Topical Administration of Melatonin-Loaded Extracellular Vesicle-Mimetic Nanovesicles Improves 2,4-Dinitrofluorobenzene-Induced Atopic Dermatitis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Generation of Melatonin-Loaded Extracellular Vesicle-Mimetic NVs
2.3. Cryo-Electron Microscopy and Nanoparticle Tracking Analysis
2.4. Western Blotting
2.5. Quantification of Melatonin in Extracellular Vesicle-Mimetic NVs
2.6. In Vitro β-Hexosaminidase Release Assay
2.7. Animal Care
2.8. Atopic Dermatitis-Like Mouse Model
2.9. Enzyme-Linked Immunosorbent Assay (ELISA)
2.10. Histological Analysis
2.11. Statistical Analysis
3. Results
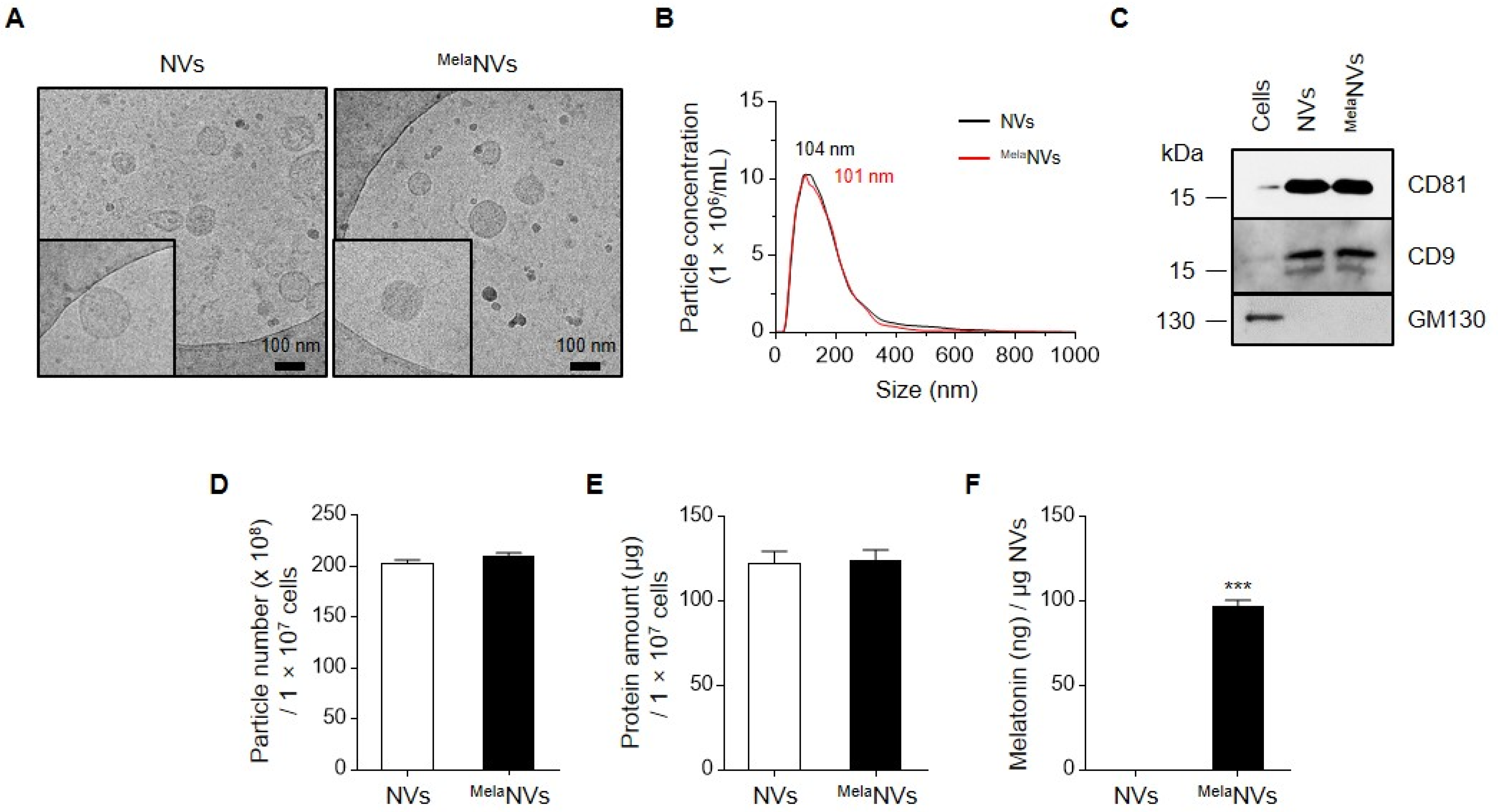
3.1. Generation and Characterization of NVs and Melatonin-Loaded NVs
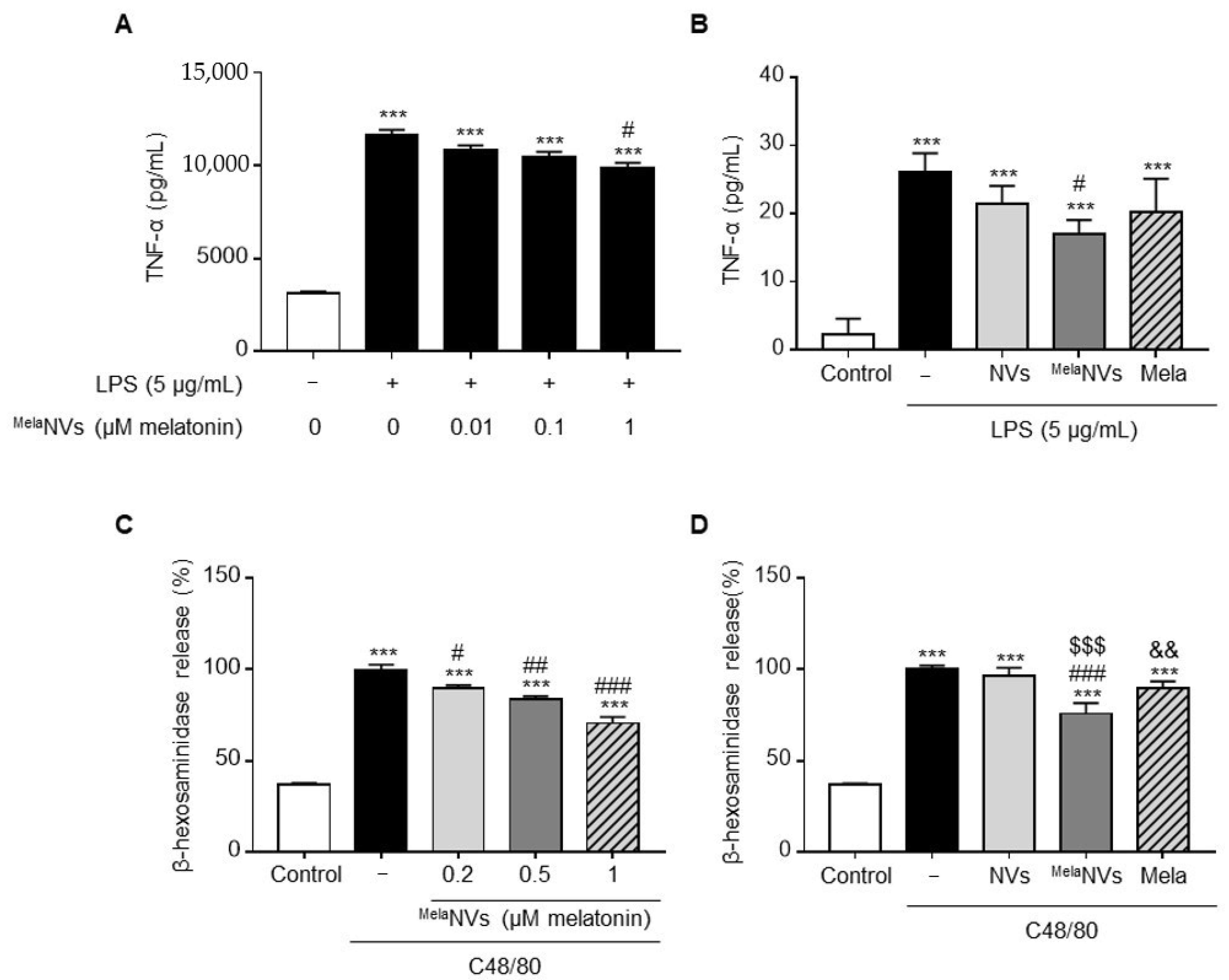
3.2. In Vitro Anti-Inflammatory Effect of MelaNVs in RAW264.7 and RBL-2H3 Cells
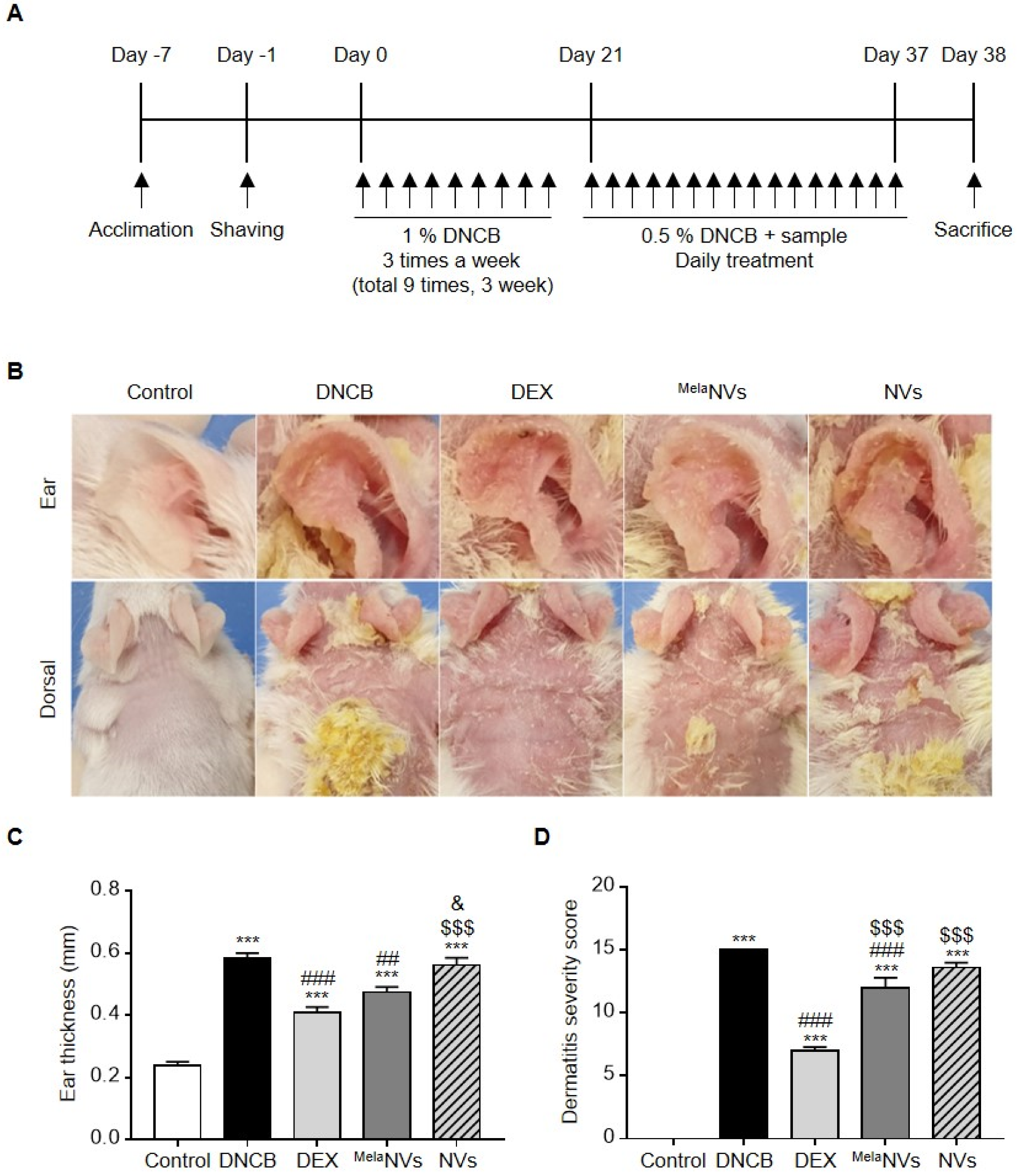
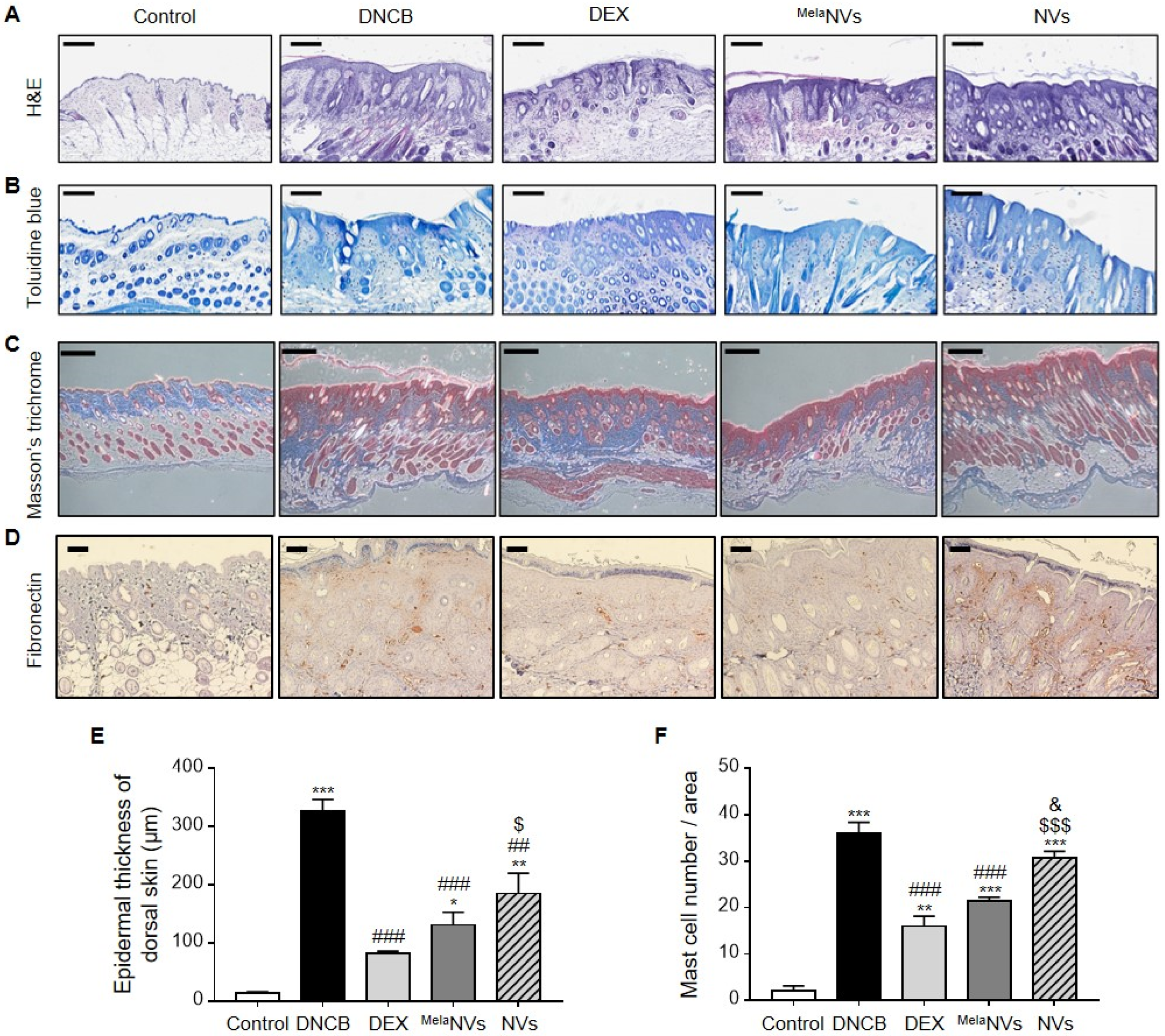
3.3. MelaNVs Ameliorate DNCB-Induced AD and Suppress Mast Cell Infiltration and Fibrosis in AD-like Skin Lesions
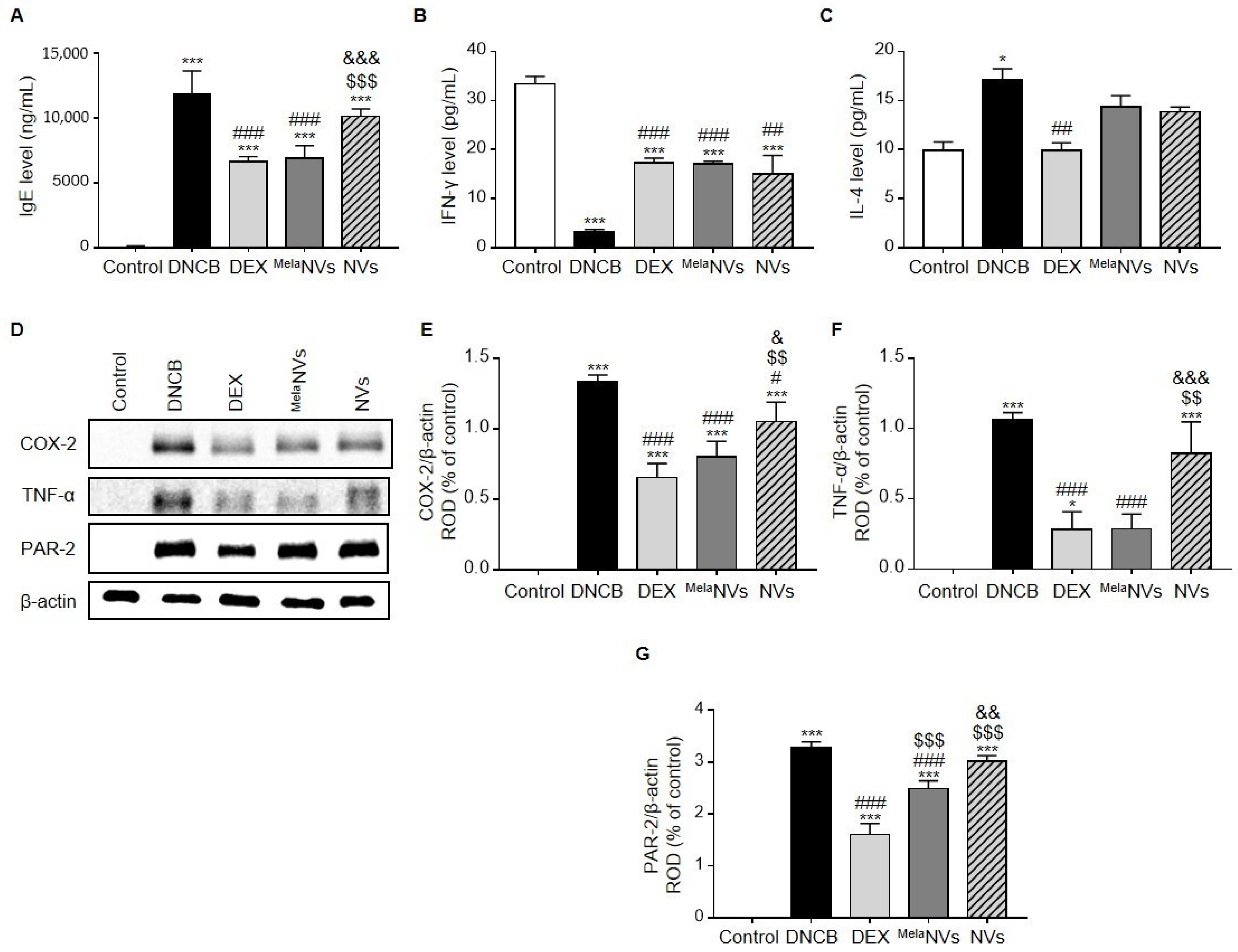
3.4. MelaNVs Decrease Serum IgE Levels, Restore Th1/Th2 Cytokine Balance, and Suppress the Release of Inflammatory Cytokines in AD-like Skin Lesions
4. Discussion
- The anti-inflammatory effects of MelaNVs in LPS-stimulated RAW264.7 cells and C48/80-stimulated RBL-2H3 cells.
- We demonstrated that MelaNVs could improve AD-like symptoms and suppress mast cell infiltration and fibrosis in AD-like skin lesions. MelaNVs decreased serum IgE, restored IFN-γ and IL-4 levels, and inhibited COX-2, TNF-α, and PAR-2 expression.
- These results suggest that MelaNVs have an excellent anti-atopic effect and can be used for the treatment of AD.
- As MelaNVs are an excellent melatonin delivery system, MelaNVs may also be used in other skin disease models, including decubitus and eye disease, to deliver melatonin.
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Avena-Woods, C. Overview of atopic dermatitis. Am. J. Manag. Care 2017, 23, S115–S123. [Google Scholar]
- Deckers, I.A.; McLean, S.; Linssen, S.; Mommers, M.; van Schayck, C.P.; Sheikh, A. Investigating international time trends in the incidence and prevalence of atopic eczema 1990–2010: A systematic review of epidemiological studies. PLoS ONE 2012, 7, e39803. [Google Scholar] [CrossRef] [Green Version]
- Kowalska-Oledzka, E.; Czarnecka, M.; Baran, A. Epidemiology of atopic dermatitis in Europe. J. Drug Assess. 2019, 8, 126–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bieber, T. Atopic dermatitis. N. Engl. J. Med. 2008, 358, 1483–1494. [Google Scholar] [CrossRef]
- Berke, R.; Singh, A.; Guralnick, M. Atopic dermatitis: An overview. Am. Fam. Physician 2012, 86, 35–42. [Google Scholar] [PubMed]
- Drucker, A.M.; Wang, A.R.; Li, W.Q.; Sevetson, E.; Block, J.K.; Qureshi, A.A. The Burden of Atopic Dermatitis: Summary of a Report for the National Eczema Association. J. Investig. Dermatol. 2017, 137, 26–30. [Google Scholar] [CrossRef] [Green Version]
- van den Oord, R.A.; Sheikh, A. Filaggrin gene defects and risk of developing allergic sensitisation and allergic disorders: Systematic review and meta-analysis. BMJ 2009, 339, b2433. [Google Scholar] [CrossRef] [Green Version]
- Sandilands, A.; Sutherland, C.; Irvine, A.D.; McLean, W.H. Filaggrin in the frontline: Role in skin barrier function and disease. J. Cell Sci. 2009, 122, 1285–1294. [Google Scholar] [CrossRef] [Green Version]
- Gittler, J.K.; Shemer, A.; Suarez-Farinas, M.; Fuentes-Duculan, J.; Gulewicz, K.J.; Wang, C.Q.; Mitsui, H.; Cardinale, I.; de Guzman Strong, C.; Krueger, J.G.; et al. Progressive activation of T(H)2/T(H)22 cytokines and selective epidermal proteins characterizes acute and chronic atopic dermatitis. J. Allergy Clin. Immunol. 2012, 130, 1344–1354. [Google Scholar] [CrossRef] [Green Version]
- Thomsen, S.F. Atopic dermatitis: Natural history, diagnosis, and treatment. ISRN Allergy 2014, 2014, 354250. [Google Scholar] [CrossRef] [Green Version]
- Tordjman, S.; Chokron, S.; Delorme, R.; Charrier, A.; Bellissant, E.; Jaafari, N.; Fougerou, C. Melatonin: Pharmacology, Functions and Therapeutic Benefits. Curr. Neuropharmacol. 2017, 15, 434–443. [Google Scholar] [CrossRef]
- Zisapel, N. New perspectives on the role of melatonin in human sleep, circadian rhythms and their regulation. Br. J. Pharmacol. 2018, 175, 3190–3199. [Google Scholar] [CrossRef]
- Turek, F.W.; Gillette, M.U. Melatonin, sleep, and circadian rhythms: Rationale for development of specific melatonin agonists. Sleep Med. 2004, 5, 523–532. [Google Scholar] [CrossRef]
- Tan, D.X.; Manchester, L.C.; Esteban-Zubero, E.; Zhou, Z.; Reiter, R.J. Melatonin as a Potent and Inducible Endogenous Antioxidant: Synthesis and Metabolism. Molecules 2015, 20, 18886–18906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, S.C.; Hsieh, M.J.; Yang, W.E.; Chung, W.H.; Reiter, R.J.; Yang, S.F. Cancer metastasis: Mechanisms of inhibition by melatonin. J. Pineal Res. 2017, 62. [Google Scholar] [CrossRef]
- Tarocco, A.; Caroccia, N.; Morciano, G.; Wieckowski, M.R.; Ancora, G.; Garani, G.; Pinton, P. Melatonin as a master regulator of cell death and inflammation: Molecular mechanisms and clinical implications for newborn care. Cell Death Dis. 2019, 10, 317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Navarro, A.; Gonzalez-Puga, C.; Escames, G.; Lopez, L.C.; Lopez, A.; Lopez-Cantarero, M.; Camacho, E.; Espinosa, A.; Gallo, M.A.; Acuna-Castroviejo, D. Cellular mechanisms involved in the melatonin inhibition of HT-29 human colon cancer cell proliferation in culture. J. Pineal Res. 2007, 43, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Jung, J.A.; Kim, G.D.; Jang, A.H.; Ahn, H.J.; Park, Y.S.; Park, C.S. Melatonin inhibits the development of 2,4-dinitrofluorobenzene-induced atopic dermatitis-like skin lesions in NC/Nga mice. J. Pineal Res. 2009, 47, 324–329. [Google Scholar] [CrossRef]
- Fischer, T.W.; Zbytek, B.; Sayre, R.M.; Apostolov, E.O.; Basnakian, A.G.; Sweatman, T.W.; Wortsman, J.; Elsner, P.; Slominski, A. Melatonin increases survival of HaCaT keratinocytes by suppressing UV-induced apoptosis. J. Pineal Res. 2006, 40, 18–26. [Google Scholar] [CrossRef]
- Kilanczyk, E.; Bryszewska, M. The effect of melatonin on antioxidant enzymes in human diabetic skin fibroblasts. Cell Mol. Biol. Lett. 2003, 8, 333–336. [Google Scholar] [PubMed]
- Thery, C.; Ostrowski, M.; Segura, E. Membrane vesicles as conveyors of immune responses. Nat. Rev. Immunol. 2009, 9, 581–593. [Google Scholar] [CrossRef]
- Simons, M.; Raposo, G. Exosomes—Vesicular carriers for intercellular communication. Curr. Opin. Cell Biol. 2009, 21, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Bunggulawa, E.J.; Wang, W.; Yin, T.; Wang, N.; Durkan, C.; Wang, Y.; Wang, G. Recent advancements in the use of exosomes as drug delivery systems. J. Nanobiotechnol. 2018, 16, 81. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, J.P.K.; Stevens, M.M. Strategic design of extracellular vesicle drug delivery systems. Adv. Drug Deliv. Rev. 2018, 130, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Gyorgy, B.; Hung, M.E.; Breakefield, X.O.; Leonard, J.N. Therapeutic applications of extracellular vesicles: Clinical promise and open questions. Annu. Rev. Pharmacol. Toxicol. 2015, 55, 439–464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luan, X.; Sansanaphongpricha, K.; Myers, I.; Chen, H.; Yuan, H.; Sun, D. Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacol. Sin. 2017, 38, 754–763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, D.; Zhuang, X.; Zhang, S.; Deng, Z.B.; Grizzle, W.; Miller, D.; Zhang, H.G. Exosomes are endogenous nanoparticles that can deliver biological information between cells. Adv. Drug Deliv. Rev. 2013, 65, 342–347. [Google Scholar] [CrossRef]
- Jang, S.C.; Kim, O.Y.; Yoon, C.M.; Choi, D.S.; Roh, T.Y.; Park, J.; Nilsson, J.; Lotvall, J.; Kim, Y.K.; Gho, Y.S. Bioinspired exosome-mimetic nanovesicles for targeted delivery of chemotherapeutics to malignant tumors. ACS Nano 2013, 7, 7698–7710. [Google Scholar] [CrossRef]
- Go, G.; Lee, J.; Choi, D.S.; Kim, S.S.; Gho, Y.S. Extracellular Vesicle-Mimetic Ghost Nanovesicles for Delivering Anti-Inflammatory Drugs to Mitigate Gram-Negative Bacterial Outer Membrane Vesicle-Induced Systemic Inflammatory Response Syndrome. Adv. Healthc. Mater. 2019, 8, e1801082. [Google Scholar] [CrossRef]
- Kenari, A.N.; Cheng, L.; Hill, A.F. Methods for loading therapeutics into extracellular vesicles and generating extracellular vesicles mimetic-nanovesicles. Methods 2020, 177, 103–113. [Google Scholar] [CrossRef]
- Gao, J.; Dong, X.; Wang, Z. Generation, purification and engineering of extracellular vesicles and their biomedical applications. Methods 2020, 177, 114–125. [Google Scholar] [CrossRef]
- Gangadaran, P.; Ahn, B.C. Extracellular Vesicle- and Extracellular Vesicle Mimetics-Based Drug Delivery Systems: New Perspectives, Challenges, and Clinical Developments. Pharmaceutics 2020, 12, 442. [Google Scholar] [CrossRef]
- Kim, Y.-Y.; Je, I.-G.; Kim, M.J.; Kang, B.-C.; Choi, Y.-A.; Baek, M.-C.; Lee, B.; Choi, J.K.; Park, H.R.; Shin, T.-Y.; et al. 2-Hydroxy-3-methoxybenzoic acid attenuates mast cell-mediated allergic reaction in mice via modulation of the FcεRI signaling pathway. Acta Pharmacol. Sin. 2017, 38, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Ryu, A.R.; Lee, M.-Y. Ameliorative effect of chlorin e6-mediated photodynamic therapy on DNCB-induced atopic dermatitis-like skin lesions in mice. Mol. Cell. Toxicol. 2019, 15, 265–270. [Google Scholar] [CrossRef]
- Leung, D.Y.; Hirsch, R.L.; Schneider, L.; Moody, C.; Takaoka, R.; Li, S.H.; Meyerson, L.A.; Mariam, S.G.; Goldstein, G.; Hanifin, J.M. Thymopentin therapy reduces the clinical severity of atopic dermatitis. J. Allergy Clin. Immunol. 1990, 85, 927–933. [Google Scholar] [CrossRef]
- Buhl, T.; Ikoma, A.; Kempkes, C.; Cevikbas, F.; Sulk, M.; Buddenkotte, J.; Akiyama, T.; Crumrine, D.; Camerer, E.; Carstens, E.; et al. Protease-Activated Receptor-2 Regulates Neuro-Epidermal Communication in Atopic Dermatitis. Front. Immunol. 2020, 11, 1740. [Google Scholar] [CrossRef] [PubMed]
- Egawa, G.; Weninger, W. Pathogenesis of atopic dermatitis: A short review. Cogent Biol. 2015, 1, 1103459. [Google Scholar] [CrossRef]
- Cikler, E.; Ercan, F.; Cetinel, S.; Contuk, G.; Sener, G. The protective effects of melatonin against water avoidance stress-induced mast cell degranulation in dermis. Acta Histochem. 2005, 106, 467–475. [Google Scholar] [CrossRef]
- Han, Y.S.; Yoon, Y.M.; Go, G.; Lee, J.H.; Lee, S.H. Melatonin Protects Human Renal Proximal Tubule Epithelial Cells Against High Glucose-Mediated Fibrosis via the Cellular Prion Protein-TGF-beta-Smad Signaling Axis. Int. J. Med. Sci. 2020, 17, 1235–1245. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.M.; Go, G.; Yun, C.W.; Lim, J.H.; Lee, J.H.; Lee, S.H. Melatonin Suppresses Renal Cortical Fibrosis by Inhibiting Cytoskeleton Reorganization and Mitochondrial Dysfunction through Regulation of miR-4516. Int. J. Mol. Sci. 2020, 21, 5323. [Google Scholar] [CrossRef]
- Bessone, C.D.V.; Martinez, S.M.; Luna, J.D.; Marquez, M.A.; Ramirez, M.L.; Allemandi, D.A.; Carpentieri, A.R.; Quinteros, D.A. Neuroprotective effect of melatonin loaded in ethylcellulose nanoparticles applied topically in a retinal degeneration model in rabbits. Exp. Eye Res. 2020, 200, 108222. [Google Scholar] [CrossRef]
- Siahdasht, F.N.; Farhadian, N.; Karimi, M.; Hafizi, L. Enhanced delivery of melatonin loaded nanostructured lipid carriers during in vitro fertilization: NLC formulation, optimization and IVF efficacy. RSC Adv. 2020, 10, 9462–9475. [Google Scholar] [CrossRef] [Green Version]
- Massella, D.; Leone, F.; Peila, R.; Barresi, A.A.; Ferri, A. Functionalization of Cotton Fabrics with Polycaprolactone Nanoparticles for Transdermal Release of Melatonin. J. Funct. Biomater. 2017, 9, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, S.C.; Rui, B.Y.; Wang, Q.Y.; Zhou, D.; Zhang, Y.; Guo, S.C. Extracellular vesicle-mimetic nanovesicles transport LncRNA-H19 as competing endogenous RNA for the treatment of diabetic wounds. Drug Deliv. 2018, 25, 241–255. [Google Scholar] [CrossRef] [Green Version]
- Park, K.S.; Svennerholm, K.; Shelke, G.V.; Bandeira, E.; Lasser, C.; Jang, S.C.; Chandode, R.; Gribonika, I.; Lotvall, J. Mesenchymal stromal cell-derived nanovesicles ameliorate bacterial outer membrane vesicle-induced sepsis via IL-10. Stem. Cell Res. Ther. 2019, 10, 231. [Google Scholar] [CrossRef] [Green Version]
- Elias, P.M.; Wakefield, J.S. Mechanisms of abnormal lamellar body secretion and the dysfunctional skin barrier in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2014, 134, 781–791.e1. [Google Scholar] [CrossRef] [Green Version]
- Shpacovitch, V.M.; Varga, G.; Strey, A.; Gunzer, M.; Mooren, F.; Buddenkotte, J.; Vergnolle, N.; Sommerhoff, C.P.; Grabbe, S.; Gerke, V.; et al. Agonists of proteinase-activated receptor-2 modulate human neutrophil cytokine secretion, expression of cell adhesion molecules, and migration within 3-D collagen lattices. J. Leukoc. Biol. 2004, 76, 388–398. [Google Scholar] [CrossRef]
- Cevikbas, F.; Seeliger, S.; Fastrich, M.; Hinte, H.; Metze, D.; Kempkes, C.; Homey, B.; Steinhoff, M. Role of protease-activated receptors in human skin fibrosis and scleroderma. Exp. Dermatol. 2011, 20, 69–71. [Google Scholar] [CrossRef]
- Seiberg, M.; Paine, C.; Sharlow, E.; Andrade-Gordon, P.; Costanzo, M.; Eisinger, M.; Shapiro, S.S. The protease-activated receptor 2 regulates pigmentation via keratinocyte-melanocyte interactions. Exp. Cell Res. 2000, 254, 25–32. [Google Scholar] [CrossRef]
- Cattaruzza, F.; Amadesi, S.; Carlsson, J.F.; Murphy, J.E.; Lyo, V.; Kirkwood, K.; Cottrell, G.S.; Bogyo, M.; Knecht, W.; Bunnett, N.W. Serine proteases and protease-activated receptor 2 mediate the proinflammatory and algesic actions of diverse stimulants. Br. J. Pharmacol. 2014, 171, 3814–3826. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.E.; Jeong, S.K.; Lee, S.H. Protease and protease-activated receptor-2 signaling in the pathogenesis of atopic dermatitis. Yonsei Med. J. 2010, 51, 808–822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawagoe, J.; Takizawa, T.; Matsumoto, J.; Tamiya, M.; Meek, S.E.; Smith, A.J.; Hunter, G.D.; Plevin, R.; Saito, N.; Kanke, T.; et al. Effect of protease-activated receptor-2 deficiency on allergic dermatitis in the mouse ear. Jpn. J. Pharmacol. 2002, 88, 77–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinhoff, M.; Neisius, U.; Ikoma, A.; Fartasch, M.; Heyer, G.; Skov, P.S.; Luger, T.A.; Schmelz, M. Proteinase-activated receptor-2 mediates itch: A novel pathway for pruritus in human skin. J. Neurosci. 2003, 23, 6176–6180. [Google Scholar] [CrossRef] [PubMed]
- Briot, A.; Deraison, C.; Lacroix, M.; Bonnart, C.; Robin, A.; Besson, C.; Dubus, P.; Hovnanian, A. Kallikrein 5 induces atopic dermatitis-like lesions through PAR2-mediated thymic stromal lymphopoietin expression in Netherton syndrome. J. Exp. Med. 2009, 206, 1135–1147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sales, K.U.; Masedunskas, A.; Bey, A.L.; Rasmussen, A.L.; Weigert, R.; List, K.; Szabo, R.; Overbeek, P.A.; Bugge, T.H. Matriptase initiates activation of epidermal pro-kallikrein and disease onset in a mouse model of Netherton syndrome. Nat. Genet. 2010, 42, 676–683. [Google Scholar] [CrossRef] [Green Version]
- Bäckman, A.; Ny, A.; Edlund, M.; Ekholm, E.; Hammarström, B.E.; Törnell, J.; Wallbrandt, P.; Egelrud, T.; Hansson, L.; Wennbo, H. Epidermal Overexpression of Stratum Corneum Chymotryptic Enzyme in Mice: A Model for Chronic Itchy Dermatitis. J. Investig. Dermatol. 2002, 118, 444–449. [Google Scholar] [CrossRef] [Green Version]
- Oikonomopoulou, K.; Hansen, K.K.; Saifeddine, M.; Tea, I.; Blaber, M.; Blaber, S.I.; Scarisbrick, I.; Andrade-Gordon, P.; Cottrell, G.S.; Bunnett, N.W.; et al. Proteinase-activated receptors, targets for kallikrein signaling. J. Biol. Chem. 2006, 281, 32095–32112. [Google Scholar] [CrossRef] [Green Version]
- Rothmeier, A.S.; Ruf, W. Protease-activated receptor 2 signaling in inflammation. Semin. Immunopathol. 2012, 34, 133–149. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.S.; Go, G.; Yun, C.-W.; Yea, J.-H.; Yoon, S.; Han, S.-Y.; Lee, G.; Lee, M.-Y.; Lee, S.H. Topical Administration of Melatonin-Loaded Extracellular Vesicle-Mimetic Nanovesicles Improves 2,4-Dinitrofluorobenzene-Induced Atopic Dermatitis. Biomolecules 2021, 11, 1450. https://doi.org/10.3390/biom11101450
Kim YS, Go G, Yun C-W, Yea J-H, Yoon S, Han S-Y, Lee G, Lee M-Y, Lee SH. Topical Administration of Melatonin-Loaded Extracellular Vesicle-Mimetic Nanovesicles Improves 2,4-Dinitrofluorobenzene-Induced Atopic Dermatitis. Biomolecules. 2021; 11(10):1450. https://doi.org/10.3390/biom11101450
Chicago/Turabian StyleKim, Yoon Seon, Gyeongyun Go, Chul-Won Yun, Ji-Hye Yea, Sungtae Yoon, Su-Yeon Han, Gaeun Lee, Mi-Young Lee, and Sang Hun Lee. 2021. "Topical Administration of Melatonin-Loaded Extracellular Vesicle-Mimetic Nanovesicles Improves 2,4-Dinitrofluorobenzene-Induced Atopic Dermatitis" Biomolecules 11, no. 10: 1450. https://doi.org/10.3390/biom11101450
APA StyleKim, Y. S., Go, G., Yun, C.-W., Yea, J.-H., Yoon, S., Han, S.-Y., Lee, G., Lee, M.-Y., & Lee, S. H. (2021). Topical Administration of Melatonin-Loaded Extracellular Vesicle-Mimetic Nanovesicles Improves 2,4-Dinitrofluorobenzene-Induced Atopic Dermatitis. Biomolecules, 11(10), 1450. https://doi.org/10.3390/biom11101450





