Mangiferin as New Potential Anti-Cancer Agent and Mangiferin-Integrated Polymer Systems—A Novel Research Direction
Abstract
1. Introduction
2. Anti-Cancer Effects of Mangiferin and Mechanisms of Anti-Cancer Action
2.1. Antioxidant and Anti-Inflammatory Effects of Mangiferin
2.2. Mouse Melanoma
2.3. Acute Myeloid Leukemia
2.4. Glioma Cells
2.5. Prostate Cancer
2.6. Colon Carcinoma
2.7. Hepatocellular Carcinoma
2.8. Breast Carcinoma
2.9. Lung Carcinoma
2.10. Other Types of Cancer Diseases
2.11. Synergic Action
2.12. Summary of Anti-Cancer Activities of Mangiferin
3. Mangiferin-Integrated Polymer Systems
3.1. Spray-Drying Technique
- -
- The largest average diameter was observed in SD3 (15 μm), the smallest was in SD4 (2.9 μm), the difference between the two formulae with the same polymer components SD1 (7.2 μm) and SD2 (10.2 μm) was thought to be due to the presence of Tween 80.
- -
- Tween 80 was also thought to cause the surface of the SD2 particles to be smoother and more uniform than SD1 particles. The SD3 formula gave irregular spherical shape with some set type. Meanwhile, the SD4 were spherical particles with a rough surface.
- -
- Tween 80 used in the SD2 formula also helped to increase the compatibility of mangiferin in the polymer.
- -
- Values of mangiferin concentration in the capsules correlated to the particle sizes: the highest one was in SD3 and the lowest was in SD4. These values were 29, 41, 49 and 16 (μg/mg) respectively for SD1-SD4 formulations.
- -
- Similar to the previous research, the majority of particles had a spherical shape, rough surface, heterogeneous size—there were microparticles, nanoparticles, broken particles and some aggregates. The average diameter of microparticles was 2.64 μm (equivalent to the smallest diameter of SD4 in the previous work) and that of nanoparticles was 460.54 nm.
- -
- In nanoparticles, chitosan content was higher than in microparticles. The average mangiferin content was 136 μg/mg of particles (much higher than the SD4 sample in the previous publication).
- -
- When chitosan-mangiferin systems were dissolved in a 1.0% acetic acid solution, the hydraulic average diameter of the particles became 467.7 nm. As such, 1.0% acetic acid promotes the dissolution of chitosan and creates self-assembled nanoparticles.
- -
- In chitosan-mangiferin particles, the chitosan amino group was protonated and Tween 80 had the effect of promoting the formation of intermolecular bonds.
- -
- Comparing the ability to adsorb and remove Cr (VI) with chitosan, the chitosan-mangiferin systems showed the collection at pH values 1–7 (different environments of the gastrointestinal tract) and especially at pH 5.0, the improved efficiency of the system reached 57.34% higher than that of free chitosan.
- -
- Cr (VI) absorption and removal efficiency increased over time and reached a maximum at approximately 120 min and had also shown a rapid effect in removing and reducing Cr (VI) during the first 30 min.
3.2. Simple Solvent-Evaporation Technique
- -
- Mangiferin content of the complex was as high as 35.02% (w/w).
- -
- In the complex, mangiferin was dispersed evenly, interacting with the polar parts of the phospholipid molecule, the non-polar part of the phospholipid was unchanged, and the hydrogen carbon chains in phospholipids could rotate freely, then encapsulated polar parts of mangiferin.
- -
- In the three media (pH 1.2, pH 6.8 and water), the solubility efficiency of mangiferin from the phospholipid complex was higher than pure mangiferin.
- -
- Compared to pure mangiferin and its physical mixture with phospholipids, mangiferin-phospholipid complexes had higher solubility in water or n-octanol. Especially in n-octanol, the solubility of mangiferin-phospholipid was about 30 times higher, the solubility in water was 1.4 times higher when compared with pure mangiferin and the water-oil partition coefficient of the complex was 6.2 times higher than with mangiferin. As a result, the complex increased mangiferin solubility in the lipid phase, which would increase intestinal permeability thereby increasing the oral bioavailability of mangiferin.
- -
- Compared to pure mangiferin at the duodenum, jejunum, ileum and colon, the mangiferin phospholipid complex had higher rates of constraint of drug absorption at 4.9, 4.8, 5.9 and 18.7 times, respectively, and the effective permeability values were also 11.4, 13.25, 9.2 and 64.6 times higher, respectively.
- -
- Pharmacokinetic studies in mice showed that after administration, comparing the physical mixture of mangiferin and phospholipid, their complex showed that the average Cmax increased from 180.90 to 377.66 μg/mL, the elimination half-life of mangiferin increased from 4.49 to 9.31 h and the relative bioavailability also increased by 230.00%.
3.3. Emulsion Solvent Evaporation Technique
- -
- Surfactants helped mangiferin particles to disperse finely in ethylene vinyl acetate films, preventing them from agglomeration (which is observed in films without surfactants).
- -
- The low molecular weight sorbitan esters consist of the hydrophilic heads and hydrophobic tails, while the high molecular weight polymeric surfactants contain a number of functional groups to interact with the particles. Span®20 helped to produce particles that were (39.86 ± 20.71 µm) smaller than the size (82.88 ± 30.05 μm) in the Pluronic®P 123 system.
- -
- Compared to Pluronic®P 123, Span®20 produced smaller particles and more stable microemulsions, the produced films had the same flexibility as ethylene vinyl acetate films without surfactants.
- -
- Vinyl acetate contents had a significant influence on film characteristics: increased degree of crystallization and melting temperature; reduced flexibility and degree of mangiferin release into 95% ethanol with reduced vinyl acetate contents. Vinyl acetate content only mildly affected the glass transition temperature. Oxygen permeability (the highest oxygen barrier), which also played an important role in the suppression of oxidative reactions related to the antioxidant activity, increased with the increasing of vinyl acetate content.
- -
- Compared to the ethylene vinyl acetate films combined with mangiferin without any surfactants, the addition of Span®20 only slightly affected the mechanical and barrier characteristics of the films, but markedly increased the mangiferin release from the ethylene vinyl acetate matrix, thereby significantly increasing the activity of antioxidants except in 40% vinyl acetate film.
- -
- Encapsulation efficiency increased with increasing stirring speed and increasing mangiferin concentration, but the increase in concentration also promoted the saturation of the system leading to reduced efficiency again. According to the results of statistical analysis, the most important factor affecting encapsulation efficiency is speed, followed by the influence of concentration ratio and interaction among them. Accordingly, under optimal encapsulation conditions with 15 mg of PLGA, 300 μg mangiferin was stirred for 10 min with a stirring speed of 6000 rpm and obtained 77% and 97% trap efficiency.
- -
- The optimal formulation was obtained with a response surface methodology. The average size was 176.7 ± 1.021 nm with a polydispersity index value of 0.153, and mangiferin packaging efficiency was about 55%.
- -
- Under in vitro conditions, mangiferin is gradually released over a period of 15 to 180 min under acidic conditions (pH 1.5).
- -
- Fingerprint studies have found a change in the maximum absorption wavelength of both the polymer and the mangiferin. This means that intermolecular bond formation has occurred and also explains the transition of the polymer state from crystal to amorphous.
- -
- This encapsulation product was resistant to in vitro digestion (1.5 h) without the metabolism of healthy cells as well as without modulation of their biological activities.
- -
- The results of the toposiomerase resistance tests have shown that the optimal formulation at 25 μg/mL has anticoagulant activity. Even at a high concentration of 2500 μg/mL, this formulation does not alter morphology and could not reduce the viability of the BEAS-2B and HEPG2 cell lines.
3.4. Supercritical Antisolvent Technique
- -
- In the SAS1 process, both the polymer and the mangiferin were dissolved in the same solution in different proportions (from 1:1 to 1:10), then it was sprayed into the flask via one nozzle. The CO2 flow rate was 30 g/min, the solution flow rate was 5 mL/min and the nozzle diameter 100 μm.
- -
- In the SAS2 process, the polymer and mangiferin solutions were prepared separately and they were sprayed into the same tank through two different nozzles. The SAS2 process was performed under the same conditions.
- -
- In the SAS1 process, the average size of particles ranged from 0.25 to 0.41 μm with a narrow particle size distribution, the ratio between the mangiferin and the polymer did not affect the particle size and morphology of the collected precipitate and higher mangiferin loadings were found for the lower proportion of mangiferin in the system.
- -
- In the SAS2 process, two different particle morphologies were formed, consisting of the precipitates formed by a large number of very small mangiferin fibers and microspheres by cellulose acetate phthalate in the range 0.2–1.0 μm. The size of the system increased as its polymer ratio increased.
- -
- After the SAS processes, the systems were in an amorphous state, losing their crystallinity because they could not be restructured into a crystal under the effect of supercritical CO2 flow and unmodified mangiferin and polymer chemical change. This helped to improve the solubility of mangiferin in water.
- -
- In two liquids simulating intestinal and gastric juice, after three minutes almost 100% mangiferin-cellulose acetate phthalate was dissolved, greatly improved compared to commercial mangiferin (dissolved about 15% in 3 min and 95% after two hours).
- -
- Investigation of mangiferin release level of SAS1 products showed that the presence of cellulose acetate phthalate slowed the process in gastric juice and did not affect the release in the intestinal juice. However, instead of the polymer ratios in the system, their high flow rate during SAS2 was the factor that reduced the release of mangiferin in the digestive environment.
3.5. Nanoemulsion Technique
- -
- Nanoemulsions had droplet sizes ranging from 194.5 to 397.9 nm, their mean size was 296 nm with a single dispersion distribution (PI ≤ 0.30). The zeta potential was very negative (<−30 mV). They were all physically stable for up to 30 days under 4 °C storage conditions.
- -
- The mean size and zeta potential of the oil core depend on the molecular weight of hyaluronic acid. Short-chain hyaluronic acid nanoparticles had smaller particle size and more negative zeta potential; they were similar to nanoemulsions without polymers.
- -
- The solubility of mangiferin-phospholipid-polysorbate 80 (0.71 ± 0.03 mg/mL) was higher than that of mangiferin (0.10 ± 0.01 mg/mL) and mangiferin-phospholipid (0.25 ± 0.02 mg/mL). The partition coefficient of mangiferin-phospholipid-polysorbate 80 (5.23 ± 0.17) was also higher than that of mangiferin (0.12 ± 0.01) and mangiferin-phospholipid (2.10 ± 0.12).
- -
- High molecular weight hyaluronic acid formed nanoemulsions with high viscosity. All the nanoemulsions had pseudo-plasticity properties (s~0.40), which were unchanged with or without Transcutol-P.
- -
- The lower molecular weight hyaluronic acid formed the nanoemulsion that had more improvement in mangiferin permeability and it was further improved in the presence of Transcutol-P.
- -
- Compared with the demonstrated hollow nanoemulsion, all mangiferin-containing nanoemulsions strongly inhibited edema and decreased myeloperoxidase activity. Treatment with the mangiferin formula showed a significant improvement in animals’ skin, they exhibited inhibitory edema and leukocytosis activity (p < 0.01).
3.6. Sol-Gel Synthesis Technique
- -
- Similar to other studies on integrating mangiferin into polymer systems, this group of authors has also made a comment about the formation of intra-molecular and inter-molecular hydrogen bonds. However, in this work, by using attenuated total reflection FTIR and SEM with energy dispersive X-ray analysis, the authors not only validated intra-molecular and inter-molecular hydrogen bonds, but also proposed the schemes for interactions between homopolymers and mangiferin as follows (Figure 2 and Figure 3):
- -
- Thus, the cumulative release of mangiferin from binary and ternary mixtures indicated that the release depends on the hydrogel composition as well as pH. The mangiferin release was lower when its matrix was simpler, either its hydrogel had higher chitosan content or was carried out in a higher pH environment.
- -
- Preliminary results from swelling behavior and in vitro release studies revealed that hydrogel M90PV/5CHI/5GEL-T2 (1.8 g polyvinyl alcohol, 0.1 g chitosan, 0.1 g gelatin, 60 μg siloxane) integrated 0.05 g mangiferin was the most appropriate matrix for the controlled release of mangiferin.
3.7. Thin Film-Sonication Technique
- -
- Mangiferin loading micelles showed a spherical morphology with a diameter of 14.26 ± 0.52 nm and a zeta potential of −2.89 ± 1.70 mV. The micelle mixtures were neutral particles (±10 mV) with a general feature of high stability against agglomeration. This feature could allow them to diffuse readily through the mucus layer and deeper into the intestinal epithelium. Therefore, mixed micelles were considered stealth bags with special biological stability such as their ability to avoid rapid uptake by the monocytic phagocytic system and maintain circulation time.
- -
- Mangiferin was loaded in both hydrophilic and hydrophobic parts of the micelle mixes by directing its xanthone portion back towards the core and, its glucoside portion was close to corona.
- -
- Analysis of calculations by the Design Expert software obtained the optimal ratio of mangiferin loading into copolymers of F127/P123/TPGS of 0.120/0.328/0.552.
- -
- In the simulated digestive environment, while mangiferin was insoluble, all of its micelles showed excellent solubility and sustainable release.
3.8. Summary of the General Characteristics of the Polymer-Mangiferin Systems
- -
- Mangiferin prefers to combine with hydrophilic polymers with smaller molecular sizes than hydrophobic polymers or bulky molecules.
- -
- Mangiferin interacts closely with positively charged polymers (such as chitosan) to facilitate the development of a sustainable polymer system but this made it difficult to release the drug. Research is needed to have an appropriate ratio when combining these polymers with mangiferin.
- -
- Bonding formed in mangiferin loading polymer systems is mainly intermolecular and intra-molecular hydrogen.
- -
- Mangiferin can bind to both hydrophilic and hydrophilic parts of polymer systems, which greatly enhances the encapsulation efficiency and its loading efficiency, and its solubility and permeability are also greatly improved.
- -
- Most of the polymer systems of mangiferin dissolve better in an acidic environment than in a base environment, this solubility could be adjusted by changing the proportion of polymers in the systems.
- -
- Usually, the size of the system increases as the proportion of polymers and molecular sizes of the polymers in its composition increase.
- -
- The surfactant chemical structure also affects the characteristics of mangiferin-polymer systems including particle size, distribution of mangiferin and active ingredients in the matrix or their total properties. The presence of surfactants accelerates the release of active ingredients from the polymer systems.
4. Summary
- -
- use in other various encapsulation techniques;
- -
- more complete encapsulation techniques have to be implemented;
- -
- create new polymer systems with higher encapsulation quality, better drug loading and biological effects;
- -
- further in vitro and in vivo experiments, aiming the application of these advances at the treatment of life-threatening diseases in humans.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALP | alkaline phosphatase |
| AML | acute myeloid leukemia |
| ARE | antioxidant response element |
| AST | Aspartate Aminotransferase |
| ATR | Ataxia Telangiectasia and Rad3-related protein |
| Bax | Bcl-2 associated X protein |
| bcl-2 | B Cell Lymphoma-2 |
| bcl-xL | B Cell Lymphoma-extra large |
| Chk1 | Checkpoint kinase 1 |
| CDK1 | Cyclin-Dependent Kinase 1 |
| COX-2 | Cyclooxygenase-2 |
| DEN | diethylnitrosamine |
| DLS | dynamic light scattering |
| DSC | differential scanning calorimetry |
| EMT | Epithelial to Mesenchymal Transition |
| ERK | Extracellular signal-Regulated Kinase |
| ESI-MS | electrospray ionization mass spectrometry |
| FTIR | Fourier transform infrared spectra |
| GSH | glutathione S-transferase |
| H2O2 | hydrogen peroxide |
| HPLC | High-performance liquid chromatography |
| JNK | c-Jun terminal kinases |
| IKK-alpha | Inhibitor of NF-kB Kinase subunit-alpha |
| IKK-beta | Inhibitor of NF-kB Kinase subunit-beta |
| IL-1R | Interleukin-1 Receptors |
| IL-6 | Interleukin-6 |
| IL-8 | Interleukin-8 |
| i.m. | intramuscular route of administration |
| i.p. | intraperitoneal injection |
| IRAK1 | Interleukin-1 Receptor Activated Kinase 1 |
| LEF1 | Lymphoid Enhancer Binding Factor 1 |
| MAPK | Mitogen Activated Protein Kinase |
| MDSC | modulated differential scanning calorimeter |
| MMP | matrix metalloproteinase |
| MYC | MYC Proto-Oncogene, BHLH Transcription Factor |
| NF-kB | Nuclear Factor k-light-chain-enhancer of activated B cells |
| NIK | NCK Interacting Kinase |
| NMR | Nuclear Magnetic Resonance |
| NQO1 | NAD(P)H: quinine reductases |
| Nrf2 | Nuclear factor erythroid 2-Related Factor 2 |
| PARP | Poly ADP ribose polymerase-1 |
| PDG | Peptidoglycan |
| PG | Prostaglandin |
| PK | Pharmacokinetics |
| PKC | protein kinase C |
| PLGA | poly (lactic-co-glycolic) poly nanoparticles |
| PMN | polymorphonuclear cells |
| PPAR | Peroxisome Proliferator Activated Receptor Gamma |
| PVA | polyvinyl alcohol |
| QR | quinone reductase |
| ROS | reactive oxygen species |
| SAS | supercritical antisolvent process |
| SEAP | Secreted Embryonic Alkaline Phosphatase |
| SEM | scanning electron microscope |
| TEM | transmission electron microscopy |
| TNF | Tumor Necrosis Factor |
| TNFR | Tumor Necrosis Factor Receptor |
| TRADD | TNFR with Tumor Necrosis Factor Receptor type-1-Associated Death Domain protein |
| UV-Vis | Ultraviolet-visible |
| VEGF | Vascular Endothelial Growth Factor |
| VLA | very late antigens |
| XIAP | X linked Inhibitor of Apoptosis Protein |
References
- Nott, P.E.; Roberts, J.C. The structure of mangiferin. Phytochemistry 1967, 6, 741–747. [Google Scholar] [CrossRef]
- Bhatia, V.K.; Ramanathan, J.D.; Seshadri, T.R. Constitution of mangiferin. Tetrahedron 1967, 23, 1363–1368. [Google Scholar] [CrossRef]
- Nott, P.E.; Roberts, J.C. A synthesis of mangiferin. Phytochemistry 1967, 6, 1597–1599. [Google Scholar] [CrossRef]
- Faizi, S.; Zikr-Ur-Rehman, S.; Versiani, M.; Naz, A. Temperature and solvent dependent NMR studies on mangiferin and complete NMR spectral assignments of its acyl and methyl derivatives. Magn. Reson. Chem. 2006, 44, 838–844. [Google Scholar] [CrossRef] [PubMed]
- da Cruz, J.W., Jr; de Moraes, L.R.; dos Santos, M.H.; da Silva, G.A.; Brigagão, M.R.P.L.; Ellena, J.; Doriguetto, A.C. Crystalline structure of mangiferin, a C-Glycosyl-Substituted 9H-Xanthen-9-one isolated from the stem bark of mangifera indica. Helv. Chim. Acta 2008, 91, 144–154. [Google Scholar] [CrossRef]
- Wiechowski, W. Phytochemical and pharmacological investigations on mangiferin. Lotos 1908, 56, 61. [Google Scholar]
- Gold-Smith, F.; Fernandez, A.; Bishop, K. Mangiferin and cancer: Mechanisms of action. Nutrients 2016, 8, 396. [Google Scholar] [CrossRef]
- Barreto, J.C.; Trevisan, M.T.S.; Hull, W.E.; Erben, G.; de Brito, E.S.; Pfundstein, B.; Würtele, G.; Spiegelhalder, B.; Owen, R.W. Characterization and quantitation of polyphenolic compounds in bark, kernel, leaves, and peel of mango (Mangifera indica L.). J. Agric. Food Chem. 2008, 56, 5599–5610. [Google Scholar] [CrossRef]
- Acosta, J.; Sevilla, I.; Salomón, S.; Nuevas, L.; Romero, A.; Amaro, D. Determination of mangiferin solubility in solvents used in the biopharmaceutical industry. J. Pharm. Pharm. Res. 2016, 4, 49–53. [Google Scholar]
- da Rocha Ferreira, F.; Valentima, L.B.; Luís Catarí Ramones, E.; Salles Trevisan, M.T.; Olea-Azar, C.; Perez-Cruz, F.; et al. Antioxidant activity of the mangiferin inclusion complex with β-cyclodextrin. LWT Food Sci Technol. 2013, 51, 129–134. [Google Scholar] [CrossRef]
- Wada, M. Foodstuffs Compounding Agent for Treating Diabetes Comprises Glycoside Having Xanthone Structure. Japanese Patent JP2007204462-A, 2007. [Google Scholar] [CrossRef]
- Matkowski, A.; Kuś, P.; Góralska, E.; Woźniak, D. Mangiferin–a Bioactive Xanthonoid, not only from Mango and not just Antioxidant. Mini Rev. Med. Chem. 2012, 13, 439–455. [Google Scholar] [CrossRef]
- Kavitha, M.; Jagadeesan, N.; Musthafa, M.E.; Memon, M.; Manivasagam, T. Mangiferin attenuates MPTP induced dopaminergic neurodegeneration and improves motor impairment, redox balance and Bcl-2/Bax expression in experimental Parkinson’s disease mice. Chem. Biol. Interact. 2013, 206, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Tolosa, L.; Rodeiro, I.; Donato, M.T.; Herrera, J.A.; Delgado, R.; Castell, J.V.; Gómez Lechón, M.J. Multiparametric evaluation of the cytoprotective effect of the Mangifera indica L. stem bark extract and mangiferin in HepG2 cells. J Pharm Pharm. 2013, 65, 1073–1082. [Google Scholar] [CrossRef] [PubMed]
- Zou, T.; Wu, H.; Li, H.; Jia, Q.; Song, G. Comparison of microwave-assisted and conventional extraction of mangiferin from mango (Mangifera indica L.) leaves. J. Sep. Sci. 2013, 36, 3457–3462. [Google Scholar] [CrossRef] [PubMed]
- Cooper, G.M. The Cell: A Molecular Approach. In The Cell; Sinauer Associates, Boston Univ.: Sunderland, MA, USA, 2000; p. 689. ISBN 0-87893-106-6. [Google Scholar]
- Hu, X.; Moscinski, L.C. Cdc2: A monopotent or pluripotent CDK? Cell Prolif. 2011, 44, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.G.; Yao, Y.B.; Yang, J.; Tang, Y.L.; Huang, X. Mangiferin induces cell cycle arrest at G2/M phase through ATR-Chk1 pathway in HL-60 leukemia cells. Genet. Mol. Res. 2015, 14, 4989–5002. [Google Scholar] [CrossRef]
- du Plessis-Stoman, D.; du Preez, J.; van de Venter, M. Combination treatment with oxaliplatin and mangiferin causes increased apoptosis and downregulation of NFκB in cancer cell lines. Afr. J. Tradit. Complement. Altern. Med. AJTCAM 2011, 8, 177–184. [Google Scholar] [CrossRef]
- Lv, J.; Wang, Z.; Zhang, L.; Wang, H.-L.; Liu, Y.; Li, C.; Deng, J.; Yi-Wang; Bao, J.-K. Mangiferin induces apoptosis and cell Cycle arrest in MCF-7 cells both in vitro and in vivo. J. Anim. Vet. Adv. 2013, 12, 352–359. [Google Scholar] [CrossRef]
- Dolcet, X.; Llobet, D.; Pallares, J.; Matias-Guiu, X. NF-kB in development and progression of human cancer. Virchows Arch. 2005, 446, 475–482. [Google Scholar] [CrossRef]
- Garcia-Rivera, D.; Delgado Hernandez, R.; Bougarne, N.; Haegeman, G.; Berghe, W. Gallic acid indanone and mangiferin xanthone are strong determinants of immunosuppressive anti-tumour effects of Mangifera indica L. bark in MDA-MB231 breast cancer cell. Cancer Lett. 2011, 305, 21–31. [Google Scholar] [CrossRef]
- Shi, W.; Deng, J.; Tong, R.; Yang, Y.; He, X.; Lv, J.; Wang, H.; Deng, S.; Qi, P.; Zhang, D.; et al. Molecular mechanisms underlying mangiferin-induced apoptosis and cell cycle arrest in A549 human lung carcinoma cells. Mol. Med. Rep. 2016, 13, 3423–3432. [Google Scholar] [CrossRef] [PubMed]
- Telang, M.; Dhulap, S.; Mandhare, A.; Hirwani, R. Therapeutic and cosmetic applications of mangiferin: A patent review. Expert Opin. Ther. Pat. 2013, 23, 1561–1580. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, H.; Toyoda, M.; Shinohara, H.; Okuda, J.; Watanabe, I.; Yamamoto, T.; Tanaka, K.; Tenjo, T.; Tanigawa, N. Expression of survivin correlates with apoptosis, proliferation, and angiogenesis during human colorectal tumorigenesis. Cancer 2001, 91, 2026–2032. [Google Scholar] [CrossRef]
- Kim, H.; Kim, H.; Mosaddik, A.; Gyawali, R.; Ahn, K.S.; Cho, S.K. Induction of apoptosis by ethanolic extract of mango peel and comparative analysis of the chemical constitutes of mango peel and flesh. Food Chem. 2012, 133, 416–422. [Google Scholar] [CrossRef]
- Zou, B.; Wang, H.; Liu, Y.; Qi, P.; Lei, T.; Sun, M.; Wang, Y. Mangiferin induces apoptosis in human ovarian adenocarcinoma OVCAR3 cells via the regulation of Notch3. Oncol. Rep. 2017, 38, 1431–1441. [Google Scholar] [CrossRef]
- Stoilova, I.; Jirovetz, L.; Stoyanova, A.; Krastanov, A.; Gargova, S.; Ho, L. Antioxidant activity of the polyphenol mangiferin. Electron. J. Environ. Agric. Food Chem. 2008, 7, 2706–2716. [Google Scholar]
- Saha, S.; Sadhukhan, P.; Sil, P. Mangiferin: A xanthonoid with multipotent anti-inflammatory potential. Biofactors 2016, 42, 459–474. [Google Scholar] [CrossRef]
- Rajendran, P.; Ganapathy, E.; Sakthisekaran, D. Effect of mangiferin on benzo(a)pyrene induced lung carcinogenesis in experimental Swiss albino mice. Nat. Prod. Res. 2008, 22, 672–680. [Google Scholar] [CrossRef]
- Venugopal, R.; Dhanapal, S.; Rajkapoor, B.; Ikuo, N. In vitro Protective Effect of Mangiferin Against Glycated Protein-Iron Chelate Induced toxicity in human umbilical vein endothelial cells. J. Biol. Sci. 2007, 7, 1227–1232. [Google Scholar] [CrossRef]
- Pardo-Andreu, G.; Delgado Hernandez, R.; Velho, J.; Curti, C.; Vercesi, A. Iron complexing activity of mangiferin, a naturally occurring glucosylxanthone, inhibits mitochondrial lipid peroxidation induced by Fe 2+-citrate. Eur. J. Pharm. 2005, 513, 47–55. [Google Scholar] [CrossRef]
- García, D.; Escalante, M.; Delgado, R.; Ubeira, F.M.; Leiro, J. Anthelminthic and antiallergic activities of Mangifera indica L. stem bark components Vimang and mangiferin. Phytother. Res. 2003, 17, 1203–1208. [Google Scholar] [CrossRef] [PubMed]
- Garrido, G.; Gonzalez, D.; Delporte, C.; Backhouse, N.; Quintero, G.; Nunez Selles, A.; Morales, M. Analgesic and anti-inflammatory effects of Mangifera indica L. extract (Vimang). Phytother. Res. 2001, 15, 18–21. [Google Scholar] [CrossRef]
- Hu, H.G.; Wang, M.J.; Zhao, Q.J.; Yu, S.C.; Liu, C.M.; Wu, Q.Y. Synthesis of mangiferin derivates and study their potent PTP1B inhibitory activity. Chin. Chem. Lett. 2007, 18, 1323–1326. [Google Scholar] [CrossRef]
- Prashanth, D.; Agarwal, A.; Samiulla, D.; Asha, M.; Rani, P. α-Glucosidase inhibitory activity of Mangifera indica bark. Fitoterapia 2001, 72, 686–688. [Google Scholar] [CrossRef]
- Zhu, X.; Cheng, Y.; Du, L.; Li, Y.; Zhang, F.; Guo, H.; Liu, Y.-W.; Yin, X. Mangiferin attenuates renal fibrosis through down-regulation of osteopontin in diabetic rats. Phyther. Res. 2015, 29, 295–302. [Google Scholar] [CrossRef]
- Dineshkumar, B.; Mitra, A.; Mahadevappa, M. Studies on the anti-diabetic and hypolipidemic potentials of mangiferin (Xanthone Glucoside) in streptozotocin-induced Type 1 and Type 2 diabetic model rats. Int. J. Adv. Pharm. Sci. 2010, 1, 75–85. [Google Scholar] [CrossRef][Green Version]
- Sellamuthu, P.; Arulselvan, P.; Fakurazi, S. Beneficial effects of mangiferin isolated from Salacia chinensis on biochemical and hematological parameters in rats with streptozotocin-induced diabetes. Pak. J. Pharm. Sci. 2014, 27, 161–167. [Google Scholar]
- Du, S.; Liu, H.; Lei, T.; Xie, X.; Wang, H.; He, X.; Tong, R.; Wang, Y. Mangiferin: An effective therapeutic agent against several disorders (Review). Mol. Med. Rep. 2018, 18, 4775–4786. [Google Scholar] [CrossRef]
- Prabhu, S.; Jainu, M.; Sabitha, K.E.; Devi, C.S.S. Role of mangiferin on biochemical alterations and antioxidant status in isoproterenol-induced myocardial infarction in rats. J. Ethnopharmacol 2006, 107, 126–133. [Google Scholar] [CrossRef]
- Prabhu, S.; Jainu, M.; Sabitha, K.E.; Devi, C.S.S. Cardioprotective effect of mangiferin on isoproterenol induced myocardial infarction in rats. Indian J. Exp. Biol. 2006, 44, 209–215. [Google Scholar]
- Prabhu, S.; Jainu, M.; Sabitha, K.E.; Shyamala Devi, C.S. Effect of mangiferin on mitochondrial energy production in experimentally induced myocardial infarcted rats. Vasc. Pharm. 2006, 44, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, S.; Naraya, Sh.; Shyamala Devi, C.S. Mechanism of protective action of mangiferin on suppression of inflammatory response and lysosomal instability in rat model of myocardial infarction. Phytother. Res. 2009, 23, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Arozal, W.; Suyatna, F.; Juniantito, V.; Rosdiana, D.; Arumugam, S.; Aulia, R.; Monayo, E.; Siswandi, R. The effects of mangiferin (Mangifera indica L) in doxorubicin-induced cardiotoxicity in rats. Drug Res. (Stuttg.) 2014, 65, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Hou, J.; Xiao, Y.; Zhao, Z.; Chen, L. Cardioprotective effect of mangiferin on left ventricular remodeling in rats. Pharmacology 2012, 90, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Wei, B.; Song, X.; An, N.; Zhou, Y.; Jin, X.; Zhang, Y. Edaravone’s free radical scavenging mechanisms of neuroprotection against cerebral ischemia: Review of the literature. Int. J. Neurosci. 2014, 125, 555–565. [Google Scholar] [CrossRef]
- Abdul-Muneer, P.M.; Chandra, N.; Haorah, J. Interactions of oxidative stress and neurovascular inflammation in the pathogenesis of traumatic brain injury. Mol. Neurobiol. 2014, 51, 966–979. [Google Scholar] [CrossRef]
- Peng, Sh.; Hou, Y.; Yao, J.; Fang, J. Neuroprotection of mangiferin against oxidative damage via arousing Nrf2 signaling pathway in PC12 cells. Biofactors 2019, 45, 381–392. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, S.; Wang, J.; Shen, Y.; Zhang, J.; Wu, Q. Nrf2/HO-1 mediates the neuroprotective effect of mangiferin on early brain injury after subarachnoid hemorrhage by attenuating mitochondria-related apoptosis and neuroinflammation. Sci. Rep. 2017, 7, 11883. [Google Scholar] [CrossRef]
- Zheng, M.S.; Lu, Z.Y. Antiviral effect of mangiferin and isomangiferin on herpes simplex virus. Chin. Med. J. (Engl.) 1990, 103, 160–165. [Google Scholar]
- Wang, R.-R.; Gao, Y.-D.; Ma, C.-H.; Zhang, X.-J.; Huang, C.; Huang, J.; Zheng, Y.T. Mangiferin, an anti-HIV-1 agent targeting protease and effective against resistant strains. Molecules 2011, 16, 4264–4277. [Google Scholar] [CrossRef]
- Al-rawi, A.; Dulaimi, H.; Rawi, M. Antiviral activity of mangifera extract on influenza virus cultivated in different cell cultures. J. Pure Appl. Microbiol. 2019, 13, 455–458. [Google Scholar] [CrossRef]
- Rechenchoski, D.; Galhardi, L.; Cunha, A.; Ricardo, N.; Nozawa, C.; Linhares, R. Antiviral potential of mangiferin against poliovirus. Int. J. Pharmacol. Res. 2018, 8, 34–39. [Google Scholar] [CrossRef]
- Chattopadhyay, U.; Das, S.; Guha, S.; Ghosal, S. Activation of lymphocytes of normal and tumor bearing mice by mangiferin, a naturally occurring glucosylxanthone. Cancer Lett. 1987, 37, 293–299. [Google Scholar] [CrossRef]
- Guha, S.; Chattopadhyay, U.; Ghosal, S. Activation of peritoneal macrophages by mangiferin, a naturally occurring xanthone. Phyther. Res. 1993, 7, 107–110. [Google Scholar] [CrossRef]
- Rivera, D.; Delgado Hernandez, R.; Ubeira, F.; Leiro, J. Modulation of rat macrophage function by the Mangifera indica L. Extracts Vimang and mangiferin. Int. Immunopharmacol. 2002, 2, 797–806. [Google Scholar] [CrossRef]
- Makare, N.; Bodhankar, S.; Rangari, V. Immunomodulatory activity of alcoholic extract of Mangifera indica Linn in mice. J. Ethnopharmacol. 2001, 78, 133–137. [Google Scholar] [CrossRef]
- Rivera, D.; Leiro, J.; Delgado, R.; Sanmartín, L.M.; Ubeira, F.M. Mangifera indica L. Extract (Vimang) and mangiferin modulate Mouse humoral immune responses. Phytother. Res. 2004, 17, 1182–1187. [Google Scholar] [CrossRef]
- Garrido, G.; Blanco-Molina, M.; Sancho, R.; Macho, A.; Delgado, R.; Muñoz, E. An aqueous stem bark extract of Mangifera indica (Vimang®) inhibits T cell proliferation and TNF-induced activation of nuclear transcription factor NF-κB. Phytother. Res. 2005, 19, 211–215. [Google Scholar] [CrossRef]
- Carvalho, A.C.; Guedes, M.M.; Souza, A.L.; Trevisan, M.T.; Lima, A.F.; Santos, F.A.; Rao, V.S. Gastroprotective effect of mangiferin, a xanthonoid from mangifera indica, against gastric injury induced by ethanol and indomethacin in rodents. Planta Med. 2007, 73, 1372–1376. [Google Scholar] [CrossRef]
- Dar, A.; Faizi, S.; Naqvi, S.; Roome, T.; Zikr-Ur-Rehman, S.; Ali, M.; Firdous, S.; Moin, S.T. Analgesic and antioxidant activity of mangiferin and its derivatives: The structure activity relationship. Biol. Pharm. Bull. 2005, 28, 596–600. [Google Scholar] [CrossRef]
- Jagetia, G.C.; Baliga, M.S. Radioprotection by mangiferin in DBAxC57BL mice: A preliminary study. Phytomedicine 2005, 12, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Jagetia, G.C.; Venkatesha, V.A. Effect of mangiferin on radiation-induced micronucleus formation in cultured human peripheral blood lymphocytes. Environ. Mol. Mutagen. 2005, 46, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Menkovic, N.; Juranic, Z.; Stanojkovic, T.; Raonic-Stevanovic, T.; Šavikin, K.; Zduni´c, G.; Borojevic, N. Radioprotective activity of gentiana lutea extract and mangiferin. Phytother. Res. 2010, 24, 1693–1696. [Google Scholar] [CrossRef] [PubMed]
- Matute Almau, C.; Sanchez Gomez, M.V.; Campos Esparza, R.; Alberdi Alfonso, E.; Gottlieb, M.; IBarretxe Bilbao, G.; Delgado Garcia, J.-M.; Gruart, I.; Masso, A.; Leal Campanario, R. Compounds Having Neuroprotective Properties. WIPO Patent WO2007077279A1, 12 July 2007. [Google Scholar]
- Rivera, D.; Hernández, I.; Alvarez, A.; Hernández, B.; Montiel, L.; Garrido, G.; Cuzzocrea, S.; Delgado Hernandez, R. Anti-allergic properties of Mangifera indica L. extract (Vimang) and contribution of its glucosylxanthone mangiferin. J. Pharm. Pharmacol. 2006, 58, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Rivera, D.G.; Hernández, I.; Merino, N.; Luque, Y.; Álvarez, A.; Martín, Y.; Amador, A.; Nuevas, L.; Delgado, R. Mangifera indica L. extract (Vimang) and mangiferin reduce the airway inflammation and Th2 cytokines in murine model of allergic asthma. J. Pharm. Pharmacol. 2011, 63, 1336–1345. [Google Scholar] [CrossRef]
- Das, J.; Ghosh, J.; Roy, A.; Sil, P. Mangiferin exerts hepatoprotective activity against D-galactosamine induced acute toxicity and oxidative/nitrosative stress via Nrf2–NFκB pathways. Toxicol. Appl. Pharmacol. 2012, 260, 35–47. [Google Scholar] [CrossRef]
- Muruganandan, S.; Srinivasan, K.; Gupta, S.; Gupta, P.K.; Lal, J. Effect of mangiferin on hyperglycemia and atherogenicity in streptozotocin diabetic rats. J. Ethnopharmacol. 2005, 97, 497–501. [Google Scholar] [CrossRef]
- Lim, J.; Liu, Z.; Apontes, P.; Feng, D.; Pessin, J.E.; Sauve, A.A.; Angeletti, R.H.; Chi, Y. Dual mode action of mangiferin in mouse liver under high fat diet. PLoS ONE 2014, 9, e90137. [Google Scholar] [CrossRef]
- Jangra, A.; Lukhi, M.; Sulakhiya, K.; Baruah, C.; Lahkar, M. Protective effect of mangiferin against lipopolysaccharide-induced depressive and anxiety-like behavior in mice. Eur. J. Pharmacol. 2014, 740, 337–345. [Google Scholar] [CrossRef]
- Sethiya, N.; Nahata, A.; Dixit, V. Anxiolytic activity of canscora decussata in albino rats. J. Complement. Integr. Med. 2010, 7, Art.19. [Google Scholar] [CrossRef]
- Pardo-Andreu, G.L.; Maurmann, N.; Reolon, G.K.; Farias, C.B.; Schwartsmann, G.; Delgado, R.; Roesler, R. Mangiferin, a naturally occurring glucoxilxanthone improves long-term object recognition memory in rats. Eur. J. Pharmacol. 2010, 635, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, H.S.; Candelario-Jalil, E.; de Oliveira, A.C.P.; Olajide, O.A.; Martínez-Sánchez, G.; Fiebich, B.L. Mangiferin inhibits cyclooxygenase-2 expression and prostaglandin E2 production in activated rat microglial cells. Arch. Biochem. Biophys. 2008, 477, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Pal, P.B.; Sinha, K.; Sil, P.C. Mangiferin, a natural xanthone, protects murine liver in Pb(II) induced hepatic damage and cell death via MAP kinase, NF-κB and mitochondria dependent pathways. PLoS ONE 2013, 8, e56894. [Google Scholar] [CrossRef] [PubMed]
- Rasool, M.; Sabina, E.P.; Mahinda, P.S.; Gnanaselvi, B.C. Mangiferin, a natural polyphenol protects the hepatic damage in mice caused by CCl4 intoxication. Comp. Clin. Pathol. 2012, 21, 865–872. [Google Scholar] [CrossRef]
- Pan, C.-W.; Pan, Z.-Z.; Hu, J.-J.; Chen, W.; Zhou, G.; Lin, W.; Jin, L.; Xu, C. Mangiferin alleviates Lipopolysaccharide and d-galactosamine-induced acute liver injury by activating the Nrf2 pathway and inhibiting NLRP3 inflammasome activation. Eur. J. Pharmacol. 2015, 770, 85–91. [Google Scholar] [CrossRef]
- Xing, X.; Li, D.; Chen, D.; Zhou, L.; Chonan, R.; Yamahara, J.; Wang, J.; Li, Y. Mangiferin treatment inhibits hepatic expression of acyl-coenzyme A: Diacylglycerol acyltransferase-2 in fructose-fed spontaneously hypertensive rats: A link to amelioration of fatty liver. Toxicol. Appl. Pharmacol. 2014, 280, 207–215. [Google Scholar] [CrossRef]
- Andreu, G.L.P.; Delgado, R.; Velho, J.A.; Curti, C.; Vercesi, A.E. Mangiferin, a natural occurring glucosyl xanthone, increases susceptibility of rat liver mitochondria to calcium-induced permeability transition. Arch. Biochem. Biophys. 2005, 439, 184–193. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Wu, Z.; Liu, E.; Shi, P.; Han, L.; Guo, L.; Gao, X.; Wang, T. Acute and Long-Term Toxicity of Mango Leaves Extract in Mice and Rats. Evid.-Based Complement. Altern. Med. 2014, 2014, 691574. [Google Scholar] [CrossRef]
- Shah, K.A.; Patel, M.B.; Patel, R.J.; Parmar, P.K. Mangifera indica (mango). Pharmacogn. Rev. 2010, 4, 42–48. [Google Scholar] [CrossRef]
- González, J.E.; Rodríguez, M.D.; Rodeiro, I.; Morffi, J.; Guerra, E.; Leal, F.; García, H.; Goicochea, E.; Guerrero, S.; Garrido, G.; et al. Lack of in vivo embryotoxic and genotoxic activities of orally administered stem bark aqueous extract of Mangifera indica L. (Vimang®). Food Chem. Toxicol. 2008, 45, 2526–2532. [Google Scholar] [CrossRef]
- Reddeman, R.A.; Glávits, R.; Endres, J.R.; Clewell, A.E.; Hirka, G.; Vértesi, A.; Béres, E.; Szakonyiné, I.P. A toxicological evaluation of mango leaf extract (Mangifera indica) containing 60% mangiferin. J. Toxicol. 2019, 2019, 4763015. [Google Scholar] [CrossRef] [PubMed]
- Rodeiro, I.; Hernandez, S.; Morffi, J.; Herrera, J.; Gómez-Lechón, M.J.; Delgado Hernandez, R.; Espinosa-aguirre, J. Evaluation of genotoxicity and DNA protective effects of mangiferin, a glucosylxanthone isolated from Mangifera indica L. stem bark extract. Food Chem. Toxicol. 2012, 50, 3360–3366. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, M.; Nikolova, I.; Benbasat, N.; Kitanov, G.; Danchev, N. Acute toxicity, antidepressive and mao inhibitory activity of mangiferin isolated from hypericum aucheri. Biotechnol. Biotechnol. Equip. 2011, 25, 2668–2671. [Google Scholar] [CrossRef]
- Matsushima, T.; Araki, A.; Yagame, O.; Muramatsu, M.; Koyama, K.; Ohsawa, K.; Natori, S.; Tomimori, H. Mutagenicities of xanthone derivatives in Salmonella typhimurium TA100, TA98, TA97, and TA2637. Mutat. Res. 1985, 150, 141–146. [Google Scholar] [CrossRef]
- Shanmugam, M.K.; Kannaiyan, R.; Sethi, G. Targeting Cell Signaling and Apoptotic Pathways by Dietary Agents: Role in the Prevention and Treatment of Cancer. Nutr. Cancer 2011, 63, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, M.G.; Candelario-Jalil, E.; Giuliani, A.; León, O.-S.; Sam, S.; Delgado, R.; Nunez Selles, A. Mangifera indica L. extract (QF808) reduces ischaemia-induced neuronal loss and oxidative damage in the gerbil brain. Free Radic. Res. 2001, 35, 465–473. [Google Scholar] [CrossRef]
- Hou, Y.I.; Fan, S.J.; Zhang, H.; Gu, Y.Q.; Yu, X.H.; Li, B.X. Pharmacokinetic study of mangiferin in rat plasma and retina using high-performance liquid chromatography. Mol. Vis. 2010, 16, 1659–1668. [Google Scholar]
- Hou, S.Y.; Wang, F.; Li, Y.M.; Li, Y.; Wang, M.Q.; Sun, D.J.; Sun, C.H. Pharmacokinetic study of mangiferin in human plasma after oral administration. Food Chem. 2012, 132, 289–294. [Google Scholar] [CrossRef]
- Wang, H.; Ye, G.; Tang, Y.H.; Zhu, H.Y.; Ma, R.R.; Sun, Z.L.; Huang, C.G. High-performance liquid chromatographic method for the determination of mangiferin in rat plasma and urine. Biomed. Chromatogr. 2006, 20, 1304–1308. [Google Scholar] [CrossRef]
- Bock, C.; Waldmann, K.H.; Ternes, W. Mangiferin and hesperidin metabolites are absorbed from the gastrointestinal tract of pigs after oral ingestion of a Cyclopia genistoides (honeybush tea) extract. Nutr. Res. 2009, 28, 879–891. [Google Scholar] [CrossRef]
- Han, D.; Chen, C.; Zhang, C.; Zhang, Y.; Tang, X. Determination of mangiferin in rat plasma by liquid-liquid extraction with UPLC-MS/MS. J. Pharm. Biomed. Anal. 2009, 51, 260–263. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Liu, Z.; Zhang, C.; Zhang, B. Gelucire44/14 as a novel absorption enhancer for drugs with different hydrophilicities: In Vitro and in vivo improvement on transcorneal permeation. J. Pharm. Sci. 2011, 100, 3186–3195. [Google Scholar] [CrossRef] [PubMed]
- Andreu, G.P.; Delgado, R.; Velho, J.; Inada, N.M.; Curti, C.; Vercesi, A.E. Mangifera indica L. extract (Vimang) inhibits Fe2+-citrate-induced lipoperoxidation in isolated rat liver mitochondria. Pharmacol. Res. 2005, 51, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Martínez, G.; Delgado, R.; Pérez, G.; Garrido, G.; Núñez Sellés, A.J.; León, O.S. Evaluation of the in vitro antioxidant activity of Mangifera indica L. extract (Vimang). Phytother. Res. 2000, 14, 424–427. [Google Scholar] [CrossRef]
- Sánchez, G.M.; Re, L.; Giuliani, A.; Nunez Selles, A.J.; Davison, G.P.; León-Fernández, O.S. Protective effects of Mangifera indica L. extract, Mangiferin and selected antioxidants against TPA-induced biomolecules oxidation and peritoneal macrophage activation in mice. Pharmacol. Res. 2001, 42, 565–573. [Google Scholar] [CrossRef]
- Leiro, J.; Arranz, J.A.; Yáñez, M.; Ubeira, F.M.; Sanmartín, M.L.; Orallo, F. Expression profiles of genes involved in the mouse nuclear factor-kappa B signal transduction pathway are modulated by mangiferin. Int. Immunopharmacol. 2004, 4, 763–778. [Google Scholar] [CrossRef]
- Naik, E.; Dixit, V.M. Mitochondrial reactive oxygen species drive proinflammatory cytokine production. J. Exp. Med. 2011, 208, 417–420. [Google Scholar] [CrossRef]
- Leiro, J.M.; Alvarez, E.; Arranz, J.A.; Siso, I.G.; Orallo, F. In vitro effects of mangiferin on superoxide concentrations and expression of the inducible nitric oxide synthase, tumour necrosis factor-alpha and transforming growth factor-beta genes. Biochem. Pharmacol. 2003, 65, 1361–1371. [Google Scholar] [CrossRef]
- Rodríguez, J.; Di Pierro, D.; Gioia, M.; Monaco, S.; Delgado, R.; Coletta, M.; Marini, S. Effects of a natural extract from Mangifera indica L, and its active compound, mangiferin, on energy state and lipid peroxidation of red blood cells. Biochim. Biophys. Acta 2006, 1760, 1333–1342. [Google Scholar] [CrossRef]
- Sarkar, A.; Sreenivasan, Y.; Ramesh, G.T.; Manna, S.K. beta-D-Glucoside suppresses tumor necrosis factor-induced activation of nuclear transcription factor kappaB but potentiates apoptosis. J. Biol. Chem. 2004, 279, 33768–33781. [Google Scholar] [CrossRef]
- Sahoo, B.K.; Zaidi, A.H.; Gupta, P.; Mokhamatam, R.B.; Raviprakash, N.; Mahali, S.; Manna, S.K. A natural xanthone increases catalase activity but decreases NF-kappa B and lipid peroxidation in U-937 and HepG2 cell lines. Eur. J. Pharmacol. 2015, 764, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.J.; Jang, S.E.; Hyam, S.R.; Han, M.J.; Kim, D.H. Mangiferin ameliorates colitis by inhibiting IRAK1 phosphorylation in NF-κB and MAPK pathways. Eur. J. Pharmacol. 2014, 740, 652–661. [Google Scholar] [CrossRef] [PubMed]
- Garrido, G.; González, D.; Lemus, Y.; Delporte, C.; Delgado, R. Protective effects of a standard extract of Mangifera indica L. (VIMANG) against mouse ear edemas and its inhibition of eicosanoid production in J774 murine macrophages. Phytomedicine 2006, 13, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Jain, A.; Marleau, S.; Clish, C.; Kantarci, A.; Behbehani, B.; Colgan, S.P.; Stahl, G.L.; Merched, A.; Petasis, N.A.; et al. Reduced inflammation and tissue damage in transgenic rabbits overexpressing 15-Lipoxygenase and endogenous anti-inflammatory lipid mediators 1. J. Immunol. 2004, 171, 6856–6865. [Google Scholar] [CrossRef]
- Dou, W.; Zhang, J.; Ren, G.; Ding, L.; Sun, A.; Deng, C.; Wu, X.-J.; Wei, X.; Mani, S.; Wang, Z. Mangiferin attenuates the symptoms of dextran sulfate sodium-induced colitis in mice via NF-κB and MAPK signaling inactivation. Int. Immunopharmacol. 2014, 23, 170–178. [Google Scholar] [CrossRef]
- Mahmoud-Awny, M.; Attia, A.S.; Abd-Ellah, M.F.; El-Abhar, H.S. Mangiferin mitigates gastric ulcer in ischemia/ reperfused rats: Involvement of PPAR-γ, NF-κB and Nrf2/HO-1 signaling pathways. PLoS ONE 2015, 10, e0132497. [Google Scholar] [CrossRef]
- Pardo-Andreu, G.L.; Sánchez-Baldoquín, C.; Avila-González, R.; Delgado, R.; Naal, Z.; Curti, C. Fe(III) improves antioxidant and cytoprotecting activities of mangiferin. Eur. J. Pharmacol. 2006, 547, 31–36. [Google Scholar] [CrossRef]
- Rajendran, P.; Rengarajan, T.; Natarajan, N.; Divya, H.; Nishigaki, I. Mangiferin in cancer chemoprevention and treatment: Pharmacokinetics and molecular targets. J. Recept. Signal Transduct. Res. 2014, 35, 1–9. [Google Scholar] [CrossRef]
- Wilkinson, A.S.; Taing, M.-W.; Pierson, J.T.; Lin, C.-N.; Dietzgen, R.G.; Shaw, P.N.; Gidley, M.J.; Monteith, G.R.; Roberts-Thomson, S.J. Estrogen modulation properties of mangiferin and quercetin and the mangiferin metabolite norathyriol. Food Funct. 2015, 6, 1847–1854. [Google Scholar] [CrossRef]
- Guha, S.; Ghosal, S.; Chattopadhyay, U. Antitumor, immunomodulatory and anti-HIV effect of mangiferin, a naturally occurring glucosylxanthone. Chemotherapy 1996, 42, 443–451. [Google Scholar] [CrossRef]
- Rajendran, P.; Ekambaram, G.; Sakthisekaran, D. Protective role of mangiferin against Benzo(a)pyrene induced lung carcinogenesis in experimental animals. Biol. Pharm. Bull. 2008, 31, 1053–1058. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, P.; Ganapathy, E.; Sakthisekaran, D. Cytoprotective Effect of mangiferin on Benzo(a)pyrene-induced lung carcinogenesis in swiss albino mice. Basic Clin. Pharmacol. Toxicol. 2008, 103, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Huang, J.; Yang, B.; Xiang, T.; Yin, X.; Peng, W.; Cheng, W.; Wan, J.; Luo, F.; Li, H.; et al. Mangiferin exerts antitumor activity in breast cancer cells by regulating matrix metalloproteinases, epithelial to mesenchymal transition, and β-catenin signaling pathway. Toxicol. Appl. Pharmacol. 2013, 272, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Takeda, T.; Tsubaki, M.; Sakamoto, K.; Ichimura, E.; Enomoto, A.; Suzuki, Y.; Itoh, T.; Imano, M.; Tanabe, G.; Muraoka, O.; et al. Mangiferin, a novel nuclear factor kappa B-inducing kinase inhibitor, suppresses metastasis and tumor growth in a mouse metastatic melanoma model. Toxicol. Appl. Pharmacol. 2016, 306, 105–112. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, B.; Li, S.; Zeng, L.; Chen, Y.; Fang, J. Mangiferin increases Nrf2 protein stability by inhibiting its ubiquitination and degradation in human HL60 myeloid leukemia cells. Int. J. Mol. Med. 2014, 33, 1348–1354. [Google Scholar] [CrossRef]
- Zhang, B.P.; Zhao, J.; Li, S.S.; Yang, L.J.; Zeng, L.L.; Chen, Y.; Fang, J. Mangiferin activates Nrf2-antioxidant response element signaling without reducing the sensitivity to etoposide of human myeloid leukemia cells in vitro. Acta Pharmacol. Sin. 2014, 35, 257–266. [Google Scholar] [CrossRef]
- Zhang, B.P.; Zhao, J.; Li, S.S.; Zeng, L.L.; Chen, Y.; Fang, J. Mangiferin activates the Nrf2-ARE pathway and reduces etoposide-induced DNA damage in human umbilical cord mononuclear blood cells. Pharm. Biol. 2015, 53, 503–511. [Google Scholar] [CrossRef]
- Shoji, K.; Tsubaki, M.; Yamazoe, Y.; Satou, T.; Itoh, T.; Kidera, Y.; Tanimori, Y.; Yanae, M.; Matsuda, H.; Taga, A.; et al. Mangiferin induces apoptosis by suppressing Bcl-xL and XIAP expressions and nuclear entry of NF-κB in HL-60 cells. Arch. Pharm. Res. 2011, 34, 469–475. [Google Scholar] [CrossRef]
- Peng, Z.G.; Luo, J.; Xia, L.H.; Chen, Y.; Song, S.J. CML cell line K562 cell apoptosis induced by mangiferin. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2004, 12, 590–594. [Google Scholar]
- Cheng, P.; Peng, Z.G.; Yang, J.; Song, S.J. The effect of mangiferin on telomerase activity and apoptosis in Leukemic K562 cells. Zhong Yao Cai 2007, 30, 306–309. [Google Scholar]
- Yao, Y.B.; Peng, Z.G.; Liu, Z.F.; Yang, J.; Luo, J. Effects of mangiferin on cell cycle status and CDC2/Cyclin B1 expression of HL-60 cells. Zhong Yao Cai 2010, 33, 81–85. [Google Scholar] [PubMed]
- Xiao, J.; Liu, L.; Zhong, Z.; Xiao, C.; Zhang, J. Mangiferin regulates proliferation and apoptosis in glioma cells by induction of microRNA-15b and inhibition of MMP-9 expression. Oncol. Rep. 2015, 33, 2815–2820. [Google Scholar] [CrossRef] [PubMed]
- Dilshara, M.; Kang, C.-H.; Choi, Y.-H.; Gi Young, K. Mangiferin inhibits tumor necrosis factor-α-induced matrix metalloproteinase-9 expression and cellular invasion by suppressing nuclear factor-κB activity. BMB Rep. 2015, 48. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Yang, J.; Moses, M.A. Matrix metalloproteinases as novel biomarkers and potential therapeutic targets in human cancer. J. Clin. Oncol. 2009, 27, 5287–5297. [Google Scholar] [CrossRef]
- Jung, J.S.; Jung, K.; Kim, D.H.; Kim, H.S. Selective inhibition of MMP-9 gene expression by mangiferin in PMA-stimulated human astroglioma cells: Involvement of PI3K/Akt and MAPK signaling pathways. Pharm. Res. 2012, 66, 95–103. [Google Scholar] [CrossRef]
- Li, M.; Ma, H.; Yang, L.; Li, P. Mangiferin inhibition of proliferation and induction of apoptosis in human prostate cancer cells is correlated with downregulation of B-cell lymphoma-2 and upregulation of microRNA-182. Oncol Lett. 2016, 11, 817–822. [Google Scholar] [CrossRef]
- Yoshimi, N.; Matsunaga, K.; Katayama, M.; Yamada, Y.; Kuno, T.; Qiao, Z.; Hara, A.; Yamahara, J.; Mori, H. The inhibitory effects of mangiferin, a naturally occurring glucosylxanthone, in bowel carcinogenesis of male F344 rats. Cancer Lett. 2001, 163, 163–170. [Google Scholar] [CrossRef]
- Rao, S.; Sreedevi, M.V.; Rao, B. Cytoprotective and antigenotoxic potential of Mangiferin, a glucosylxanthone against cadmium chloride induced toxicity in HepG2 cells. Food Chem. Toxicol. 2008, 47, 592–600. [Google Scholar] [CrossRef]
- Huang, H.; Nong, C.; Guo, L. The proliferation inhibition effect and apoptosis induction of Mangiferin on BEL-7404 human hepatocellular carcinoma cell. Chinese J. Dig. 2002, 6, 341–343. [Google Scholar]
- Yang, G.; Shang, X.; Guozhen Cui, G.; Zhao, L.; Zhao, H.; Wang, N. Mangiferin attenuated diethynitrosamine-induced hepatocellular carcinoma in sprague-dawley rats via alteration of oxidative stress and apoptotic pathway. J Environ. Pathol. Toxicol. Oncol. 2019, 38, 1–12. [Google Scholar] [CrossRef]
- Saranya, M.; Maheswari, R. Mangiferin a bioactive compound of mangifera indica l on oxidative damage and antioxidant status in n-diethylnitrosoamine induced hepatocellular carcinoma in animal model. J. Pharm. Biol. Sci. 2018, 6, 114–124. [Google Scholar] [CrossRef]
- Tan, H.-Y.; Wang, N.; ·Li, S.; ·Hong, M.; ·Guo, W.; ·Man, K.; ·Cheng, C.; ·Chen, Z.; ·Feng, Y. Repression of WT1-mediated LEF1 transcription by mangiferin governs β-catenin-independent Wnt signalling inactivation in hepatocellular carcinoma. Cell Physiol. Biochem. 2018, 47, 1819–1834. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, P.; Jayakumar, T.; Nishigaki, I.; Ekambaram, G.; Nishigaki, Y.; Vetriselvi, J.; Sakthisekaran, D. Immunomodulatory effect of mangiferin in experimental animals with Benzo(a)Pyrene-induced lung carcinogenesis. Int. J. Biomed. Sci. 2013, 9, 68–74. [Google Scholar] [PubMed]
- Rajendran, P.; Ekambaram, G.; Magesh, V.; Sakthisekaran, D. Chemopreventive efficacy of mangiferin against benzo(a)pyrene induced lung carcinogenesis in experimental animals. Environ. Toxicol. Pharmacol. 2008, 26, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, P.; Venugopal, R.; Ganapathy, E.; Aadithya, A.; Sakthisekaran, D. Rehabilating activity of mangiferin in benzo(a) pyrene induced lung carcinogenesis. Asian J. Biochem. 2008, 3, 118–125. [Google Scholar] [CrossRef]
- Rajendran, P.; Rengarajan, T.; Nishigaki, I.; Ekambaram, G.; Sakthisekaran, D. Potent chemopreventive effect of mangiferin on lung carcinogenesis in experimental Swiss albino mice. J. Cancer Res. Ther. 2014, 10, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.L.; Wang, A.Y.; Huang, Y.Q.; Luo, Y.; Ling, M. Mangiferin induces apoptosis by regulating Bcl-2 and Bax expression in the CNE2 nasopharyngeal carcinoma cell line. Asian Pac. J. Cancer Prev. 2014, 15, 7065–7068. [Google Scholar] [CrossRef]
- Das, S.; Rao, B.; Rao, S. Mangiferin attenuates methylmercury induced cytotoxicity against IMR-32, human neuroblastoma cells by the inhibition of oxidative stress and free radical scavenging potential. Chem. Biol. Interact. 2011, 193, 129–140. [Google Scholar] [CrossRef]
- Ahmad, A.; Padhye, S.; Sarkar, F. Role of Novel nutraceuticals garcinol, plumbagin and mangiferin in the prevention and therapy of human malignancies: Mechanisms of anticancer activity. In Nutraceuticals and Cancer; Springer: Berlin/Heidelberg, Germany, 2012; pp. 179–199. ISBN 9789400726291. [Google Scholar]
- Bartoszewski, R.; Hering, A.; Marszałł, M.; Stefanowicz Hajduk, J.; Bartoszewska, S.; Kapoor, N.; Kochan, K.; Ochocka, R. Mangiferin Has an Additive Effect on the Apoptotic Properties of Hesperidin in Cyclopia sp. Tea Extracts. PLoS ONE 2014, 9, e92128. [Google Scholar] [CrossRef]
- Louisa, M.; Soediro, T.M.; Suyatna, F.D. In vitro modulation of P-glycoprotein, MRP-1 and BCRP expression by mangiferin in doxorubicin-treated MCF-7 cells. Asian Pac J Cancer Prev. 2014, 15, 1639–1642. [Google Scholar] [CrossRef]
- de Souza, J.R.R.; Feitosa, J.P.A.; Ricardo, N.M.P.S.; Trevisan, M.T.S.; de Paula, H.C.B.; Ulrich, C.M.; Owen, R.W. Spray-drying encapsulation of mangiferin using natural polymers. Food Hydrocoll. 2013, 33, 10–18. [Google Scholar] [CrossRef]
- de Sampaio, C.G.; Frota, L.S.; Magalhães, H.S.; Dutra, L.M.U.; Queiroz, D.C.; Araújo, R.S.; Becker, H.; de Souza, J.R.R.; Ricardo, N.M.P.S.; Trevisan, M.T.S. Chitosan/mangiferin particles for Cr(VI) reduction and removal. Int. J. Biol. Macromol. 2015, 78, 273–279. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ma, H.; Chen, H.; Sun, L.; Tong, L.; Zhang, T. Improving permeability and oral absorption of mangiferin by phospholipid complexation. Fitoterapia 2014, 93, 54–61. [Google Scholar] [CrossRef]
- Boonnattakorn, R.; Sane, A.; Chonhenchob, V. Antioxidant Microemulsion-based Ethylene Vinyl Acetate Film Containing Mangiferin and Surfactants. MATEC Web Conf. 2016, 67, 6101. [Google Scholar] [CrossRef]
- Razura-Carmona, F.F.; Pérez-Larios, A.; González-Silva, N.; Herrera-Martínez, M.; Medina-Torres, L.; Sáyago-Ayerdi, S.G.; Sánchez-Burgos, J.A. Mangiferin-loaded polymeric nanoparticles: Optical characterization, effect of anti-topoisomerase I., and cytotoxicity. Cancers 2019, 11, 1965. [Google Scholar] [CrossRef]
- García-Casas, I.; Montes, A.; Pereyra, C.; Martinez de la Ossa, E. Co-precipitation of mangiferin with cellulose acetate phthalate by Supercritical antisolvent process. J. CO2 Util. 2017, 22, 197–207. [Google Scholar] [CrossRef]
- Pleguezuelos-Villa, M.; Nácher, A.; Hernández, M.J.; Ofelia Vila Buso, M.A.; Ruiz Sauri, A.; Díez-Sales, O. Mangiferin nanoemulsions in treatment of inflammatory disorders and skin regeneration. Int. J. Pharm. 2019, 564, 299–307. [Google Scholar] [CrossRef]
- Pipattanawarothai, A.; Athipornchai, A.; Sripreechasak, P.; Trakulsujaritchok, T. Development of polymeric hydrogels for potential biomedical applications: Sol-gel synthesis and in vitro release of mangiferin. Burapha Sci. J. 2019, 24, 885–900. [Google Scholar]
- Thanitwatthanasak, S.; Sagis, L.; Chitprasert, P. Pluronic F127/Pluronic P123/vitamin E TPGS mixed micelles for oral delivery of mangiferin and quercetin: Mixture-design optimization, micellization, and solubilization behavior. J. Mol. Liq. 2019, 274, 223–238. [Google Scholar] [CrossRef]
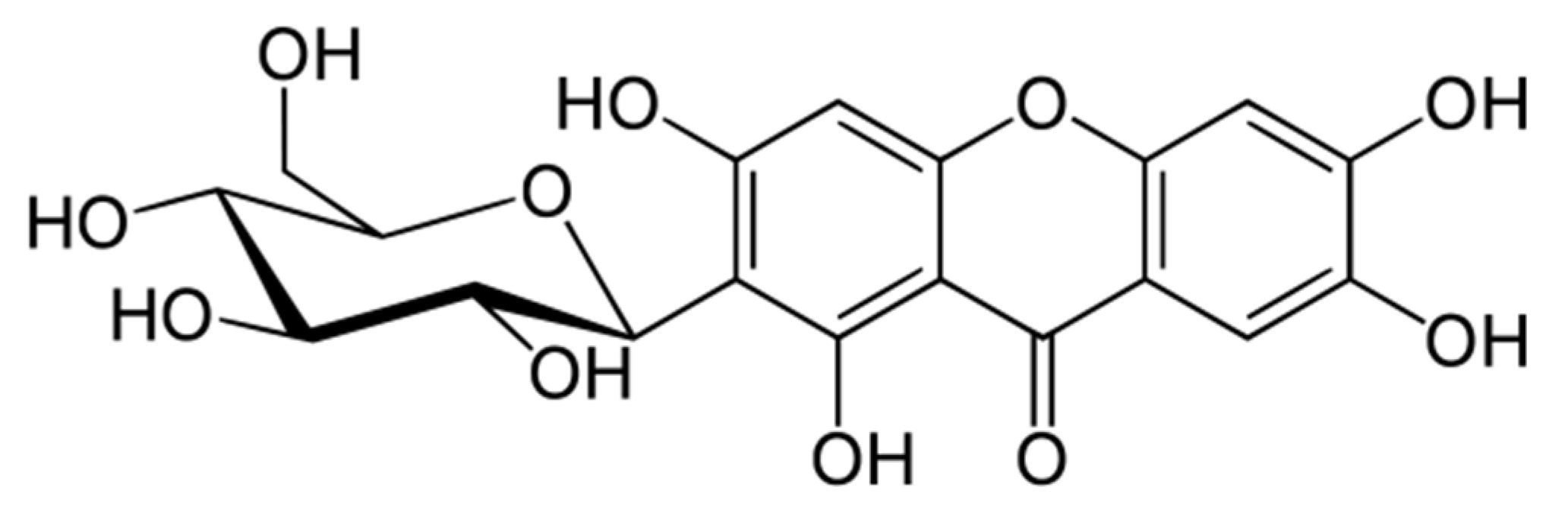
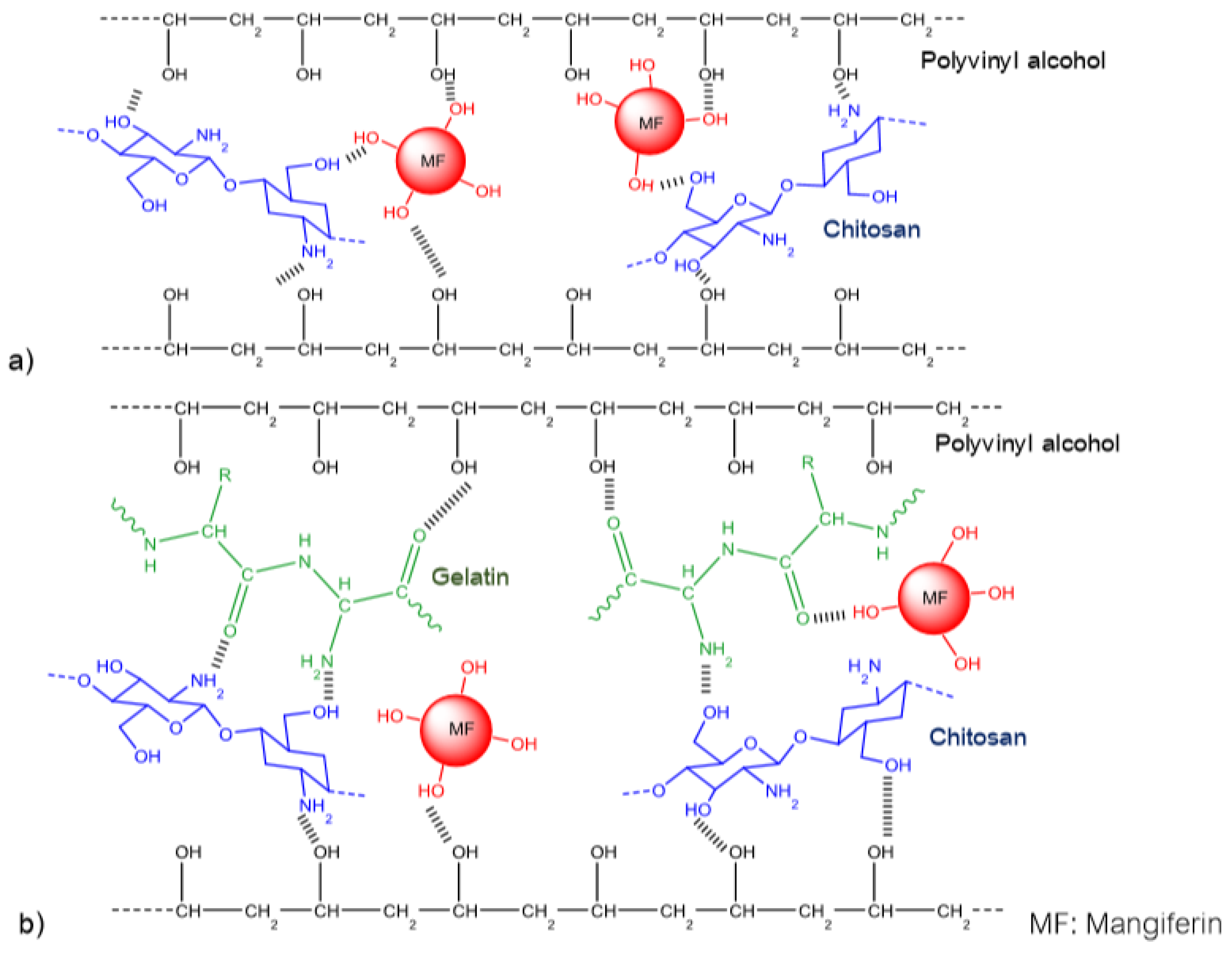
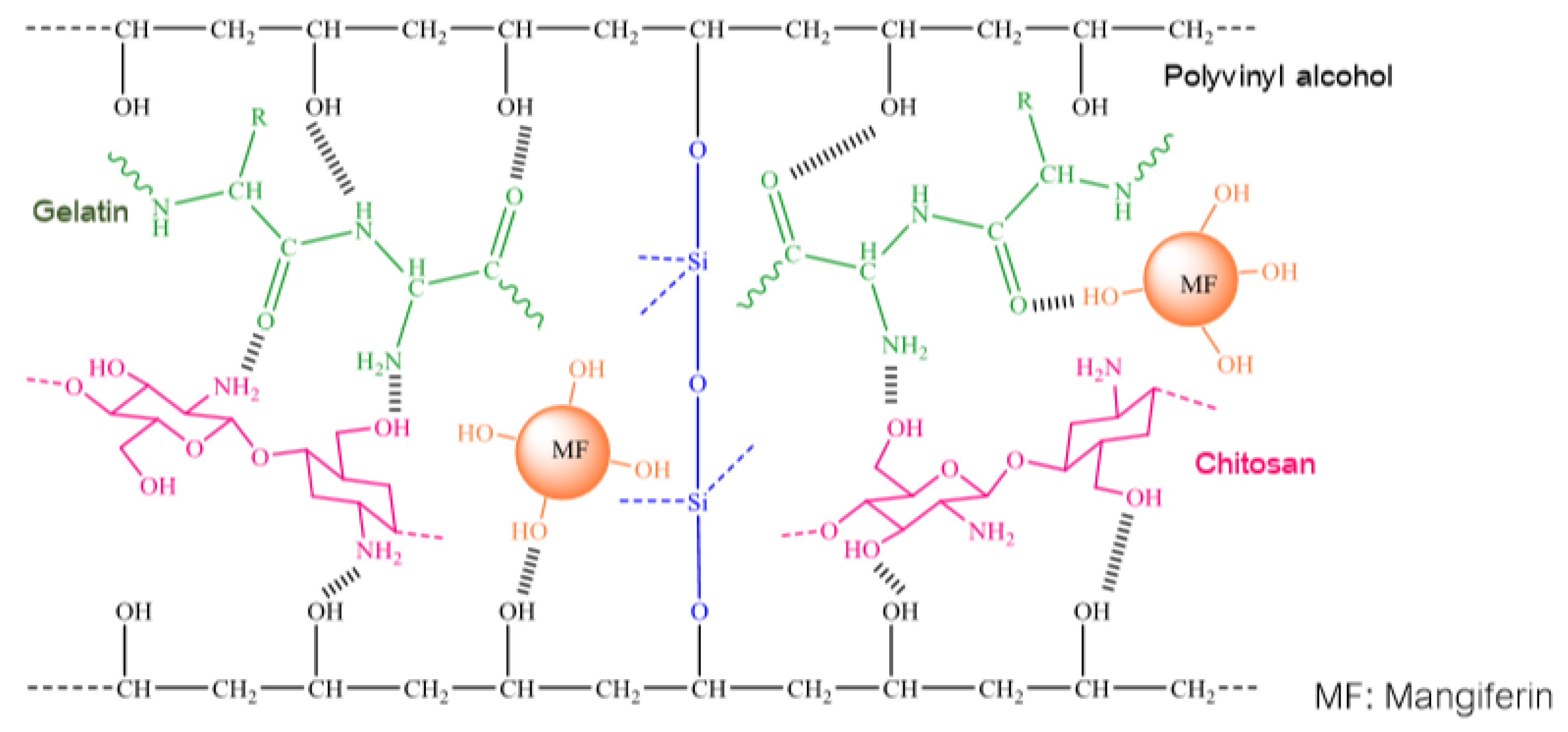
| Type of Cancer | Molecular Targets | Model | Doses, Regime | Reference |
|---|---|---|---|---|
| Breast cancer | Activation of NF-κB | MCF-7 cell line | [103,104] | |
| Inhibits P-gp activity | MCF-7 | 10, 25 or 50 μM | [144] | |
| Downregulates the CDK1-cyclin Bl signaling pathway; induces G2/M phase cell-cycle arrest; increases caspase-3, -8, -9; decreases expression of procaspase-3, -8, -9 activity | MCF-7 xenograft rat model | [20] | ||
| Triple negative breast cancer | Induces decreasing of MMP-7 and -9, and EMT; significantly inhibits the activation of β-catenin pathway; decreases tumor volume, weight and proliferation, increases apoptosis; lowers expression of MMP-7 and -9, vimentin and active β-catenin, and higher expression of E-cadherin | MDA-MB-231 and BT-549 cells; MCF-7 and T47D MDA-MB-231 xenograft mice | 300 μM 100 mg/kg | [116] |
| Extends lifespan | C57BL/6J mice | 100 mg/kg | [20] | |
| Triple negative breast cancer | PPARgamma, COX-2 | MDA-MB-231 cell line | [24] | |
| Lymphoma | Activation of NF-κB | U-937 cell line | 10 μg/mL | [103,104] |
| Cervical cancer | Activation of NF-κB; downregulates protein expression of BH3, Bcl 2 and pro-caspase-3 and pro-caspase-8, thereby activating caspase-3, -7, -8 and -9; induces a delay in the S phase | HeLa cell line | 10 μg/mL | [26] [19] |
| Mouse melanoma | Suppresses the nuclear translocation of NF-κB; minimizes the expression of phosphorylated NIK, IKK, IκB; inhibits MMP-1, MMP-2, MMP-9, MMP-14 and VLA-4, VLA-5 and VLA-6; enhances the expression of cleaved caspase-3, cleaved PARP-1, p53 proteins; reduces the expression of Survivin and Bcl-xL proteins in vivo | highly metastatic malignant cancer B16BL6 model | 50, 100 and 200 mg/kg (mice, orally, 21 days) | [117] |
| Acute myeloid leukemia | Activates the G2/M phase cell cycle arrest by modulating CDK1-cyclin B1 signaling pathway; induces Wee1 mRNA expression; significantly suppresses mRNA expression of Chk1 and cdc25C; remarkably inhibits the phosphorylation of Ataxia Telangiectasia and Rad3-related protein (ATR), Chk1, Wee1, Akt and Erk1/2; decreases the activation of cyclin B1 and cdc25C, and protein expression levels of Akt and Wee1; increases Nrf2 expression and protein stabilization; enhances Nrf2 binding of antioxidant response element (ARE); modulates NQO1 expression; restricts intracellular ROS levels; reduces the nuclear penetration of NF-κB p65; blocks the expressions of Bcl-xL and XIAP | HL-60 leukemia cells | 50 μM 100 μg/mL | [18] [118,119] |
| Glioma | Promotes miR-15b; inhibits MMP-7, MMP-9 and EMT | U87, U373MG, CRT-MG cells | [128] | |
| Prostate cancer | Significantly reduces TNFα-induced MMP-9 activity, relieved NF-κB activity, inhibits nuclear translocation of the NF-κB subunits p65 and p50 | LNCaP prostate carcinoma cells | [126] | |
| Promotes apoptosis and induces the caspase-3 activity; significantly reduces Bcl-2 expression levels and enhances miR-182 expression | PC3 human prostate cancer cells | 20 µM, 40 µM | [129] | |
| Hepatocellular carcinoma | Enhances the expression of CDC2 and CCNB1 mRNA; β-catenin-independent Wnt pathway, LEF1 gene; downregulation of MYC, axin2, MMP-2 and CCND1; decreases AST, ALT, ALP and LDH levels; | Sprague Dawley rats treated with 0.01% diethylnitrosamine (DEN) MHCC97L, HLF cells; the orthotopic HCC implantation murine model (mice); male albino rats of Wistar strain | 80 μM 50 mg/kg/2 days, orally 120 µg/mL 10, 20, 30 mg/kg | [124] [133] [135] [136] [132] |
| Lung carcinoma | Induces G2/M phase cell cycle arrest through the CDK1-cyclin B1 signaling pathway; inhibits PKC-NF-κB pathway; increases levels of glutathione, catalase (CAT), superoxide dismutase, glutathione reductase, glutathione peroxidase, vitamin E and vitamin C; enhances lipid peroxidation; decreases activity of catalase and superoxide dismutase; decreases the activities of GST, quinone reductase (QR) and uridin 5′-diphosphate-glucuronosyl transferase (UDP-GT); significantly decreases the levels of polyamines, protein carbonyl, nucleic acid content and lipid peroxidation; decreases activity of lysosomal enzymes β-glucuronidase, acidphosphatase, β-galactosidase and N-acetyl glucosaminidase | A549 cells (25 µg/mL), A549 xenograft mice | oral, 100 mg/kg oral, twice a week, 4 weeks | [23,115] [114,115] [136] [138] [139] [136] |
| Colon cancer | Causes a reduction of NF-kB activation; increases in delay in the S phase; increases Nrf2 and manganese superoxide dismutase (MnSOD) | HT29 HT29, Caco-2, HCT116 | 2.81 mg/100 g | [19] [130] |
| Number | Technique | Polymer Material | Formulations | Results | Characterization Methods | Reference |
|---|---|---|---|---|---|---|
| 1 | Spray-drying technique | Citric pectin, pumpkin pectin, chitosan | SD1 (Citric pectin/mangiferin); SD2 (Citric pectin/mangiferin/Tween 80), SD3 (Pumpkin pectin/mangiferin/Tween 80), SD4 (Chitosan/mangiferin/Tween 80) | Size (mangiferin concentration): SD1—7.2 μm (29 μg/mg), SD2—10.2 μm (41 μg/mg), SD3—15 μm (49 μg/mg), SD4—2.9 μm (16 μg/mg) | FTIR SEM HPLC–ESI-MS ESI-MS | [145] |
| Chitosan | Chitosan 0.5 g; 100 mL acetic acid 1%; mangiferin 50 mg; Tween 80 0.1% | - Sizes: from nano to micrometers; quantification mangiferin: 136 μg/mg; - Cr(VI) removal pH dependent, maximum at pH 5.0 | FTIR, SEM, HPLC-UV, DLS and adsorption studies | [146] | ||
| 2 | Simple solvent-evaporation technique | Lipoid E80 (phospholipid 80%) | Mangiferin/phospholipid (molar ratio 1:1) | Mangiferin-phospholipid complex was in semi-solid state; mangiferin content: 35.02%; solubility increasing: 1.4 times in water; 30 times in n-octanol; oil-water partition coefficient improving: 6.2 times; intestinal permeability was enhanced significantly; - Cmax increasing: 2.3-fold | IR, SEM, HPLC-UV, DSC | [147] |
| 3 | Emulsion solvent evaporation technique | Copolymer of ethylene vinyl acetate and vinyl acetate (12%, 18%, 25%, 40%) | Mangiferin emulsion: toluene + surfactant + mangiferin (in tetrahydrofuran); ethylene vinyl acetate solution: ethylene vinyl acetate containing vinyl acetate + toluene; Mangiferin emulsion + EVA solution ⇨ film | - Increased concentration of vinyl acetate: increased tensile strength and decreased oxygen resistance, increased mangiferin clearance, increased antioxidant activity; - Span®20: slightly affects mechanical and barrier properties, significantly increases mangiferin release and antioxidant activity | DSC, MDSC, oxygen permeation analyzer, Instron Testing machine, UV-visible | [148] |
| PLGA (75:25), PVA | Aqueous phase: PVA + different quantities of mangiferin. Organic phase: PLGA + dichloromethane. The aqueous phase + the organic phase ⇨ Nanoparticle | Nanoparticle size: 176.7 ± 1.021 nm Polydispersity index: 0.153 Encapsulation efficiency: 55% Entrapment efficiency: 97% Formulation (MG4, 25 µg/mL) had antiproliferative activity; Gastric digestion resistance to 1.5 h; No effect on healthy cells | SEM, UV, X-ray Diffraction, Assessment of Anti-Topoisomerase Activity and Cell Viability | [149] | ||
| 4 | Supercritical antisolvent technique | Cellulose acetate phthalate | Mass ratios of mangiferin/cellulose acetate phthalate from 1:1 to 1:10 | Submicron and microparticles: In SAS1, particle size is 0.25–0.41 μm, not affected by the mangiferin ratio; cellulose acetate phthalate retards the release of mangiferin in gastric fluids, has no effect on the release in the intestinal fluids In SAS2, the tiny thread and microsphere size is 0.2–1.0 μm, increases with the mangiferin content; the flow rate of the two injections slows down the mangiferin release | FTIR, nanoSEM, UV, X-ray Diffraction | [150] |
| 5 | Nanoemulsion technique | HA of different molecular weights | Aqueous phase: mangiferin + glycerin + HA + water; Oil phase: Lipoid® S75 + polysorbate 8 + tocopherol + almond oil + Transcutol-P; The aqueous phase + the oil phase ⇨ nanoemulsions. NE 0 (control without HA), NE I (HA, high molecular weight), NE II (HA, high molecular weight with TranscutolP), NE III (HA, low molecular weight), NE IV (HA, low molecular weight with Transcutol-P) | - Oil droplets average size 296 nm; monodisperses distribution (PI ≤ 0.30); - zeta potential is −30 mV; - pseudoplastic behavior (s~0.4) in presence of HA; - mangiferin release depends on HA molecular weight; - nanoemulsion permeability is improved with low molecular weight HA in the presence of Transcutol-P; - appropriate anti-inflammatory effect | HPLC, TEM microscope, Photon Correlation Spectroscopy, electrophoretic light scattering in thermostatic cell, FTIR, Rheological measurements, Franz diffuse cells | [151] |
| 6 | Sol-Gel synthesis technique | PVA, chitosan and gelatin | PVA, chitosan and gelatin including binary (vinyl alcohol, chitosan), ternary (PVA, chitosan and gelatin) and hybrid-ternary (ternary system associated with siloxane) | Hydrogel M90PV/5CHI/5GEL-T2 (1.8 g PVA, 0.1 g chitosan, 0.1 g gelatin, 60 μg siloxane) integrated 0.05 g mangiferin was the most appropriate matrix to release controlled mangiferin | 1H-NMR, UV-Vis, SEM-EDX and ATR-FTIR | [152] |
| 7 | Thin film-sonication technique | Mixtures of Pluronic F127 (F127), Pluronic p123 (P123), and Vitamin E TPGS (TPGS) copolymers | Mangiferin/copolymer mixed micelles (in the copolymer, total proportion of weight fractions of F127 (X1), P123 (X2) and TPGS (X3) is 1; X1 + X2 + X3 = 1) | Micelles with a spherical morphology; diameter—14.26 ± 0.52 nm; zeta potential—−2.89 ± 1.70 mV; encapsulation efficacy: 91.72 (F127), 75.65 (P123), 92.33% (TPGS); excellent solubility and sustainable release in digestive environment | HPLC, DLS, measurement of profile analysis tensiometry Zetasizer, TEM; FTIR, DSC, NMR | [153] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morozkina, S.N.; Nhung Vu, T.H.; Generalova, Y.E.; Snetkov, P.P.; Uspenskaya, M.V. Mangiferin as New Potential Anti-Cancer Agent and Mangiferin-Integrated Polymer Systems—A Novel Research Direction. Biomolecules 2021, 11, 79. https://doi.org/10.3390/biom11010079
Morozkina SN, Nhung Vu TH, Generalova YE, Snetkov PP, Uspenskaya MV. Mangiferin as New Potential Anti-Cancer Agent and Mangiferin-Integrated Polymer Systems—A Novel Research Direction. Biomolecules. 2021; 11(1):79. https://doi.org/10.3390/biom11010079
Chicago/Turabian StyleMorozkina, Svetlana N., Thi Hong Nhung Vu, Yuliya E. Generalova, Petr P. Snetkov, and Mayya V. Uspenskaya. 2021. "Mangiferin as New Potential Anti-Cancer Agent and Mangiferin-Integrated Polymer Systems—A Novel Research Direction" Biomolecules 11, no. 1: 79. https://doi.org/10.3390/biom11010079
APA StyleMorozkina, S. N., Nhung Vu, T. H., Generalova, Y. E., Snetkov, P. P., & Uspenskaya, M. V. (2021). Mangiferin as New Potential Anti-Cancer Agent and Mangiferin-Integrated Polymer Systems—A Novel Research Direction. Biomolecules, 11(1), 79. https://doi.org/10.3390/biom11010079







