Nicotinamide N-Methyltransferase in Head and Neck Tumors: A Comprehensive Review
Abstract
:1. Introduction
2. Oral Squamous Cell Carcinoma
3. Upper Aerodigestive Tract Cancer
4. Other Tumors of Maxillo-Facial Region
5. Thyroid Cancer
6. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Bernier, J. (Ed.) Head and Neck Cancer: Multimodality Management, 2nd ed.; Springer: New York, NY, USA, 2016. [Google Scholar]
- El-Naggar, A.K.; Chan, J.K.C.; Grandis, J.R.; Takata, T.; Slootweg, P.J. (Eds.) WHO Classification of Head and Neck Tumours, 4th ed.; IARC Press: Lyon, France, 2017; Volume 9. [Google Scholar]
- Mascitti, M.; Togni, L.; Troiano, G.; Caponio, V.C.A.; Sabatucci, A.; Balercia, A.; Rubini, C.; Lo Muzio, L.; Santarelli, A. Odontogenic tumours: A 25-year epidemiological study in the Marche region of Italy. Eur. Arch. Otorhinolaryngol. 2020, 277, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B.; Edge, S.B.; Greene, F.L.; Byrd, D.R.; Brookland, R.K.; Washington, M.; Gershenwald, J.; Compton, C.; Hess, K.; Sullivan, D.C.; et al. (Eds.) AJCC Cancer Staging Manual, 8th ed.; Springer International Publishing: New York, NY, USA, 2017. [Google Scholar]
- Sandulache, V.C.; Myers, J.N. Altered metabolism in head and neck squamous cell carcinoma: An opportunity for identification of novel biomarkers and drug targets. Head Neck 2012, 34, 282–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Haren, M.J.; Sastre Torano, J.; Sartini, D.; Emanuelli, M.; Parsons, R.B.; Martin, N.I. A Rapid and Efficient Assay for the Characterization of Substrates and Inhibitors of Nicotinamide N-Methyltransferase. Biochemistry 2016, 55, 5307–5315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rini, J.; Szumlanski, C.; Guerciolini, R.; Weinshilboum, R.M. Human liver nicotinamide N-methyltransferase: Ion-pairing radiochemical assay, biochemical properties and individual variation. Clin. Chim. Acta 1990, 186, 359–374. [Google Scholar] [CrossRef]
- Jung, J.; Kim, L.J.; Wang, X.; Wu, Q.; Sanvoranart, T.; Hubert, C.G.; Prager, B.C.; Wallace, L.C.; Jin, X.; Mack, S.C.; et al. Nicotinamide metabolism regulates glioblastoma stem cell maintenance. JCI Insight 2017, 2, e90019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sartini, D.; Muzzonigro, G.; Milanese, G.; Pierella, F.; Rossi, V.; Emanuelli, M. Identification of nicotinamide N-methyltransferase as a novel tumor marker for renal clear cell carcinoma. J. Urol. 2006, 176, 2248–2254. [Google Scholar] [CrossRef]
- Xie, X.; Yu, H.; Wang, Y.; Zhou, Y.; Li, G.; Ruan, Z.; Li, F.; Wang, X.; Liu, H.; Zhang, J. Nicotinamide N-methyltransferase enhances the capacity of tumorigenesis associated with the promotion of cell cycle progression in human colorectal cancer cells. Arch. Biochem. Biophys. 2014, 564, 52–66. [Google Scholar] [CrossRef]
- Wu, Y.; Siadaty, M.S.; Berens, M.E.; Hampton, G.M.; Theodorescu, D. Overlapping gene expression profiles of cell migration and tumor invasion in human bladder cancer identify metallothionein 1E and nicotinamide N-methyltransferase as novel regulators of cell migration. Oncogene 2008, 27, 6679–6689. [Google Scholar] [CrossRef] [Green Version]
- Tang, S.W.; Yang, T.C.; Lin, W.C.; Chang, W.H.; Wang, C.C.; Lai, M.K.; Lin, J.Y. Nicotinamide N-methyltransferase induces cellular invasion through activating matrix metalloproteinase-2 expression in clear cell renal cell carcinoma cells. Carcinogenesis 2011, 32, 138–145. [Google Scholar] [CrossRef] [Green Version]
- Cui, Y.; Zhang, L.; Wang, W.; Ma, S.; Liu, H.; Zang, X.; Zhang, Y.; Guan, F. Downregulation of nicotinamide N-methyltransferase inhibits migration and epithelial-mesenchymal transition of esophageal squamous cell carcinoma via Wnt/beta-catenin pathway. Mol. Cell. Biochem. 2019, 460, 93–103. [Google Scholar] [CrossRef]
- Ogawa, A.; Griffin, R.J.; Song, C.W. Effect of a combination of mild-temperature hyperthermia and nicotinamide on the radiation response of experimental tumors. Radiat. Res. 2000, 153, 327–331. [Google Scholar] [CrossRef]
- Kassem, H.; Sangar, V.; Cowan, R.; Clarke, N.; Margison, G.P. A potential role of heat shock proteins and nicotinamide N-methyl transferase in predicting response to radiation in bladder cancer. Int. J. Cancer 2002, 101, 454–460. [Google Scholar] [CrossRef]
- Li, H.F.; Kim, J.S.; Waldman, T. Radiation-induced Akt activation modulates radioresistance in human glioblastoma cells. Radiat. Oncol. 2009, 4, 43. [Google Scholar] [CrossRef] [Green Version]
- Xia, S.; Zhao, Y.; Yu, S.; Zhang, M. Activated PI3K/Akt/COX-2 pathway induces resistance to radiation in human cervical cancer HeLa cells. Cancer Biother. Radiopharm. 2010, 25, 317–323. [Google Scholar] [CrossRef]
- Cui, Y.; Yang, D.; Wang, W.; Zhang, L.; Liu, H.; Ma, S.; Guo, W.; Yao, M.; Zhang, K.; Li, W.; et al. Nicotinamide N-methyltransferase decreases 5-fluorouracil sensitivity in human esophageal squamous cell carcinoma through metabolic reprogramming and promoting the Warburg effect. Mol. Carcinog. 2020, 59, 940–954. [Google Scholar] [CrossRef] [PubMed]
- Sartini, D.; Pozzi, V.; Renzi, E.; Morganti, S.; Rocchetti, R.; Rubini, C.; Santarelli, A.; Lo Muzio, L.; Emanuelli, M. Analysis of tissue and salivary nicotinamide N-methyltransferase in oral squamous cell carcinoma: Basis for the development of a noninvasive diagnostic test for early-stage disease. Biol. Chem. 2012, 393, 505–511. [Google Scholar] [CrossRef]
- Sartini, D.; Muzzonigro, G.; Milanese, G.; Pozzi, V.; Vici, A.; Morganti, S.; Rossi, V.; Mazzucchelli, R.; Montironi, R.; Emanuelli, M. Upregulation of tissue and urinary nicotinamide N-methyltransferase in bladder cancer: Potential for the development of a urine-based diagnostic test. Cell Biochem. Biophys. 2013, 65, 473–483. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2017. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Global Burden of Disease Cancer Collaboration; Fitzmaurice, C.; Allen, C.; Barber, R.M.; Barregard, L.; Bhutta, Z.A.; Brenner, H.; Dicker, D.J.; Chimed-Orchir, O.; Dandona, R.; et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived with Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2017, 3, 524–548. [Google Scholar] [CrossRef]
- Mascitti, M.; Zhurakivska, K.; Togni, L.; Caponio, V.C.A.; Almangush, A.; Balercia, P.; Balercia, A.; Rubini, C.; Lo Muzio, L.; Santarelli, A.; et al. Addition of the tumour-stroma ratio to the 8th edition American Joint Committee on Cancer staging system improves survival prediction for patients with oral tongue squamous cell carcinoma. Histopathology 2020, 77, 810–822. [Google Scholar] [CrossRef] [PubMed]
- Mascitti, M.; Tempesta, A.; Togni, L.; Capodiferro, S.; Troiano, G.; Rubini, C.; Maiorano, E.; Santarelli, A.; Favia, G.; Limongelli, L. Histological features and survival in young patients with HPV-negative oral squamous cell carcinoma. Oral Dis. 2020, 26, 1640–1648. [Google Scholar] [CrossRef]
- Sartini, D.; Santarelli, A.; Rossi, V.; Goteri, G.; Rubini, C.; Ciavarella, D.; Lo Muzio, L.; Emanuelli, M. Nicotinamide N-methyltransferase upregulation inversely correlates with lymph node metastasis in oral squamous cell carcinoma. Mol. Med. 2007, 13, 415–421. [Google Scholar] [CrossRef]
- Roberg, K.; Ceder, R.; Farnebo, L.; Norberg-Spaak, L.; Grafstrom, R.C. Multiple genotypic aberrances associate to terminal differentiation-deficiency of an oral squamous cell carcinoma in serum-free culture. Differentiation 2008, 76, 868–880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pozzi, V.; Sartini, D.; Morganti, S.; Giuliante, R.; Di Ruscio, G.; Santarelli, A.; Rocchetti, R.; Rubini, C.; Tomasetti, M.; Giannatempo, G.; et al. RNA-mediated gene silencing of nicotinamide N-methyltransferase is associated with decreased tumorigenicity in human oral carcinoma cells. PLoS ONE 2013, 8, e71272. [Google Scholar] [CrossRef] [Green Version]
- Seta, R.; Mascitti, M.; Campagna, R.; Sartini, D.; Fumarola, S.; Santarelli, A.; Giuliani, M.; Cecati, M.; Muzio, L.L.; Emanuelli, M. Overexpression of nicotinamide N-methyltransferase in HSC-2 OSCC cell line: Effect on apoptosis and cell proliferation. Clin. Oral. Investig. 2019, 23, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Emanuelli, M.; Santarelli, A.; Sartini, D.; Ciavarella, D.; Rossi, V.; Pozzi, V.; Rubini, C.; Lo Muzio, L. Nicotinamide N-Methyltransferase upregulation correlates with tumour differentiation in oral squamous cell carcinoma. Histol. Histopathol. 2010, 25, 15–20. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Multani, S.; Dabholkar, J.; Saranath, D. Whole genome expression profiling in chewing-tobacco-associated oral cancers: A pilot study. Med. Oncol. 2015, 32, 60. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Yu, Y.C.; Ding, X.J.; Luo, Y.; Ruan, H. Bioinformatics analysis reveals significant genes and pathways to target for oral squamous cell carcinoma. Asian Pac. J. Cancer Prev. 2014, 15, 2273–2278. [Google Scholar] [CrossRef] [Green Version]
- Liao, K.A.; Tsay, Y.G.; Huang, L.C.; Huang, H.Y.; Li, C.F.; Wu, T.F. Search for the tumor-associated proteins of oral squamous cell carcinoma collected in Taiwan using proteomics strategy. J. Proteome Res. 2011, 10, 2347–2358. [Google Scholar] [CrossRef]
- Win, K.T.; Lee, S.W.; Huang, H.Y.; Lin, L.C.; Lin, C.Y.; Hsing, C.H.; Chen, L.T.; Li, C.F. Nicotinamide N-methyltransferase overexpression is associated with Akt phosphorylation and indicates worse prognosis in patients with nasopharyngeal carcinoma. Tumour Biol. 2013, 34, 3923–3931. [Google Scholar] [CrossRef]
- Pozzi, V.; Mazzotta, M.; Lo Muzio, L.; Sartini, D.; Santarelli, A.; Renzi, E.; Rocchetti, R.; Tomasetti, M.; Ciavarella, D.; Emanuelli, M. Inhibiting proliferation in KB cancer cells by RNA interference-mediated knockdown of nicotinamide N-methyltransferase expression. Int. J. Immunopathol. Pharmacol. 2011, 24, 69–77. [Google Scholar] [CrossRef] [Green Version]
- Ishibashi, K.; Ishii, K.; Sugiyama, G.; Sumida, T.; Sugiura, T.; Kamata, Y.U.; Seki, K.; Fujinaga, T.; Kumamaru, W.; Kobayashi, Y.; et al. Deregulation of Nicotinamide N-Methyltransferase and Gap Junction Protein Alpha-1 Causes Metastasis in Adenoid Cystic Carcinoma. Anticancer Res. 2018, 38, 187–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mascitti, M.; Sartini, D.; Togni, L.; Pozzi, V.; Rubini, C.; Santarelli, A.; Emanuelli, M. Differential expression of nicotinamide N-methyltransferase in primary and recurrent ameloblastomas and odontogenic keratocysts. Eur. J. Clin. Investig. 2020, 50, e13220. [Google Scholar] [CrossRef] [PubMed]
- Mascitti, M.; Santarelli, A.; Sartini, D.; Rubini, C.; Colella, G.; Salvolini, E.; Ganzetti, G.; Offidani, A.; Emanuelli, M. Analysis of nicotinamide N-methyltransferase in oral malignant melanoma and potential prognostic significance. Melanoma Res. 2019, 29, 151–156. [Google Scholar] [CrossRef]
- Xu, J.; Capezzone, M.; Xu, X.; Hershman, J.M. Activation of nicotinamide N-methyltransferase gene promoter by hepatocyte nuclear factor-1beta in human papillary thyroid cancer cells. Mol. Endocrinol. 2005, 19, 527–539. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Moatamed, F.; Caldwell, J.S.; Walker, J.R.; Kraiem, Z.; Taki, K.; Brent, G.A.; Hershman, J.M. Enhanced expression of nicotinamide N-methyltransferase in human papillary thyroid carcinoma cells. J. Clin. Endocrinol. Metab. 2003, 88, 4990–4996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.; Hershman, J.M. Histone deacetylase inhibitor depsipeptide represses nicotinamide N-methyltransferase and hepatocyte nuclear factor-1beta gene expression in human papillary thyroid cancer cells. Thyroid 2006, 16, 151–160. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, X.Y.; Yang, S.W.; Wang, J.; He, C. Nicotinamide N-methyltransferase protein expression in renal cell cancer. J. Zhejiang Univ. Sci. B 2010, 11, 136–143. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.Q.; Coucke, P.A.; Mirimanoff, R.O.; Buchegger, F. Fractionated irradiation combined with carbogen breathing and nicotinamide of two human glioblastomas grafted in nude mice. Radiat. Res. 2001, 155, 26–31. [Google Scholar] [CrossRef]
- Overgaard, J.; Horsman, M.R. Modification of Hypoxia-Induced Radioresistance in Tumors by the Use of Oxygen and Sensitizers. Semin. Radiat. Oncol. 1996, 6, 10–21. [Google Scholar] [CrossRef]
- Droller, M.J. Hypoxic radiosensitizers in radical radiotherapy for patients with bladder carcinoma: Hyperbaric oxygen, misonidazole, and accelerated radiotherapy, carbogen and nicotinamide. J. Urol. 2000, 163, 1600. [Google Scholar]
- Troiano, G.; Rubini, C.; Togni, L.; Caponio, V.C.A.; Zhurakivska, K.; Santarelli, A.; Cirillo, N.; Lo Muzio, L.; Mascitti, M. The immune phenotype of tongue squamous cell carcinoma predicts early relapse and poor prognosis. Cancer Med. 2020, 9, 8333–8344. [Google Scholar] [CrossRef]
- Gao, Y.; van Haren, M.J.; Moret, E.E.; Rood, J.J.M.; Sartini, D.; Salvucci, A.; Emanuelli, M.; Craveur, P.; Babault, N.; Jin, J.; et al. Bisubstrate Inhibitors of Nicotinamide N-Methyltransferase (NNMT) with Enhanced Activity. J. Med. Chem. 2019, 62, 6597–6614. [Google Scholar] [CrossRef] [Green Version]
- Neelakantan, H.; Wang, H.Y.; Vance, V.; Hommel, J.D.; McHardy, S.F.; Watowich, S.J. Structure-Activity Relationship for Small Molecule Inhibitors of Nicotinamide N-Methyltransferase. J. Med. Chem. 2017, 60, 5015–5028. [Google Scholar] [CrossRef] [PubMed]
- Policarpo, R.L.; Decultot, L.; May, E.; Kuzmic, P.; Carlson, S.; Huang, D.; Chu, V.; Wright, B.A.; Dhakshinamoorthy, S.; Kannt, A.; et al. High-Affinity Alkynyl Bisubstrate Inhibitors of Nicotinamide N-Methyltransferase (NNMT). J. Med. Chem. 2019, 62, 9837–9873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, D.; Li, L.; Diaz, K.; Iyamu, I.D.; Yadav, R.; Noinaj, N.; Huang, R. Novel Propargyl-Linked Bisubstrate Analogues as Tight-Binding Inhibitors for Nicotinamide N-Methyltransferase. J. Med. Chem. 2019, 62, 10783–10797. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; van Haren, M.J.; Buijs, N.; Innocenti, P.; Zhang, Y.; Sartini, D.; Campagna, R.; Emanuelli, M.; Parsons, R.B.; Jespers, W.; et al. Potent Inhibition of Nicotinamide N-Methyltransferase by Alkene-Linked Bisubstrate Mimics Bearing Electron Deficient Aromatics. J. Med. Chem. 2021, 64, 12938–12963. [Google Scholar] [CrossRef]
- Gao, Y.; Martin, N.I.; van Haren, M.J. Nicotinamide N-methyl transferase (NNMT): An emerging therapeutic target. Drug Discov. Today 2021, S1359-6446(21)00242-7. [Google Scholar] [CrossRef]
| Author, Year [ref] | Tumor Type | Methods | Materials | Main Results |
|---|---|---|---|---|
| Sartini D et al., 2007 [25] | OSCC | IHC | Rabbit polyclonal anti-human NNMT Ab, 1:500 | NNMT expression in N0 OSCC is higher than N+ OSCC and normal tissues. Inverse association between NNMT expression and tumor size and pathological staging. |
| Roberg K et al., 2008 [26] | OSCC | Genomic study | Genomic microarray [Affymetrix HG-Focus chip] (LK0412 cell line) | NNMT expression is 74-fold change higher in OSCC than in normal oral keratinocytes. |
| Emanuelli M et al., 2010 [29] | OSCC | IHC | Rabbit polyclonal anti-human NNMT Ab, 1:500 | NNMT immunostaining is higher in tumor tissue compared to normal mucosa. Moreover, there is an inverse relationship between NNMT expression and histological grading. |
| Liao KA et al., 2011 [32] | OSCC | Proteomic study | Liquid chromatography-mass spectrometry [LTQ-Orbitrap hybrid tandem mass spectrometer and Agilent 1200 nanoflow HPLC] (OSCC and adjacent non tumor tissue) | A total of 17 proteins were differentially expressed in OSCC compared to non-tumor tissue, including NNMT. |
| IHC | Rabbit polyclonal anti-human NNMT Ab, 1:50 | The expression of three proteins, including NNMT, confirmed the proteomic data. | ||
| Sartini D et al., 2012 [19] | OSCC | In vitro study | Frozen OSCC tissue samples | Increase of NNMT enzyme activity from non-tumor tissue to OSCC and from N0 to N+ OSCC. |
| Clinical study | Saliva from OSCC patients and healthy subjects | Salivary NNMT levels higher in OSCC patients than in healthy subjects. | ||
| Pozzi V et al., 2013 [27] | OSCC | In vitro study | PE/CA PJ-15 cell line | NNMT gene silencing reduce cell proliferation and colony formation ability. |
| In vivo study | BALB/c nude mice | NNMT silencing reduce tumor volume. | ||
| Jiang Q et al., 2014 [31] | OSCC | Bioinformatic study | Bioinformatic analysis based on genomic microarray [Affymetrix Human Genome U133A Array] | NNMT found to be overexpressed (>1-fold change) compared to normal oral mucosa. |
| Chakrabarti S et al., 2015 [30] | OSCC | Genomic study | Genomic microarray [IlluminaSentrix Human Ref-8 v2 Expression BeadChip arrays] (OSCC patients and healthy subjects) | NNMT expression in OSCC is higher (2.39-fold change) than in normal mucosa. |
| Seta R et al., 2019 [28] | OSCC | In vitro study | HSC-2 cell line | NNMT overexpression increase cell growth in vitro. Moreover, NNMT is associated to survivin-ΔEx3 isoform expression. |
| Ishibashi K et al., 2018 [35] | AdCC | In vitro study | ACCS-GFP, ACCS-M-GFP, ACCS-LN-GFP cell lines | NNMT mRNA and protein levels increase from ACCS-GFP line to ACCS-M-GFP and to ACCS-LN-GFP cell line. |
| Genomic study | Genomic microarray [60K Agilent 60-mer oligomicroarray] (ACCS-M-GFP, ACCS-LN-GFP cell lines) | NNMT expression is higher (2.0-fold change) in ACCS-LN-GFP than in ACCS-M-GFP. | ||
| Mascitti M et al., 2019 [37] | OMM | IHC | Rabbit polyclonal anti-human NNMT Ab, 1:1500 | NNMT intensity is higher in ulcerated OMM compared to no-ulcerated OMM. NNMT overexpression negatively affects the DFS. |
| Mascitti M et al., 2020 [36] | OT | IHC | Rabbit polyclonal anti-human NNMT Ab, 1:1500 | NNMT expression is higher in recurrent than primary lesions. |
| Xu J et al., 2003 [39] | thyroid cancer | In vitro study | BHP2-7, BHP5-16, BHP 7-13, BHP 10-3, BHP 14-9, BHP 15-3, BHP 17-10, BHP 18-21, ARO 81-1, DRO 90-1, HRO 85-1, DRO 81-1, WRO 82-1, TC1 cell lines | NNMT mRNA and catalytic activity are higher in BHP 2-7, BHP 7-13, BHP 10-3, BHP 18-21 and TPC1, compared to other cancer cell types and thyroid primary cells (higher NNMT expression detected on BHP 2-7 cell line). |
| IHC | Rabbit polyclonal anti-human NNMT Ab, 1:3000 | PTCs overexpress NNMT, normal tissues and benign thyroid lesions do not express NNMT. | ||
| Xu J et al., 2005 [38] | PTC | In vitro study | BHP 2-7, BHP 7-13, BHP 10-3, BHP 18-21, TPC 1, NPA 87, BHP 5-16, BHP 14-9, BHP 15-3, WRO 82-1, ML-1A, ML-1B, FTC133, FTC238, XTC-1, O4 PC, HX5 PC, Hep G2, LNCaP, MCF-7 cell lines | Higher HNF-1β expression is detected in papillary cell lines with high NNMT activity. |
| Xu J et al., 2006 [40] | PTC | In vitro study | BHP 18-21, BHP 2-7, BHP 14-9, Hep G2 cell lines | The HNF-1β binding site mutation significantly decreases the NNMT promoter activity in BHP 2-7 cells compare to Hep G2 cells. The depsipeptide downregulates NNMT and HNF-1β gene expression in BHP 18-21 cell line. |
| Win T et al., 2013 [33] | NPC | IHC | Mouse monoclonal anti-human NNMT Ab, 1:200 | NNMT overexpression is an independent prognostic factor of worse DSS and MeFS. |
| Pozzi V et al., 2011 [34] | LSCC | In vitro study | KB cell line | KB cells show higher NNMT expression levels compare to mock cell. NNMT levels significantly decreases after silencing. NNMT downregulation significantly reduces the cell proliferation. |
| Cui Y et al., 2019 [13] | ESCC | IHC | Rabbit polyclonal anti-human NNMT Ab, 1:75 | NNMT expression is significantly higher in ESCCs compared to the adjacent normal tissues. NNMT overexpression significantly correlated with lymph node metastasis. |
| In vitro study | EC1, EC9706, TE1, TE13, Eca109 cell lines | EC9706 and TE1 cell lines show highest NNMT expression. NNMT silencing suppresses tumor cells growth, proliferation, and migration. | ||
| Cui Y et al., 2020 [18] | ESCC | In vitro study | EC1, TE1, Eca109 cell lines | NNMT silencing enhances 5-FU inhibitory effect on cell viability and colony formation and increases 5-FU-induced proapoptotic protein levels in the TE1 cells. TE1-siNNMT tumors are significantly smaller and more sensitivity to 5-FU respect to TE1-NC tumors. In TE1-siNNMT tumors, the 5-FU promotes the apoptosis and the tumoral necrosis. |
| Metabolomic study | Gas chromatography-mass spectrometry [Agilent Technologies] (EC1, TE1, Eca109 cell lines) | Nicotinate and nicotinamide metabolism and tricarboxylic acid cycle in TE1 cells are significantly different from those in EC1 and Eca109 cells. |
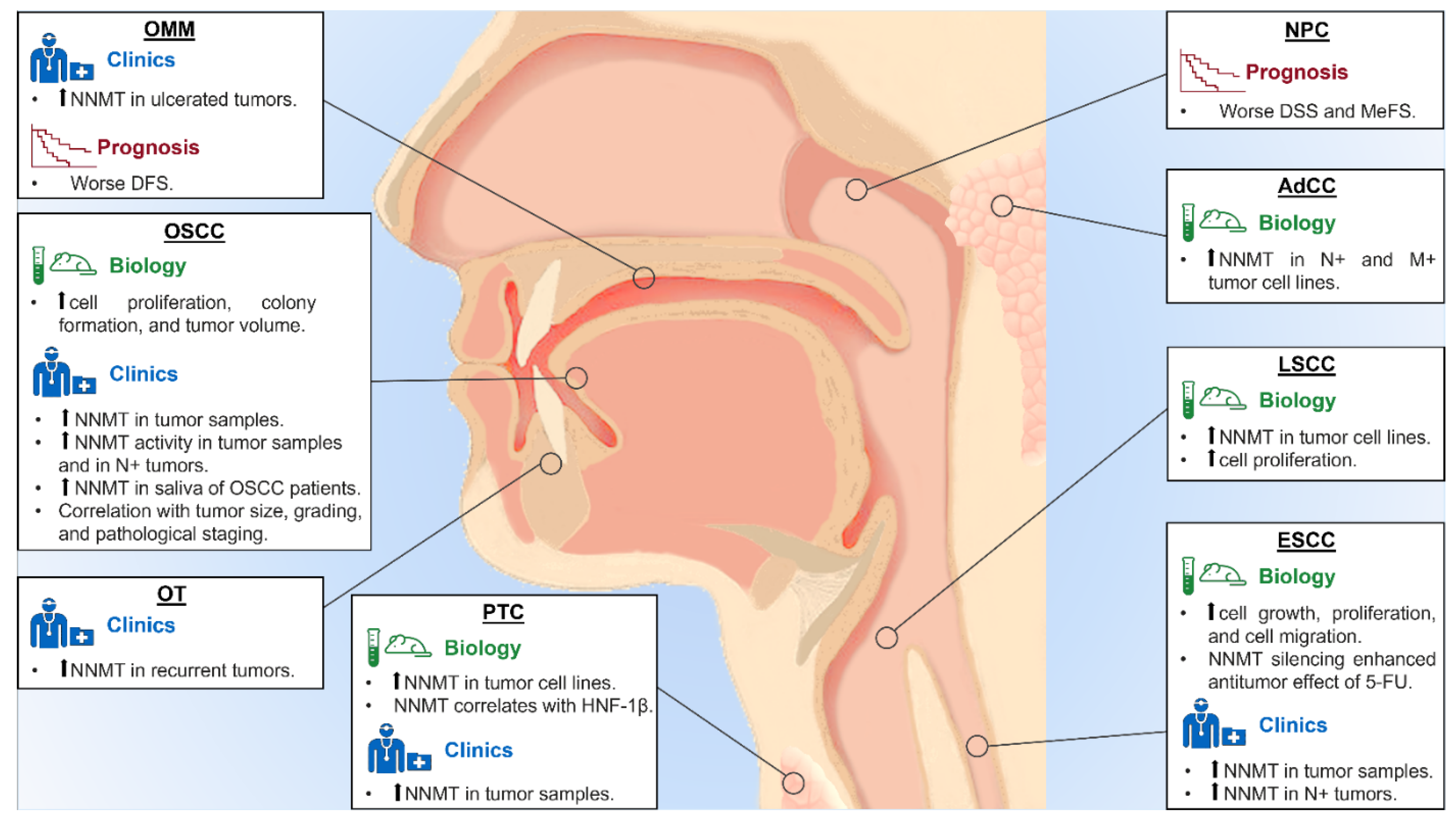
| Tumor Type | Biological and Clinical Role of NNMT |
|---|---|
| OSCC | - Increase in cell proliferation, colony formation, and tumor volume; - Overexpression in tumor samples; - Association with tumor size, histological grading, and pathological staging; - Higher enzyme activity in N+ OSCC; - Higher salivary levels in OSCC patients. |
| AdCC | - Higher expression in N+ and M+ AdCC cell lines. |
| OT | - Overexpression in recurrent lesions. |
| OMM | - Overexpression in ulcerated OMM; - Association with worse DFS. |
| PTC | - Overexpression in PTC samples and cell lines; - Correlation between NNMT and HNF-1β overexpression. |
| NPC | - Association with worse DSS and MeFS. |
| LSCC | - Overexpression in cell lines; - Increase in cell proliferation. |
| ESCC | - Overexpression in tumor samples - Overexpression in N+ ESCC; - Increase in cells growth, proliferation, and migration; - Increase tumor cell viability. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Togni, L.; Mascitti, M.; Sartini, D.; Campagna, R.; Pozzi, V.; Salvolini, E.; Offidani, A.; Santarelli, A.; Emanuelli, M. Nicotinamide N-Methyltransferase in Head and Neck Tumors: A Comprehensive Review. Biomolecules 2021, 11, 1594. https://doi.org/10.3390/biom11111594
Togni L, Mascitti M, Sartini D, Campagna R, Pozzi V, Salvolini E, Offidani A, Santarelli A, Emanuelli M. Nicotinamide N-Methyltransferase in Head and Neck Tumors: A Comprehensive Review. Biomolecules. 2021; 11(11):1594. https://doi.org/10.3390/biom11111594
Chicago/Turabian StyleTogni, Lucrezia, Marco Mascitti, Davide Sartini, Roberto Campagna, Valentina Pozzi, Eleonora Salvolini, Annamaria Offidani, Andrea Santarelli, and Monica Emanuelli. 2021. "Nicotinamide N-Methyltransferase in Head and Neck Tumors: A Comprehensive Review" Biomolecules 11, no. 11: 1594. https://doi.org/10.3390/biom11111594
APA StyleTogni, L., Mascitti, M., Sartini, D., Campagna, R., Pozzi, V., Salvolini, E., Offidani, A., Santarelli, A., & Emanuelli, M. (2021). Nicotinamide N-Methyltransferase in Head and Neck Tumors: A Comprehensive Review. Biomolecules, 11(11), 1594. https://doi.org/10.3390/biom11111594










