J. 2005, 11, 9–17. [Google Scholar] [CrossRef] [Green Version]
- Thayer, P.; Martinez, H.; Gatenholm, E. History and Trends of 3D Bioprinting. In 3D Bioprinting: Principles and Protocols; Crooki, J.M., Ed.; Springer: New York, NY, USA, 2020; pp. 3–18. [Google Scholar]
- Pardo, L.; Wilson, W.C.; Boland, T. Characterization of Patterned Self-Assembled Monolayers and Protein Arrays Generated by the Ink-Jet Method. Langmuir 2003, 19, 1462–1466. [Google Scholar] [CrossRef]
- Roth, E.A.; Xu, T.; Das, M.; Gregory, C.; Hickman, J.J.; Boland, T. Inkjet printing for high-throughput cell patterning. Biomaterials 2004, 25, 3707–3715. [Google Scholar] [CrossRef] [PubMed]
- Lee, V.K.; Dias, A.; Ozturk, M.S.; Chen, K.; Tricomi, B.; Corr, D.T.; Intes, X.; Dai, G. 3D Bioprinting and 3D Imaging for Stem Cell Engineering. In Bioprinting in Regenerative Medicine; Turksen, K., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 33–66. [Google Scholar]
- Sears, N.A.; Seshadri, D.R.; Dhavalikar, P.S.; Cosgriff-Hernandez, E. A Review of Three-Dimensional Printing in Tissue Engineering. Tissue Eng. Part B Rev. 2016, 22, 298–310. [Google Scholar] [CrossRef]
- Billiet, T.; Gevaert, E.; de Schryver, T.; Cornelissen, M.; Dubruel, P. The 3D printing of gelatin methacrylamide cell-laden tissue-engineered constructs with high cell viability. Biomaterials 2014, 35, 49–62. [Google Scholar] [CrossRef]
- Li, M.; Tian, X.; Zhu, N.; Schreyer, D.J.; Chen, X. Modeling process-induced cell damage in the biodispensing process. Tissue Eng. Part C Methods 2010, 16, 533–542. [Google Scholar] [CrossRef]
- Tirella, A.; Ahluwalia, A. The impact of fabrication parameters and substrate stiffness in direct writing of living constructs. Biotechnol. Prog. 2012, 28, 1315–1320. [Google Scholar]
- Buyukhatipoglu, K.; Jo, W.; Sun, W.; Morss, C.A. The role of printing parameters and scaffold biopolymer properties in the efficacy of a new hybrid nano-bioprinting system. Biofabrication 2009, 1, 035003. [Google Scholar] [CrossRef] [PubMed]
- Paxton, N.C.; Smolan, W.; Böck, T.; Ferry, P.W.M.; Groll, J.; Juengst, T. Proposal to assess printability of bioinks for extrusion-based bioprinting and evaluation of rheological properties governing bioprintability. Biofabrication 2017, 9, 044107. [Google Scholar] [CrossRef] [PubMed]
- Hazur, J.; Detsch, R.; Karakaya, E.; Kaschta, J.; Teßmar, J.; Schneidereit, D.; Friedrich, O.; Schubert, D.W.; Boccaccini, A.R. Improving alginate printability for biofabrication: Establishment of a universal and homogeneous pre-crosslinking technique. Biofabrication 2020, 12, 045004. [Google Scholar] [CrossRef]
- Ouyang, L.; Highley, C.B.; Sun, W.; Burdick, J.A. A Generalizable Strategy for the 3D Bioprinting of Hydrogels from Nonviscous Photo-crosslinkable Inks. Adv. Mater. 2017, 29, 1604983. [Google Scholar] [CrossRef]
- Kim, E.; Seok, J.M.; Bae, S.B.; Park, S.A.; Park, W.H. Silk Fibroin Enhances Cytocompatibilty and Dimensional Stability of Alginate Hydrogels for Light-Based Three-Dimensional Bioprinting. Biomacromolecules 2021, 22, 1921–1931. [Google Scholar] [CrossRef] [PubMed]
- Shanjani, Y.; Pan, C.C.; Elomaa, L.; Yang, Y. A novel bioprinting method and system for forming hybrid tissue engineering constructs. Biofabrication 2015, 7, 045008. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.B.; Tomov, M.L.; Buikema, J.W.; Chen, C.; Mahmoudi, M.; Wu, S.M.; Serpooshan, V. Cardiovascular tissue bioprinting: Physical and chemical processes. Appl. Phys. Rev. 2018, 5, 041106. [Google Scholar] [CrossRef]
- Abelardo, E. 7-Synthetic material bioinks. In 3D Bioprinting for Reconstructive Surgery; Thomas, D.J., Jessop, Z.M., Whitaker, I.S., Eds.; Woodhead Publishing: Sawston, UK, 2018; pp. 137–144. [Google Scholar]
- Mayer, H.K.; Fiechter, G. Chapter 10—Electrophoretic Techniques. In Comprehensive Analytical Chemistry; de la Guardia, M., Gonzálvez, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 251–278. [Google Scholar]
- Fedorovich, N.E.; de Wijn, J.R.; Verbout, A.J.; Alblas, J.; Dhert, W.J. Three-dimensional fiber deposition of cell-laden, viable, patterned constructs for bone tissue printing. Tissue Eng. Part A 2008, 14, 127–133. [Google Scholar] [CrossRef]
- Livoti, C.M.; Morgan, J.R. Self-assembly and tissue fusion of toroid-shaped minimal building units. Tissue Eng. Part A 2010, 16, 2051–2061. [Google Scholar] [CrossRef] [Green Version]
- López-Marcial, G.R.; Zeng, A.Y.; Osuna, C.; Dennis, J.; García, J.M.; O’Connell, G.D. Agarose-Based Hydrogels as Suitable Bioprinting Materials for Tissue Engineering. ACS Biomater. Sci. Eng. 2018, 4, 3610–3616. [Google Scholar] [CrossRef]
- Forget, A.; Blaeser, A.; Miessmer, F.; Köpf, M.; Campos, D.F.D.; Voelcker, N.H.; Blencowe, A.; Fischer, H.; Shastri, V.P. Mechanically Tunable Bioink for 3D Bioprinting of Human Cells. Adv. Healthc. Mater. 2017, 6, 1700255. [Google Scholar] [CrossRef] [PubMed]
- Arya, N.; Forget, A.; Sarem, M.; Shastri, V.P. RGDSP functionalized carboxylated agarose as extrudable carriers for chondrocyte delivery. Mater. Sci. Eng. C 2019, 99, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Forget, A.; Derme, T.; Mitterberger, D.; Heiny, M.; Sweeney, C.; Mudili, L.; Dargaville, T.R.; Shastri, V.P. Architecture-inspired paradigm for 3D bioprinting of vessel-like structures using extrudable carboxylated agarose hydrogels. Emergent Mater. 2019, 2, 233–243. [Google Scholar] [CrossRef]
- Mirdamadi, E.; Muselimyan, N.; Koti, P.; Asfour, H.; Sarvazyan, N. Agarose Slurry as a Support Medium for Bioprinting and Culturing Freestanding Cell-Laden Hydrogel Constructs. 3D Print. Addit. Manuf. 2019, 6, 158–164. [Google Scholar] [CrossRef]
- Tariverdian, T.; Navaei, T.; Milan, P.B.; Samadikuchaksaraei, A.; Mozafari, M. Chapter 16—Functionalized polymers for tissue engineering and regenerative medicines. In Advanced Functional Polymers for Biomedical Applications; Mozafari, M., Chauhan, N.P.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 323–357. [Google Scholar]
- Hariyadi, D.M.; Lin, S.C.; Wang, Y.; Bostrom, T.; Turner, M.S.; Bhandari, B.; Coombes, A.G. Diffusion loading and drug delivery characteristics of alginate gel microparticles produced by a novel impinging aerosols method. J. Drug Target 2010, 18, 831–841. [Google Scholar] [CrossRef] [PubMed]
- Mirtič, J.; Ilaš, J.; Kristl, J. Influence of different classes of crosslinkers on alginate polyelectrolyte nanoparticle formation, thermodynamics and characteristics. Carbohydr. Polym. 2018, 18, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Mørch, Ý.A.; Donati, I.; Strand, B.L.; Skjåk-Bræk, G. Effect of Ca2+, Ba2+, and Sr2+ on Alginate Microbeads. Biomacromolecules 2006, 7, 1471–1480. [Google Scholar] [CrossRef] [PubMed]
- Straccia, M.C.; d’Ayala, G.G.; Romano, I.; Laurienzo, P. Novel zinc alginate hydrogels prepared by internal setting method with intrinsic antibacterial activity. Carbohydr. Polym. 2015, 125, 103–112. [Google Scholar] [CrossRef]
- Gopinathan, J.; Noh, I. Recent trends in bioinks for 3D printing. Biomater. Res. 2018, 22, 11. [Google Scholar] [CrossRef] [Green Version]
- Moradali, M.; Ghods, S.; Rehm, B. Alginate Biosynthesis and Biotechnological Production; Springer: Singapore, 2018; pp. 1–25. [Google Scholar]
- Draget, K.I.; Bræk, G.S.; Smidsrød, O. Alginic acid gels: The effect of alginate chemical composition and molecular weight. Carbohydr. Polym. 1994, 25, 31–38. [Google Scholar] [CrossRef]
- Aarstad, O.; Heggset, E.B.; Pedersen, I.S.; Bjørnøy, S.H.; Syverud, K.; Strand, B.L. Mechanical Properties of Composite Hydrogels of Alginate and Cellulose Nanofibrils. Polymers 2017, 9, 378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Q.; He, Y.; Fu, J.Z.; Liu, A.; Ma, L. Coaxial nozzle-assisted 3D bioprinting with built-in microchannels for nutrients delivery. Biomaterials 2015, 61, 203–215. [Google Scholar] [CrossRef]
- Gao, G.; Kim, H.; Kim, B.; Kong, J.; Lee, J.Y.; Park, B.W.; Chae, S.; Kim, J.; Ban, K.; Jang, J.; et al. Tissue-engineering of vascular grafts containing endothelium and smooth-muscle using triple-coaxial cell printing. Appl. Phys. Rev. 2019, 6, 041402. [Google Scholar] [CrossRef]
- Ahlfeld, T.; Cidonio, G.; Kilian, D.; Duin, S.; Akkineni, A.R.; Dawson, J.I.; Yang, S.; Lode, A.; Oreffo, R.O.C.; Gelinsky, M. Development of a clay based bioink for 3D cell printing for skeletal application. Biofabrication 2017, 9, 034103. [Google Scholar] [CrossRef]
- Markstedt, K.; Mantas, A.; Tournier, I.; Ávila, H.M.; Hägg, D.; Gatenholm, P. 3D Bioprinting Human Chondrocytes with Nanocellulose-Alginate Bioink for Cartilage Tissue Engineering Applications. Biomacromolecules 2015, 16, 1489. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Gungor-Ozkerim, P.S.; Zhang, Y.S.; Yue, K.; Zhu, K.; Liu, W.; Pi, Q.; Byambaa, B.; Dokmeci, M.R.; Shin, S.R.; et al. Direct 3D bioprinting of perfusable vascular constructs using a blend bioink. Biomaterials 2016, 106, 58–68. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Zhang, Y.; Martin, J.A.; Ozbolat, I.T. Evaluation of cell viability and functionality in vessel-like bioprintable cell-laden tubular channels. J. Biomech. Eng. 2013, 135, 91011. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Yu, Y.; Chen, H.; Ozbolat, I.T. Characterization of printable cellular micro-fluidic channels for tissue engineering. Biofabrication 2013, 5, 025004. [Google Scholar] [CrossRef]
- Attalla, R.; Ling, C.; Selvaganapathy, P. Fabrication and characterization of gels with integrated channels using 3D printing with microfluidic nozzle for tissue engineering applications. Biomed. Microdevices 2016, 18, 17. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, Y.; Ozbolat, I.T. Direct Bioprinting of Vessel-Like Tubular Microfluidic Channels. J. Nanotechnol. Eng. Med. 2013, 4, 0210011–0210017. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, Y.; Akkouch, A.; Dababneh, A.; Dolati, F.; Ozbolat, I.T. In Vitro Study of Directly Bioprinted Perfusable Vasculature Conduits. Biomater. Sci. 2015, 3, 134–143. [Google Scholar] [CrossRef]
- Gao, G.; Lee, J.H.; Jang, J.; Lee, D.H.; Kong, J.S.; Kim, B.S.; Choi, Y.J.; Jang, W.B.; Hong, Y.J.; Kwon, S.M.; et al. Tissue Engineered Bio-Blood-Vessels Constructed Using a Tissue-Specific Bioink and 3D Coaxial Cell Printing Technique: A Novel Therapy for Ischemic Disease. Adv. Funct. Mater. 2017, 27, 1700798. [Google Scholar] [CrossRef]
- Liu, J.; Chi, J.; Wang, K.; Liu, X.; Gu, F. Full-Thickness Wound Healing Using 3D Bioprinted Gelatin-Alginate Scaffolds in Mice: A Histopathological Study; Washington University School of Medicine: Washington, DC, USA, 2016; Volume 9, pp. 11197–11205. [Google Scholar]
- Shi, L.; Xiong, L.; Hu, Y.; Li, W.; Chen, Z.; Liu, K.; Zhang, X. Three-dimensional printing alginate/gelatin scaffolds as dermal substitutes for skin tissue engineering. Polym. Eng. Sci. 2018, 58, 1782–1790. [Google Scholar] [CrossRef]
- Liu, P.; Shen, H.; Zhi, Y.; Si, J.; Shi, J.; Guo, L.; Shen, S.G. 3D bioprinting and in vitro study of bilayered membranous construct with human cells-laden alginate/gelatin composite hydrogels. Colloids Surf. B Biointerfaces 2019, 181, 1026–1034. [Google Scholar] [CrossRef]
- Dutta, S.D.; Hexiu, J.; Patel, D.K.; Ganguly, K.; Lim, K.T. 3D-printed bioactive and biodegradable hydrogel scaffolds of alginate/gelatin/cellulose nanocrystals for tissue engineering. Int. J. Biol. Macromol. 2021, 167, 644–658. [Google Scholar] [CrossRef]
- Verma, D.; Fortunati, E. 1-Biopolymer processing and its composites: An introduction. In Biomass, Biopolymer-Based Materials, and Bioenergy; Verma, D., Fortunati, E., Jain, S., Zhang, X., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 3–23. [Google Scholar]
- Croisier, F.; Jérôme, C. Chitosan-based biomaterials for tissue engineering. Eur. Polym. J. 2013, 49, 780–792. [Google Scholar] [CrossRef] [Green Version]
- Boza, A.M.; Wlodarczyk-Biegun, M.; Campo, A.; Vázquez-Lasal, B.; Roman, J.S. Chitosan-based inks: 3D printing and bioprinting strategies to improve shape fidelity, mechanical properties, and biocompatibility of 3D scaffolds. Biomecánica 2019, 27, 7–16. [Google Scholar]
- Wu, Q.; Therriault, D.; Heuzey, M.-C. Processing and Properties of Chitosan Inks for 3D Printing of Hydrogel Microstructures. ACS Biomater. Sci. Eng. 2018, 4, 2643–2652. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Allardyce, B.J.; Rajkhowa, R.; Zhao, Y.; Dilley, R.J.; Redmond, S.L.; Wang, X.; Liu, X. 3D Printing of Silk Particle-Reinforced Chitosan Hydrogel Structures and Their Properties. ACS Biomater. Sci. Eng. 2018, 4, 3036–3046. [Google Scholar] [CrossRef]
- Almeida, C.R.; Serra, T.; Oliveira, M.I.; Planell, J.A.; Barbosa, M.A.; Navarro, M. Impact of 3-D printed PLA- and chitosan-based scaffolds on human monocyte/macrophage responses: Unraveling the effect of 3-D structures on inflammation. Acta Biomater. 2014, 10, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Fu, H.; Wang, Z.; Meng, Q.; Liu, S.; Wang, H.; Zheng, X.; Dai, J.; Zhang, Z. BMSCs-laden gelatin/sodium alginate/carboxymethyl chitosan hydrogel for 3D bioprinting. RSC Adv. 2016, 6, 108423–108430. [Google Scholar] [CrossRef]
- Gu, Q.; Tomaskovic-Crook, E.; Wallace, G.G.; Crook, J.M. 3D Bioprinting Human Induced Pluripotent Stem Cell Constructs for In Situ Cell Proliferation and Successive Multilineage Differentiation. Adv. Healthc. Mater. 2017, 6, 1700175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roehm, K.D.; Madihally, S.V. Bioprinted chitosan-gelatin thermosensitive hydrogels using an inexpensive 3D printer. Biofabrication 2017, 10, 015002. [Google Scholar] [CrossRef] [PubMed]
- Demirtaş, T.T.; Irmak, G.; Gümüşderelioğlu, M. A bioprintable form of chitosan hydrogel for bone tissue engineering. Biofabrication 2017, 9, 035003. [Google Scholar] [CrossRef]
- Lee, D.; Park, J.P.; Koh, M.-Y.; Kim, P.; Lee, J.; Shin, M.; Lee, H. Chitosan-catechol: A writable bioink under serum culture media. Biomater. Sci. 2018, 6, 1040–1047. [Google Scholar] [CrossRef]
- Maxson, E.L.; Young, M.D.; Noble, C.; Go, J.L.; Heidari, B.; Khorramirouz, R.; Morse, D.W.; Lerman, A. In vivo remodeling of a 3D-Bioprinted tissue engineered heart valve scaffold. Bioprinting 2019, 16, e00059. [Google Scholar] [CrossRef]
- Skardal, A.; Devarasetty, M.; Kang, H.-W.; Mead, I.; Bishop, C.; Shupe, T.; Lee, S.J.; Jackson, J.; Yoo, J.; Soker, S.; et al. A hydrogel bioink toolkit for mimicking native tissue biochemical and mechanical properties in bioprinted tissue constructs. Acta Biomater. 2015, 25, 24–34. [Google Scholar] [CrossRef]
- Yu, C.; Ma, X.; Zhu, W.; Wang, P.; Miller, K.L.; Stupin, J.; Koroleva-Maharajh, A.; Hairabedian, A.; Chen, S. Scanningless and continuous 3D bioprinting of human tissues with decellularized extracellular matrix. Biomaterials 2019, 194, 1–13. [Google Scholar] [CrossRef]
- Jorgensen, A.M.; Varkey, M.; Gorkun, A.; Clouse, C.; Xu, L.; Chou, Z.; Murphy, S.V.; Molnar, J.; Lee, S.J.; Yoo, J.J.; et al. Bioprinted Skin Recapitulates Normal Collagen Remodeling in Full-Thickness Wounds. Tissue Eng. Part A 2020, 26, 512–526. [Google Scholar] [CrossRef]
- Rastin, H.; Ormsby, R.T.; Atkins, G.J.; Losic, D. 3D Bioprinting of Methylcellulose/Gelatin-Methacryloyl (MC/GelMA) Bioink with High Shape Integrity. ACS Appl. Bio Mater. 2020, 3, 1815–1826. [Google Scholar] [CrossRef]
- Pescosolido, L.; Schuurman, W.; Malda, J.; Matricardi, P.; Alhaique, F.; Coviello, T.; van Weeren, P.R.; Dhert, W.J.A.; Hennink, W.E.; Vermonden, T. Hyaluronic Acid and Dextran-Based Semi-IPN Hydrogels as Biomaterials for Bioprinting. Biomacromolecules 2011, 12, 1831–1838. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Yeon, Y.K.; Lee, J.M.; Chao, J.R.; Lee, Y.J.; Seo, Y.B.; Sultan, M.T.; Lee, O.J.; Lee, J.S.; Yoon, S.-I.; et al. Precisely printable and biocompatible silk fibroin bioink for digital light processing 3D printing. Nat. Commun. 2018, 9, 1620. [Google Scholar] [CrossRef] [PubMed]
- Hospodiuk, M.; Dey, M.; Sosnoski, D.; Ozbolat, I.T. The bioink: A comprehensive review on bioprintable materials. Biotechnol. Adv. 2017, 35, 217–239. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, A.M.; Gentile, P.; Sartori, S.; Pagliano, C.; Cabrele, C.; Chiono, V.; Ciardelli, G. Biomimetic soluble collagen purified from bones. Biotechnol. J. 2012, 7, 1386–1394. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, G. Three-Dimensional Hierarchical Nanofibrous Collagen Scaffold Fabricated Using Fibrillated Collagen and Pluronic F-127 for Regenerating Bone Tissue. ACS Appl. Mater. Interfaces 2018, 10, 35801–35811. [Google Scholar] [CrossRef]
- Homenick, C.M.; de Silveira, G.; Sheardown, H.; Adronov, A. Pluronics as crosslinking agents for collagen: Novel amphiphilic hydrogels. Polym. Int. 2011, 60, 458–465. [Google Scholar] [CrossRef]
- Shi, Y.; Xing, T.L.; Zhang, H.B.; Yin, R.X.; Yang, S.M.; Wei, J.; Zhang, W.J. Tyrosinase-doped bioink for 3D bioprinting of living skin constructs. Biomed Mater 2018, 13, 035008. [Google Scholar] [CrossRef]
- Chen, C.; Zhao, M.L.; Zhang, R.K.; Lu, G.; Zhao, C.Y.; Fu, F.; Sun, H.T.; Zhang, S.; Tu, Y.; Li, X.H. Collagen/heparin sulfate scaffolds fabricated by a 3D bioprinter improved mechanical properties and neurological function after spinal cord injury in rats. J. Biomed. Mater. Res. Part A 2017, 105, 1324–1332. [Google Scholar] [CrossRef]
- Yang, X.; Lu, Z.; Wu, H.; Li, W.; Zheng, L.; Zhao, J. Collagen-alginate as bioink for three-dimensional (3D) cell printing based cartilage tissue engineering. Mater. Sci. Eng. C 2018, 83, 195–201. [Google Scholar] [CrossRef]
- Kim, W.J.; Yun, H.-S.; Kim, G.H. An innovative cell-laden α-TCP/collagen scaffold fabricated using a two-step printing process for potential application in regenerating hard tissues. Sci. Rep. 2017, 7, 3181. [Google Scholar] [CrossRef] [Green Version]
- Mazzocchi, A.; Devarasetty, M.; Huntwork, R.; Soker, S.; Skardal, A. Optimization of collagen type I-hyaluronan hybrid bioink for 3D bioprinted liver microenvironments. Biofabrication 2018, 11, 015003. [Google Scholar] [CrossRef]
- Tytgat, L.; Dobos, A.; Markovic, M.; van Damme, L.; van Hoorick, J.; Bray, F.; Thienpont, H.; Ottevaere, H.; Dubruel, P.; Ovsianikov, A.; et al. High-Resolution 3D Bioprinting of Photo-Cross-linkable Recombinant Collagen to Serve Tissue Engineering Applications. Biomacromolecules 2020, 21, 3997–4007. [Google Scholar] [CrossRef]
- Yue, B. Biology of the extracellular matrix: An overview. J Glaucoma 2014, 23, S20–S23. [Google Scholar] [CrossRef] [PubMed]
- Ott, H.C.; Matthiesen, T.S.; Goh, S.-K.; Black, L.D.; Kren, S.M.; Netoff, T.I.; Taylor, D.A. Perfusion-decellularized matrix: Using nature’s platform to engineer a bioartificial heart. Nat. Med. 2008, 14, 213–221. [Google Scholar] [CrossRef]
- Pati, F.; Jang, J.; Ha, D.-H.; Kim, S.W.; Rhie, J.-W.; Shim, J.-H.; Kim, D.-H.; Cho, D.-W. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat. Commun. 2014, 5, 3935. [Google Scholar] [CrossRef] [Green Version]
- Jang, J.; Kim, T.G.; Kim, B.S.; Kim, S.-W.; Kwon, S.-M.; Cho, D.-W. Tailoring mechanical properties of decellularized extracellular matrix bioink by vitamin B2-induced photo-crosslinking. Acta Biomater. 2016, 33, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kang, B.; Cui, X.; Lee, S.-H.; Lee, K.; Cho, D.-W.; Hwang, W.; Woodfield, T.B.F.; Lim, K.S.; Jang, J. Light-Activated Decellularized Extracellular Matrix-Based Bioinks for Volumetric Tissue Analogs at the Centimeter Scale. Adv. Funct. Mater. 2021, 31, 2011252. [Google Scholar] [CrossRef]
- Kim, W.; Lee, H.; Lee, J.; Atala, A.; Yoo, J.J.; Lee, S.J.; Kim, G.H. Efficient myotube formation in 3D bioprinted tissue construct by biochemical and topographical cues. Biomaterials 2020, 230, 119632. [Google Scholar] [CrossRef] [PubMed]
- Janmey, P.A.; Winer, J.P.; Weisel, J.W. Fibrin gels and their clinical and bioengineering applications. J. R. Soc. Interface 2009, 6, 1–10. [Google Scholar] [CrossRef]
- Skardal, A.; Mack, D.; Kapetanovic, E.; Atala, A.; Jackson, J.D.; Yoo, J.; Soker, S. Bioprinted amniotic fluid-derived stem cells accelerate healing of large skin wounds. Stem Cells Transl. Med. 2012, 1, 792–802. [Google Scholar] [CrossRef]
- de Melo, B.A.G.; Jodat, Y.A.; Cruz, E.M.; Benincasa, J.C.; Shin, S.R.; Porcionatto, M.A. Strategies to use fibrinogen as bioink for 3D bioprinting fibrin-based soft and hard tissues. Acta Biomater. 2020, 117, 60–76. [Google Scholar] [CrossRef]
- Arulmoli, J.; Wright, H.J.; Phan, D.T.T.; Sheth, U.; Que, R.A.; Botten, G.A.; Keating, M.; Botvinick, E.L.; Pathak, M.M.; Zarembinski, T.I.; et al. Combination scaffolds of salmon fibrin, hyaluronic acid, and laminin for human neural stem cell and vascular tissue engineering. Acta Biomater. 2016, 43, 122–138. [Google Scholar] [CrossRef] [Green Version]
- Snyder, T.N.; Madhavan, K.; Intrator, M.; Dregalla, R.C.; Park, D. A fibrin/hyaluronic acid hydrogel for the delivery of mesenchymal stem cells and potential for articular cartilage repair. J. Biol. Eng. 2014, 8, 10. [Google Scholar] [CrossRef] [Green Version]
- Wei, X.; Xiaohong, W.; Yongnian, Y.; Wei, Z.; Zhuo, X.; Feng, L.; Rendong, W.; Renji, Z. Rapid Prototyping Three-Dimensional Cell/Gelatin/Fibrinogen Constructs for Medical Regeneration. J. Bioact. Compat. Polym. 2007, 22, 363–377. [Google Scholar] [CrossRef]
- Anil Kumar, S.; Alonzo, M.; Allen, S.C.; Abelseth, L.; Thakur, V.; Akimoto, J.; Ito, Y.; Willerth, S.M.; Suggs, L.; Chattopadhyay, M.; et al. A Visible Light-Cross-Linkable, Fibrin–Gelatin-Based Bioprinted Construct with Human Cardiomyocytes and Fibroblasts. ACS Biomater. Sci. Eng. 2019, 5, 4551–4563. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, X. Fluid and cell behaviors along a 3D printed alginate/gelatin/fibrin channel. Biotechnol. Bioeng. 2015, 112, 1683–1695. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yao, R.; Ouyang, L.; Ding, H.; Zhang, T.; Zhang, K.; Cheng, S.; Sun, W. Three-dimensional printing of Hela cells for cervical tumor model in vitro. Biofabrication 2014, 6, 035001. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.D.; Chen, A.; Ernst, L.A.; Waggoner, A.S.; Campbell, P.G. Immobilization of Aprotinin to Fibrinogen as a Novel Method for Controlling Degradation of Fibrin Gels. Bioconjugate Chem. 2007, 18, 695–701. [Google Scholar] [CrossRef]
- Lorentz, K.M.; Kontos, S.; Frey, P.; Hubbell, J.A. Engineered aprotinin for improved stability of fibrin biomaterials. Biomaterials 2011, 32, 430–438. [Google Scholar] [CrossRef]
- Ning, L.; Sun, H.; Lelong, T.; Guilloteau, R.; Zhu, N.; Schreyer, D.J.; Chen, X. 3D bioprinting of scaffolds with living Schwann cells for potential nerve tissue engineering applications. Biofabrication 2018, 10, 035014. [Google Scholar] [CrossRef]
- Kang, H.-W.; Lee, S.J.; Ko, I.K.; Kengla, C.; Yoo, J.J.; Atala, A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat. Biotechnol. 2016, 34, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Chiou, B.-S.; Avena-Bustillos, R.J.; Bechtel, P.J.; Jafri, H.; Narayan, R.; Imam, S.H.; Glenn, G.M.; Orts, W.J. Cold water fish gelatin films: Effects of cross-linking on thermal, mechanical, barrier, and biodegradation properties. Eur. Polym. J. 2008, 44, 3748–3753. [Google Scholar] [CrossRef]
- Sakai, S.; Hirose, K.; Taguchi, K.; Ogushi, Y.; Kawakami, K. An injectable, in situ enzymatically gellable, gelatin derivative for drug delivery and tissue engineering. Biomaterials 2009, 30, 3371–3377. [Google Scholar] [CrossRef]
- Hinton, T.J.; Jallerat, Q.; Palchesko, R.N.; Park, J.H.; Grodzicki, M.S.; Shue, H.-J.; Ramadan, M.H.; Hudson, A.R.; Feinberg, A.W. Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Science Adv. 2015, 1, e1500758. [Google Scholar] [CrossRef] [Green Version]
- Lee, A.; Hudson, A.R.; Shiwarski, D.J.; Tashman, J.W.; Hinton, T.J.; Yerneni, S.; Bliley, J.M.; Campbell, P.G.; Feinberg, A.W. 3D bioprinting of collagen to rebuild components of the human heart. Science 2019, 365, 482. [Google Scholar] [CrossRef] [PubMed]
- Irvine, S.A.; Agrawal, A.; Lee, B.H.; Chua, H.Y.; Low, K.Y.; Lau, B.C.; Machluf, M.; Venkatraman, S. Printing cell-laden gelatin constructs by free-form fabrication and enzymatic protein crosslinking. Biomed. Microdevices 2015, 17, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, H.; Illsley, N.P.; Chang, R.C. 3D Bioprinted GelMA Based Models for the Study of Trophoblast Cell Invasion. Sci. Rep. 2019, 9, 18854. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Zhu, W.; Nowicki, M.; Miao, S.; Cui, H.; Holmes, B.; Glazer, R.I.; Zhang, L.G. 3D Bioprinting a Cell-Laden Bone Matrix for Breast Cancer Metastasis Study. ACS Appl. Mater. Interfaces 2016, 8, 30017–30026. [Google Scholar] [CrossRef]
- Kilic Bektas, C.; Hasirci, V. Cell Loaded 3D Bioprinted GelMA Hydrogels for Corneal Stroma Engineering. Biomater. Sci. 2019, 8, 438–449. [Google Scholar] [CrossRef]
- Ruiz-Cantu, L.; Gleadall, A.; Faris, C.; Segal, J.; Shakesheff, K.; Yang, J. Multi-material 3D bioprinting of porous constructs for cartilage regeneration. Mater. Sci. Eng. C 2020, 109, 110578. [Google Scholar] [CrossRef] [PubMed]
- Mouser, V.H.; Melchels, F.P.; Visser, J.; Dhert, W.J.; Gawlitta, D.; Malda, J. Yield stress determines bioprintability of hydrogels based on gelatin-methacryloyl and gellan gum for cartilage bioprinting. Biofabrication 2016, 8, 035003. [Google Scholar] [CrossRef] [PubMed]
- Bejleri, D.; Streeter, B.W.; Nachlas, A.L.Y.; Brown, M.E.; Gaetani, R.; Christman, K.L.; Davis, M.E. A Bioprinted Cardiac Patch Composed of Cardiac-Specific Extracellular Matrix and Progenitor Cells for Heart Repair. Adv. Healthc. Mater. 2018, 7, e1800672. [Google Scholar] [CrossRef] [PubMed]
- Koti, P.; Muselimyan, N.; Mirdamadi, E.; Asfour, H.; Sarvazyan, N.A. Use of GelMA for 3D printing of cardiac myocytes and fibroblasts. J. 3D Print. Med. 2019, 3, 11–22. [Google Scholar] [CrossRef]
- Bhise, N.S.; Manoharan, V.; Massa, S.; Tamayol, A.; Ghaderi, M.; Miscuglio, M.; Lang, Q.; Zhang, Y.S.; Shin, S.R.; Calzone, G.; et al. A liver-on-a-chip platform with bioprinted hepatic spheroids. Biofabrication 2016, 8, 014101. [Google Scholar] [CrossRef]
- Ma, X.; Qu, X.; Zhu, W.; Li, Y.-S.; Yuan, S.; Zhang, H.; Liu, J.; Wang, P.; Lai, C.S.E.; Zanella, F.; et al. Deterministically patterned biomimetic human iPSC-derived hepatic model via rapid 3D bioprinting. Proc. Natl. Acad. Sci. USA 2016, 113, 2206–2211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuvellier, M.; Ezan, F.; Oliveira, H.; Rose, S.; Fricain, J.C.; Langouët, S.; Legagneux, V.; Baffet, G. 3D culture of HepaRG cells in GelMa and its application to bioprinting of a multicellular hepatic model. Biomaterials 2021, 269, 120611. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, J.; Athanasiou, K.A. The role of tissue engineering in articular cartilage repair and regeneration. Crit. Rev. Biomed. Eng. 2009, 37, 1–57. [Google Scholar] [CrossRef]
- Zhong, S.P.; Campoccia, D.; Doherty, P.J.; Williams, R.L.; Benedetti, L.; Williams, D.F. Biodegradation of hyaluronic acid derivatives by hyaluronidase. Biomaterials 1994, 15, 359–365. [Google Scholar] [CrossRef]
- Migliore, A.; Granata, M. Intra-articular use of hyaluronic acid in the treatment of osteoarthritis. Clin. Interv. Aging 2008, 3, 365–369. [Google Scholar] [CrossRef] [Green Version]
- Bowman, S.; Awad, M.E.; Hamrick, M.W.; Hunter, M.; Fulzele, S. Recent advances in hyaluronic acid based therapy for osteoarthritis. Clin. Transl. Med. 2018, 7, 6. [Google Scholar] [CrossRef] [Green Version]
- Migliore, A.; Tormenta, S.; Massafra, U.; Bizzi, E.; Iannessi, F.; Alimonti, A.; Granata, M. Intra-articular administration of hylan G-F 20 in patients with symptomatic hip osteoarthritis: Tolerability and effectiveness in a large cohort study in clinical practice. Curr. Med. Res. Opin. 2008, 24, 1309–1316. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Kirker, K.R.; Prestwich, G.D. Cross-linked hyaluronic acid hydrogel films: New biomaterials for drug delivery. J. Control. Release 2000, 69, 169–184. [Google Scholar] [CrossRef]
- Khunmanee, S.; Jeong, Y.; Park, H. Crosslinking method of hyaluronic-based hydrogel for biomedical applications. J. Tissue Eng. 2017, 8, 2041731417726464. [Google Scholar] [CrossRef] [Green Version]
- Skardal, A.; Zhang, J.; McCoard, L.; Xu, X.; Oottamasathien, S.; Prestwich, G.D. Photocrosslinkable hyaluronan-gelatin hydrogels for two-step bioprinting. Tissue Eng. Part A 2010, 16, 2675–2685. [Google Scholar] [CrossRef] [Green Version]
- Skardal, A.; Zhang, J.; McCoard, L.; Oottamasathien, S.; Prestwich, G.D. Dynamically Crosslinked Gold Nanoparticle–Hyaluronan Hydrogels. Adv. Mater. 2010, 22, 4736–4740. [Google Scholar] [CrossRef] [PubMed]
- Stichler, S.; Böck, T.; Paxton, N.; Bertlein, S.; Levato, R.; Schill, V.; Smolan, W.; Malda, J.; Teßmar, J.; Blunk, T. Double printing of hyaluronic acid/poly(glycidol) hybrid hydrogels with poly(ε-caprolactone) for MSC chondrogenesis. Biofabrication 2017, 9, 044108. [Google Scholar] [CrossRef]
- Shie, M.-Y.; Chang, W.-C.; Wei, L.-J.; Huang, Y.-H.; Chen, C.-H.; Shih, C.-T.; Chen, Y.-W.; Shen, Y.-F. 3D Printing of Cytocompatible Water-Based Light-Cured Polyurethane with Hyaluronic Acid for Cartilage Tissue Engineering Applications. Materials 2017, 10, 136. [Google Scholar] [CrossRef]
- Sakai, S.; Ohi, H.; Hotta, T.; Kamei, H.; Taya, M. Differentiation potential of human adipose stem cells bioprinted with hyaluronic acid/gelatin-based bioink through microextrusion and visible light-initiated crosslinking. Biopolymers 2018, 109, e23080. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Moncal, K.K.; Li, J.; Peng, W.; Rivero, I.; Martin, J.A.; Ozbolat, I.T. Three-dimensional bioprinting using self-assembling scalable scaffold-free “tissue strands” as a new bioink. Sci. Rep. 2016, 6, 28714. [Google Scholar] [CrossRef] [PubMed]
- Akkouch, A.; Yu, Y.; Ozbolat, I.T. Microfabrication of scaffold-free tissue strands for three-dimensional tissue engineering. Biofabrication 2015, 7, 031002. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Hospodiuk, M.; Peng, W.; Gudapati, H.; Neuberger, T.; Koduru, S.; Ravnic, D.J.; Ozbolat, I.T. Porous tissue strands: Avascular building blocks for scalable tissue fabrication. Biofabrication 2018, 11, 015009. [Google Scholar] [CrossRef]
- Oliveira, J.M. Current and future trends of silk fibroin-based bioinks in 3D printing. J. 3D Print. Med. 2020, 4, 69–73. [Google Scholar] [CrossRef]
- Gupta, S.; Alrabaiah, H.; Christophe, M.; Rahimi-Gorji, M.; Nadeem, S.; Bit, A. Evaluation of silk-based bioink during pre and post 3D bioprinting: A review. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 279–293. [Google Scholar] [CrossRef]
- Matsumoto, A.; Chen, J.; Collette, A.L.; Kim, U.-J.; Altman, G.H.; Cebe, P.; Kaplan, D.L. Mechanisms of Silk Fibroin Sol−Gel Transitions. J. Phys. Chem. B 2006, 110, 21630–21638. [Google Scholar] [CrossRef]
- Rodriguez, M.J.; Dixon, T.A.; Cohen, E.; Huang, W.; Omenetto, F.G.; Kaplan, D.L. 3D freeform printing of silk fibroin. Acta Biomater. 2018, 71, 379–387. [Google Scholar] [CrossRef]
- Panilaitis, B.; Altman, G.H.; Chen, J.; Jin, H.J.; Karageorgiou, V.; Kaplan, D.L. Macrophage responses to silk. Biomaterials 2003, 24, 3079–3085. [Google Scholar] [CrossRef]
- Terada, S.; Nishimura, T.; Sasaki, M.; Yamada, H.; Miki, M. Sericin, a protein derived from silkworms, accelerates the proliferation of several mammalian cell lines including a hybridoma. Cytotechnology 2002, 40, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Terada, S.; Sasaki, M.; Yanagihara, K.; Yamada, H. Preparation of silk protein sericin as mitogenic factor for better mammalian cell culture. J. Biosci. Bioeng. 2005, 100, 667–671. [Google Scholar] [CrossRef]
- Aramwit, P.; Palapinyo, S.; Srichana, T.; Chottanapund, S.; Muangman, P. Silk sericin ameliorates wound healing and its clinical efficacy in burn wounds. Arch. Derm. Res. 2013, 305, 585–594. [Google Scholar] [CrossRef]
- Aramwit, P.; Sangcakul, A. The effects of sericin cream on wound healing in rats. Biosci. Biotechnol. Biochem. 2007, 71, 2473–2477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandal, B.B.; Priya, A.S.; Kundu, S.C. Novel silk sericin/gelatin 3-D scaffolds and 2-D films: Fabrication and characterization for potential tissue engineering applications. Acta Biomater. 2009, 5, 3007–3020. [Google Scholar] [CrossRef] [PubMed]
- Teramoto, H.; Nakajima, K.; Takabayashi, C. Preparation of elastic silk sericin hydrogel. Biosci. Biotechnol. Biochem. 2005, 69, 845–847. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Wu, J.; Liu, M.; Wang, H.; Li, C.; Rodriguez, M.J.; Li, G.; Wang, X.; Kaplan, D.L. 3D Bioprinting of Self-Standing Silk-Based Bioink. Adv. Healthc. Mater. 2018, 7, e1701026. [Google Scholar] [CrossRef]
- Singh, Y.P.; Bandyopadhyay, A.; Mandal, B.B. 3D Bioprinting Using Cross-Linker-Free Silk–Gelatin Bioink for Cartilage Tissue Engineering. ACS Appl. Mater. Interfaces 2019, 11, 33684–33696. [Google Scholar] [CrossRef]
- Ribeiro, V.P.; Pina, S.; Costa, J.B.; Cengiz, I.F.; García-Fernández, L.; Fernández-Gutiérrez, M.D.M.; Paiva, O.C.; Oliveira, A.L.; San-Román, J.; Oliveira, J.M.; et al. Enzymatically Cross-Linked Silk Fibroin-Based Hierarchical Scaffolds for Osteochondral Regeneration. ACS Appl. Mater. Interfaces 2019, 11, 3781–3799. [Google Scholar] [CrossRef]
- Chen, C.-S.; Zeng, F.; Xiao, X.; Wang, Z.; Li, X.-L.; Tan, R.-W.; Liu, W.-Q.; Zhang, Y.-S.; She, Z.-D.; Li, S.-J. Three-Dimensionally Printed Silk-Sericin-Based Hydrogel Scaffold: A Promising Visualized Dressing Material for Real-Time Monitoring of Wounds. ACS Appl. Mater. Interfaces 2018, 10, 33879–33890. [Google Scholar] [CrossRef]
- Sanz-Fraile, H.; Amoros, S.; Mendizabal, I.; Galvez-Monton, C.; Prat-Vidal, C.; Bayes-Genis, A.; Navajas, D.; Farre, R.; Otero, J. Silk-Reinforced Collagen Hydrogels with Raised Multiscale Stiffness for Mesenchymal Cells 3D Culture. Tissue Eng. Part A 2020, 26, 358–370. [Google Scholar] [CrossRef]

| Bioink | Advantages | Disadvantages |
|---|---|---|
| Agarose | Tunable strength | Low rates of cellular proliferation |
| Tunable melting temperatures | Low cell adhesion/spreading | |
| Alginate | Tunable strength through alteration of monomer percentages | Biologically inert |
| Rapid ionic crosslinking | Limited biodegradability | |
| Chitosan | Mucoadhesion | Poor solubility |
| Hemostatic activity | Poor shape fidelity post printing | |
| Interactions with cell membrane | ||
| Antimicrobial/analgesic effects | ||
| Controllable degradation | ||
| Collagen | Enhanced cellular attachment/growth | Gelation at higher temperatures, liquid form at lower temperatures |
| Extracellular Matrix | Tissue specific | May be difficult to source |
| Multitude of growth factors/cell adhesion points | Difficult to characterize | |
| Batch to batch variability | ||
| Mechanically unstable | ||
| Fibrin | Enzymatic crosslinking | Rapid degradation profile |
| Non-linear elasticity: high deformation potential | Host source may result in immune reaction | |
| High cell adhesion/growth/development | Poor shape fidelity pre-crosslinking | |
| Natural degradation | Highly viscous post crosslinking | |
| Gelatin | Thermo-reversible gelation | Many crosslinking options are cytotoxic |
| High cell adhesion/growth/development | ||
| Can act as thickening agent/support material for other bioinks | ||
| Hyaluronic Acid | High biocompatibility | Poor mechanical properties |
| Reproducible/tunable formation and degradation profiles | Slow gelation rate | |
| Rapid degradation profile | ||
| Scaffold-Free | High cell density | Complicated manufacturing techniques |
| Rapid strand fusion | High cell density (sourcing/expanding) | |
| Self-assembly | Cannot be used with DLP systems | |
| Silk (Fibroin) | Biocompatible | Hydrophobic |
| Adjustable degradation | Slow gelation rate | |
| Mechanically stable | ||
| Self-assembly | ||
| Silk (Sericin) | Immunologically inert | Poor mechanical properties |
| Stimulated cell migration/proliferation | ||
| Gelation at low concentrations |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Willson, K.; Atala, A.; Yoo, J.J. Bioprinting Au Natural: The Biologics of Bioinks. Biomolecules 2021, 11, 1593. https://doi.org/10.3390/biom11111593
Willson K, Atala A, Yoo JJ. Bioprinting Au Natural: The Biologics of Bioinks. Biomolecules. 2021; 11(11):1593. https://doi.org/10.3390/biom11111593
Chicago/Turabian StyleWillson, Kelsey, Anthony Atala, and James J. Yoo. 2021. "Bioprinting Au Natural: The Biologics of Bioinks" Biomolecules 11, no. 11: 1593. https://doi.org/10.3390/biom11111593
APA StyleWillson, K., Atala, A., & Yoo, J. J. (2021). Bioprinting Au Natural: The Biologics of Bioinks. Biomolecules, 11(11), 1593. https://doi.org/10.3390/biom11111593







