Antioxidant and Anti-Inflammatory Properties of Bioavailable Protein Hydrolysates from Lupin-Derived Agri-Waste
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of Lupin Protein Hydrolysate
2.3. Analytical Methods
2.4. Caco-2 Culture
2.5. THP-1 Culture
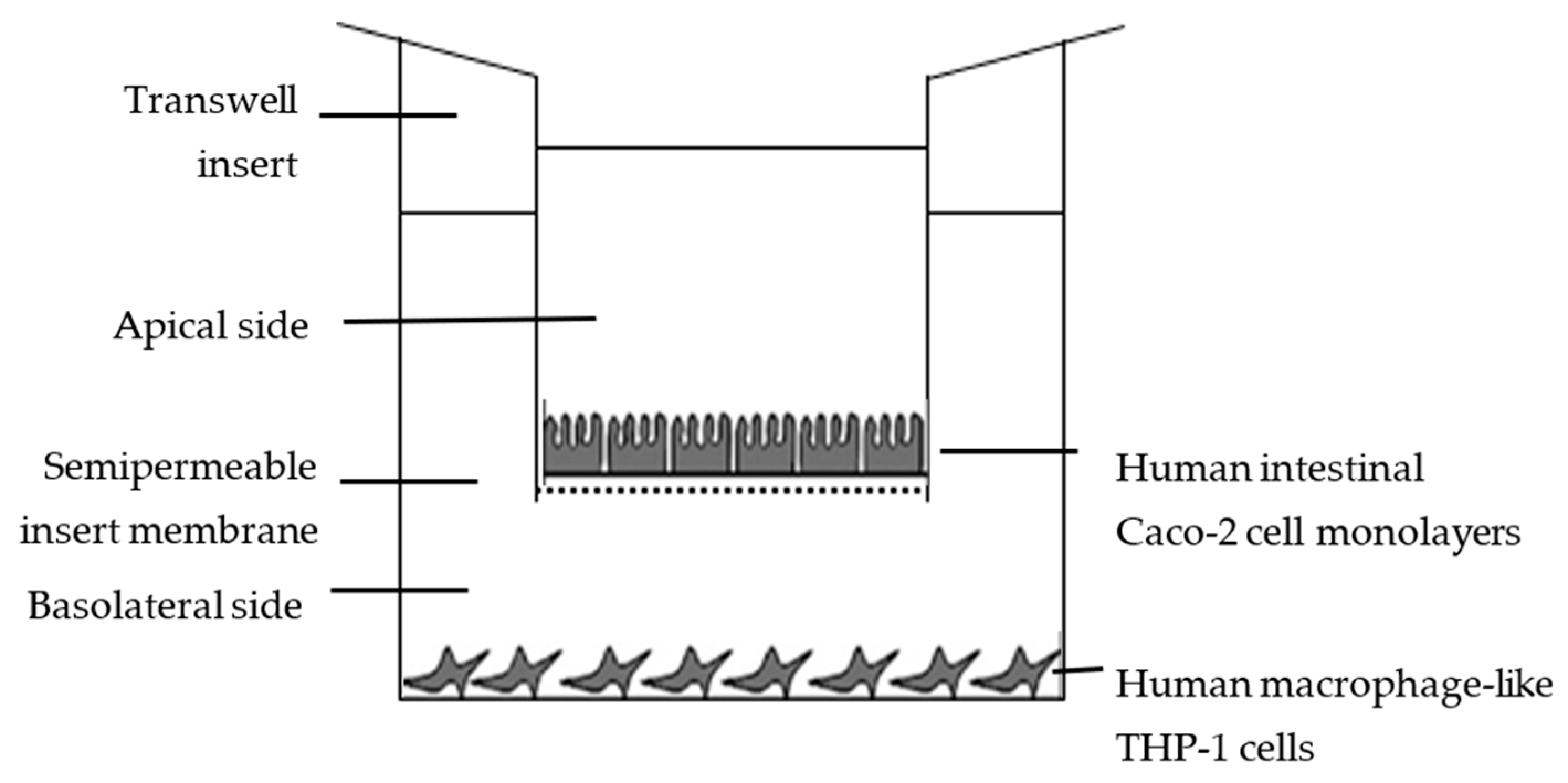
2.6. In Vitro Availability Using Caco-2/THP-1 Co-Cultures
2.7. Reactive Oxygen Species Determination
2.8. Nitric Oxide Assay
2.9. RNA Extraction and Analysis by RT-qPCR
2.10. Enzyme-Linked Immunosorbent Assay (ELISA)
2.11. Statistical Analysis
3. Results and Discussion
3.1. Chemical Characterization of LPH
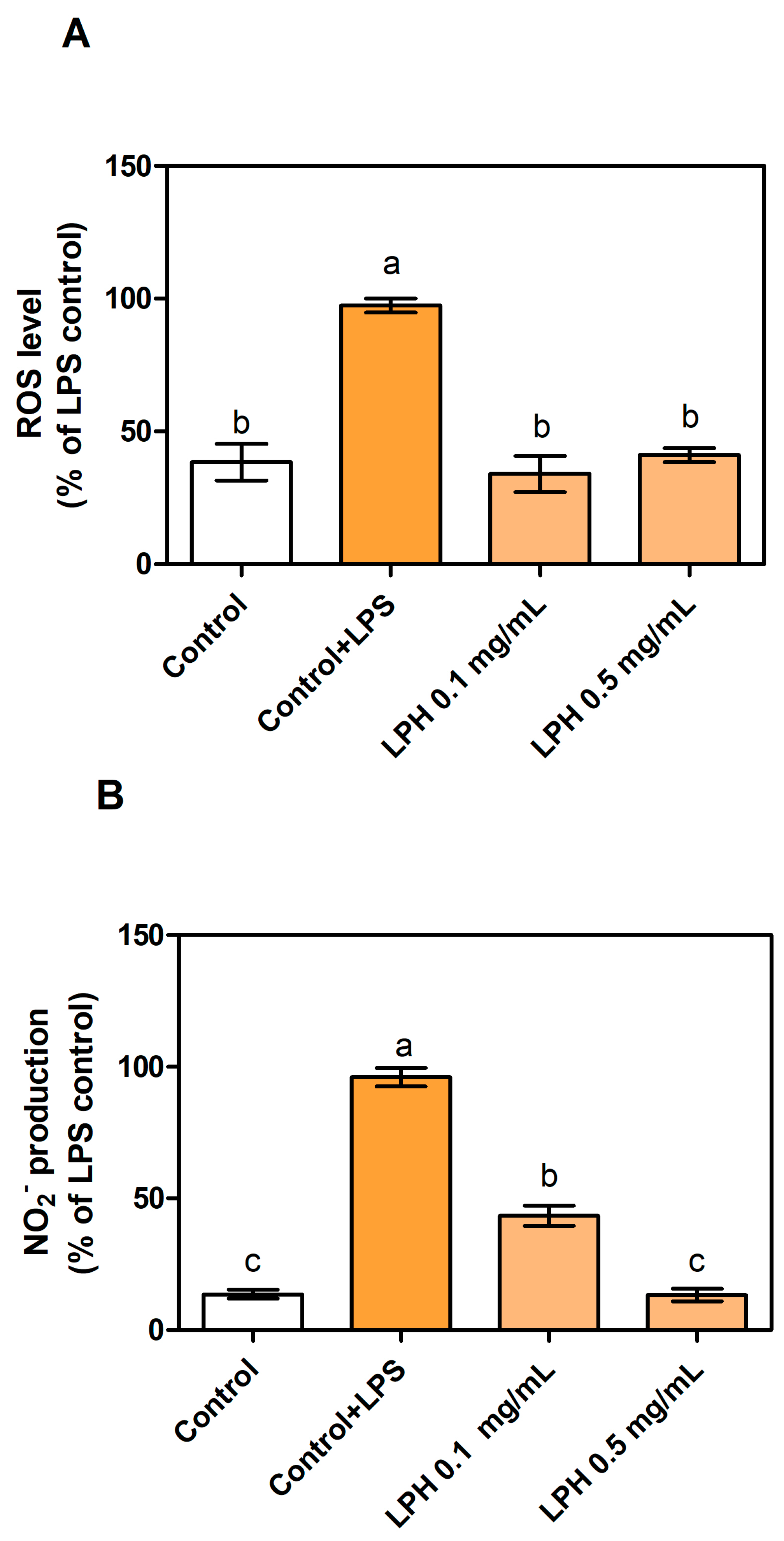
3.2. LPH Reduces Production of ROS and Nitrites in LPS-Stimulated THP-1-Derived Macrophages
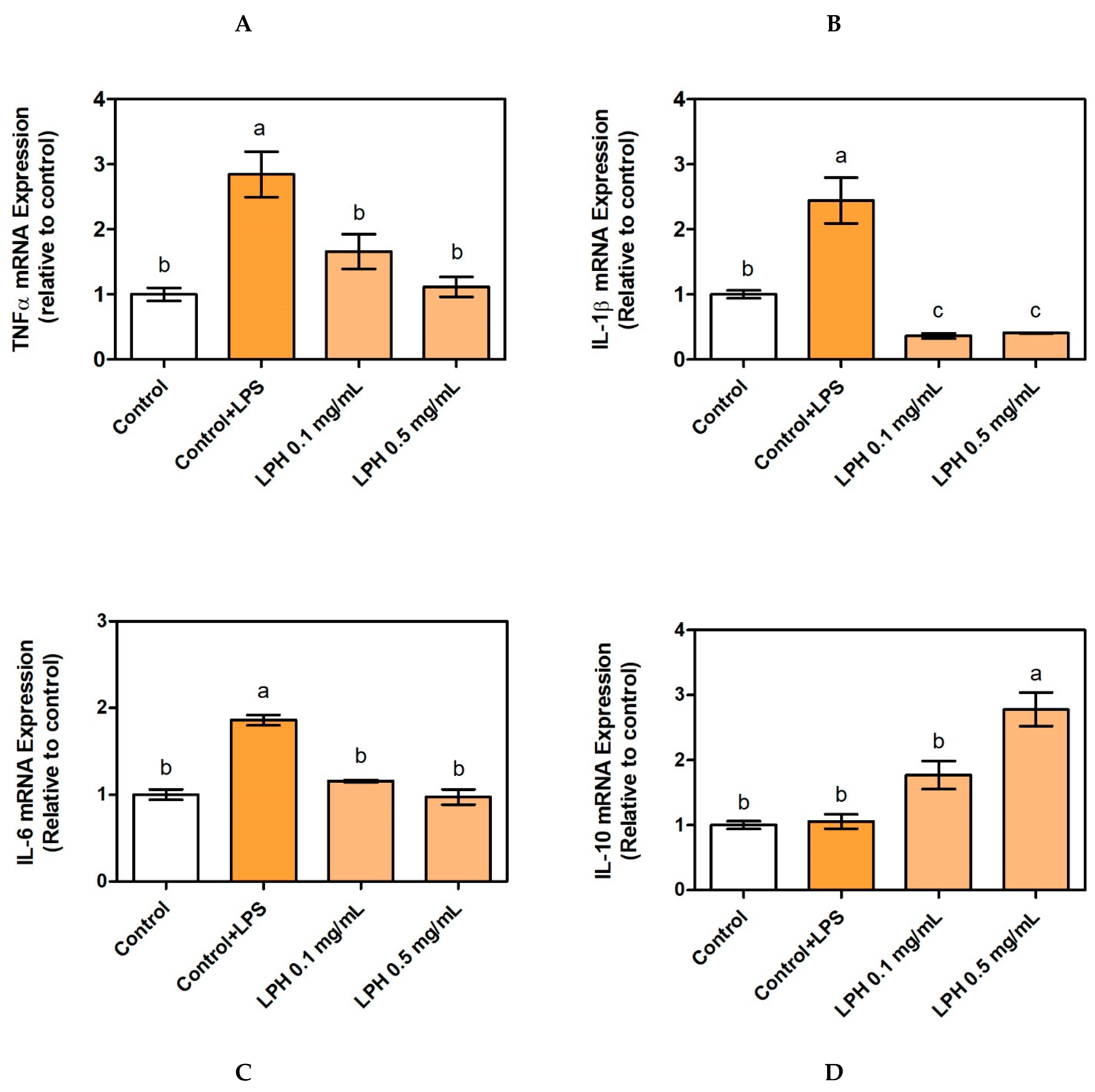
3.3. Effect of LPH on mRNA Expression of Pro-Inflammatory Cytokines
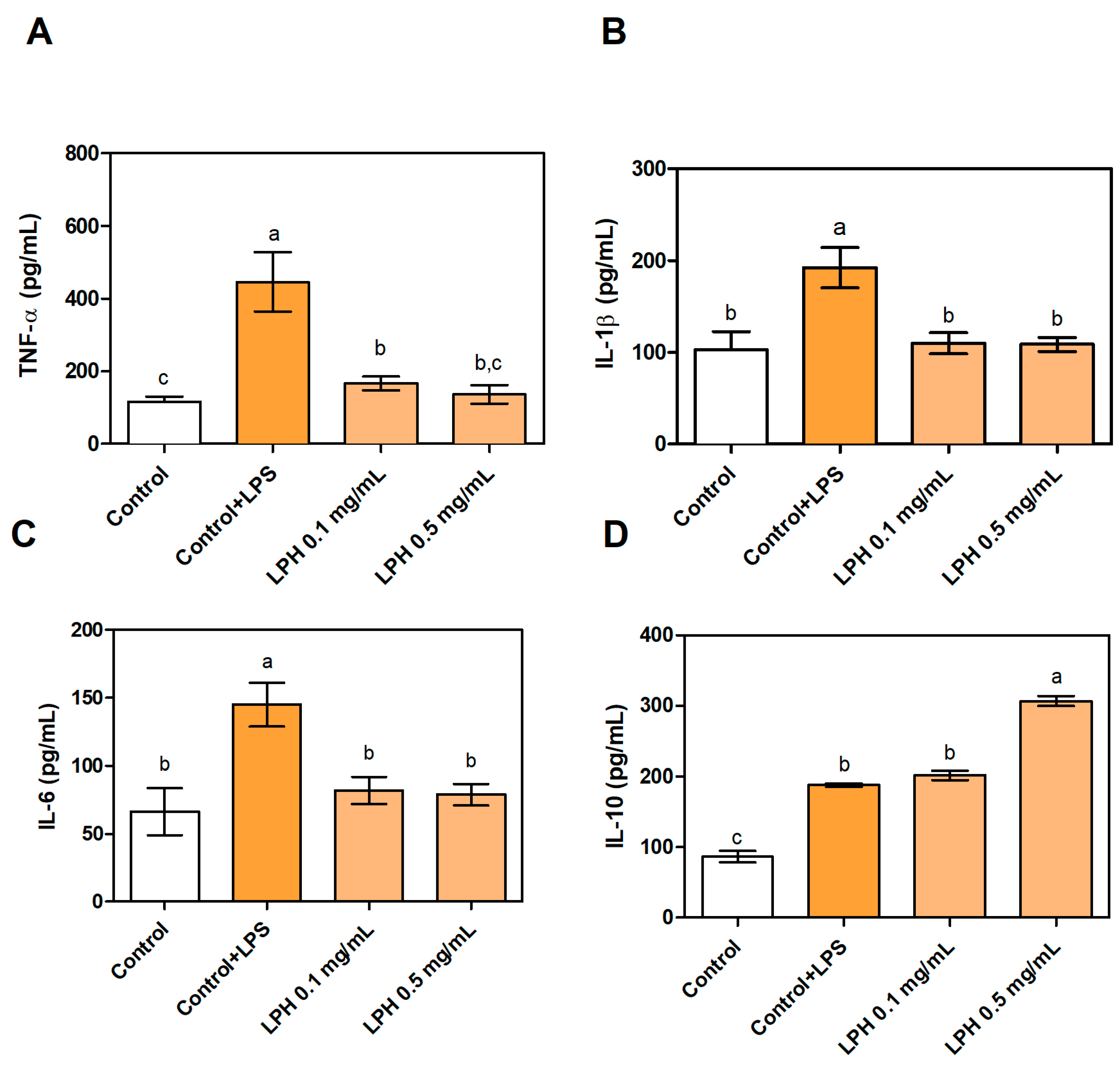
3.4. Effect of LPH on the Production of Pro- and Anti-Inflammatory Cytokines
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kamal, H.; Le, C.F.; Salter, A.M.; Ali, A. Extraction of protein from food waste: An overview of current status and opportunities. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2455–2475. [Google Scholar] [CrossRef]
- Joehnke, M.S.; Jeske, S.; Ispiryan, L.; Zannini, E.; Arendt, E.K.; Bez, J.; Sørensen, J.C.; Petersen, I.L. Nutritional and anti-nutritional properties of lentil (Lens culinaris) protein isolates prepared by pilot-scale processing. Food Chem. X 2021, 9, 100112. [Google Scholar] [CrossRef] [PubMed]
- Akharume, F.U.; Aluko, R.E.; Adedeji, A.A. Modification of plant proteins for improved functionality: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 198–224. [Google Scholar] [CrossRef] [PubMed]
- Lupin Market Global Industry Trends and Forecast to 2027 Data Bridge Market Research. Available online: https://www.databridgemarketresearch.com/reports/global-lupin-market (accessed on 5 September 2021).
- Bartkiene, E.; Sakiene, V.; Bartkevics, V.; Juodeikiene, G.; Lele, V.; Wiacek, C.; Braun, P.G. Modulation of the nutritional value of lupine wholemeal and protein isolates using submerged and solid-state fermentation with Pediococcus pentosaceus strains. Int. J. Food Sci. Technol. 2018, 53, 1896–1905. [Google Scholar] [CrossRef]
- Łopusiewicz, Ł.; Drozłowska, E.; Trocer, P.; Kwiatkowski, P.; Bartkowiak, A.; Gefrom, A.; Sienkiewicz, M. The Effect of Fermentation with Kefir Grains on the Physicochemical and Antioxidant Properties of Beverages from Blue Lupin (Lupinus angustifolius L.) Seeds. Molecules 2020, 25, 5791. [Google Scholar] [CrossRef] [PubMed]
- Karamać, M.; Orak, H.H.; Amarowicz, R.; Orak, A.; Piekoszewski, W. Phenolic contents and antioxidant capacities of wild and cultivated white lupin (Lupinus albus L.) seeds. Food Chem. 2018, 258, 1–7. [Google Scholar] [CrossRef]
- Khan, M.K.; Karnpanit, W.; Nasar-Abbas, S.M.; Huma, Z.-E.; Jayasena, V. Phytochemical composition and bioactivities of lupin: A review. Int. J. Food Sci. Technol. 2015, 50, 2004–2012. [Google Scholar] [CrossRef]
- Abraham, E.M.; Ganopoulos, I.; Madesis, P.; Mavromatis, A.; Mylona, P.; Nianiou-Obeidat, I.; Parissi, Z.; Polidoros, A.; Tani, E.; Vlachostergios, D. The Use of Lupin as a Source of Protein in Animal Feeding: Genomic Tools and Breeding Approaches. Int. J. Mol. Sci. 2019, 20, 851. [Google Scholar] [CrossRef] [Green Version]
- Mota, J.; Lima, A.; Ferreira, R.B.; Raymundo, A. Lupin Seed Protein Extract Can Efficiently Enrich the Physical Properties of Cookies Prepared with Alternative Flours. Foods 2020, 9, 1064. [Google Scholar] [CrossRef]
- Ruiz López, F.J. Hidrolizado de Proteínas de Chícharo (Pisum sativum) y Arroz (Oryza sativa) y Su Efecto Antiadipogénico in Silico. Ph.D. Thesis, Universidad Autónoma de Nuevo León, San Nicolás de los Garza, Mexico, 2020. [Google Scholar]
- Fleit, H. Chronic Inflammation. Pathobiol. Hum. Dis. A Dyn. Encycl. Dis. Mech. 2014, 2014, 300–314. [Google Scholar] [CrossRef]
- Majumder, K.; Mine, Y.; Wu, J. The potential of food protein-derived anti-inflammatory peptides against various chronic inflammatory diseases. J. Sci. Food Agric. 2015, 96, 2303–2311. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Jahandideh, F.; Wu, J. Food-Derived Bioactive Peptides on Inflammation and Oxidative Stress. BioMed Res. Int. 2014, 2014, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Rizzello, C.G.; Tagliazucchi, D.; Babini, E.; Rutella, G.S.; Saa, D.L.T.; Gianotti, A. Bioactive peptides from vegetable food matrices: Research trends and novel biotechnologies for synthesis and recovery. J. Funct. Foods 2016, 27, 549–569. [Google Scholar] [CrossRef]
- Sánchez, A.; Vázquez, A. Bioactive peptides: A review. Food Qual. Saf. 2017, 1, 29–46. [Google Scholar] [CrossRef]
- Guha, S.; Alvarez, S.; Majumder, K. Transport of Dietary Anti-Inflammatory Peptide, γ-Glutamyl Valine (γ-EV), across the Intestinal Caco-2 Monolayer. Nutrients 2021, 13, 1448. [Google Scholar] [CrossRef] [PubMed]
- Görgüç, A.; Gençdağ, E.; Yılmaz, F.M. Bioactive peptides derived from plant origin by-products: Biological activities and techno-functional utilizations in food developments A review. Food Res. Int. 2020, 136, 109504. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, S.; Guha, S.; Majumder, K. Food-Derived Bioactive Peptides in Human Health: Challenges and Opportunities. Nutrients 2018, 10, 1738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallego, M.; Grootaert, C.; Mora, L.; Aristoy, M.C.; Van Camp, J.; Toldrá, F. Transepithelial transport of dry-cured ham peptides with ACE inhibitory activity through a Caco-2 cell monolayer. J. Funct. Foods 2016, 21, 388–395. [Google Scholar] [CrossRef]
- Lammi, C.; Aiello, G.; Vistoli, G.; Zanoni, C.; Arnoldi, A.; Sambuy, Y.; Ferruzza, S.; Ranaldi, G. A multidisciplinary investigation on the bioavailability and activity of peptides from lupin protein. J. Funct. Foods 2016, 24, 297–306. [Google Scholar] [CrossRef]
- Xu, Q.; Hong, H.; Wu, J.; Yan, X. Bioavailability of bioactive peptides derived from food proteins across the intestinal epithelial membrane: A review. Trends Food Sci. Technol. 2019, 86, 399–411. [Google Scholar] [CrossRef]
- Millán-Linares, M.D.C.; Yust, M.D.M.; Hidalgo, J.M.A.; Millán, F.; Pedroche, J. Lupine protein hydrolysates inhibit enzymes involved in the inflammatory pathway. Food Chem. 2014, 151, 141–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Millán-Linares, M.D.C.; Bermúdez, B.; Yust, M.D.M.; Millán, F.; Pedroche, J. Anti-inflammatory activity of lupine (Lupinus angustifolius L.) protein hydrolysates in THP-1-derived macrophages. J. Funct. Foods 2014, 8, 224–233. [Google Scholar] [CrossRef] [Green Version]
- Millán-Linares, M.D.C.; Lemus-Conejo, A.; Yust, M.M.; Pedroche, J.; Vico, A.C.; Millan, F.; La Paz, S.M.-D. GPETAFLR, a novel bioactive peptide from Lupinus angustifolius L. protein hydrolysate, reduces osteoclastogenesis. J. Funct. Foods 2018, 47, 299–303. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.C.; Prosky, L.; De Vries, J.W. Determination of Total, Soluble, and Insoluble Dietary Fiber in Foods—Enzymatic-Gravimetric Method, MES-TRIS Buffer: Collaborative Study. J. AOAC Int. 1992, 75, 395–416. [Google Scholar] [CrossRef]
- Thiex, N. Evaluation of Analytical Methods for the Determination of Moisture, Crude Protein, Crude Fat, and Crude Fiber in Distillers Dried Grains with Solubles. J. AOAC Int. 2009, 92, 61–73. [Google Scholar] [CrossRef] [Green Version]
- Moores, R.G.; Mcdermott, D.L.; Wood, T.R. Determination of Chlorogenic Acid in Coffee millimicrons of a mixture of phenolic compounds, including chlorogenic acid, which they found in sweet potatoes. Purification and properties of chlorogenic ACID. Anal. Chem. 1948, 28, 620–624. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Alaiz, M.; Navarro, J.L.; Giron-Calle, J.; Vioque, E. Amino acid analysis by high-performance liquid chromatography after derivatization with diethyl ethoxymethylenemalonate. J. Chromatogr. A 1992, 591, 181–186. [Google Scholar] [CrossRef]
- Yust, M.D.M.; Pedroche, J.; Giron-Calle, J.; Vioque, J.; Millán, F.; Alaiz, M. Determination of tryptophan by high-performance liquid chromatography of alkaline hydrolysates with spectrophotometric detection. Food Chem. 2004, 85, 317–320. [Google Scholar] [CrossRef]
- Castellano, J.M.; Garcia-Rodriguez, S.; Espinosa, J.M.; Millan-Linares, M.C.; Rada, M.; Perona, J.S. Oleanolic Acid Exerts a Neuroprotective Effect Against Microglial Cell Activation by Modulating Cytokine Release and Antioxidant Defense Systems. Biomolecules 2019, 9, 683. [Google Scholar] [CrossRef] [Green Version]
- Lqari, H.; Vioque, J.; Pedroche, J.; Millán, F. Lupinus angustifolius protein isolates: Chemical composition, functional properties and protein characterization. Food Chem. 2002, 76, 349–356. [Google Scholar] [CrossRef]
- Berghout, J.; Venema, P.; Boom, R.; van der Goot, A.J. Comparing functional properties of concentrated protein isolates with freeze-dried protein isolates from lupin seeds. Food Hydrocoll. 2015, 51, 346–354. [Google Scholar] [CrossRef]
- Yust, M.D.M.; Pedroche, J.; Millán-Linares, M.D.C.; Hidalgo, J.M.A.; Millán, F. Improvement of functional properties of chickpea proteins by hydrolysis with immobilised Alcalase. Food Chem. 2010, 122, 1212–1217. [Google Scholar] [CrossRef]
- Muranyi, I.S.; Volke, D.; Hoffmann, R.; Eisner, P.; Herfellner, T.; Brunnbauer, M.; Koehler, P.; Schweiggert-Weisz, U. Protein distribution in lupin protein isolates from Lupinus angustifolius L. prepared by various isolation techniques. Food Chem. 2016, 207, 6–15. [Google Scholar] [CrossRef]
- Khalid, I.I.; Elhardallou, S.B.; Gubouri, A.A. Amino Acid Composition and Physicochemical Properties of Bitter Lupine (Lupinustermis) Seed Flour. Orient. J. Chem. 2016, 32, 3175–3182. [Google Scholar] [CrossRef] [Green Version]
- FAO. Dietary Protein Quality Evaluation in Human Nutrition Report of an FAO Expert Consultation; FAO Food and Nutrition Paper, 92; FAO: Rome, Italy, 2013. [Google Scholar]
- Artursson, P.; Palm, K.; Luthman, K. Caco-2 monolayers in experimental and theoretical predictions of drug transport. Adv. Drug Deliv. Rev. 2012, 64, 280–289. [Google Scholar] [CrossRef]
- Shi, Y.; Kovacs-Nolan, J.; Jiang, B.; Tsao, R.; Mine, Y. Antioxidant activity of enzymatic hydrolysates from eggshell membrane proteins and its protective capacity in human intestinal epithelial Caco-2 cells. J. Funct. Foods 2014, 10, 35–45. [Google Scholar] [CrossRef]
- Kuerban, A.; Al-Malki, A.L.; Kumosani, T.A.; Sheikh, R.A.; Al-Abbasi, F.A.M.; Alshubaily, F.A.; Abulnaja, K.O.; Moselhy, S.S. Identification, protein antiglycation, antioxidant, antiproliferative, and molecular docking of novel bioactive peptides produced from hydrolysis of Lens culinaris. J. Food Biochem. 2020, 44, e13494. [Google Scholar] [CrossRef] [PubMed]
- Tonolo, F.; Moretto, L.; Ferro, S.; Folda, A.; Scalcon, V.; Sandre, M.; Fiorese, F.; Marin, O.; Bindoli, A.; Rigobello, M.P. Insight into antioxidant properties of milk-derived bioactive peptides in vitro and in a cellular model. J. Pept. Sci. 2019, 25, e3162. [Google Scholar] [CrossRef]
- Tonolo, F.; Sandre, M.; Ferro, S.; Folda, A.; Scalcon, V.; Scutari, G.; Feller, E.; Marin, O.; Bindoli, A.; Rigobello, M.P. Milk-derived bioactive peptides protect against oxidative stress in a Caco-2 cell model. Food Funct. 2018, 9, 1245–1253. [Google Scholar] [CrossRef]
- Admassu, H.; Gasmalla, M.A.A.; Yang, R.; Zhao, W. Bioactive Peptides Derived from Seaweed Protein and Their Health Benefits: Antihypertensive, Antioxidant, and Antidiabetic Properties. J. Food Sci. 2017, 83, 6–16. [Google Scholar] [CrossRef] [Green Version]
- La Paz, S.M.-D.; Rodriguez-Martin, N.M.; Villanueva, A.; Pedroche, J.; Cruz-Chamorro, I.; Millan, F.; Millan-Linares, M.C. Evaluation of Anti-Inflammatory and Atheroprotective Properties of Wheat Gluten Protein Hydrolysates in Primary Human Monocytes. Foods 2020, 9, 854. [Google Scholar] [CrossRef]
- Rodriguez-Martin, N.M.; La Paz, S.M.-D.; Toscano, R.; Grao-Cruces, E.; Villanueva, A.; Pedroche, J.; Millan, F.; Millan-Linares, M.C. Hemp (Cannabis sativa L.) Protein Hydrolysates Promote Anti-Inflammatory Response in Primary Human Monocytes. Biomolecules 2020, 10, 803. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.D.; Nedjai, B.; Hurst, T.; Pennington, D.J. Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochim. Biophys. Acta Bioenerg. 2014, 1843, 2563–2582. [Google Scholar] [CrossRef] [Green Version]
- Zanoni, C.; Aiello, G.; Arnoldi, A.; Lammi, C. Investigations on the hypocholesterolaemic activity of LILPKHSDAD and LTFPGSAED, two peptides from lupin β-conglutin: Focus on LDLR and PCSK9 pathways. J. Funct. Foods 2017, 32, 1–8. [Google Scholar] [CrossRef]
- Millán-Linares, M.D.C.; Millán, F.; Pedroche, J.; Yust, M.D.M. GPETAFLR: A new anti-inflammatory peptide from Lupinus angustifolius L. protein hydrolysate. J. Funct. Foods 2015, 18, 358–367. [Google Scholar] [CrossRef] [Green Version]
| (%) | LPI | LPH |
|---|---|---|
| Protein | 86.72 ± 0.13 | 83.70 ± 0.09 |
| Ash | 0.78 ± 0.13 | 8.98 ± 0.09 |
| Fibre | 5.97 ± 0.34 | 0.97 ± 0.02 |
| Oil | 5.14 ± 0.17 | 1.15 ± 0.01 |
| Soluble sugars | 0.04 ± 0.00 | 0.02 ± 0.00 |
| Polyphenols | 0.01 ± 0.00 | 0.06 ± 0.00 |
| Others 1 | 1.28 | 5.12 |
| LPI | LPH | FAO/WHO [38] | |
|---|---|---|---|
| Asp+ Asn | 10.68 ± 0.27 | 10.27 ± 0.12 | |
| Glu+Gln | 23.06 ± 0.40 | 24.47 ± 0.17 | |
| Ser | 5.96 ± 0.05 | 5.97 ± 0.16 | |
| His | 2.39 ± 0.15 | 2.36 ± 0.01 | 1.5 |
| Gly | 4.47 ± 0.17 | 4.52 ± 0.06 | |
| Thr | 3.88 ± 0.19 | 4.05 ± 0.02 | 2.3 |
| Arg | 11.78 ± 0.02 | 11.60 ± 0.06 | |
| Ala | 3.80 ± 0.04 | 3.89 ± 0.07 | |
| Pro | 0.75 ± 0.01 | 0.75 ± 0.01 | |
| Tyr | 4.27 ± 0.39 | 4.42 ± 0.12 | |
| Val | 3.98 ± 0.47 | 3.47 ± 0.07 | 3.9 |
| Met | 0.37 ± 0.00 | 0.44 ± 0.15 | 2.2 1 |
| Cys | 0.78 ± 0.13 | 0.56 ± 0.19 | |
| Ile | 4.83 ± 0.04 | 4.45 ± 0.01 | 3.0 |
| Leu | 8.71 ± 0.03 | 8.55 ± 0.05 | 5.9 |
| Phe | 5.06 ± 0.01 | 4.95 ± 0.01 | 3.8 2 |
| Lys | 4.87 ± 0.00 | 4.92 ± 0.02 | 4.5 |
| Trp | 0.38 ± 0.03 | 0.35 ± 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montserrat-de la Paz, S.; Villanueva, A.; Pedroche, J.; Millan, F.; Martin, M.E.; Millan-Linares, M.C. Antioxidant and Anti-Inflammatory Properties of Bioavailable Protein Hydrolysates from Lupin-Derived Agri-Waste. Biomolecules 2021, 11, 1458. https://doi.org/10.3390/biom11101458
Montserrat-de la Paz S, Villanueva A, Pedroche J, Millan F, Martin ME, Millan-Linares MC. Antioxidant and Anti-Inflammatory Properties of Bioavailable Protein Hydrolysates from Lupin-Derived Agri-Waste. Biomolecules. 2021; 11(10):1458. https://doi.org/10.3390/biom11101458
Chicago/Turabian StyleMontserrat-de la Paz, Sergio, Alvaro Villanueva, Justo Pedroche, Francisco Millan, Maria E. Martin, and Maria C. Millan-Linares. 2021. "Antioxidant and Anti-Inflammatory Properties of Bioavailable Protein Hydrolysates from Lupin-Derived Agri-Waste" Biomolecules 11, no. 10: 1458. https://doi.org/10.3390/biom11101458
APA StyleMontserrat-de la Paz, S., Villanueva, A., Pedroche, J., Millan, F., Martin, M. E., & Millan-Linares, M. C. (2021). Antioxidant and Anti-Inflammatory Properties of Bioavailable Protein Hydrolysates from Lupin-Derived Agri-Waste. Biomolecules, 11(10), 1458. https://doi.org/10.3390/biom11101458








