The Histamine H4 Receptor Participates in the Anti-Neuropathic Effect of the Adenosine A3 Receptor Agonist IB-MECA: Role of CD4+ T Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Chronic Constriction Injury (CCI)-Induced Neuropathic Pain
2.3. Administration of Compounds
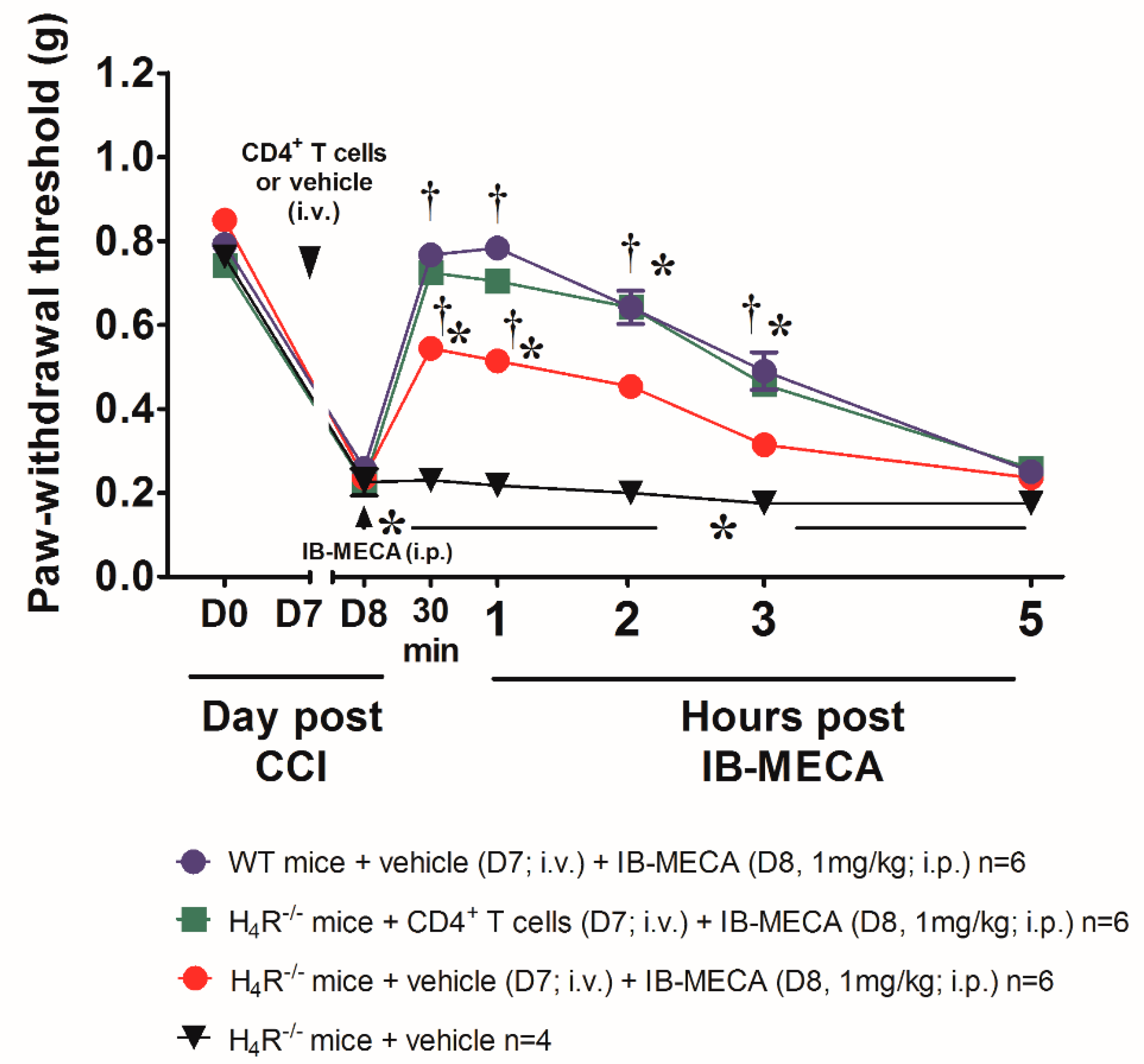
2.4. T Cells Isolation and Adoptive Transfer
2.5. Von Frey Test
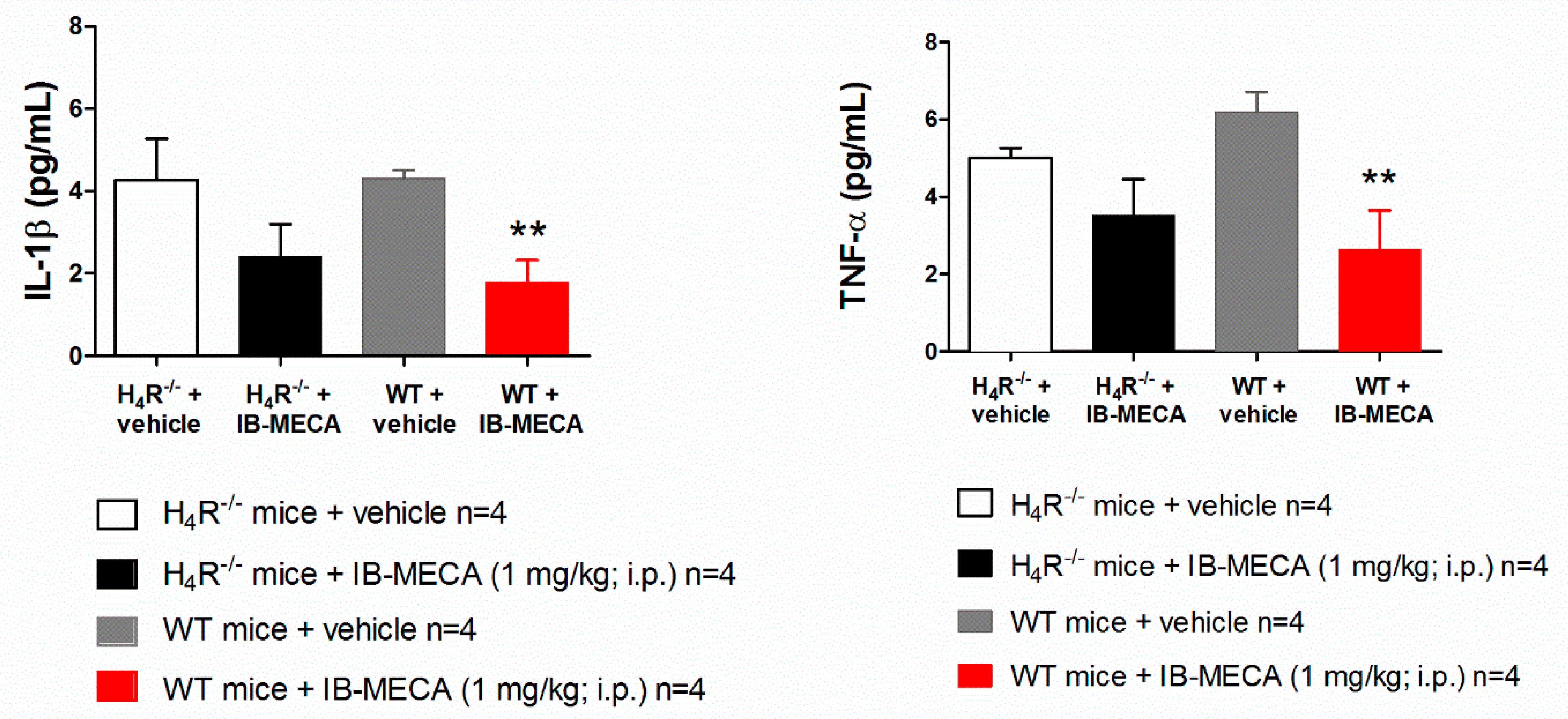
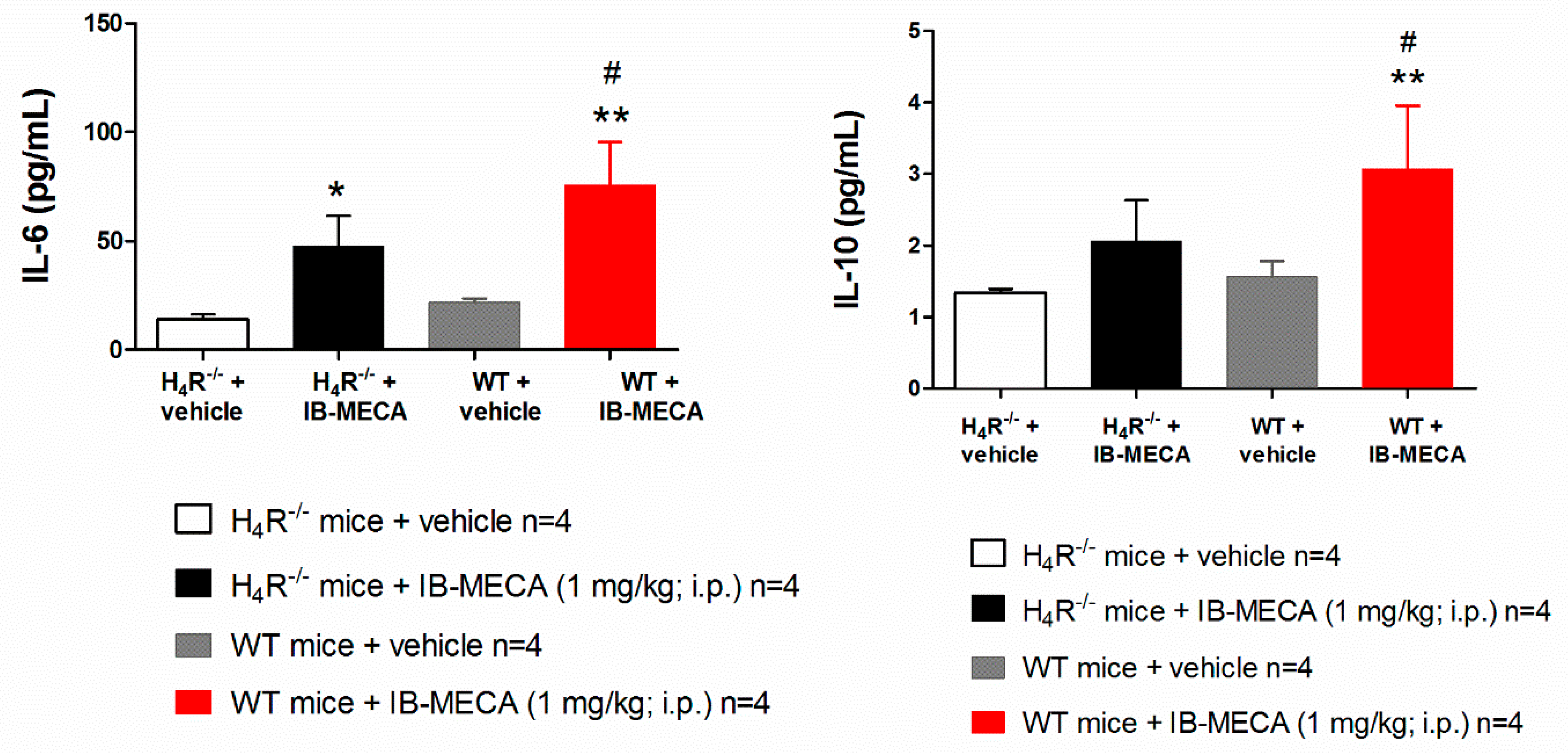
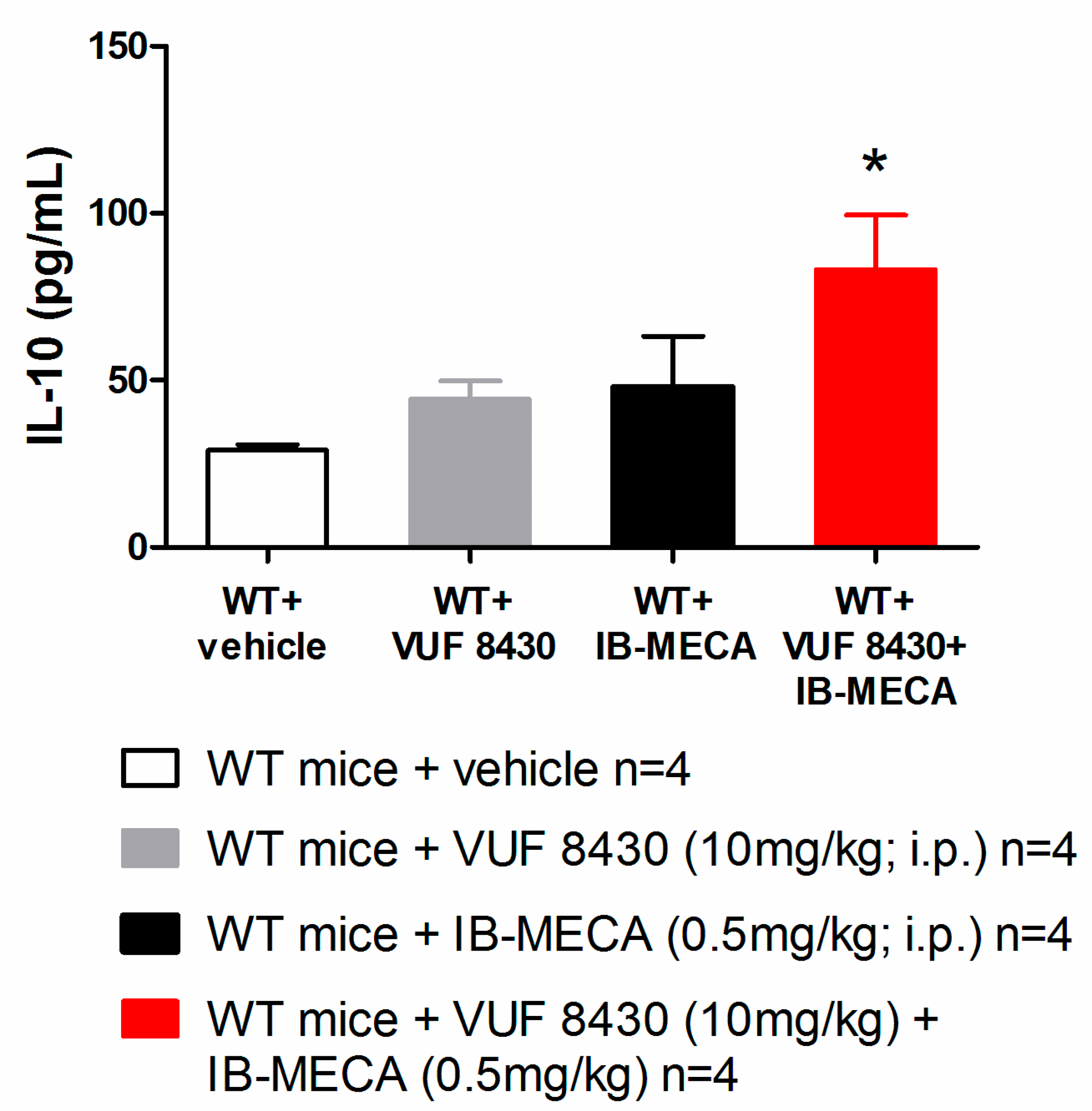
2.6. Cytokine Measurements
2.7. Statistical Analysis
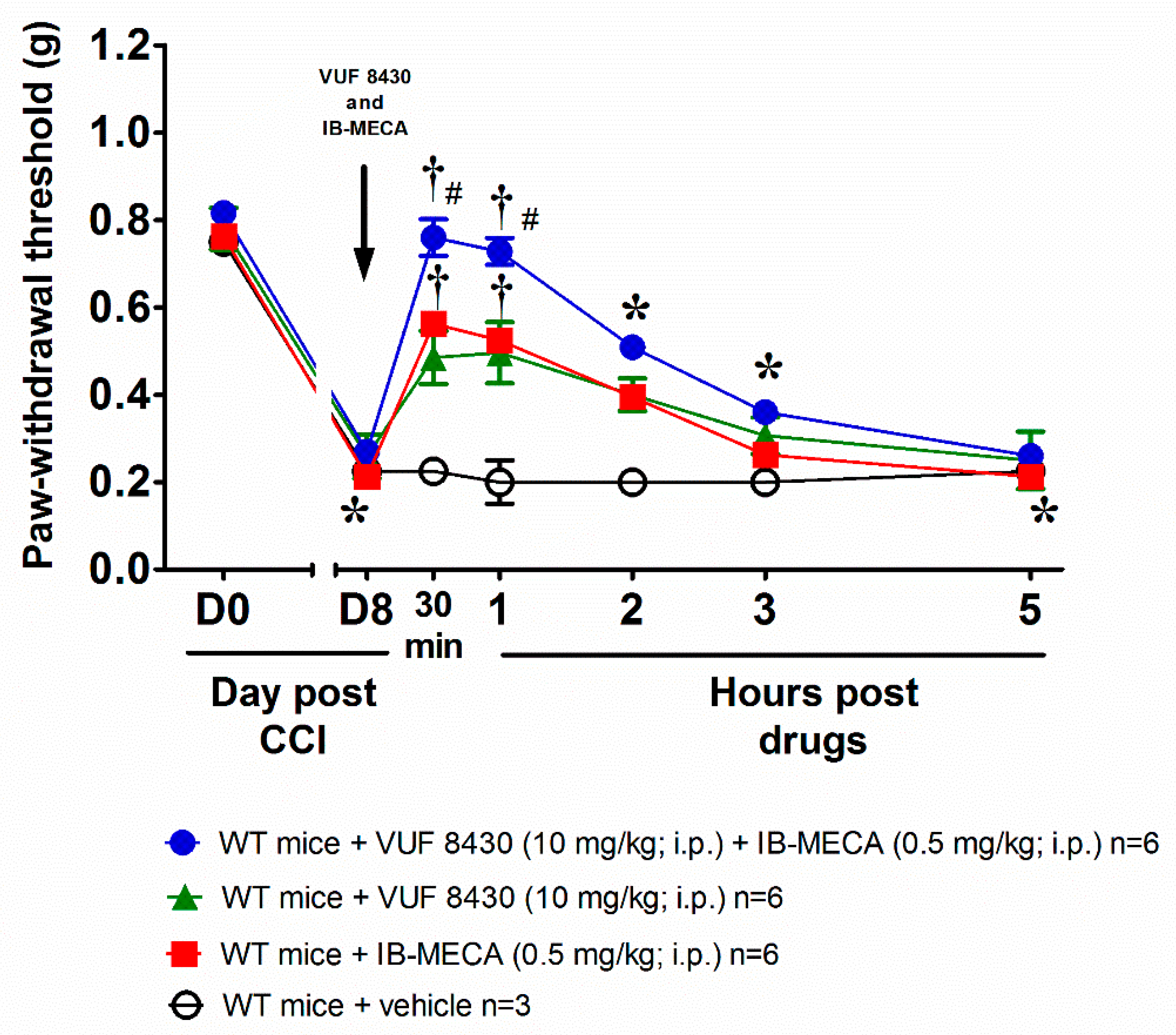
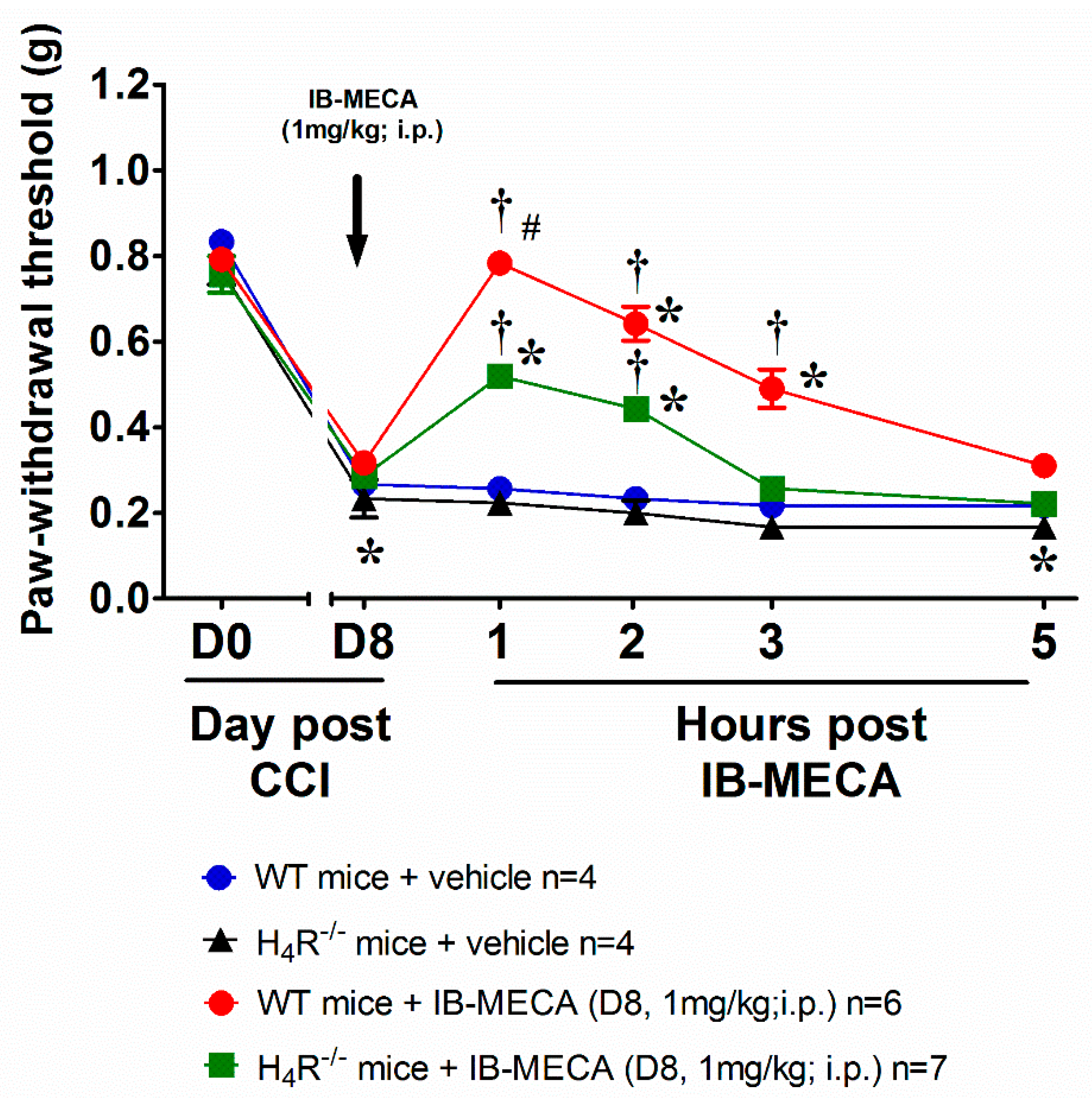
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- van Hecke, O.; Austin, S.K.; Khan, R.A.; Smith, B.H.; Torrance, N. Neuropathic pain in the general population: A systematic review of epidemiological studies. Pain 2014, 155, 654–662. [Google Scholar] [CrossRef]
- Gilron, I.; Jensen, T.S.; Dickenson, A.H. Combination pharmacotherapy for management of chronic pain: From bench to bedside. Lancet Neurol. 2013, 12, 1084–1095. [Google Scholar] [CrossRef]
- Gierthmühlen, J.; Baron, R. Neuropathic Pain. Semin. Neurol. 2016, 36, 462–468. [Google Scholar] [CrossRef] [Green Version]
- Finnerup, N.B.; Attal, N.; Haroutounian, S.; McNicol, E.; Baron, R.; Dworkin, R.H.; Gilron, I.; Haanpää, M.; Hansson, P.; Jensen, T.S.; et al. Pharmacotherapy for neuropathic pain in adults: A systematic review and meta-analysis. Lancet Neurol. 2015, 14, 162–173. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Janes, K.; Chen, C.; Doyle, T.; Bryant, L.; Tosh, D.K.; Jacobson, K.A.; Salvemini, D. Controlling murine and rat chronic pain through A3 adenosine receptor activation. FASEB J. 2012, 26, 1855–1865. [Google Scholar] [CrossRef] [Green Version]
- Janes, K.; Little, J.W.; Li, C.; Bryant, L.; Chen, C.; Chen, Z.; Kamocki, K.; Doyle, T.; Snider, A.; Esposito, E.; et al. The Development and Maintenance of Paclitaxel-induced Neuropathic Pain Require Activation of the Sphingosine 1-Phosphate Receptor Subtype 1. J. Biol. Chem. 2014, 289, 21082–21097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durante, M.; Squillace, S.; Lauro, F.; Giancotti, L.A.; Coppi, E.; Cherchi, F.; Di Cesare Mannelli, L.; Ghelardini, C.; Kolar, G.; Wahlman, C.; et al. Adenosine A3 agonists reverse neuropathic pain via T cell—Mediated production of IL-10. J. Clin. Investig. 2021, 131, e139299. [Google Scholar] [CrossRef] [PubMed]
- Ru, F.; Surdenikova, L.; Brozmanova, M.; Kollarik, M. Adenosine-induced activation of esophageal nociceptors. Am. J. Physiol. Liver Physiol. 2011, 300, G485–G493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopes, L.V.; Rebola, N.; Pinheiro, P.C.; Richardson, P.J.; Oliveira, C.R.; Cunha, R.A. Adenosine A3 receptors are located in neurons of the rat hippocampus. Neuroreport 2003, 14, 1645–1648. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.T.; Hendrix, S.; Boato, F.; Smirnov, I.; Zheng, J.; Lukens, J.R.; Gadani, S.; Hechler, D.; Gölz, G.; Rosenberger, K.; et al. MHCII-independent CD4+ T cells protect injured CNS neurons via IL-4. J. Clin. Investig. 2015, 125, 699–714. [Google Scholar] [CrossRef] [PubMed]
- Hoskin, D.W.; Butler, J.J.; Drapeau, D.; Haeryfar, S.M.M.; Blay, J. Adenosine acts through an A3 receptor to prevent the induction of murine anti-CD3-activated killer T cells. Int. J. Cancer 2002, 99, 386–395. [Google Scholar] [CrossRef]
- Sanna, M.D.; Stark, H.; Lucarini, L.; Ghelardini, C.; Masini, E.; Galeotti, N. Histamine H4 receptor activation alleviates neuropathic pain through differential regulation of ERK, JNK, and P38 MAPK phosphorylation. Pain 2015, 156, 2492–2504. [Google Scholar] [CrossRef]
- Sanna, M.D.; Lucarini, L.; Durante, M.; Ghelardini, C.; Masini, E.; Galeotti, N. Histamine H4 receptor agonist-induced relief from painful peripheral neuropathy is mediated by inhibition of spinal neuroinflammation and oxidative stress: Neuronal H4 receptors in neuropathic pain. Br. J. Pharmacol. 2017, 174, 28–40. [Google Scholar] [CrossRef] [Green Version]
- Connelly, W.; Shenton, F.; Lethbridge, N.; Leurs, R.; Waldvogel, H.; Faull, R.; Lees, G.; Chazot, P. The histamine H4 receptor is functionally expressed on neurons in the mammalian CNS: Histamine H4 receptor functionally expressed on neurons in the mammalian CNS. Br. J. Pharmacol. 2009, 157, 55–63. [Google Scholar] [CrossRef] [Green Version]
- Kajihara, Y.; Murakami, M.; Imagawa, T.; Otsuguro, K.; Ito, S.; Ohta, T. Histamine potentiates acid-induced responses mediating transient receptor potential V1 in mouse primary sensory neurons. Neuroscience 2010, 166, 292–304. [Google Scholar] [CrossRef]
- Sanna, M.D.; Ghelardini, C.; Thurmond, R.L.; Masini, E.; Galeotti, N. Behavioural phenotype of histamine H4 receptor knockout mice: Focus on central neuronal functions. Neuropharmacology 2017, 114, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Gutzmer, R.; Mommert, S.; Gschwandtner, M.; Zwingmann, K.; Stark, H.; Werfel, T. The histamine H4 receptor is functionally expressed on TH2 cells. J. Allergy Clin. Immunol. 2009, 123, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Dunford, P.J.; O’Donnell, N.; Riley, J.P.; Williams, K.N.; Karlsson, L.; Thurmond, R.L. The Histamine H4 Receptor Mediates Allergic Airway Inflammation by Regulating the Activation of CD4+ TCells. J. Immunol. 2006, 176, 7062–7070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgan, R.K.; McAllister, B.; Cross, L.; Green, D.S.; Kornfeld, H.; Center, D.M.; Cruikshank, W.W. Histamine 4 Receptor Activation Induces Recruitment of FoxP3 T Cells and Inhibits Allergic Asthma in a Murine Model. J. Immunol. 2007, 178, 8081–8089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGrath, J.C.; Lilley, E. Implementing guidelines on reporting research using animals (ARRIVE etc.): New requirements for publication in BJP. Br. J. Pharmacol. 2015, 172, 3189–3193. [Google Scholar] [CrossRef] [Green Version]
- Bennett, G.J.; Xie, Y.-K. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 1988, 33, 87–107. [Google Scholar] [CrossRef]
- Sakurai, M.; Egashira, N.; Kawashiri, T.; Yano, T.; Ikesue, H.; Oishi, R. Oxaliplatin-induced neuropathy in the rat: Involvement of oxalate in cold hyperalgesia but not mechanical allodynia. Pain 2009, 147, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Mannelli, L.D.C.; Micheli, L.; Maresca, M.; Cravotto, G.; Bellumori, M.; Innocenti, M.; Mulinacci, N.; Ghelardini, C. Anti-neuropathic effects of Rosmarinus officinalis L. terpenoid fraction: Relevance of nicotinic receptors. Sci. Rep. 2016, 6, 34832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ford, A.; Castonguay, A.; Cottet, M.; Little, J.W.; Chen, Z.; Symons-Liguori, A.M.; Doyle, T.; Egan, T.M.; Vanderah, T.W.; De Koninck, Y.; et al. Engagement of the GABA to KCC2 Signaling Pathway Contributes to the Analgesic Effects of A3AR Agonists in Neuropathic Pain. J. Neurosci. 2015, 35, 6057–6067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haskó, G.; Szabó, C.; Németh, Z.H.; Kvetan, V.; Pastores, S.M.; Vizi, E.S. Adenosine receptor agonists differentially regulate IL-10, TNF-alpha, and nitric oxide production in RAW 264.7 macrophages and in endotoxemic mice. J. Immunol. 1996, 157, 4634–4640. [Google Scholar]
- Asadullah, K.; Sterry, W.; Volk, H.D. Interleukin-10 Therapy—Review of a New Approach. Pharmacol. Rev. 2003, 55, 241–269. [Google Scholar] [CrossRef]
- Jankovic, D.; Kugler, D.G.; Sher, A. IL-10 production by CD4+ effector T cells: A mechanism for self-regulation. Mucosal Immunol. 2010, 3, 239–246. [Google Scholar] [CrossRef]
- Watkins, L.R.; Hutchinson, M.R.; Ledeboer, A.; Wieseler-Frank, J.; Milligan, E.D.; Maier, S.F. Glia as the “bad guys”: Implications for improving clinical pain control and the clinical utility of opioids. Brain. Behav. Immun. 2007, 21, 131–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiorentino, D.F.; Bond, M.W.; Mosmann, T.R. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J. Exp. Med. 1989, 170, 2081–2095. [Google Scholar] [CrossRef]
- Wagner, R.; Janjigian, M.; Myers, R.R. Anti-inflammatory interleukin-10 therapy in CCI neuropathy decreases thermal hyperalgesia, macrophage recruitment, and endoneurial TNF-α expression. Pain 1998, 74, 35–42. [Google Scholar] [CrossRef]
- Johnston, I.N.; Milligan, E.D.; Wieseler-Frank, J.; Frank, M.G.; Zapata, V.; Campisi, J.; Langer, S.; Martin, D.; Green, P.; Fleshner, M.; et al. A role for proinflammatory cytokines and fractalkine in analgesia, tolerance, and subsequent pain facilitation induced by chronic intrathecal morphine. J. Neurosci. 2004, 24, 7353–7365. [Google Scholar] [CrossRef] [Green Version]
- Parimi, M.; Buelter, J.; Thanugundla, V.; Condoor, S.; Parkar, N.; Danon, S.; King, W. Feasibility and Validity of Printing 3D Heart Models from Rotational Angiography. Pediatr. Cardiol. 2018, 39, 653–658. [Google Scholar] [CrossRef]
- White, S.C.; Sedler, J.; Jones, T.W.; Seckeler, M. Utility of three-dimensional models in resident education on simple and complex intracardiac congenital heart defects: 3D Printing in Pediatric Resident Education. Congenit. Heart Dis. 2018, 13, 1045–1049. [Google Scholar] [CrossRef] [PubMed]
- Andres-Hernando, A.; Okamura, K.; Bhargava, R.; Kiekhaefer, C.M.; Soranno, D.; Kirkbride-Romeo, L.A.; Gil, H.; Altmann, C.; Faubel, S. Circulating IL-6 upregulates IL-10 production in splenic CD4+ T cells and limits acute kidney injury–induced lung inflammation. Kidney Int. 2017, 91, 1057–1069. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Micheli, L.; Durante, M.; Lucarini, E.; Sgambellone, S.; Lucarini, L.; Di Cesare Mannelli, L.; Ghelardini, C.; Masini, E. The Histamine H4 Receptor Participates in the Anti-Neuropathic Effect of the Adenosine A3 Receptor Agonist IB-MECA: Role of CD4+ T Cells. Biomolecules 2021, 11, 1447. https://doi.org/10.3390/biom11101447
Micheli L, Durante M, Lucarini E, Sgambellone S, Lucarini L, Di Cesare Mannelli L, Ghelardini C, Masini E. The Histamine H4 Receptor Participates in the Anti-Neuropathic Effect of the Adenosine A3 Receptor Agonist IB-MECA: Role of CD4+ T Cells. Biomolecules. 2021; 11(10):1447. https://doi.org/10.3390/biom11101447
Chicago/Turabian StyleMicheli, Laura, Mariaconcetta Durante, Elena Lucarini, Silvia Sgambellone, Laura Lucarini, Lorenzo Di Cesare Mannelli, Carla Ghelardini, and Emanuela Masini. 2021. "The Histamine H4 Receptor Participates in the Anti-Neuropathic Effect of the Adenosine A3 Receptor Agonist IB-MECA: Role of CD4+ T Cells" Biomolecules 11, no. 10: 1447. https://doi.org/10.3390/biom11101447
APA StyleMicheli, L., Durante, M., Lucarini, E., Sgambellone, S., Lucarini, L., Di Cesare Mannelli, L., Ghelardini, C., & Masini, E. (2021). The Histamine H4 Receptor Participates in the Anti-Neuropathic Effect of the Adenosine A3 Receptor Agonist IB-MECA: Role of CD4+ T Cells. Biomolecules, 11(10), 1447. https://doi.org/10.3390/biom11101447






