Interactions of HMGB Proteins with the Genome and the Impact on Disease
Abstract
1. Introduction
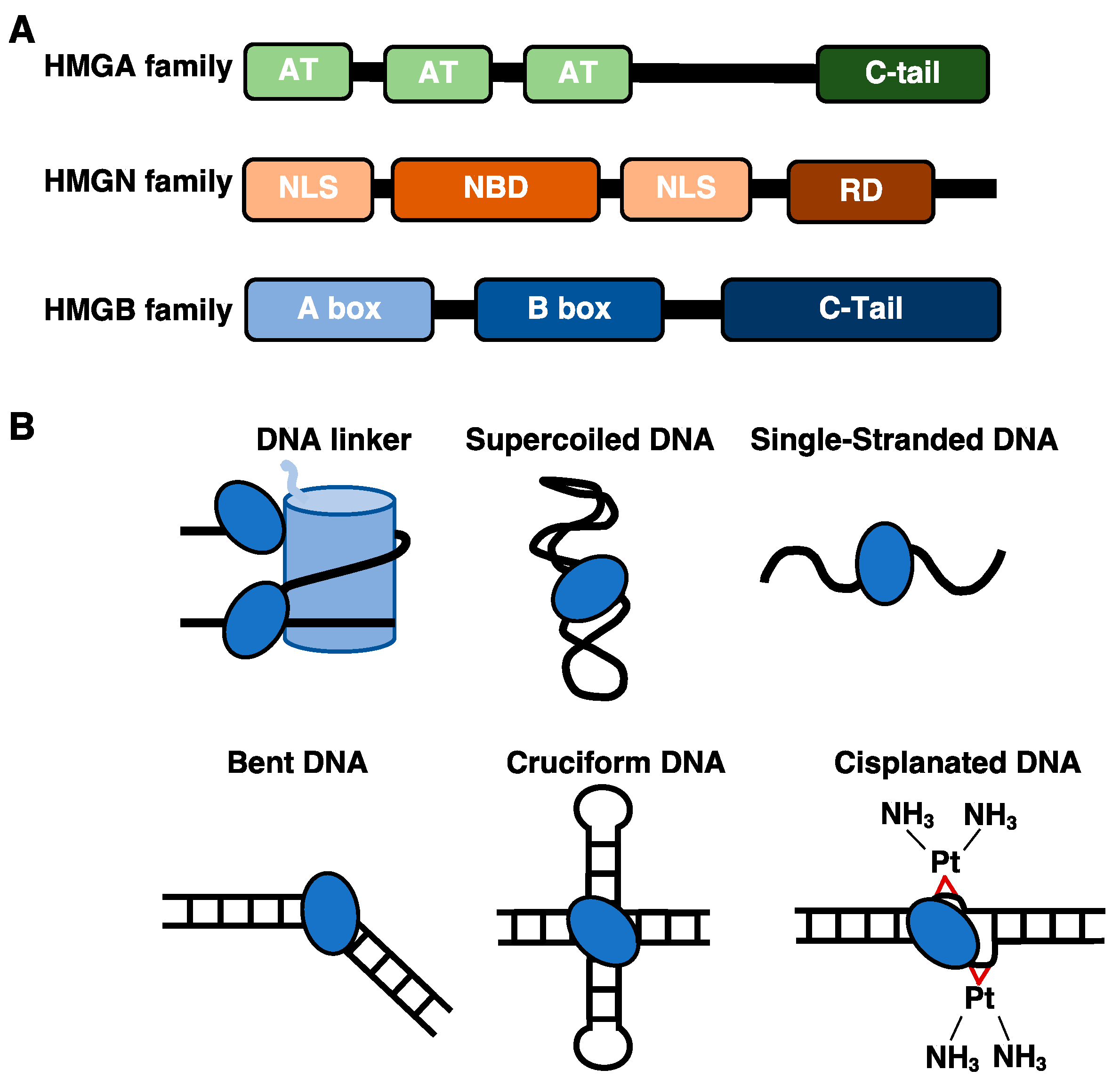
1.1. The DNA and Nucleosome Binding Properties of HMGA and HMGN Proteins
1.2. The HMGB Proteins Bind Diverse DNA Structures and Interact with Histone Proteins
2. HMGB Proteins Regulate Genome Organization
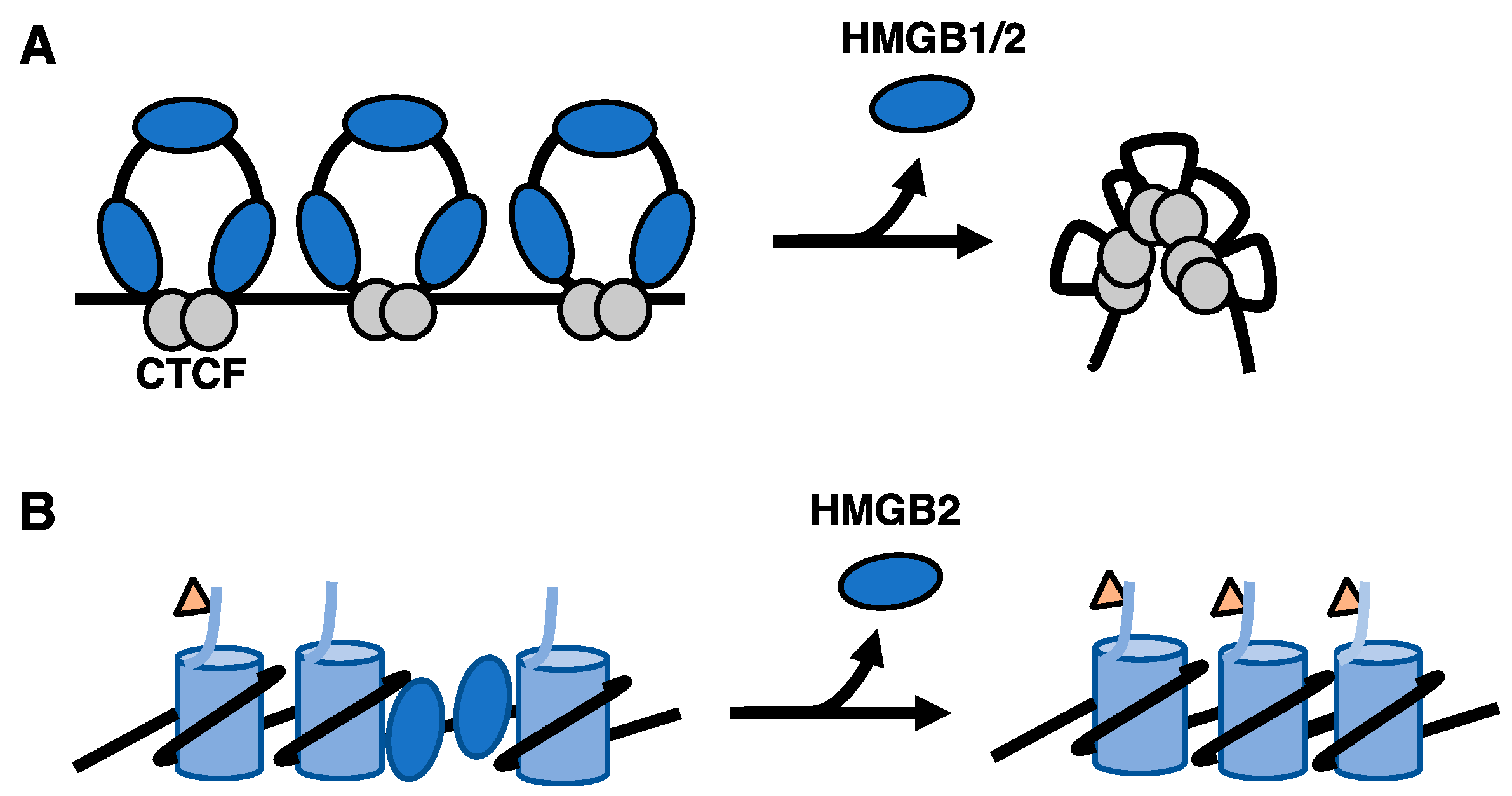
2.1. Regulation of Chromatin Organization and Structure during Senescence
2.2. Regulation of the Cardiac Genome by HMGB2
2.3. Regulation of Genome Organization in a Human Malaria Parasite
3. HMGB Proteins Bind G-Quadruplexes with Potential Effects on Cancer
3.1. The HMGB1 Protein Binds Telomeric G-Quadruplex DNA and Affects the Activity of Telomerase
3.2. HMGB1 Binds a G-Quadruplex in the Promoter of the KRAS Oncogene and Regulates Its Transcription
4. A New Regulatory Role for HMGB Proteins as RNA Binding Factors
4.1. HMGB1 Coordinates RNA Metabolism during Senescence Entry
4.2. HMGB1 Interacts with Long Noncoding RNAs to Control Disease States
5. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goodwin, G.H.; Sanders, C.; Johns, E.W. A New Group of Chromatin-Associated Proteins with a High Content of Acidic and Basic Amino Acids. Eur. J. Biochem. 1973, 38, 14–19. [Google Scholar] [CrossRef]
- Reeves, R. Nuclear Functions of the HMG Proteins. Biochim. Biophys. Acta BBA-Gene Regul. Mech. 2010, 1799, 3–14. [Google Scholar] [CrossRef]
- Hock, R.; Furusawa, T.; Ueda, T.; Bustin, M. HMG Chromosomal Proteins in Development and Disease. Trends Cell Biol. 2007, 17, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Vignali, R.; Marracci, S. HMGA Genes and Proteins in Development and Evolution. Int. J. Mol. Sci. 2020, 21, 654. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.N.S.; Zajicek, J.; Nissen, M.S.; Munske, G.; Smith, V.; Reeves, R. 1H and 13C NMR Assignments and Molecular Modelling of a Minor Groove DNA-Binding Peptide from the HMG-I Protein. Int. J. Pept. Protein Res. 1995, 45, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, N.; Singh, I.; Mehta, A.; Braun, T.; Barreto, G. HMGA Proteins as Modulators of Chromatin Structure during Transcriptional Activation. Front. Cell Dev. Biol. 2014, 2, 5. [Google Scholar] [CrossRef]
- Reeves, R.; Nissen, M.S. The AT-DNA-Binding Domain of Mammalian High Mobility Group I Chromosomal Proteins. A Novel Peptide Motif for Recognizing DNA Structure. J. Biol. Chem. 1990, 265, 8573–8582. [Google Scholar] [CrossRef]
- Winter, N.; Nimzyk, R.; Bösche, C.; Meyer, A.; Bullerdiek, J. Chromatin Immunoprecipitation to Analyze DNA Binding Sites of HMGA2. PLoS ONE 2011, 6, e18837. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Catez, F.; Yang, H.; Tracey, K.J.; Reeves, R.; Misteli, T.; Bustin, M. Network of Dynamic Interactions between Histone H1 and High-Mobility-Group Proteins in Chromatin. Mol. Cell. Biol. 2004, 24, 4321–4328. [Google Scholar] [CrossRef] [PubMed]
- Kishi, Y.; Fujii, Y.; Hirabayashi, Y.; Gotoh, Y. HMGA Regulates the Global Chromatin State and Neurogenic Potential in Neocortical Precursor Cells. Nat. Neurosci. 2012, 15, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; van Ingen, H.; Zhou, B.-R.; Feng, H.; Bustin, M.; Kay, L.E.; Bai, Y. Architecture of the High Mobility Group Nucleosomal Protein 2-Nucleosome Complex as Revealed by Methyl-Based NMR. Proc. Natl. Acad. Sci. USA 2011, 108, 12283–12288. [Google Scholar] [CrossRef] [PubMed]
- Mallik, R.; Kundu, A.; Chaudhuri, S. High Mobility Group Proteins: The Multifaceted Regulators of Chromatin Dynamics. Nucleus 2018, 61, 213–226. [Google Scholar] [CrossRef]
- Nanduri, R.; Furusawa, T.; Bustin, M. Biological Functions of HMGN Chromosomal Proteins. Int. J. Mol. Sci. 2020, 21, 449. [Google Scholar] [CrossRef] [PubMed]
- Štros, M. HMGB Proteins: Interactions with DNA and Chromatin. Biochim. Biophys. Acta BBA-Gene Regul. Mech. 2010, 1799, 101–113. [Google Scholar] [CrossRef]
- Travers, A.A. Priming the Nucleosome: A Role for HMGB Proteins? EMBO Rep. 2003, 4, 131–136. [Google Scholar] [CrossRef]
- Catena, R.; Escoffier, E.; Caron, C.; Khochbin, S.; Martianov, I.; Davidson, I. HMGB4, a Novel Member of the HMGB Family, Is Preferentially Expressed in the Mouse Testis and Localizes to the Basal Pole of Elongating Spermatids1. Biol. Reprod. 2009, 80, 358–366. [Google Scholar] [CrossRef]
- Richard, S.A.; Jiang, Y.; Xiang, L.H.; Zhou, S.; Wang, J.; Su, Z.; Xu, H. Post-Translational Modifications of High Mobility Group Box 1 and Cancer. Am. J. Transl. Res. 2017, 9, 5181–5196. [Google Scholar]
- Kwak, M.S.; Kim, H.S.; Lee, B.; Kim, Y.H.; Son, M.; Shin, J.-S. Immunological Significance of HMGB1 Post-Translational Modification and Redox Biology. Front. Immunol. 2020, 11, 1189. [Google Scholar] [CrossRef]
- Hardman, C.H.; Broadhurst, R.W.; Raine, A.R.C.; Grasser, K.D.; Thomas, J.O.; Laue, E.D. Structure of the A-Domain of HMG1 and Its Interaction with DNA as Studied by Heteronuclear Three- and Four-Dimensional NMR Spectroscopy. Biochemistry 1995, 34, 16596–16607. [Google Scholar] [CrossRef]
- Weir, H.M.; Kraulis, P.J.; Hill, C.S.; Raine, A.R.; Laue, E.D.; Thomas, J.O. Structure of the HMG Box Motif in the B-Domain of HMG1. EMBO J. 1993, 12, 1311–1319. [Google Scholar] [CrossRef]
- Read, C.M.; Cary, P.D.; Crane-Robinson, C.; Driscoll, P.C.; Norman, D.G. Solution Structure of a DNA-Binding Domain from HMG1. Nucleic Acids Res. 1993, 21, 3427–3436. [Google Scholar] [CrossRef]
- Ohndorf, U.-M.; Rould, M.A.; He, Q.; Pabo, C.O.; Lippard, S.J. Basis for Recognition of Cisplatin-Modified DNA by High-Mobility-Group Proteins. Nature 1999, 399, 708–712. [Google Scholar] [CrossRef]
- Bianchi, M.; Beltrame, M.; Paonessa, G. Specific Recognition of Cruciform DNA by Nuclear Protein HMG1. Science 1989, 243, 1056–1059. [Google Scholar] [CrossRef]
- Isackson, P.J.; Fishback, J.L.; Bidney, D.L.; Reeck, G.R. Preferential Affinity of High Molecular Weight High Mobility Group Non-Histone Chromatin Proteins for Single-Stranded DNA. J. Biol. Chem. 1979, 254, 5569–5572. [Google Scholar] [CrossRef]
- Hamada, H.; Bustin, M. Hierarchy of Binding Sites for Chromosomal Proteins HMG 1 and 2 in Supercoiled Deoxyribonucleic Acid. Biochemistry 1985, 24, 1428–1433. [Google Scholar] [CrossRef]
- Gaillard, C.; Strauss, F. High Affinity Binding of Proteins HMG1 and HMG2 to Semicatenated DNA Loops. BMC Mol. Biol. 2000, 1, 1. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Webb, M.; Payet, D.; Lee, K.-B.; Travers, A.A.; Thomas, J.O. Structural Requirements for Cooperative Binding of HMG1 to DNA Minicircles. J. Mol. Biol. 2001, 309, 79–88. [Google Scholar] [CrossRef]
- Brázda, V.; Laister, R.C.; Jagelská, E.B.; Arrowsmith, C. Cruciform Structures Are a Common DNA Feature Important for Regulating Biological Processes. BMC Mol. Biol. 2011, 12, 33. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Wu, K.; Jia, F.; Chen, L.; Wang, Z.; Zhang, Y.; Luo, Q.; Liu, S.; Qi, L.; Li, N.; et al. Single Cell Imaging Reveals Cisplatin Regulating Interactions between Transcription (Co)Factors and DNA. Chem. Sci. 2021, 12, 5419–5429. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.O.; Travers, A.A. HMG1 and 2, and Related ‘Architectural’ DNA-Binding Proteins. Trends Biochem. Sci. 2001, 26, 167–174. [Google Scholar] [CrossRef]
- Knapp, S.; Müller, S.; Digilio, G.; Bonaldi, T.; Bianchi, M.E.; Musco, G. The Long Acidic Tail of High Mobility Group Box 1 (HMGB1) Protein Forms an Extended and Flexible Structure That Interacts with Specific Residues within and between the HMG Boxes. Biochemistry 2004, 43, 11992–11997. [Google Scholar] [CrossRef]
- Watson, M.; Stott, K.; Thomas, J.O. Mapping Intramolecular Interactions between Domains in HMGB1 Using a Tail-Truncation Approach. J. Mol. Biol. 2007, 374, 1286–1297. [Google Scholar] [CrossRef] [PubMed]
- Stott, K.; Watson, M.; Howe, F.S.; Grossmann, J.G.; Thomas, J.O. Tail-Mediated Collapse of HMGB1 Is Dynamic and Occurs via Differential Binding of the Acidic Tail to the A and B Domains. J. Mol. Biol. 2010, 403, 706–722. [Google Scholar] [CrossRef]
- Lee, K.-B.; Thomas, J.O. The Effect of the Acidic Tail on the DNA-Binding Properties of the HMG1,2 Class of Proteins: Insights from Tail Switching and Tail Removal. J. Mol. Biol. 2000, 304, 135–149. [Google Scholar] [CrossRef] [PubMed]
- Štros, M.; Štokrová, J.; Thomas, J.O. DNA Looping by the HMG-Box Domains of HMG1 and Modulation of DNA Binding by the Acidic C-Terminal Domain. Nucleic Acids Res. 1994, 22, 1044–1051. [Google Scholar] [CrossRef] [PubMed]
- Blair, R.H.; Horn, A.E.; Pazhani, Y.; Grado, L.; Goodrich, J.A.; Kugel, J.F. The HMGB1 C-Terminal Tail Regulates DNA Bending. J. Mol. Biol. 2016, 428, 4060–4072. [Google Scholar] [CrossRef] [PubMed]
- Belgrano, F.S.; de Abreu da Silva, I.C.; Bastos de Oliveira, F.M.; Fantappié, M.R.; Mohana-Borges, R. Role of the Acidic Tail of High Mobility Group Protein B1 (HMGB1) in Protein Stability and DNA Bending. PLoS ONE 2013, 8, e79572. [Google Scholar]
- Ueda, T.; Chou, H.; Kawase, T.; Shirakawa, H.; Yoshida, M. Acidic C-Tail of HMGB1 Is Required for Its Target Binding to Nucleosome Linker DNA and Transcription Stimulation. Biochemistry 2004, 43, 9901–9908. [Google Scholar] [CrossRef]
- Aizawa, S.; Nishino, H.; Saito, K.; Kimura, K.; Shirakawa, H.; Yoshida, M. Stimulation of Transcription in Cultured Cells by High Mobility Group Protein 1: Essential Role of the Acidic Carboxyl-Terminal Region. Biochemistry 1994, 33, 14690–14695. [Google Scholar] [CrossRef]
- An, W.; van Holde, K.; Zlatanova, J. The Non-Histone Chromatin Protein HMG1 Protects Linker DNA on the Side Opposite to That Protected by Linker Histones. J. Biol. Chem. 1998, 273, 26289–26291. [Google Scholar] [CrossRef]
- Cato, L.; Stott, K.; Watson, M.; Thomas, J.O. The Interaction of HMGB1 and Linker Histones Occurs Through Their Acidic and Basic Tails. J. Mol. Biol. 2008, 384, 1262–1272. [Google Scholar] [CrossRef]
- Štros, M.; Polanská, E.; Kučírek, M.; Pospíšilová, Š. Histone H1 Differentially Inhibits DNA Bending by Reduced and Oxidized HMGB1 Protein. PLoS ONE 2015, 10, e0138774. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.O.; Stott, K. H1 and HMGB1: Modulators of Chromatin Structure. Biochem. Soc. Trans. 2012, 40, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Watson, M.; Stott, K.; Fischl, H.; Cato, L.; Thomas, J.O. Characterization of the Interaction between HMGB1 and H3--a Possible Means of Positioning HMGB1 in Chromatin. Nucleic Acids Res. 2014, 42, 848–859. [Google Scholar] [CrossRef]
- Bonaldi, T. The DNA Chaperone HMGB1 Facilitates ACF/CHRAC-Dependent Nucleosome Sliding. EMBO J. 2002, 21, 6865–6873. [Google Scholar] [CrossRef] [PubMed]
- Lange, S.S.; Vasquez, K.M. HMGB1: The Jack-of-All-Trades Protein Is a Master DNA Repair Mechanic. Mol. Carcinog. 2009, 48, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Jayaraman, L.; Moorthy, N.C.; Murthy, K.G.; Manley, J.L.; Bustin, M.; Prives, C. High Mobility Group Protein-1 (HMG-1) Is a Unique Activator of P53. Genes Dev. 1998, 12, 462–472. [Google Scholar] [CrossRef]
- Das, D.; Peterson, R.C.; Scovell, W.M. High Mobility Group B Proteins Facilitate Strong Estrogen Receptor Binding to Classical and Half-Site Estrogen Response Elements and Relax Binding Selectivity. Mol. Endocrinol. 2004, 18, 2616–2632. [Google Scholar] [CrossRef][Green Version]
- Ueda, T.; Yoshida, M. HMGB Proteins and Transcriptional Regulation. Biochim. Biophys. Acta BBA-Gene Regul. Mech. 2010, 1799, 114–118. [Google Scholar] [CrossRef]
- Sofiadis, K.; Josipovic, N.; Nikolic, M.; Kargapolova, Y.; Übelmesser, N.; Varamogianni-Mamatsi, V.; Zirkel, A.; Papadionysiou, I.; Loughran, G.; Keane, J.; et al. HMGB1 Coordinates SASP-related Chromatin Folding and RNA Homeostasis on the Path to Senescence. Mol. Syst. Biol. 2021, 17, e9760. [Google Scholar] [CrossRef]
- Zirkel, A.; Nikolic, M.; Sofiadis, K.; Mallm, J.-P.; Brackley, C.A.; Gothe, H.; Drechsel, O.; Becker, C.; Altmüller, J.; Josipovic, N.; et al. HMGB2 Loss upon Senescence Entry Disrupts Genomic Organization and Induces CTCF Clustering across Cell Types. Mol. Cell 2018, 70, 730–744.e6. [Google Scholar] [CrossRef] [PubMed]
- Aird, K.M.; Iwasaki, O.; Kossenkov, A.V.; Tanizawa, H.; Fatkhutdinov, N.; Bitler, B.G.; Le, L.; Alicea, G.; Yang, T.-L.; Johnson, F.B.; et al. HMGB2 Orchestrates the Chromatin Landscape of Senescence-Associated Secretory Phenotype Gene Loci. J. Cell Biol. 2016, 215, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, A.; Gil, J. HMGB2 Holds the Key to the Senescence-Associated Secretory Phenotype. J. Cell Biol. 2016, 215, 297–299. [Google Scholar] [CrossRef] [PubMed]
- Davalos, A.R.; Kawahara, M.; Malhotra, G.K.; Schaum, N.; Huang, J.; Ved, U.; Beausejour, C.M.; Coppe, J.-P.; Rodier, F.; Campisi, J. P53-Dependent Release of Alarmin HMGB1 Is a Central Mediator of Senescent Phenotypes. J. Cell Biol. 2013, 201, 613–629. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Suarez, J.S.; Minaai, M.; Li, S.; Gaudino, G.; Pass, H.I.; Carbone, M.; Yang, H. HMGB1 as a Therapeutic Target in Disease. J. Cell. Physiol. 2021, 236, 3406–3419. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Kang, R.; Livesey, K.M.; Cheh, C.-W.; Farkas, A.; Loughran, P.; Hoppe, G.; Bianchi, M.E.; Tracey, K.J.; Zeh, H.J.; et al. Endogenous HMGB1 Regulates Autophagy. J. Cell Biol. 2010, 190, 881–892. [Google Scholar] [CrossRef]
- Tang, D.; Kang, R.; Livesey, K.M.; Kroemer, G.; Billiar, T.R.; Van Houten, B.; Zeh, H.J.; Lotze, M.T. High-Mobility Group Box 1 Is Essential for Mitochondrial Quality Control. Cell Metab. 2011, 13, 701–711. [Google Scholar] [CrossRef]
- Ito, H.; Fujita, K.; Tagawa, K.; Chen, X.; Homma, H.; Sasabe, T.; Shimizu, J.; Shimizu, S.; Tamura, T.; Muramatsu, S.; et al. HMGB1 Facilitates Repair of Mitochondrial DNA Damage and Extends the Lifespan of Mutant Ataxin-1 Knock-in Mice. EMBO Mol. Med. 2015, 7, 78–101. [Google Scholar] [CrossRef]
- Kamagata, K.; Itoh, Y.; Tan, C.; Mano, E.; Wu, Y.; Mandali, S.; Takada, S.; Johnson, R.C. Testing Mechanisms of DNA Sliding by Architectural DNA-Binding Proteins: Dynamics of Single Wild-Type and Mutant Protein Molecules in Vitro and in vivo. Nucleic Acids Res. 2021, 49, 8642–8664. [Google Scholar] [CrossRef]
- Kamagata, K.; Ouchi, K.; Tan, C.; Mano, E.; Mandali, S.; Wu, Y.; Takada, S.; Takahashi, S.; Johnson, R.C. The HMGB Chromatin Protein Nhp6A Can Bypass Obstacles When Traveling on DNA. Nucleic Acids Res. 2020, 48, 10820–10831. [Google Scholar] [CrossRef]
- Yen, Y.-M.; Wong, B.; Johnson, R.C. Determinants of DNA Binding and Bending by TheSaccharomyces Cerevisiae High Mobility Group Protein NHP6A That Are Important for Its Biological Activities. J. Biol. Chem. 1998, 273, 4424–4435. [Google Scholar] [CrossRef] [PubMed]
- Masse, J.E.; Wong, B.; Yen, Y.-M.; Allain, F.H.-T.; Johnson, R.C.; Feigon, J. The S. Cerevisiae Architectural HMGB Protein NHP6A Complexed with DNA: DNA and Protein Conformational Changes upon Binding. J. Mol. Biol. 2002, 323, 263–284. [Google Scholar] [CrossRef]
- Szabo, Q.; Bantignies, F.; Cavalli, G. Principles of Genome Folding into Topologically Associating Domains. Sci. Adv. 2019, 5, eaaw1668. [Google Scholar] [CrossRef] [PubMed]
- Felsenfeld, G.; Boyes, J.; Chung, J.; Clark, D.; Studitsky, V. Chromatin Structure and Gene Expression. Proc. Natl. Acad. Sci. USA 1996, 93, 9384–9388. [Google Scholar] [CrossRef]
- Morrison, O.; Thakur, J. Molecular Complexes at Euchromatin, Heterochromatin and Centromeric Chromatin. Int. J. Mol. Sci. 2021, 22, 6922. [Google Scholar] [CrossRef]
- Liu, X.; Ding, J.; Meng, L. Oncogene-Induced Senescence: A Double Edged Sword in Cancer. Acta Pharmacol. Sin. 2018, 39, 1553–1558. [Google Scholar] [CrossRef]
- Narita, M.; Nuñez, S.; Heard, E.; Narita, M.; Lin, A.W.; Hearn, S.A.; Spector, D.L.; Hannon, G.J.; Lowe, S.W. Rb-Mediated Heterochromatin Formation and Silencing of E2F Target Genes during Cellular Senescence. Cell 2003, 113, 703–716. [Google Scholar] [CrossRef]
- Zhang, R.; Poustovoitov, M.V.; Ye, X.; Santos, H.A.; Chen, W.; Daganzo, S.M.; Erzberger, J.P.; Serebriiskii, I.G.; Canutescu, A.A.; Dunbrack, R.L.; et al. Formation of MacroH2A-Containing Senescence-Associated Heterochromatin Foci and Senescence Driven by ASF1a and HIRA. Dev. Cell 2005, 8, 19–30. [Google Scholar] [CrossRef]
- Franklin, S.; Chen, H.; Mitchell-Jordan, S.; Ren, S.; Wang, Y.; Vondriska, T.M. Quantitative Analysis of the Chromatin Proteome in Disease Reveals Remodeling Principles and Identifies High Mobility Group Protein B2 as a Regulator of Hypertrophic Growth. Mol. Cell. Proteom. 2012, 11, M111–014258. [Google Scholar] [CrossRef]
- Monte, E.; Rosa-Garrido, M.; Karbassi, E.; Chen, H.; Lopez, R.; Rau, C.D.; Wang, J.; Nelson, S.F.; Wu, Y.; Stefani, E.; et al. Reciprocal Regulation of the Cardiac Epigenome by Chromatin Structural Proteins Hmgb and Ctcf. J. Biol. Chem. 2016, 291, 15428–15446. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Liu, M.; Gu, L.; Li, Y.; Shen, S.; Guo, G.; Wang, F.; He, X.; Zhao, Y.; Shang, X.; et al. The Architectural Factor HMGB1 Is Involved in Genome Organization in the Human Malaria Parasite Plasmodium Falciparum. mBio 2021, 12, e00148-21. [Google Scholar] [CrossRef] [PubMed]
- Deitsch, K.W.; Dzikowski, R. Variant Gene Expression and Antigenic Variation by Malaria Parasites. Annu. Rev. Microbiol. 2017, 71, 625–641. [Google Scholar] [CrossRef]
- Batugedara, G.; Lu, X.M.; Bunnik, E.M.; Le Roch, K.G. The Role of Chromatin Structure in Gene Regulation of the Human Malaria Parasite. Trends Parasitol. 2017, 33, 364–377. [Google Scholar] [CrossRef] [PubMed]
- Bunnik, E.M.; Cook, K.B.; Varoquaux, N.; Batugedara, G.; Prudhomme, J.; Cort, A.; Shi, L.; Andolina, C.; Ross, L.S.; Brady, D.; et al. Changes in Genome Organization of Parasite-Specific Gene Families during the Plasmodium Transmission Stages. Nat. Commun. 2018, 9, 1910. [Google Scholar] [CrossRef] [PubMed]
- Bunnik, E.M.; Venkat, A.; Shao, J.; McGovern, K.E.; Batugedara, G.; Worth, D.; Prudhomme, J.; Lapp, S.A.; Andolina, C.; Ross, L.S.; et al. Comparative 3D Genome Organization in Apicomplexan Parasites. Proc. Natl. Acad. Sci. USA 2019, 116, 3183–3192. [Google Scholar] [CrossRef]
- Polanská, E.; Dobšáková, Z.; Dvořáčková, M.; Fajkus, J.; Štros, M. HMGB1 Gene Knockout in Mouse Embryonic Fibroblasts Results in Reduced Telomerase Activity and Telomere Dysfunction. Chromosoma 2012, 121, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Pagano, B.; Margarucci, L.; Zizza, P.; Amato, J.; Iaccarino, N.; Cassiano, C.; Salvati, E.; Novellino, E.; Biroccio, A.; Casapullo, A.; et al. Identification of Novel Interactors of Human Telomeric G-Quadruplex DNA. Chem. Commun. 2015, 51, 2964–2967. [Google Scholar] [CrossRef]
- Amato, J.; Cerofolini, L.; Brancaccio, D.; Giuntini, S.; Iaccarino, N.; Zizza, P.; Iachettini, S.; Biroccio, A.; Novellino, E.; Rosato, A.; et al. Insights into Telomeric G-Quadruplex DNA Recognition by HMGB1 Protein. Nucleic Acids Res. 2019, 47, 9950–9966. [Google Scholar] [CrossRef]
- Bochman, M.L.; Paeschke, K.; Zakian, V.A. DNA Secondary Structures: Stability and Function of G-Quadruplex Structures. Nat. Rev. Genet. 2012, 13, 770–780. [Google Scholar] [CrossRef]
- Spiegel, J.; Adhikari, S.; Balasubramanian, S. The Structure and Function of DNA G-Quadruplexes. Trends Chem. 2020, 2, 123–136. [Google Scholar] [CrossRef]
- Chambers, V.S.; Marsico, G.; Boutell, J.M.; Di Antonio, M.; Smith, G.P.; Balasubramanian, S. High-Throughput Sequencing of DNA G-Quadruplex Structures in the Human Genome. Nat. Biotechnol. 2015, 33, 877–881. [Google Scholar] [CrossRef]
- Huppert, J.L.; Balasubramanian, S. G-Quadruplexes in Promoters throughout the Human Genome. Nucleic Acids Res. 2007, 35, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, S.; Hurley, L.H.; Neidle, S. Targeting G-Quadruplexes in Gene Promoters: A Novel Anticancer Strategy? Nat. Rev. Drug Discov. 2011, 10, 261–275. [Google Scholar] [CrossRef]
- Moye, A.L.; Porter, K.C.; Cohen, S.B.; Phan, T.; Zyner, K.G.; Sasaki, N.; Lovrecz, G.O.; Beck, J.L.; Bryan, T.M. Telomeric G-Quadruplexes Are a Substrate and Site of Localization for Human Telomerase. Nat. Commun. 2015, 6, 7643. [Google Scholar] [CrossRef]
- Dogan, F.; Forsyth, N.R. Telomerase Regulation: A Role for Epigenetics. Cancers 2021, 13, 1213. [Google Scholar] [CrossRef] [PubMed]
- Ke, S.; Zhou, F.; Yang, H.; Wei, Y.; Gong, J.; Mei, Z.; Wu, L.; Yu, H.; Zhou, Y. Down-regulation of High Mobility Group Box 1 Modulates Telomere Homeostasis and Increases the Radiosensitivity of Human Breast Cancer Cells. Int. J. Oncol. 2015, 46, 1051–1058. [Google Scholar] [CrossRef]
- Li, J. Not so Crystal Clear: The Structure of the Human Telomere G-Quadruplex in Solution Differs from That Present in a Crystal. Nucleic Acids Res. 2005, 33, 4649–4659. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, N.; Murugan, A.K.; Grieco, M. Kirsten Ras* Oncogene: Significance of Its Discovery in Human Cancer Research. Oncotarget 2016, 7, 46717–46733. [Google Scholar] [CrossRef]
- Cogoi, S.; Xodo, L.E. G-Quadruplex Formation within the Promoter of the KRAS Proto-Oncogene and Its Effect on Transcription. Nucleic Acids Res. 2006, 34, 2536–2549. [Google Scholar] [CrossRef]
- Kaiser, C.E.; Van Ert, N.A.; Agrawal, P.; Chawla, R.; Yang, D.; Hurley, L.H. Insight into the Complexity of the I-Motif and G-Quadruplex DNA Structures Formed in the KRAS Promoter and Subsequent Drug-Induced Gene Repression. J. Am. Chem. Soc. 2017, 139, 8522–8536. [Google Scholar] [CrossRef] [PubMed]
- Paramasivam, M.; Membrino, A.; Cogoi, S.; Fukuda, H.; Nakagama, H.; Xodo, L.E. Protein HnRNP A1 and Its Derivative Up1 Unfold Quadruplex DNA in the Human KRAS Promoter: Implications for Transcription. Nucleic Acids Res. 2009, 37, 2841–2853. [Google Scholar] [CrossRef]
- Amato, J.; Madanayake, T.W.; Iaccarino, N.; Novellino, E.; Randazzo, A.; Hurley, L.H.; Pagano, B. HMGB1 Binds to the KRAS Promoter G-Quadruplex: A New Player in Oncogene Transcriptional Regulation? Chem. Commun. 2018, 54, 9442–9445. [Google Scholar] [CrossRef]
- Arimondo, P.B.; Gelus, N.; Hamy, F.; Payet, D.; Travers, A.; Bailly, C. The Chromosomal Protein HMG-D Binds to the TAR and RBE RNA of HIV-1. FEBS Lett. 2000, 485, 47–52. [Google Scholar] [CrossRef]
- Bell, A.J.; Chauhan, S.; Woodson, S.A.; Kallenbach, N.R. Interactions of Recombinant HMGB Proteins with Branched RNA Substrates. Biochem. Biophys. Res. Commun. 2008, 377, 262–267. [Google Scholar] [CrossRef]
- Castello, A.; Fischer, B.; Frese, C.K.; Horos, R.; Alleaume, A.-M.; Foehr, S.; Curk, T.; Krijgsveld, J.; Hentze, M.W. Comprehensive Identification of RNA-Binding Domains in Human Cells. Mol. Cell 2016, 63, 696–710. [Google Scholar] [CrossRef]
- He, C.; Sidoli, S.; Warneford-Thomson, R.; Tatomer, D.C.; Wilusz, J.E.; Garcia, B.A.; Bonasio, R. High-Resolution Mapping of RNA-Binding Regions in the Nuclear Proteome of Embryonic Stem Cells. Mol. Cell 2016, 64, 416–430. [Google Scholar] [CrossRef]
- Kargapolova, Y.; Levin, M.; Lackner, K.; Danckwardt, S. SCLIP—an Integrated Platform to Study RNA–Protein Interactomes in Biomedical Research: Identification of CSTF2tau in Alternative Processing of Small Nuclear RNAs. Nucleic Acids Res. 2017, 45, 6074–6086. [Google Scholar] [CrossRef]
- Barreiro-Alonso, A.; Lamas-Maceiras, M.; García-Díaz, R.; Rodríguez-Belmonte, E.; Yu, L.; Pardo, M.; Choudhary, J.S.; Cerdán, M.E. Delineating the HMGB1 and HMGB2 Interactome in Prostate and Ovary Epithelial Cells and Its Relationship with Cancer. Oncotarget 2018, 9, 19050–19064. [Google Scholar] [CrossRef]
- Moura-Alves, P.; Neves-Costa, A.; Raquel, H.; Pacheco, T.R.; D’Almeida, B.; Rodrigues, R.; Cadima-Couto, I.; Chora, Â.; Oliveira, M.; Gama-Carvalho, M.; et al. An ShRNA-Based Screen of Splicing Regulators Identifies SFRS3 as a Negative Regulator of IL-1β Secretion. PLoS ONE 2011, 6, e19829. [Google Scholar] [CrossRef]
- Lau, L.; Porciuncula, A.; Yu, A.; Iwakura, Y.; David, G. Uncoupling the Senescence-Associated Secretory Phenotype from Cell Cycle Exit via Interleukin-1 Inactivation Unveils Its Protumorigenic Role. Mol. Cell. Biol. 2019, 39, e00586-18. [Google Scholar] [CrossRef]
- Shang, D.; Hong, Y.; Xie, W.; Tu, Z.; Xu, J. Interleukin-1β Drives Cellular Senescence of Rat Astrocytes Induced by Oligomerized Amyloid β Peptide and Oxidative Stress. Front. Neurol. 2020, 11, 929. [Google Scholar] [CrossRef] [PubMed]
- Kopp, F.; Mendell, J.T. Functional Classification and Experimental Dissection of Long Noncoding RNAs. Cell 2018, 172, 393–407. [Google Scholar] [CrossRef] [PubMed]
- Lou, M.-M.; Tang, X.-Q.; Wang, G.-M.; He, J.; Luo, F.; Guan, M.-F.; Wang, F.; Zou, H.; Wang, J.-Y.; Zhang, Q.; et al. Long Noncoding RNA BS-DRL1 Modulates the DNA Damage Response and Genome Stability by Interacting with HMGB1 in Neurons. Nat. Commun. 2021, 12, 4075. [Google Scholar] [CrossRef]
- Gao, D.; Lv, A.; Li, H.; Han, D.; Zhang, Y. LncRNA MALAT-1 Elevates HMGB1 to Promote Autophagy Resulting in Inhibition of Tumor Cell Apoptosis in Multiple Myeloma. J. Cell. Biochem. 2017, 118, 3341–3348. [Google Scholar] [CrossRef]
- Tang, X.; Li, P.-H.; Chen, H.-Z. Cardiomyocyte Senescence and Cellular Communications Within Myocardial Microenvironments. Front. Endocrinol. 2020, 11, 280. [Google Scholar] [CrossRef] [PubMed]
- Kang, R.; Chen, R.; Zhang, Q.; Hou, W.; Wu, S.; Cao, L.; Huang, J.; Yu, Y.; Fan, X.-G.; Yan, Z.; et al. HMGB1 in Health and Disease. Mol. Aspects Med. 2014, 40, 1–116. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Vasquez, K.M. Targeting Chromosomal Architectural HMGB Proteins Could Be the Next Frontier in Cancer Therapy. Cancer Res. 2020, 80, 2075–2082. [Google Scholar] [CrossRef]
- Yanai, H.; Matsuda, A.; An, J.; Koshiba, R.; Nishio, J.; Negishi, H.; Ikushima, H.; Onoe, T.; Ohdan, H.; Yoshida, N.; et al. Conditional Ablation of HMGB1 in Mice Reveals Its Protective Function against Endotoxemia and Bacterial Infection. Proc. Natl. Acad. Sci. USA 2013, 110, 20699–20704. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Voong, C.K.; Goodrich, J.A.; Kugel, J.F. Interactions of HMGB Proteins with the Genome and the Impact on Disease. Biomolecules 2021, 11, 1451. https://doi.org/10.3390/biom11101451
Voong CK, Goodrich JA, Kugel JF. Interactions of HMGB Proteins with the Genome and the Impact on Disease. Biomolecules. 2021; 11(10):1451. https://doi.org/10.3390/biom11101451
Chicago/Turabian StyleVoong, Calvin K., James A. Goodrich, and Jennifer F. Kugel. 2021. "Interactions of HMGB Proteins with the Genome and the Impact on Disease" Biomolecules 11, no. 10: 1451. https://doi.org/10.3390/biom11101451
APA StyleVoong, C. K., Goodrich, J. A., & Kugel, J. F. (2021). Interactions of HMGB Proteins with the Genome and the Impact on Disease. Biomolecules, 11(10), 1451. https://doi.org/10.3390/biom11101451




