Role of Retinoic Acid Signaling, FGF Signaling and Meis Genes in Control of Limb Development
Abstract
1. Introduction
2. Requirement of Retinoic Acid for Forelimb Bud Initiation
2.1. Mechanism of Retinoic Acid (RA) Signaling
2.2. Function of RA during Forelimb Budding
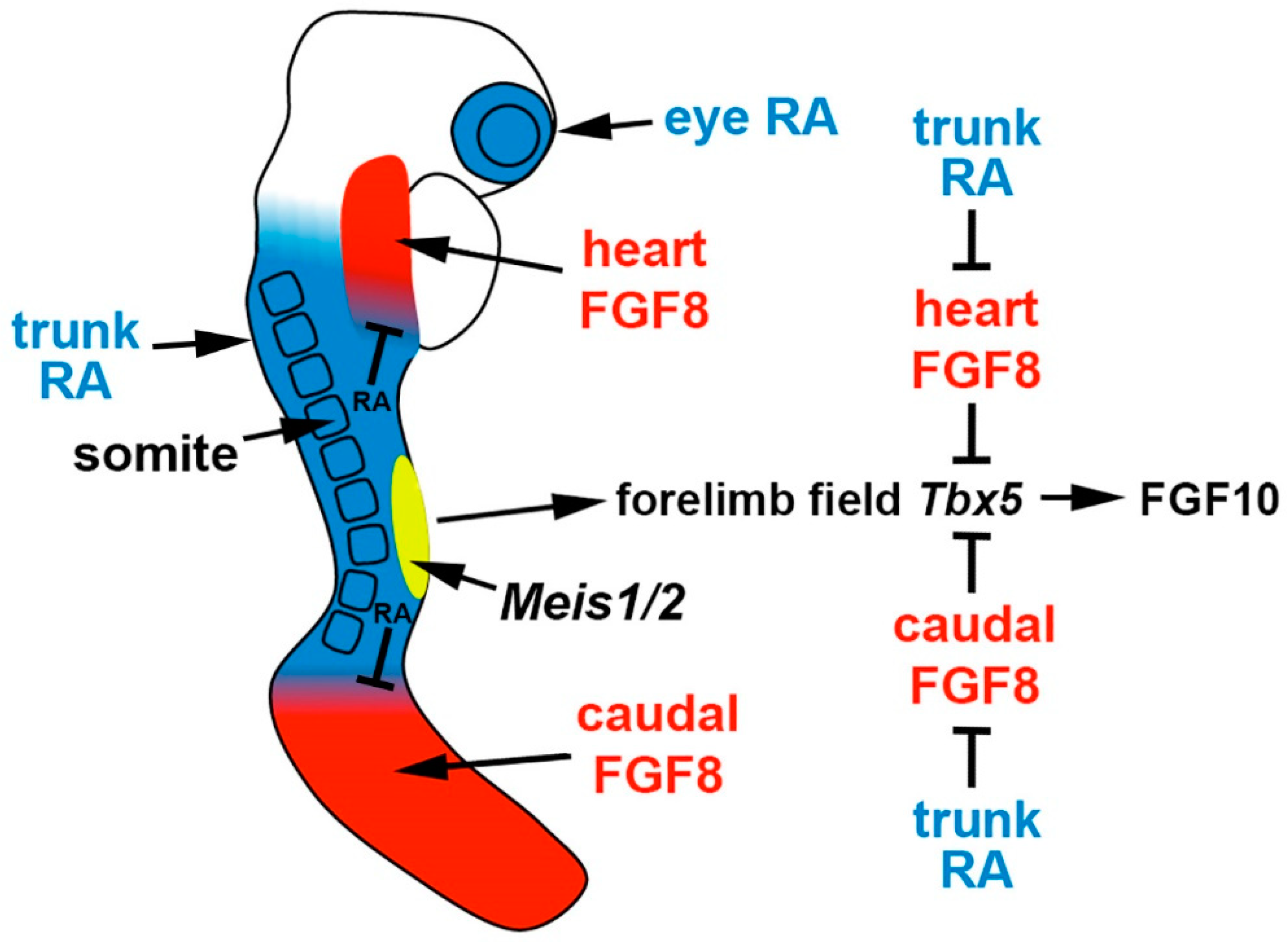
2.3. Mechanism through Which RA Represses Fgf8 in the Developing Trunk
2.4. Loss of Trunk RA Signaling Does Not Affect Hindlimb Bud Initiation
3. Requirement of Meis Genes for Forelimb Bud Initiation
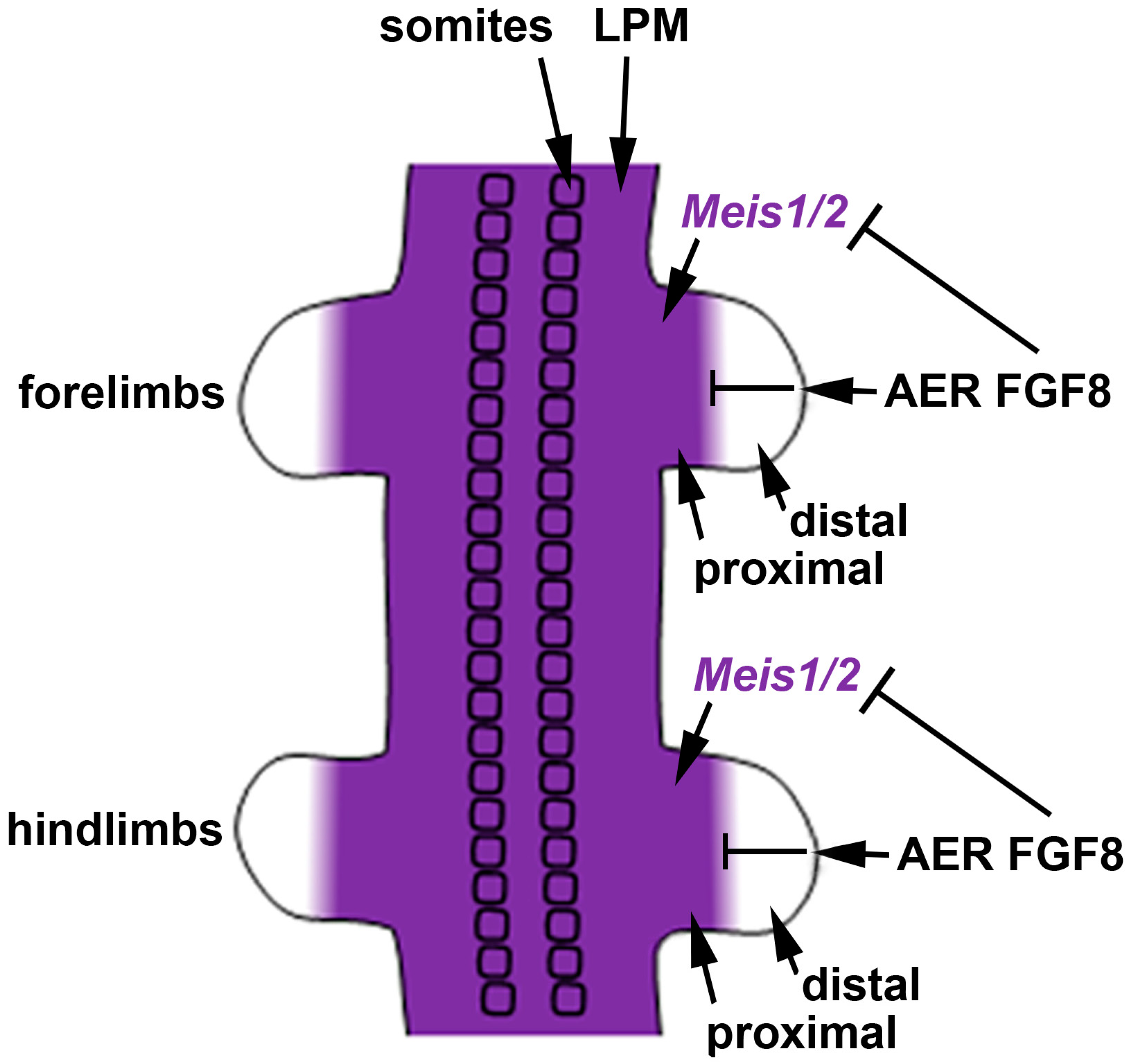
4. Meis Genes and FGF Signaling Are Required for Limb Proximodistal Patterning but RA Signaling Is Dispensable
4.1. Chick Studies Support a Two-Signal Model for Limb Proximodistal Patterning
4.2. Mouse Genetic Studies Support a One-Signal Model for Limb Proximodistal Patterning
5. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rabinowitz, A.H.; Vokes, S.A. Integration of the transcriptional networks regulating limb morphogenesis. Dev. Biol. 2012, 368, 165–180. [Google Scholar] [CrossRef] [PubMed]
- Sekine, K.; Ohuchi, H.; Fujiwara, M.; Yamasaki, M.; Yoshizawa, T.; Sato, T.; Yagishita, N.; Matsui, D.; Koga, Y.; Itoh, N.; et al. Fgf10 is essential for limb and lung formation. Nat. Genet. 1999, 21, 138–141. [Google Scholar] [CrossRef] [PubMed]
- Moon, A.M.; Capecchi, M.R. Fgf8 is required for outgrowth and patterning of the limbs. Nat. Genet. 2000, 26, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Lewandoski, M.; Sun, X.; Martin, G.R. Fgf8 signalling from the AER is essential for normal limb development. Nat. Genet. 2000, 26, 460–463. [Google Scholar] [CrossRef] [PubMed]
- Ahn, D.-G.; Kourakis, M.J.; Rohde, L.A.; Silver, L.M.; Ho, R.K. T-box gene Tbx5 is essential for formation of the pectoral limb bud. Nature 2002, 417, 754–758. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.; Wylie, J.N.; Galceran, J.; Arkhitko, O.; Li, C.; Deng, C.; Grosschedl, R.; Bruneau, B.G. Tbx5 is essential for forelimb bud initiation following patterning of the limb field in the mouse embryo. Development 2003, 130, 623–633. [Google Scholar] [CrossRef]
- Rallis, C.; Bruneau, B.G.; Del Buono, J.; Seidman, C.E.; Seidman, J.G.; Nissim, S.; Tabin, C.J.; Logan, M.P.O. Tbx5 is required for forelimb bud formation and continued outgrowth. Development 2003, 130, 2741–2751. [Google Scholar] [CrossRef]
- Zhao, X.; Sirbu, I.O.; Mic, F.A.; Molotkova, N.; Molotkov, A.; Kumar, S.; Duester, G. Retinoic acid promotes limb induction through effects on body axis extension but is unnecessary for limb patterning. Curr. Biol. 2009, 19, 1050–1057. [Google Scholar] [CrossRef]
- Logan, M.; Tabin, C.J. Role of Pitx1 upstream of Tbx4 in specification of hindlimb identity. Science 1999, 283, 1736–1739. [Google Scholar] [CrossRef]
- Mercader, N.; Leonardo, E.; Piedra, M.E.; Martínez-A, C.; Ros, M.A.; Torres, M. Opposing RA and FGF signals control proximodistal vertebrate limb development through regulation of Meis genes. Development 2000, 127, 3961–3970. [Google Scholar]
- Cooper, K.L.; Hu, J.K.; ten Berge, D.; Fernandez-Teran, M.; Ros, M.A.; Tabin, C.J. Initiation of proximal-distal patterning in the vertebrate limb by signals and growth. Science 2011, 332, 1083–1086. [Google Scholar] [CrossRef] [PubMed]
- Rosello-Diez, A.; Ros, M.A.; Torres, M. Diffusible signals, not autonomous mechanisms, determine the main proximodistal limb subdivision. Science 2011, 332, 1086–1088. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, T.J.; Chatzi, C.; Sandell, L.L.; Trainor, P.A.; Duester, G. Rdh10 mutants deficient in limb field retinoic acid signaling exhibit normal limb patterning but display interdigital webbing. Dev. Dyn. 2011, 240, 1142–1150. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, T.J.; Zhao, X.; Sandell, L.L.; Evans, S.M.; Trainor, P.A.; Duester, G. Antagonism between retinoic acid and fibroblast growth factor signaling during limb development. Cell Rep. 2013, 3, 1503–1511. [Google Scholar] [CrossRef]
- Sandell, L.L.; Sanderson, B.W.; Moiseyev, G.; Johnson, T.; Mushegian, A.; Young, K.; Rey, J.P.; Ma, J.X.; Staehling-Hampton, K.; Trainor, P.A. RDH10 is essential for synthesis of embryonic retinoic acid and is required for limb, craniofacial, and organ development. Genes Dev. 2007, 21, 1113–1124. [Google Scholar] [CrossRef]
- Ghyselinck, N.B.; Duester, G. Retinoic acid signaling pathways. Development 2019, 146, dev167502. [Google Scholar] [CrossRef]
- Pennimpede, T.; Cameron, D.A.; MacLean, G.A.; Li, H.; Abu-Abed, S.; Petkovich, M. The role of CYP26 enzymes in defining appropriate retinoic acid exposure during embryogenesis. Birth Defects Res. 2010, 88, 883–894. [Google Scholar] [CrossRef]
- Cunningham, T.J.; Duester, G. Mechanisms of retinoic acid signalling and its roles in organ and limb development. Nat. Rev. Mol. Cell Biol. 2015, 16, 110–123. [Google Scholar] [CrossRef]
- Rada-Iglesias, A.; Bajpai, R.; Swigut, T.; Brugmann, S.A.; Flynn, R.A.; Wysocka, J. A unique chromatin signature uncovers early developmental enhancers in humans. Nature 2011, 470, 279–283. [Google Scholar] [CrossRef]
- Laugesen, A.; Helin, K. Chromatin repressive complexes in stem cells, development, and cancer. Cell Stem Cell 2014, 14, 735–751. [Google Scholar] [CrossRef]
- Grandel, H.; Lun, K.; Rauch, G.J.; Rhinn, M.; Piotrowski, T.; Houart, C.; Sordino, P.; Küchler, A.M.; Schulte-Merker, S.; Geisler, R.; et al. Retinoic acid signalling in the zebrafish embryo is necessary during pre-segmentation stages to pattern the anterior-posterior axis of the CNS and to induce a pectoral fin bud. Development 2002, 129, 2851–2865. [Google Scholar] [PubMed]
- Begemann, G.; Schilling, T.F.; Rauch, G.J.; Geisler, R.; Ingham, P.W. The zebrafish neckless mutation reveals a requirement for raldh2 in mesodermal signals that pattern the hindbrain. Development 2001, 128, 3081–3094. [Google Scholar] [PubMed]
- Gros, J.; Tabin, C.J. Vertebrate limb bud formation is initiated by localized epithelial-to-mesenchymal transition. Science 2014, 343, 1253–1256. [Google Scholar] [CrossRef] [PubMed]
- Stratford, T.; Horton, C.; Maden, M. Retinoic acid is required for the initiation of outgrowth in the chick limb bud. Curr. Biol. 1996, 6, 1124–1133. [Google Scholar] [CrossRef]
- White, J.C.; Shankar, V.N.; Highland, M.; Epstein, M.L.; DeLuca, P.F.; Clagett-Dame, M. Defects in embryonic hindbrain development and fetal resorption resulting from vitamin A deficiency in the rat are prevented by feeding pharmacological levels of all-trans-retinoic acid. Proc. Natl. Acad. Sci. USA 1998, 95, 13459–13464. [Google Scholar] [CrossRef]
- Koyama, E.; Golden, E.B.; Kirsch, T.; Adams, S.L.; Chandraratna, R.A.S.; Michaille, J.J.; Pacifici, M. Retinoid signaling is required for chondrocyte maturation and endochondral bone formation during limb skeletogenesis. Dev. Biol. 1999, 208, 375–391. [Google Scholar] [CrossRef][Green Version]
- Minguillon, C.; Nishimoto, S.; Wood, S.; Vendrell, E.; Gibson-Brown, J.J.; Logan, M.P. Hox genes regulate the onset of Tbx5 expression in the forelimb. Development 2012, 139, 3180–3188. [Google Scholar] [CrossRef]
- Nishimoto, S.; Wilde, S.M.; Wood, S.; Logan, M.P. RA acts in a Coherent Feed-Forward Mechanism with Tbx5 to Control Limb Bud Induction and Initiation. Cell Rep. 2015, 12, 879–891. [Google Scholar] [CrossRef]
- Cunningham, T.J.; Lancman, J.J.; Berenguer, M.; Dong, P.D.S.; Duester, G. Genomic knockout of two presumed forelimb Tbx5 enhancers reveals they are nonessential for limb development. Cell Rep. 2018, 23, 3146–3151. [Google Scholar] [CrossRef]
- Duester, G. Knocking out enhancers to enhance epigenetic research. Trends Genet. 2019, 35, 89. [Google Scholar] [CrossRef]
- Cohn, M.J.; Izpisúa-Belmonte, J.C.; Abud, H.; Heath, J.K.; Tickle, C. Fibroblast growth factors induce additional limb development from the flank of chick embryos. Cell 1995, 80, 739–746. [Google Scholar] [CrossRef]
- Crossley, P.H.; Minowada, G.; MacArthur, C.A.; Martin, G.R. Roles for FGF8 in the induction, initiation, and maintenance of chick limb development. Cell 1996, 84, 127–136. [Google Scholar] [CrossRef]
- Kumar, S.; Duester, G. Retinoic acid controls body axis extension by directly repressing Fgf8 transcription. Development 2014, 141, 2972–2977. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Cunningham, T.J.; Duester, G. Nuclear receptor corepressors Ncor1 and Ncor2 (Smrt) are required for retinoic acid-dependent repression of Fgf8 during somitogenesis. Dev. Biol. 2016, 418, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.S.; Rhinn, M.; Semprich, C.I.; Halley, P.A.; Dolle, P.; Bickmore, W.A.; Storey, K.G. FGF Signalling Regulates Chromatin Organisation during Neural Differentiation via Mechanisms that Can Be Uncoupled from Transcription. PLoS Genet. 2013, 9, e1003614. [Google Scholar] [CrossRef] [PubMed]
- Berenguer, M.; Meyer, K.F.; Yin, J.; Duester, G. Discovery of genes required for body axis and limb formation by global identification of retinoic acid-regulated epigenetic marks. PLoS Biol. 2020, 18, e3000719. [Google Scholar] [CrossRef] [PubMed]
- Naiche, L.A.; Papaioannou, V.E. Loss of Tbx4 blocks hindlimb development and affects vascularization and fusion of the allantois. Development 2003, 130, 2681–2693. [Google Scholar] [CrossRef]
- Kawakami, Y.; Marti, M.; Kawakami, H.; Itou, J.; Quach, T.; Johnson, A.; Sahara, S.; O’Leary, D.D.; Nakagawa, Y.; Lewandoski, M.; et al. Islet1-mediated activation of the beta-catenin pathway is necessary for hindlimb initiation in mice. Development 2011, 138, 4465–4473. [Google Scholar] [CrossRef]
- Mercader, N.; Leonardo, E.; Azpiazu, N.; Serrano, A.; Morata, G.; Martinez, C.; Torres, M. Conserved regulation of proximodistal limb axis development by Meis/Hth. Nature 1999, 402, 425–429. [Google Scholar] [CrossRef]
- Mason, M.K.; Hockman, D.; Curry, L.; Cunningham, T.J.; Duester, G.; Logan, M.; Jacobs, D.S.; Illing, N. Retinoic acid-independent expression of Meis2 during autopod patterning in the developing bat and mouse limb. EvoDevo 2015, 6, 6. [Google Scholar] [CrossRef][Green Version]
- Hisa, T.; Spence, S.E.; Rachel, R.A.; Fujita, M.; Nakamura, T.; Ward, J.M.; Devor-Henneman, D.E.; Saiki, Y.; Kutsuna, H.; Tessarollo, L.; et al. Hematopoietic, angiogenic and eye defects in Meis1 mutant animals. EMBO J. 2004, 23, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Machon, O.; Masek, J.; Machonova, O.; Krauss, S.; Kozmik, Z. Meis2 is essential for cranial and cardiac neural crest development. BMC Dev. Biol. 2015, 15, 40. [Google Scholar] [CrossRef] [PubMed]
- Tickle, C.; Alberts, B.M.; Wolpert, L.; Lee, J. Local application of retinoic acid to the limb bud mimics the action of the polarizing region. Nature 1982, 296, 564–565. [Google Scholar] [CrossRef] [PubMed]
- Wanek, N.; Gardiner, D.M.; Muneoka, K.; Bryant, S.V. Conversion by retinoic acid of anterior cells into ZPA cells in the chick wing bud. Nature 1991, 350, 81–83. [Google Scholar] [CrossRef]
- Noji, S.; Nohno, T.; Koyama, E.; Muto, K.; Ohyama, K.; Aoki, Y.; Tamura, K.; Ohsugi, K.; Ide, H.; Taniguchi, S.; et al. Retinoic acid induces polarizing activity but is unlikely to be a morphogen in the chick limb bud. Nature 1991, 350, 83–86. [Google Scholar] [CrossRef]
- Riddle, R.D.; Johnson, R.L.; Laufer, E.; Tabin, C. Sonic hedgehog mediates the polarizing activity of the ZPA. Cell 1993, 75, 1401–1416. [Google Scholar] [CrossRef]
- te Welscher, P.; Fernandez-Teran, M.; Ros, M.A.; Zeller, R. Mutual genetic antagonism involving GLI3 and dHAND prepatterns the vertebrate limb bud mesenchyme prior to SHH signaling. Genes Dev. 2002, 16, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Mariani, F.V.; Ahn, C.P.; Martin, G.R. Genetic evidence that FGFs have an instructive role in limb proximal-distal patterning. Nature 2008, 453, 401–405. [Google Scholar] [CrossRef]
- Lewandoski, M.; Mackem, S. Limb development: The rise and fall of retinoic acid. Curr. Biol. 2009, 19, R558–R561. [Google Scholar] [CrossRef] [PubMed]
- Germain, P.; Iyer, J.; Zechel, C.; Gronemeyer, H. Co-regulator recruitment and the mechanism of retinoic acid receptor synergy. Nature 2002, 415, 187–192. [Google Scholar] [CrossRef]
- Yashiro, K.; Zhao, X.; Uehara, M.; Yamashita, K.; Nishijima, M.; Nishino, J.; Saijoh, Y.; Sakai, Y.; Hamada, H. Regulation of retinoic acid distribution is required for proximodistal patterning and outgrowth of the developing limb. Dev. Cell 2004, 6, 411–422. [Google Scholar] [CrossRef]
- Probst, S.; Kraemer, C.; Demougin, P.; Sheth, R.; Martin, G.R.; Shiratori, H.; Hamada, H.; Iber, D.; Zeller, R.; Zuniga, A. SHH propagates distal limb bud development by enhancing CYP26B1-mediated retinoic acid clearance via AER-FGF signalling. Development 2011, 138, 1913–1923. [Google Scholar] [CrossRef]
- Pennimpede, T.; Cameron, D.A.; MacLean, G.A.; Petkovich, M. Analysis of Cyp26b1/Rarg compound-null mice reveals two genetically separable effects of retinoic acid on limb outgrowth. Dev. Biol. 2010, 339, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Dranse, H.J.; Sampaio, A.V.; Petkovich, M.; Underhill, T.M. Genetic deletion of Cyp26b1 negatively impacts limb skeletogenesis by inhibiting chondrogenesis. J. Cell Sci. 2011, 124, 2723–2734. [Google Scholar] [CrossRef] [PubMed]
- Berenguer, M.; Darnaudery, M.; Claverol, S.; Bonneu, M.; Lacombe, D.; Rooryck, C. Prenatal retinoic acid exposure reveals candidate genes for craniofacial disorders. Sci. Rep. 2018, 8, 17492. [Google Scholar] [CrossRef]
- Sakai, Y.; Meno, C.; Fujii, H.; Nishino, J.; Shiratori, H.; Saijoh, Y.; Rossant, J.; Hamada, H. The retinoic acid-inactivating enzyme CYP26 is essential for establishing an uneven distribution of retinoic acid along the anterio-posterior axis within the mouse embryo. Genes Dev. 2001, 15, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Abu-Abed, S.; Dollé, P.; Metzger, D.; Beckett, B.; Chambon, P.; Petkovich, M. The retinoic acid-metabolizing enzyme, CYP26A1, is essential for normal hindbrain patterning, vertebral identity, and development of posterior structures. Genes Dev. 2001, 15, 226–240. [Google Scholar] [CrossRef] [PubMed]
- Delgado, I.; Lopez-Delgado, A.C.; Rosello-Diez, A.; Giovinazzo, G.; Cadenas, V.; Fernandez-de-Manuel, L.; Sanchez-Cabo, F.; Anderson, M.J.; Lewandoski, M.; Torres, M. Proximo-distal positional information encoded by an Fgf-regulated gradient of homeodomain transcription factors in the vertebrate limb. Sci. Adv. 2020, 6, eaaz0742. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berenguer, M.; Duester, G. Role of Retinoic Acid Signaling, FGF Signaling and Meis Genes in Control of Limb Development. Biomolecules 2021, 11, 80. https://doi.org/10.3390/biom11010080
Berenguer M, Duester G. Role of Retinoic Acid Signaling, FGF Signaling and Meis Genes in Control of Limb Development. Biomolecules. 2021; 11(1):80. https://doi.org/10.3390/biom11010080
Chicago/Turabian StyleBerenguer, Marie, and Gregg Duester. 2021. "Role of Retinoic Acid Signaling, FGF Signaling and Meis Genes in Control of Limb Development" Biomolecules 11, no. 1: 80. https://doi.org/10.3390/biom11010080
APA StyleBerenguer, M., & Duester, G. (2021). Role of Retinoic Acid Signaling, FGF Signaling and Meis Genes in Control of Limb Development. Biomolecules, 11(1), 80. https://doi.org/10.3390/biom11010080




