Zebrafish Models of Photoreceptor Dysfunction and Degeneration
Abstract
1. Introduction
2. Photoreceptors and Their Diseases
2.1. Retinitis Pigmentosa
2.2. Leber Congenital Amaurosis
2.3. Choroideremia
2.4. Cone-Rod Dystrophy
2.5. Enhanced S-cone Syndrome
2.6. Cone Dystrophy
2.7. Achromatopsia
3. Zebrafish as a Model for Photoreceptor Disease: Photoreceptor Features, Function and Morphological Assessment, and Model Generation
3.1. The Zebrafish Visual System
| Transgene | Description | Reference |
|---|---|---|
| Tg(3.2gnat2:eGFP) | Enhanced GFP expressed in all cone photoreceptors | [45] |
| Tg(rho:eGFP) | Enhanced GFP expressed in rod photoreceptors | [46] |
| Tg(sws1:GFP) | GFP expressed in UV cones | [47] |
| Tg(sws2:mCherry) | mCherry expressed in blue cones | [48] |
3.2. Visually-Mediated Responses as Diagnostics in Zebrafish
3.3. Retinal Imaging and Functional Assessement for Zebrafish
3.4. Generation of Transgenics and Mutants
3.5. Regenerative Response
4. Zebrafish Models of Photoreceptor Disease
4.1. Light Ablation
4.2. Toxic Lesions
4.2.1. Ouabain
4.2.2. N-Methyl-N-nitrosourea
4.3. Hypoxia-Induced Neovascularization
4.4. Gene Knockdown (Morpholinos)
4.5. Transgenic Models
4.5.1. XOPS:mCFP
4.5.2. Nitroreductase Ablation
4.5.3. Disease Genes
| Photoreceptor Disease | Transgene | Photoreceptor Features | Reference |
|---|---|---|---|
| Cone-rod dystrophy | Tg(3.2gnat2:hsa.GUCY2D-E837D R838S) | Dysmorphic cones at 5 dpf. 3-month-old animals have a thin photoreceptor layer in the central retina, dysmorphic cones, and less cone and rod staining | [143] |
| Retinitis pigmentosa | Tg(rho:hsaRHO-Q344X) | Rod degeneration detectable at 5 dpf, rapid progression. | [136] |
| Retinitis pigmentosa | Tg(rho:msRho-P23H-flag) | Rod degeneration at 3 months. | [134] |
| Retinitis pigmentosa | Tg(rho:prpf3-AD5-mCherry) | Increased photoreceptor cell death at 5 months. | [138] |
| Retinitis pigmentosa | Tg(rho:adcy2b-rho-tail) | Rod degeneration noted at 14 dpf. | [136] |
| Retinitis pigmentosa | Tg(rho:hsaHTRA1) | Rod degeneration at 5 dpf. | [142] |
| Retinitis pigmentosa | Tg(XOPS:mCFP) | Rapid rod degeneration by 5 dpf. Adults do not have a rod response on ERG. | [123] |
4.6. Genetic Mutants
4.6.1. Retinitis Pigmentosa
| Gene | Photoreceptor Features | Reference |
|---|---|---|
| cerkl | Photoreceptor functional defects at 7 dpf. Rod OS defects at 3 months, cone OS defects at 7 months. Notable thinning of the photoreceptor layer and cell death by 12 months. | [158] |
| her9 | Decrease in rod photoreceptors at 5 dpf. Few double cones with short OSs at 12 dpf. | [180] |
| kif3b | Delayed OS development. Rapid rod degeneration by 5 dpf. | [160,161] |
| myo7aa | Decreased photoreceptor function at 5dpf. Reduced rods at 8 dpf. | [174] |
| rho | Rod loss observed at 6 dpf. Degeneration continues into adulthood. | [150,151] |
| rp1l1 | Rod dysfunction at 6 months. Subretinal drusenoid deposits at 11 months. Photoreceptor loss observed at 12 months. | [170] |
| rp2 | Photoreceptor functional defects at 7 dpf. Short rod OSs at 2 months; cone OS defects at 4 months; significant rod OS loss and decreased cone OSs by 7 months. | [156] |
| rpgrip1 | No rod OSs at 5 dpf. Cone dysfunction at 7 dpf. Severe rod degeneration by 3 months, followed by cone degeneration. By 23 months, majority of photoreceptors have degenerated. | [166] |
| ush2a | Decreased photoreceptor function at 5–7 dpf and increased photoreceptor apoptosis at 8 dpf. Notable rod OS degeneration at 12 months, cone OS degeneration observed at 20 months. | [177,178] |
4.6.2. Leber Congenital Amaurosis
| Gene | Photoreceptor Features | Reference |
|---|---|---|
| cluap1 | No OS development, rapid photoreceptor degeneration. | [185] |
| gdf6a | Short, dysmorphic cones and expanded, disorganized rods at 7 dpf. | [199,200] |
| ift57 | Short OSs with normal disc stacking at 4 dpf. Central retina photoreceptor degeneration at 5 dpf. | [184,186] |
| ift88 | No OS development, rapid photoreceptor degeneration. | [181,182,183,184] |
| ift122 | Normal photoreceptor OS development. Degeneration starting at 7 dpf, severe degeneration by 10 dpf. | [187] |
| ift172 | No OS development, rapid photoreceptor degeneration. | [181,182,184] |
| kiaa0586 | Fewer OSs observed at 3 dpf, photoreceptor degeneration observed at 4 dpf. Decreased photoreceptor function detected at 6 dpf. | [195] |
| kif3a | No OS development and rapid rod photoreceptor degeneration by 5 dpf, subsequent cone degeneration. Extinguished photoreceptor response at 7 dpf. | [161,188,189] |
| napbb | Immediate, severe photoreceptor degeneration; cell death observed at 3 dpf, no distinct photoreceptor layer at 6 dpf, few photoreceptors in ciliary margin. | [191,192] |
| tmem216 | Short cilia and disorganized OS discs by 7 dpf, rapid degeneration. Few photoreceptors remaining by 14 dpf. | [198] |
4.6.3. Choroideremia
4.6.4. Cone-Rod Dystrophy
4.6.5. Enhanced S-cone Syndrome
| Photoreceptor Disease | Gene | Photoreceptor + RPE Features | Reference |
|---|---|---|---|
| Choroideremia | rep1 | RPE irregularity and pigment loss. Disorganized photoreceptor layers, dysmorphic photoreceptors, and significantly reduced photoreceptor responses at 5 dpf. | [24] |
| Cone-rod dystrophy | ahi1 | Disorganized, short OSs, but normal visual function at 5 dpf. Thin photoreceptor nuclear layer, cone degeneration, and dysmorphic rods at 5 months. | [205] |
| Cone-rod dystrophy | arl13b | Short OSs at 4dpf. Cone degeneration observed at 30 dpf in mosaic animals. | [206] |
| Cone-rod dystrophy | bbs2 | Short, disorganized photoreceptor OSs and visual deficits at 5 dpf. Photoreceptor degeneration observed in adulthood. | [220] |
| Cone-rod dystrophy | cc2d2a | Dysmorphic photoreceptor OSs and functional defects at 5 dpf. | [204] |
| Cone-rod dystrophy | eys | Progressive photoreceptor loss; cone degeneration observed at 6 months, rod degeneration observed at 14 months | [215,216,217] |
| Cone-rod dystrophy | kcnj13 | Increased phagosomes in the RPE at 3 months, enlarged RPE mitochondria at 6 months, and abnormal melanosome localization under dark adaptation at 12 months. Cone mitochondria abnormalities at 6 months and photoreceptor loss at 12 months. | [222] |
| Cone-rod dystrophy | lca5 | Photoreceptor functional defects at 7 dpf. Cone OS defects at 1 month, rod OS defects at 7 months, and progressive photoreceptor loss. | [208] |
| Cone-rod dystrophy | pcare1 | Dysmorphic OSs and dysfunctional photoreceptors at 5 dpf. Abnormal OS morphology and thinner photoreceptor layer at 6 months. | [210] |
| Cone-rod dystrophy | pomgnt1 | Reduction in cones and rods at 6 months. | [224] |
| Enhanced S-cone syndrome | nr2e3 | No rods. Green and red cone degeneration started at 1 month. | [225] |
| Enhanced S-cone syndrome | nrl | Rods fail to develop and UV cones are over-abundant in larvae. Adult zebrafish surprisingly have rods, but with synaptic defects. | [226] |
4.6.6. Cone Dystrophy
4.6.7. Achromatopsia
4.6.8. Age-Related Macular Degeneration
| Photoreceptor Disease | Gene | Photoreceptor, RPE, or Vascular Features | Reference |
|---|---|---|---|
| Achromatopsia | cacna1fa | Thin photoreceptor synaptic layer. Photoreceptor functional defects at 5–6 dpf. Cone synapses do not develop synaptic ribbons. | [97] |
| Achromatopsia | gnat2 | Cone functional defects under dim to moderate light intensities. | [242] |
| Achromatopsia | per2 | Visually-mediated behaviour deficiencies at 5–6 dpf. Unanchored cone ribbon synapses and decreased cone opsin expression. | [250] |
| Achromatopsia | synj1 | Thin photoreceptor synaptic layer and abnormal, unanchored cone ribbon synapses at 6 dpf. | [247,248,249] |
| Cone dystrophy | aipl1 | Thin photoreceptor layer and OS defects at 7 dpf. Severe cone degeneration by 3 months. | [235] |
| Cone dystrophy | atp6v0e1 | Hypopigmented RPE, reduced or absent visual response, decreased photoreceptor function, short OSs, and outer retinal holes at 5 dpf. | [237] |
| Cone dystrophy | cacna2d4a + cacna2d4b | Mild cone dysfunction at 20 dpf. | [96] |
| Cone dystrophy | cep290 | Cone OS abnormalities and degeneration at 3 months, severe degeneration at 12 months. Normal rods. | [231] |
| Cone dystrophy | pde6c | Cone degeneration at 4 dpf and rod subsequently become dysmorphic and degenerate in the larval retina. At 3 months, cones are degenerate and largely missing, rods are normal. | [49,192] |
| Cone dystrophy | prom1b | Progressive cone degeneration. Reduced red and green cones at 7 dpf; reduced UV and blue cones at 1 month. Overgrown rod OSs. | [230] |
| Cone dystrophy | wrb | Holes in the photoreceptor layer at 4 dpf. Vision defects at 5 dpf, photoreceptor function defects at 4–5 dpf. Small cone ribbon synapses | [239,240] |
| Retinal neovascularization | vhl | Retinal neovascularization, leaky blood vessels, retinal oedema, and retinal detachment at 7–8 dpf. | [251] |
4.7. Relevance to Human Disease
5. Contribution to Therapeutics
5.1. Pharmaceuticals
5.1.1. Anti-Angiogenic Compounds
5.1.2. Histone Deacetylase Inhibition
5.1.3. Novel Compound Screening
5.1.4. Oculotoxicity
5.2. Antisense Oligonucleotide Therapy
5.3. Photoreceptor Regenerative Pathways
5.3.1. Ciliary Marginal Zone
5.3.2. Müller Glia
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Daiger, S.P.; Rossiter, B.J.F.; Greenberg, J.; Christoffels, A.; Hide, W. RetNet. Available online: https://sph.uth.edu/RetNet/ (accessed on 10 December 2020).
- Nathans, J.; Thomas, D.; Hogness, D.S. Molecular genetics of human color vision: The genes encoding blue, green, and red pigments. Science 1985, 232, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Bowmaker, J.K.; Dartnall, H.J.A. Visual pigments of rods and cones in a human retina. J. Physiol. 1980, 298, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Hofer, H.; Carroll, J.; Neitz, J.; Neitz, M.; Williams, D.R. Organization of the human trichromatic cone mosaic. J. Neurosci. 2005, 25, 9669–9679. [Google Scholar] [CrossRef] [PubMed]
- Curcio, C.A.; Sloan, K.R.; Packer, O.; Hendrickson, A.E.; Kalina, R.E. Distribution of cones in human and monkey retina: Individual variability and radial asymmetry. Science 1987, 236, 579–582. [Google Scholar] [CrossRef] [PubMed]
- Schultze, M. Zur Anatomie and Physiologie der Retina. Ach Mikr Anat. 1866, 2, 175–286. [Google Scholar] [CrossRef]
- Strauss, O. The retinal pigment epithelium in visual function. Physiol. Rev. 2005, 85, 845–881. [Google Scholar] [CrossRef]
- Cayouette, M.; Smith, S.B.; Becerra, S.P.; Gravel, C. Pigment epithelium-derived factor delays the death of photoreceptors in mouse models of inherited retinal degenerations. Neurobiol. Dis. 1999, 6, 523–532. [Google Scholar] [CrossRef]
- Becerra, S.P.; Fariss, R.N.; Wu, Y.Q.; Montuenga, L.M.; Wong, P.; Pfeffer, B.A. Pigment epithelium-derived factor in the monkey retinal pigment epithelium and interphotoreceptor matrix: Apical secretion and distribution. Exp. Eye Res. 2004, 78, 223–234. [Google Scholar] [CrossRef]
- Ogata, N.; Wang, L.; Jo, N.; Tombran-Tink, J.; Takahashi, K.; Mrazek, D.; Matsumura, M. Pigment epithelium derived factor as a neuroprotective agent against ischemic retinal injury. Curr. Eye Res. 2001, 22, 245–252. [Google Scholar] [CrossRef]
- Reiter, J.F.; Leroux, M.R. Genes and molecular pathways underpinning ciliopathies. Nat. Rev. Mol. Cell Biol. 2017, 18, 533–547. [Google Scholar] [CrossRef]
- National Eye Institute (National Institutes of Health) AMD Data and Statistics. Available online: https://www.nei.nih.gov/learn-about-eye-health/resources-for-health-educators/eye-health-data-and-statistics/age-related-macular-degeneration-amd-data-and-statistics (accessed on 10 December 2020).
- Hamel, C. Retinitis pigmentosa. Orphanetj. Rare Dis. 2006, 1, 40. [Google Scholar] [CrossRef]
- Hanany, M.; Rivolta, C.; Sharon, D. Worldwide carrier frequency and genetic prevalence of autosomal recessive inherited retinal diseases. Proc. Natl. Acad. Sci. USA 2020, 117, 2710–2716. [Google Scholar] [CrossRef] [PubMed]
- Santer, R.; Schneppenheim, R.; Dombrowski, A.; Gotze, H.; Steinmann, B.; Schaub, J. Mutations in RPE65 cause Leber’s congenital amaurosis. Nat. Genet. 1997, 15, 57–61. [Google Scholar]
- Perrault, I.; Rozet, J.M.; Calvas, P.; Gerber, S.; Camuzat, A.; Dollfus, H.; Châtelin, S.; Souied, E.; Ghazi, I.; Leowski, C.; et al. Retinal-specific guanylate cyclase gene mutations in Leber’s congenital amaurosis. Nat. Genet. 1996, 14, 461–464. [Google Scholar] [CrossRef] [PubMed]
- den Hollander, A.I.; Koenekoop, R.K.; Yzer, S.; Lopez, I.; Arends, M.L.; Voesenek, K.E.; Zonneveld, M.N.; Strom, T.M.; Meitinger, T.; Brunner, H.G.; et al. Mutations in the CEP290 (NPHP6) gene are a frequent cause of Leber congenital amaurosis. Am. J. Hum. Genet. 2006, 79, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Chacon-Camacho, O.F. Review and update on the molecular basis of Leber congenital amaurosis. Worldj. Clin. Cases 2015, 3, 112. [Google Scholar] [CrossRef]
- Xue, L.; Gollapalli, D.R.; Maiti, P.; Jahng, W.J.; Rando, R.R. A palmitoylation switch mechanism in the regulation of the visual cycle. Cell 2004, 117, 761–771. [Google Scholar] [CrossRef]
- Wimberg, H.; Lev, D.; Yosovich, K.; Namburi, P.; Banin, E.; Sharon, D.; Koch, K.W. Photoreceptor guanylate cyclase (GUCY2D) mutations cause retinal dystrophies by severe malfunction of Ca2+-dependent cyclic GMP synthesis. Front. Mol. Neurosci. 2018, 11, 1–12. [Google Scholar] [CrossRef]
- Azadi, S.; Molday, L.L.; Molday, R.S. RD3, the protein associated with Leber congenital amaurosis type 12, is required for guanylate cyclase trafficking in photoreceptor cells. Proc. Natl. Acad. Sci. USA 2010, 107, 21158–21163. [Google Scholar] [CrossRef]
- Chang, B.; Khanna, H.; Hawes, N.; Jimeno, D.; He, S.; Lillo, C.; Parapuram, S.K.; Cheng, H.; Scott, A.; Hurd, R.E.; et al. In-frame deletion in a novel centrosomal/ciliary protein CEP290/NPHP6 perturbs its interaction with RPGR and results in early-onset retinal degeneration in the rd16 mouse. Hum. Mol. Genet. 2006, 15, 1847–1857. [Google Scholar] [CrossRef]
- Seabra, M.C.; Brown, M.S.; Goldstein, J.L. Retinal degeneration in choroideremia: Deficiency of Rab geranylgeranyl transferase. Science 1993, 259, 377–381. [Google Scholar] [CrossRef]
- Krock, B.L.; Bilotta, J.; Perkins, B.D. Noncell-autonomous photoreceptor degeneration in a zebrafish model of choroideremia. Proc. Natl. Acad. Sci. USA 2007, 104, 4600–4605. [Google Scholar] [CrossRef]
- Wavre-Shapton, S.T.; Tolmachova, T.; da Silva, M.L.; Futter, C.E.; Seabra, M.C. Conditional ablation of the choroideremia gene causes age-related changes in mouse retinal pigment epithelium. PLoS ONE 2013, 8, 1–11. [Google Scholar] [CrossRef]
- Haider, N.B.; Jacobson, S.G.; Cideciyan, A.V.; Swiderski, R.; Streb, L.M.; Searby, C.; Beck, G.; Hockey, R.; Hanna, D.B.; Gorman, S.; et al. Mutation of a nuclear receptor gene, NR2E3, causes enhanced S cone syndrome, a disorder of retinal cell fate. Nat. Genet. 2000, 24, 127–131. [Google Scholar] [CrossRef]
- Nishiguchi, K.M.; Friedman, J.S.; Sandberg, M.A.; Swaroop, A.; Berson, E.L.; Dryja, T.P. Recessive NRL mutations in patients with clumped pigmentary retinal degeneration and relative preservation of blue cone function. Proc. Natl. Acad. Sci. USA 2004, 101, 17819–17824. [Google Scholar] [CrossRef]
- Newman, H.; Blumen, S.C.; Braverman, I.; Hanna, R.; Tiosano, B.; Perlman, I.; Ben-Yosef, T. Homozygosity for a recessive loss-of-function mutation of the NRL gene is associated with a variant of enhanced S-cone syndrome. Investig. Ophthalmol. Vis. Sci. 2016, 57, 5361–5371. [Google Scholar] [CrossRef]
- Littink, K.W.; Stappers, P.T.Y.; Riemslag, F.C.C.; Talsma, H.E.; van Genderen, M.M.; Cremers, F.P.M.; Collin, R.W.J.; van den Born, L.I. Autosomal recessive NRL mutations in patients with enhanced S-cone syndrome. Genes 2018, 9, 68. [Google Scholar] [CrossRef]
- Sisk, R.A.; Hufnagel, R.B.; Laham, A.; Wohler, E.S.; Sobreira, N.; Ahmed, Z.M. Peripheral cone dystrophy: Expanded clinical spectrum, multimodal and ultrawide-field imaging, and genomic analysis. J. Ophthalmol. 2018, 2018. [Google Scholar] [CrossRef]
- Remmer, M.H.; Rastogi, N.; Ranka, M.P.; Ceisler, E.J. Achromatopsia: A review. Curr. Opin. Ophthalmol. 2015, 26, 333–340. [Google Scholar] [CrossRef]
- Viets, K.; Eldred, K.C.; Johnston, R.J. Mechanisms of photoreceptor patterning in vertebrates and invertebrates. Trends Genet. 2016, 32, 638–659. [Google Scholar] [CrossRef]
- Kostic, C.; Arsenijevic, Y. Animal modelling for inherited central vision loss. J. Pathol. 2016, 238, 300–310. [Google Scholar] [CrossRef]
- Schmitt, E.A.; Hyatt, G.A.; Dowling, J.E. Erratum: Temporal and spatial patterns of opsin gene expression in the zebrafish (Danio rerio): Corrections with additions. Vis. Neurosci. 1999, 16, 601–605. [Google Scholar] [CrossRef]
- Robinson, J.; Schmitt, E.A.; Dowling, J.E. Temporal and spatial patterns of opsin gene expression in zebrafish (Danio rerio). Vis. Neurosci. 1995, 12, 895–906. [Google Scholar] [CrossRef]
- Neuhauss, S.C.F. Behavioral genetic approaches to visual system development and function in zebrafish. J. Neurobiol. 2003, 54, 148–160. [Google Scholar] [CrossRef]
- Allison, W.T.; Barthel, L.K.; Skebo, K.M.; Takechi, M.; Kawamura, S.; Raymond, P.A. Ontogeny of cone photoreceptor mosaics in zebrafish. J. Comp. Neurol. 2010, 518, 4182–4195. [Google Scholar] [CrossRef]
- Yoshimatsu, T.; Schröder, C.; Nevala, N.E.; Berens, P.; Baden, T. Fovea-like photoreceptor specializations underlie single UV cone driven prey-capture behavior in zebrafish. Neuron 2020, 107, 320–337.e6. [Google Scholar] [CrossRef]
- Fadool, J.M. Development of a rod photoreceptor mosaic revealed in transgenic zebrafish. Dev. Biol. 2003, 258, 277–290. [Google Scholar] [CrossRef]
- Noel, N.C.L.; Allison, W.T. Connectivity of cone photoreceptor telodendria in the zebrafish retina. J. Comp. Neurol. 2018, 526, 609–625. [Google Scholar] [CrossRef]
- Morrow, J.M.; Lazic, S.; Fox, M.D.; Kuo, C.; Schott, R.K.; De Gutierrez, E.A.; Santini, F.; Tropepe, V.; Chang, B.S.W. A second visual rhodopsin gene, rh1-2, is expressed in zebrafish photoreceptors and found in other ray-finned fishes. J. Exp. Biol. 2017, 220, 294–303. [Google Scholar] [CrossRef]
- Takechi, M.; Kawamura, S. Temporal and spatial changes in the expression pattern of multiple red and green subtype opsin genes during zebrafish development. J. Exp. Biol. 2005, 208, 1337–1345. [Google Scholar] [CrossRef]
- Morrow, J.M.; Lazic, S.; Chang, B.S.W. A novel rhodopsin-like gene expressed in zebrafish retina. Vis. Neurosci. 2011, 28, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Ruzicka, L.; Howe, D.G.; Ramachandran, S.; Toro, S.; Van Slyke, C.E.; Bradford, Y.M.; Eagle, A.; Fashena, D.; Frazer, K.; Kalita, P.; et al. The Zebrafish Information Network: New support for non-coding genes, richer Gene Ontology annotations and the Alliance of Genome Resources. Nucleic Acids Res. 2019, 47, D867–D873. [Google Scholar] [CrossRef]
- Kennedy, B.N.; Alvarez, Y.; Brockerhoff, S.E.; Stearns, G.W.; Sapetto-Rebow, B.; Taylor, M.R.; Hurley, J.B. Identification of a zebrafish cone photoreceptor-specific promoter and genetic rescue of achromatopsia in the nof mutant. Investig. Ophthalmol. Vis. Sci. 2007, 48, 522–529. [Google Scholar] [CrossRef]
- Hamaoka, T.; Takechi, M.; Chinen, A.; Nishiwaki, Y.; Kawamura, S. Visualization of rod photoreceptor development using GFP-transgenic zebrafish. Genesis 2002, 34, 215–220. [Google Scholar] [CrossRef]
- Takechi, M.; Hamaoka, T.; Kawamura, S. Fluorescence visualization of ultraviolet-sensitive cone photoreceptor development in living zebrafish. Febs Lett. 2003, 553, 90–94. [Google Scholar] [CrossRef]
- DuVal, M.G.; Chung, H.; Lehmann, O.J.; Allison, W.T. Longitudinal fluorescent observation of retinal degeneration and regeneration in zebrafish using fundus lens imaging. Mol. Vis. 2013, 19, 1082–1095. [Google Scholar]
- Stearns, G.; Evangelista, M.; Fadool, J.M.; Brockerhoff, S.E. A mutation in the cone-specific pde6 gene causes rapid cone photoreceptor degeneration in zebrafish. J. Neurosci. 2007, 27, 13866–13874. [Google Scholar] [CrossRef]
- DuVal, M.G.; Oel, A.P.; Allison, W.T. gdf6a is required for cone photoreceptor subtype differentiation and for the actions of tbx2b in determining rod versus cone photoreceptor fate. PLoS ONE 2014, 9, e92991. [Google Scholar] [CrossRef]
- Yin, J.; Brocher, J.; Linder, B.; Hirmer, A.; Sundaramurthi, H.; Fischer, U.; Winkler, C. The 1D4 antibody labels outer segments of long double cone but not rod photoreceptors in zebrafish. Investig. Ophthalmol. Vis. Sci. 2012, 53, 4943–4951. [Google Scholar] [CrossRef]
- Larison, K.D.; Bremiller, R. Early onset of phenotype and cell patterning in the embryonic zebrafish retina. Development 1990, 109, 567–576. [Google Scholar]
- Ile, K.E.; Kassen, S.; Cao, C.; Vihtehlic, T.; Shah, S.D.; Mousley, C.J.; Alb, J.G., Jr.; Huijbregts, R.P.; Stearns, G.W.; Brockerhoff, S.E.; et al. Zebrafish class 1 phosphatidylinositol transfer proteins: PITPbeta and double cone cell outer segment integrity in retina. Traffic 2010, 11, 1151–1167. [Google Scholar] [CrossRef]
- Schmitt, E.A.; Dowling, J.E. Comparison of topographical patterns of ganglion and photoreceptor cell differentiation in the retina of the zebrafish, Danio rerio. J. Comp. Neurol. 1996, 371, 222–234. [Google Scholar] [CrossRef]
- Brockerhoff, S.E.; Hurley, J.B.; Janssen-Bienhold, U.; Neuhauss, S.C.F.; Driever, W.; Dowling, J.E. A behavioral screen for isolating zebrafish mutants with visual system defects. Proc. Natl. Acad. Sci. USA 1995, 92, 10545–10549. [Google Scholar] [CrossRef]
- Brockerhoff, S.E. Measuring the optokinetic response of zebrafish larvae. Nat. Protoc. 2006, 1, 2448–2451. [Google Scholar] [CrossRef]
- Easter, S.S.; Nicola, G.N. The development of eye movements in the zebrafish (Danio rerio). Dev. Psychobiol. 1997, 31, 267–276. [Google Scholar] [CrossRef]
- Orger, M.B.; Gahtan, E.; Muto, A.; Page-McCaw, P.; Smear, M.C.; Baier, H. Behavioral screening assays in zebrafish. Methods Cell Biol. 2004, 77, 53–68. [Google Scholar] [CrossRef]
- Orger, M.B.; Smear, M.C.; Anstis, S.M.; Baier, H. Perception of Fourier and non-Fourier motion by larval zebrafish. Nat. Neurosci. 2000, 3, 1128–1133. [Google Scholar] [CrossRef]
- Hagerman, G.F.; Noel, N.C.L.; Cao, S.; DuVal, M.G.; Oel, A.P.; Allison, W.T. Rapid recovery of visual function associated with blue cone ablation in zebrafish. PLoS ONE 2016, 11. [Google Scholar] [CrossRef]
- Emran, F.; Rihel, J.; Dowling, J.E. A behavioral assay to measure responsiveness of zebrafish to changes in light intensities. J. Vis. Exp. 2008, 20, 1–6. [Google Scholar] [CrossRef]
- Fernández, E.J.; Hermann, B.; Považay, B.; Unterhuber, A.; Sattmann, H.; Hofer, B.; Ahnelt, P.K.; Drexler, W. Ultrahigh resolution optical coherence tomography and pancorrection for cellular imaging of the living human retina. Opt. Express 2008, 16, 11083. [Google Scholar] [CrossRef]
- Huckenpahler, A.L.; Wilk, M.A.; Cooper, R.F.; Moehring, F.; Link, B.A.; Carroll, J.; Collery, R.F. Imaging the adult zebrafish cone mosaic using optical coherence tomography. Vis. Neurosci. 2016, 33, E011. [Google Scholar] [CrossRef]
- Collery, R.F.; Veth, K.N.; Dubis, A.M.; Carroll, J.; Link, B.A. Rapid, accurate, and non-invasive measurement of zebrafish axial length and other eye dimensions using SD-OCT allows longitudinal analysis of myopia and emmetropization. PLoS ONE 2014, 9. [Google Scholar] [CrossRef]
- Nadolski, N.J.; Wong, C.X.L.; Hocking, J.C. Electroretinogram analysis of zebrafish retinal function across development. Doc. Ophthalmol. 2020. [Google Scholar] [CrossRef]
- Van Epps, H.A.; Yim, C.M.; Hurley, J.B.; Brockerhoff, S.E. Investigations of photoreceptor synaptic transmission and light adaptation in the zebrafish visual mutant nrc. Investig. Ophthalmol. Vis. Sci. 2001, 42, 868–874. [Google Scholar]
- Makhankov, Y.V.; Rinner, O.; Neuhauss, S.C.F. An inexpensive device for non-invasive electroretinography in small aquatic vertebrates. J. Neurosci. Methods 2004, 135, 205–210. [Google Scholar] [CrossRef]
- Fleisch, V.C.; Jametti, T.; Neuhauss, S.C.F. Electroretinogram (ERG) measurements in larval zebrafish. Cold Spring Harb. Protoc. 2008, 3, 1–6. [Google Scholar] [CrossRef][Green Version]
- Brockerhoff, S.E.; Hurley, J.B.; Niemi, G.A.; Dowling, J.E. A new form of inherited red-blindness identified in zebrafish. J. Neurosci. 1997, 17, 4236–4242. [Google Scholar] [CrossRef]
- Giarmarco, M.M.; Cleghorn, W.M.; Sloat, S.R.; Hurley, J.B.; Brockerhoff, S.E. Mitochondria maintain distinct Ca2+ pools in cone photoreceptors. J. Neurosci. 2017, 37, 2061–2072. [Google Scholar] [CrossRef]
- Ma, E.Y.; Lewis, A.; Barabas, P.; Stearns, G.; Suzuki, S.; Krizaj, D.; Brockerhoff, S.E. Loss of Pde6 reduces cell body Ca2+ transients within photoreceptors. Cell Death Dis. 2013, 4, 1–10. [Google Scholar] [CrossRef]
- Bisbach, C.M.; Hutto, R.A.; Poria, D.; Cleghorn, W.M.; Abbas, F.; Vinberg, F.; Kefalov, V.J.; Hurley, J.B.; Brockerhoff, S.E. Mitochondrial Calcium Uniporter (MCU) deficiency reveals an alternate path for Ca2+ uptake in photoreceptor mitochondria. Sci. Rep. 2020, 10, 1–19. [Google Scholar] [CrossRef]
- Hutto, R.A.; Bisbach, C.M.; Abbas, F.; Brock, D.C.; Cleghorn, W.M.; Parker, E.D.; Bauer, B.H.; Ge, W.; Vinberg, F.; Hurley, J.B.; et al. Increasing Ca2+ in photoreceptor mitochondria alters metabolites, accelerates photoresponse recovery, and reveals adaptations to mitochondrial stress. Cell Death Differ. 2020, 27, 1067–1085. [Google Scholar] [CrossRef] [PubMed]
- Rosen, J.N.; Sweeney, M.F.; Mably, J.D. Microinjection of zebrafish embryos to analyze gene function. J. Vis. Exp. 2009, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, K.; Shima, A.; Kawakami, N. Identification of a functional transposase of the Tol2 element, an Ac-like element from the Japanese medaka fish, and its transposition in the zebrafish germ lineage. Proc. Natl. Acad. Sci. USA 2000, 97, 11403–11408. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, K. Tol2: A versatile gene transfer vector in vertebrates. Genome Biol. 2007, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Thermes, V.; Grabher, C.; Ristoratore, F.; Bourrat, F.; Choulika, A.; Wittbrodt, J.; Joly, J.S. I-SceI meganuclease mediates highly efficient transgenesis in fish. Mech. Dev. 2002, 118, 91–98. [Google Scholar] [CrossRef]
- Distel, M.; Wullimann, M.F.; Köster, R.W. Optimized Gal4 genetics for permanent gene expression mapping in zebrafish. Proc. Natl. Acad. Sci. USA 2009, 106, 13365–13370. [Google Scholar] [CrossRef] [PubMed]
- Akitake, C.M.; Macurak, M.; Halpern, M.E.; Goll, M.G. Transgenerational analysis of transcriptional silencing in zebrafish. Dev. Biol. 2011, 352, 191–201. [Google Scholar] [CrossRef]
- Goll, M.G.; Anderson, R.; Stainier, D.Y.R.; Spradling, A.C.; Halpern, M.E. Transcriptional silencing and reactivation in transgenic zebrafish. Genetics 2009, 182, 747–755. [Google Scholar] [CrossRef]
- Jungke, P.; Hammer, J.; Hans, S.; Brand, M. Isolation of novel CreERT2-driver lines in zebrafish using an unbiased gene trap approach. PLoS ONE 2015, 10, 1–24. [Google Scholar] [CrossRef]
- Kirchgeorg, L.; Felker, A.; van Oostrom, M.; Chiavacci, E.; Mosimann, C. Cre/lox-controlled spatiotemporal perturbation of FGF signaling in zebrafish. Dev. Dyn. 2018, 247, 1146–1159. [Google Scholar] [CrossRef]
- Lin, H.J.; Lee, S.H.; Wu, J.L.; Duann, Y.F.; Chen, J.Y. Development of Cre-loxP technology in zebrafish to study the regulation of fish reproduction. Fish Physiol. Biochem. 2013, 39, 1525–1539. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, K.; Liao, E.C. Generation and characterization of a zebrafish muscle specific inducible Cre line. Transgenic Res. 2018, 27, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Le, X.; Langenau, D.M.; Keefe, M.D.; Kutok, J.L.; Neuberg, D.S.; Zon, L.I. Heat shock-inducible Cre/Lox approaches to induce diverse types of tumors and hyperplasia in transgenic zebrafish. Proc. Natl. Acad. Sci. USA 2007, 104, 9410–9415. [Google Scholar] [CrossRef] [PubMed]
- Tabor, K.M.; Marquart, G.D.; Hurt, C.; Smith, T.S.; Geoca, A.K.; Bhandiwad, A.A.; Subedi, A.; Sinclair, J.L.; Rose, H.M.; Polys, N.F.; et al. Brain-wide cellular resolution imaging of Cre transgenic zebrafish lines for functional circuit-mapping. eLife 2019, 8, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Muto, A.; Orger, M.B.; Wehman, A.M.; Smear, M.C.; Kay, J.N.; Page-McCaw, P.S.; Gahtan, E.; Xiao, T.; Nevin, L.M.; Gosse, N.J.; et al. Forward genetic analysis of visual behavior in zebrafish. PLoS Genet. 2005, 1, e66. [Google Scholar] [CrossRef] [PubMed]
- Doyon, Y.; McCammon, J.M.; Miller, J.C.; Faraji, F.; Ngo, C.; Katibah, G.E.; Amora, R.; Hocking, T.D.; Zhang, L.; Rebar, E.J.; et al. Heritable targeted gene disruption in zebrafish using designed zinc-finger nucleases. Nat. Biotechnol. 2008, 26, 702–708. [Google Scholar] [CrossRef]
- Zu, Y.; Tong, X.; Wang, Z.; Liu, D.; Pan, R.; Li, Z.; Hu, Y.; Luo, Z.; Huang, P.; Wu, Q.; et al. TALEN-mediated precise genome modification by homologous recombination in zebrafish. Nat. Methods 2013, 10, 329–331. [Google Scholar] [CrossRef]
- Hwang, W.Y.; Fu, Y.; Reyon, D.; Maeder, M.L.; Tsai, S.Q.; Sander, J.D.; Peterson, R.T.; Yeh, J.R.J.; Joung, J.K. Efficient genome editing in zebrafish using a CRISPR-Cas system. Nat. Biotechnol. 2013, 31, 227–229. [Google Scholar] [CrossRef]
- Doench, J.G.; Fusi, N.; Sullender, M.; Hegde, M.; Vaimberg, E.W.; Donovan, K.F.; Smith, I.; Tothova, Z.; Wilen, C.; Orchard, R.; et al. Optimized sgRNA design to maximize activity and minimize off-target effects of CRISPR-Cas9. Nat. Biotechnol. 2016, 34, 184–191. [Google Scholar] [CrossRef]
- Ran, F.A.; Hsu, P.D.; Lin, C.Y.; Gootenberg, J.S.; Konermann, S.; Trevino, A.E.; Scott, D.A.; Inoue, A.; Matoba, S.; Zhang, Y.; et al. Double nicking by RNA-guided CRISPR Cas9 for enhanced genome editing specificity. Cell 2013, 154, 1380–1389. [Google Scholar] [CrossRef]
- Maruyama, T.; Dougan, S.K.; Truttmann, M.C.; Bilate, A.M.; Ingram, J.R.; Ploegh, H.L. Increasing the efficiency of precise genome editing with CRISPR-Cas9 by inhibition of nonhomologous end joining. Nat. Biotechnol. 2015, 33, 538–542. [Google Scholar] [CrossRef] [PubMed]
- Kocak, D.D.; Josephs, E.A.; Bhandarkar, V.; Adkar, S.S.; Kwon, J.B.; Gersbach, C.A. Increasing the specificity of CRISPR systems with engineered RNA secondary structures. Nat. Biotechnol. 2019, 37, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Kassahn, K.S.; Dang, V.T.; Wilkins, S.J.; Perkins, A.C.; Ragan, M.A. Evolution of gene function and regulatory control after whole-genome duplication: Comparative analyses in vertebrates. Genome Res. 2009, 19, 1404–1418. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, D.K.; Glasauer, S.M.K.; Mateos, J.M.; Barmettler, G.; Ziegler, U.; Neuhauss, S.C.F. A new zebrafish model for CACNA2D4-dysfunction. Investig. Ophthalmol. Vis. Sci. 2019, 60, 5124–5135. [Google Scholar] [CrossRef]
- Jia, S.; Muto, A.; Orisme, W.; Henson, H.E.; Parupalli, C.; Ju, B.; Baier, H.; Taylor, M.R. Zebrafish cacna1fa is required for cone photoreceptor function and synaptic ribbon formation. Hum. Mol. Genet. 2014, 23, 2981–2994. [Google Scholar] [CrossRef] [PubMed]
- Seiler, C.; Finger-Baier, K.C.; Rinner, O.; Makhankov, Y.V.; Schwarz, H.; Neuhauss, S.C.F.; Nicolson, T. Duplicated genes with split functions: Independent roles of protocadherin 15 orthologues in zebrafish hearing and vision. Development 2005, 132, 615–623. [Google Scholar] [CrossRef]
- Hitchcock, P.F.; Raymond, P.A. The teleost retina as a model for developmental and regeneration biology. Zebrafish 2004, 1, 257–271. [Google Scholar] [CrossRef]
- Wan, J.; Ramachandran, R.; Goldman, D. HB-EGF is necessary and sufficient for Müller glia dedifferentiation and retina regeneration. Dev. Cell 2012, 22, 334–347. [Google Scholar] [CrossRef]
- Ramachandran, R.; Zhao, X.F.; Goldman, D. Insm1a-mediated gene repression is essential for the formation and differentiation of Müller glia-derived progenitors in the injured retina. Nat. Cell Biol. 2012, 14, 1013–1023. [Google Scholar] [CrossRef]
- Meyers, J.R.; Hu, L.; Moses, A.; Kaboli, K.; Papandrea, A.; Raymond, P.A. β-catenin/Wnt signaling controls progenitor fate in the developing and regenerating zebrafish retina. Neural Dev. 2012, 7, 1–17. [Google Scholar] [CrossRef]
- Qin, Z.; Barthel, L.K.; Raymond, P.A. Genetic evidence for shared mechanisms of epimorphic regeneration in zebrafish. Proc. Natl. Acad. Sci. USA 2009, 106, 9310–9315. [Google Scholar] [CrossRef]
- Bernardos, R.L.; Barthel, L.K.; Meyers, J.R.; Raymond, P.A. Late-stage neuronal progenitors in the retina are radial Müller glia that function as retinal stem cells. J. Neurosci. 2007, 27, 7028–7040. [Google Scholar] [CrossRef] [PubMed]
- Penn, J.S. Effects of continuous light on the retina of a fish, Notemigonus crysoleucas. J. Comp. Neurol. 1985, 238, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Shahinfar, S.; Edward, D.P.; Tso, M.O. A pathological study of photoreceptor cell death in retinal photic injury. Curr. Eye Res. 1991, 10, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Thummel, R.; Kassen, S.C.; Enright, J.M.; Nelson, C.M.; Montgomery, J.E.; Hyde, D.R. Characterization of Müller glia and neuronal progenitors during adult zebrafish retinal regeneration. Exp. Eye Res. 2008, 87, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Vihtelic, T.S.; Soverly, J.E.; Kassen, S.C.; Hyde, D.R. Retinal regional differences in photoreceptor cell death and regeneration in light-lesioned albino zebrafish. Exp. Eye Res. 2006, 82, 558–575. [Google Scholar] [CrossRef]
- Dunn, F.A. Photoreceptor ablation initiates the immediate loss of glutamate receptors in postsynaptic bipolar cells in retina. J. Neurosci. 2015, 35, 2423–2431. [Google Scholar] [CrossRef]
- Winkler, B.S. Relative inhibitory effects of ATP depletion, ouabain and calcium on retinal photoreceptors. Exp. Eye Res. 1983, 36, 581–594. [Google Scholar] [CrossRef]
- Fimbel, S.M.; Montgomery, J.E.; Burket, C.T.; Hyde, D.R. Regeneration of inner retinal neurons after intravitreal injection of ouabain in zebrafish. J. Neurosci. 2007, 27, 1712–1724. [Google Scholar] [CrossRef]
- Maier, W.; Wolburg, H. Regeneration of the goldfish retina after exposure to different doses of ouabain. Cell Tissue Res. 1979, 202, 99–118. [Google Scholar] [CrossRef]
- Terracjni, B.; Testa, M.C. Carcinogenicity of a single administration of N-nitrosomethylurea: A comparison between newborn and 5-week-old mice and rats. Br. J. Cancer 1970, 24, 588–598. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wurdeman, R.L.; Church, K.M.; Gold, B. DNA methylation by N-methyl-N-nitrosourea, N-methyl-N’-nitro-N-nitrosoguanidine, N-nitroso(1-acetoxyethyl)methylamine and diazomethane: Mechanism for the formation of N7-methylguanine in sequence-characterized 5′-32P-end-labeled DNA. J. Am. Chem. Soc. 1989, 111, 6408–6412. [Google Scholar] [CrossRef]
- Tappeiner, C.; Balmer, J.; Iglicki, M.; Schuerch, K.; Jazwinska, A.; Enzmann, V.; Tschopp, M. Characteristics of rod regeneration in a novel zebrafish retinal degeneration model using N-methyl-N-nitrosourea (MNU). PLoS ONE 2013, 8, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Nagar, S.; Krishnamoorthy, V.; Cherukuri, P.; Jain, V.; Dhingra, N.K. Early remodeling in an inducible animal model of retinal degeneration. Neuroscience 2009, 160, 517–529. [Google Scholar] [CrossRef] [PubMed]
- Zulliger, R.; Lecaudé, S.; Eigeldinger-Berthou, S.; Wolf-Schnurrbusch, U.E.K.; Enzmann, V. Caspase-3-independent photoreceptor degeneration by N-methyl-N-nitrosourea (MNU) induces morphological and functional changes in the mouse retina. Graefe’s Arch. Clin. Exp. Ophthalmol. 2011, 249, 859–869. [Google Scholar] [CrossRef][Green Version]
- Cao, R.; Jensen, L.D.E.; Söll, I.; Hauptmann, G.; Cao, Y. Hypoxia-induced retinal angiogenesis in zebrafish as a model to study retinopathy. PLoS ONE 2008, 3, 1–9. [Google Scholar] [CrossRef]
- Nasevicius, A.; Ekker, S.C. Effective targeted gene “knockdown” in zebrafish. Nat. Genet. 2000, 26, 216–220. [Google Scholar] [CrossRef]
- Draper, B.W.; Morcos, P.A.; Kimmel, C.B. Inhibition of zebrafish fgf8 pre-mRNA splicing with morpholino oligos: A quantifiable method for gene knockdown. Genesis 2001, 30, 154–156. [Google Scholar] [CrossRef]
- Kok, F.O.; Shin, M.; Ni, C.W.; Gupta, A.; Grosse, A.S.; vanImpel, A.; Kirchmaier, B.C.; Peterson-Maduro, J.; Kourkoulis, G.; Male, I.; et al. Reverse genetic screening reveals poor correlation between morpholino-induced and mutant phenotypes in zebrafish. Dev. Cell 2015, 32, 97–108. [Google Scholar] [CrossRef]
- Rossi, A.; Kontarakis, Z.; Gerri, C.; Nolte, H.; Hölper, S.; Krüger, M.; Stainier, D.Y.R. Genetic compensation induced by deleterious mutations but not gene knockdowns. Nature 2015, 524, 230–233. [Google Scholar] [CrossRef]
- Morris, A.C.; Schroeter, E.H.; Bilotta, J.; Wong, R.O.; Fadool, J.M. Cone survival despite rod degeneration in XOPS-mCFP transgenic zebrafish. Investig. Ophthalmol. Vis. Sci. 2005, 46, 4762–4771. [Google Scholar] [CrossRef] [PubMed]
- Curado, S.; Stainier, D.Y.; Anderson, R.M. Nitroreductase-mediated cell/tissue ablation in zebrafish: A spatially and temporally controlled ablation method with applications in developmental and regeneration studies. Nat. Protoc. 2008, 3, 948–954. [Google Scholar] [CrossRef] [PubMed]
- Fraser, B.; DuVal, M.G.; Wang, H.; Allison, W.T. Regeneration of cone photoreceptors when cell ablation is primarily restricted to a particular cone subtype. PLoS ONE 2013, 8, e55410. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, J.E.; Parsons, M.J.; Hyde, D.R. A novel model of retinal ablation demonstrate that the extent of rod cell death regulates the origin of the regenerated zebrafish rod photoreceptors. J. Comp. Neurol. 2010, 518, 800–814. [Google Scholar] [CrossRef] [PubMed]
- Yoshimatsu, T.; D’Orazi, F.D.; Gamlin, C.R.; Suzuki, S.C.; Suli, A.; Kimelman, D.; Raible, D.W.; Wong, R.O. Presynaptic partner selection during retinal circuit reassembly varies with timing of neuronal regeneration in vivo. Nat. Commun. 2016, 7, 1–10. [Google Scholar] [CrossRef]
- White, D.T.; Sengupta, S.; Saxena, M.T.; Xu, Q.; Hanes, J.; Ding, D.; Ji, H.; Mumm, J.S. Immunomodulation-accelerated neuronal regeneration following selective rod photoreceptor cell ablation in the zebrafish retina. Proc. Natl. Acad. Sci. USA 2017, 114, E3719–E3728. [Google Scholar] [CrossRef]
- D’Orazi, F.D.; Suzuki, S.C.; Darling, N.; Wong, R.O.; Yoshimatsu, T. Conditional and biased regeneration of cone photoreceptor types in the zebrafish retina. J. Comp. Neurol. 2020, 528, 2816–2830. [Google Scholar] [CrossRef]
- Hanovice, N.J.; Leach, L.L.; Slater, K.; Gabriel, A.E.; Romanovicz, D.; Shao, E.; Collery, R.; Burton, E.A.; Lathrop, K.L.; Link, B.A.; et al. Regeneration of the zebrafish retinal pigment epithelium after widespread genetic ablation. PLoS Genet. 2019, 15, 1–35. [Google Scholar] [CrossRef]
- Dryja, T.P.; McGee, T.L.; Reichel, E.; Hahn, L.; Cowley, G.S.; Yandell, D.W.; Sandberg, M.A.; Berson, E.L. A point mutation of the rhodopsin gene in one form of retinitis pigmentosa. Nature 1990, 343, 364–366. [Google Scholar] [CrossRef]
- Sullivan, L.S.; Bowne, S.J.; Birch, D.G.; Hughbanks-Wheaton, D.; Heckenlively, J.R.; Lewis, R.A.; Garcia, C.A.; Ruiz, R.S.; Blanton, S.H.; Northrup, H.; et al. Prevalence of disease-causing mutations in families with autosomal dominant retinitis pigmentosa: A screen of known genes in 200 families. Investig. Ophthalmol. Vis. Sci. 2006, 47, 3052–3064. [Google Scholar] [CrossRef]
- Parmeggiani, F.S.; Sorrentino, F.; Ponzin, D.; Barbaro, V.; Ferrari, S.; Di Iorio, E. Retinitis pigmentosa: Genes and disease mechanisms. Curr. Genom. 2011, 12, 238–249. [Google Scholar] [CrossRef] [PubMed]
- Santhanam, A.; Shihabeddin, E.; Atkinson, J.A.; Nguyen, D.; Lin, Y.-P.; O’Brien, J. A zebrafish model of retinitis pigmentosa shows continuous degeneration and regeneration of rod photoreceptors. Cells 2020, 9, 2242. [Google Scholar] [CrossRef]
- Sung, C.H.; Makino, C.; Baylor, D.; Nathans, J. A rhodopsin gene mutation responsible for autosomal dominant retinitis pigmentosa results in a protein that is defective in localization to the photoreceptor outer segment. J. Neurosci. 1994, 14, 5818–5833. [Google Scholar] [CrossRef] [PubMed]
- Nakao, T.; Tsujikawa, M.; Notomi, S.; Ikeda, Y.; Nishida, K. The role of mislocalized phototransduction in photoreceptor cell death of retinitis pigmentosa. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Vithana, E.N.; Abu-Safieh, L.; Allen, M.J.; Carey, A.; Papaioannou, M.; Chakarova, C.; Al-Maghtheh, M.; Ebenezer, N.D.; Willis, C.; Moore, A.T.; et al. A human homolog of yeast pre-mRNA splicing gene, PRP31, underlies autosomal dominant retinitis pigmentosa on chromosome 19q13.4 (RP11). Mol. Cell 2001, 8, 375–381. [Google Scholar] [CrossRef]
- Yin, J.; Brocher, J.; Fischer, U.; Winkler, C. Mutant Prpf31 causes pre-mRNA splicing defects and rod photoreceptor cell degeneration in a zebrafish model for retinitis pigmentosa. Mol. Neurodegener. 2011, 6, 56. [Google Scholar] [CrossRef]
- Yang, Z.; Camp, N.J.; Sun, H.; Tong, Z.; Gibbs, D.; Cameron, D.J.; Chen, H.; Zhao, Y.; Pearson, E.; Li, X.; et al. A variant of the HTRA1 gene increases susceptibility to age-related macular degeneration. Science 2006, 314, 992–993. [Google Scholar] [CrossRef] [PubMed]
- DeWan, A.; Liu, M.; Hartman, S.; Zhang, S.S.-M.; Liu, D.T.L.; Zhao, C.; Tam, P.O.S.T.; Chan, W.M.; Lam, D.S.C.; Snyder, M.; et al. HTRA1 promoter polymorphism in wet age-related macular degeneration. Science 2006, 314, 989–993. [Google Scholar] [CrossRef]
- Jones, A.; Kumar, S.; Zhang, N.; Tong, Z.; Yang, J.H.; Watt, C.; Anderson, J.; Amrita; Fillerup, H.; McCloskey, M.; et al. Increased expression of multifunctional serine protease, HTRA1, in retinal pigment epithelium induces polypoidal choroidal vasculopathy in mice. Proc. Natl. Acad. Sci. USA 2011, 108, 14578–14583. [Google Scholar] [CrossRef]
- Oura, Y.; Nakamura, M.; Takigawa, T.; Fukushima, Y.; Wakabayashi, T.; Tsujikawa, M.; Nishida, K. High-temperature requirement A 1 causes photoreceptor cell death in zebrafish disease models. Am. J. Pathol. 2018, 188, 2729–2744. [Google Scholar] [CrossRef]
- Collery, R.F.; Cederlund, M.L.; Kennedy, B.N. Transgenic zebrafish expressing mutant human RETGC-1 exhibit aberrant cone and rod morphology. Exp. Eye Res. 2013, 108, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Link, B.A.; Collery, R.F. Zebrafish models of retinal disease. Annu. Rev. Vis. Sci. 2015, 1, 125–153. [Google Scholar] [CrossRef] [PubMed]
- Angueyra, J.M.; Kindt, K.S. Leveraging zebrafish to study retinal degenerations. Front. Cell Dev. Biol. 2018, 6, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Chhetri, J.; Jacobson, G.; Gueven, N. Zebrafish–on the move towards ophthalmological research. Eye 2014, 28, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Gross, J.M.; Perkins, B.D. Zebrafish mutants as models for congenital ocular disorders in humans. Mol. Reprod. Dev. 2008, 75, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Morris, A.C. The genetics of ocular disorders: Insights from the zebrafish. Birth Defects Res. Part. C Embryo Today Rev. 2011, 93, 215–228. [Google Scholar] [CrossRef]
- Athanasiou, D.; Aguila, M.; Bellingham, J.; Li, W.; McCulley, C.; Reeves, P.J.; Cheetham, M.E. The molecular and cellular basis of rhodopsin retinitis pigmentosa reveals potential strategies for therapy. Prog. Retin. Eye Res. 2018, 62, 1–23. [Google Scholar] [CrossRef]
- Zelinka, C.P.; Sotolongo-Lopez, M.; Fadool, J.M. Targeted disruption of the endogenous zebrafish rhodopsin locus as models of rapid rod photoreceptor degeneration. Mol. Vis. 2018, 24, 587–602. [Google Scholar]
- Eroglu, A.U.; Mulligan, T.S.; Zhang, L.; White, D.T.; Sengupta, S.; Nie, C.; Lu, N.Y.; Qian, J.; Xu, L.; Pei, W.; et al. Multiplexed CRISPR/Cas9 targeting of genes implicated in retinal regeneration and degeneration. Front. Cell Dev. Biol. 2018, 6. [Google Scholar] [CrossRef]
- Schwahn, U.; Lenzner, S.; Dong, J.; Feil, S.; Hinzmann, B.; Van Duijnhoven, G.; Kirschner, R.; Hemberger, M.; Bergen, A.A.B.; Rosenberg, T.; et al. Positional cloning of the gene for X-linked retinitis pigmentosa 2. Nat. Genet. 1998, 19, 327–332. [Google Scholar] [CrossRef]
- Hurd, T.W.; Fan, S.; Margolis, B.L. Localization of retinitis pigmentosa 2 to cilia is regulated by importin β2. J. Cell Sci. 2011, 124, 718–726. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, N.; Hardcastle, A.J.; Cheetham, M.E. Arl3 and RP2 mediated assembly and traffic of membrane associated cilia proteins. Vis. Res. 2012, 75, 2–4. [Google Scholar] [CrossRef] [PubMed]
- Evans, R.J.; Schwarz, N.; Nagel-Wolfrum, K.; Wolfrum, U.; Hardcastle, A.J.; Cheetham, M.E. The retinitis pigmentosa protein RP2 links pericentriolar vesicle transport between the Golgi and the primary cilium. Hum. Mol. Genet. 2010, 19, 1358–1367. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Chen, J.; Yu, S.; Raghupathy, R.K.; Liu, X.; Qin, Y.; Li, C.; Huang, M.; Liao, S.; Wang, J.; et al. Knockout of RP2 decreases GRK1 and rod transducin subunits and leads to photoreceptor degeneration in zebrafish. Hum. Mol. Genet. 2015, 24, 4648–4659. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tuson, M.; Marfany, G.; Gonzàlez-Duarte, R. Mutation of CERKL, a novel human ceramide kinase gene, causes autosomal recessive retinitis pigmentosa (RP26). Am. J. Hum. Genet. 2004, 74, 128–138. [Google Scholar] [CrossRef]
- Yu, S.; Li, C.; Biswas, L.; Hu, X.; Liu, F.; Reilly, J.; Liu, X.; Liu, Y.; Huang, Y.; Lu, Z.; et al. CERKL gene knockout disturbs photoreceptor outer segment phagocytosis and causes rod-cone dystrophy in zebrafish. Hum. Mol. Genet. 2017, 26, 2335–2345. [Google Scholar] [CrossRef]
- Cogné, B.; Latypova, X.; Senaratne, L.D.S.; Martin, L.; Koboldt, D.C.; Kellaris, G.; Fievet, L.; Le Meur, G.; Caldari, D.; Debray, D. Mutations in the kinesin-2 motor KIF3B cause an autosomal-dominant ciliopathy. Am. J. Hum. Genet. 2020, 106, 893–904. [Google Scholar] [CrossRef]
- Zhao, C.; Omori, Y.; Brodowska, K.; Kovach, P.; Malicki, J. Kinesin-2 family in vertebrate ciliogenesis. Proc. Natl. Acad. Sci. USA 2012, 109, 2388–2393. [Google Scholar] [CrossRef]
- Feng, D.; Chen, Z.; Yang, K.; Miao, S.; Xu, B.; Kang, Y.; Xie, H.; Zhao, C. The cytoplasmic tail of rhodopsin triggers rapid rod degeneration in kinesin-2 mutants. J. Biol. Chem. 2017, 292, 1735–17386. [Google Scholar] [CrossRef]
- Dryja, T.P.; Adams, S.M.; Grimsby, J.L.; McGee, T.L.; Hong, D.H.; Li, T.; Andréasson, S.; Berson, E.L. Null RPGRIP1 alleles in patients with Leber congenital amaurosis. Am. J. Hum. Genet. 2001, 68, 1295–1298. [Google Scholar] [CrossRef]
- Hameed, A.; Abid, A.; Aziz, A.; Ismail, M.; Mehdi, S.Q.; Khaliq, S. Evidence of RPGRIP1 gene mutations associated with recessive cone-rod dystrophy. J. Med. Genet. 2003, 40, 616–619. [Google Scholar] [CrossRef] [PubMed]
- Booij, J.C.; Florijn, R.J.; ten Brink, J.B.; Loves, W.; Meire, F.; van Schooneveld, M.J.; de Jong, P.T.; Bergen, A.A. Identification of mutations in the AIPL1, CRB1, GUCY2D, RPE65, and RPGRIP1 genes in patients with juvenile retinitis pigmentosa. J. Med. Genet. 2005, 42, 1–8. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mavlyutov, T.A.; Zhao, H.; Ferreira, P.A. Species-specific subcellular localization of RPGR and RPGRIP isoforms: Implications for the phenotypic variability of congenital retinopathies among species. Hum. Mol. Genet. 2002, 11, 1899–1907. [Google Scholar] [CrossRef] [PubMed]
- Raghupathy, R.K.; Zhang, X.; Liu, F.; Alhasani, R.H.; Biswas, L.; Akhtar, S.; Pan, L.; Moens, C.B.; Li, W.; Liu, M.; et al. Rpgrip1 is required for rod outer segment development and ciliary protein trafficking in zebrafish. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef]
- Davidson, A.E.; Sergouniotis, P.I.; Mackay, D.S.; Wright, G.A.; Waseem, N.H.; Michaelides, M.; Holder, G.E.; Robson, A.G.; Moore, A.T.; Plagnol, V.; et al. RP1L1 variants are associated with a spectrum of inherited retinal diseases including retinitis pigmentosa and occult macular dystrophy. Hum. Mutat. 2013, 34, 506–514. [Google Scholar] [CrossRef]
- Noel, N.C.L.; MacDonald, I.M. RP1L1 and inherited photoreceptor disease: A review. Surv. Ophthalmol. 2020, 65, 725–739. [Google Scholar] [CrossRef]
- Akahori, M.; Tsunoda, K.; Miyake, Y.; Fukuda, Y.; Ishiura, H.; Tsuji, S.; Usui, T.; Hatase, T.; Nakamura, M.; Ohde, H.; et al. Dominant mutations in RP1L1 are responsible for occult macular dystrophy. Am. J. Hum. Genet. 2010, 87, 424–429. [Google Scholar] [CrossRef]
- Noel, N.C.L.; Nadolski, N.J.; Hocking, J.C.; Macdonald, I.M.; Allison, W.T. Progressive photoreceptor dysfunction and age-related macular degeneration-like features in rp1l1 mutant zebrafish. Cells 2020, 9, 2214. [Google Scholar] [CrossRef]
- El-Amraoui, A.; Sahly, I.; Picaud, S.; Sahel, J.; Abitbol, M.; Petit, C. Human Usher 1B/mouse shaker-1: The retinal phenotype discrepancy explained by the presence/absence of myosin VIIA in the photoreceptor cells. Hum. Mol. Genet. 1996, 5, 1171–1178. [Google Scholar] [CrossRef]
- Zina, Z.B.; Masmoudi, S.; Ayadi, H.; Chaker, F.; Ghorbel, A.M.; Drira, M.; Petit, C. From DFNB2 to usher syndrome: Variable expressivity of the same disease. Am. J. Med. Genet. 2001, 101, 181–183. [Google Scholar] [CrossRef]
- Weil, D.; Küssel, P.; Blanchard, S.; Lévy, G.; Levi-Acobas, F.; Drira, M.; Ayadi, H.; Petit, C. The autosomal recessive isolated deafness, DFNB2, and the Usher 1B syndrome are allelic defects of the myosin-VIIA gene. Nat. Genet. 1997, 16, 191–193. [Google Scholar] [CrossRef] [PubMed]
- Wasfy, M.M.; Matsui, J.I.; Miller, J.; Dowling, J.E.; Perkins, B.D. Myosin 7aa-/- mutant zebrafish show mild photoreceptor degeneration and reduced electroretinographic responses. Exp. Eye Res. 2014, 122, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Eudy, J.D.; Weston, M.D.; Yao, S.F.; Hoover, D.M.; Rehm, H.L.; Ma-Edmonds, M.; Yan, D.; Ahmad, I.; Cheng, J.J.; Ayuso, C.; et al. Mutation of a gene encoding a protein with extracellular matrix motifs in Usher syndrome type IIa. Science 1998, 280, 1753–1757. [Google Scholar] [CrossRef] [PubMed]
- van Wijk, E.; Pennings, R.J.E.; te Brinke, H.; Claassen, A.; Yntema, H.G.; Hoefsloot, L.H.; Cremers, F.P.M.; Cremers, W.R.J.; Kremer, H. Identification of 51 Novel Exons of the Usher Syndrome Type 2A (USH2A) Gene That Encode Multiple Conserved Functional Domains and That Are Mutated in Patients with Usher Syndrome Type II. Am. J. Hum. Genet. 2004, 74, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Liu, X.; Xie, S.; Gao, M.; Liu, F.; Yu, S.; Sun, P.; Wang, C.; Archacki, S.; Lu, Z.; et al. Knockout of ush2a gene in zebrafish causes hearing impairment and late onset rod-cone dystrophy. Hum. Genet. 2018, 137, 779–794. [Google Scholar] [CrossRef]
- Dona, M.; Slijkerman, R.; Lerner, K.; Broekman, S.; Wegner, J.; Howat, T.; Peters, T.; Hetterschijt, L.; Boon, N.; de Vrieze, E.; et al. Usherin defects lead to early-onset retinal dysfunction in zebrafish. Exp. Eye Res. 2018, 173, 148–159. [Google Scholar] [CrossRef]
- Morris, A.C.; Forbes-Osborne, M.A.; Pillai, L.S.; Fadool, J.M. Microarray analysis of XOPS-mCFP zebrafish retina identifies genes associated with rod photoreceptor degeneration and regeneration. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2255–2266. [Google Scholar] [CrossRef]
- Coomer, C.E.; Wilson, S.G.; Titialii-Torres, K.F.; Bills, J.D.; Krueger, L.A.; Petersen, R.A.; Turnbaugh, E.M.; Janesch, E.L.; Morris, A.C. Her9/Hes4 is required for retinal photoreceptor development, maintenance, and survival. Sci. Rep. 2020, 10, 1–20. [Google Scholar] [CrossRef]
- Sukumaran, S.; Perkins, B.D. Early defects in photoreceptor outer segment morphogenesis in zebrafish ift57, ift88 and ift172 intraflagellar transport mutants. Vis. Res. 2009, 49, 479–489. [Google Scholar] [CrossRef]
- Doerre, G.; Malicki, J. Genetic analysis of photoreceptor cell development in the zebrafish retina. Mech. Dev. 2002, 110, 125–138. [Google Scholar] [CrossRef]
- Tsujikawa, M.; Malicki, J. Intraflagellar transport genes are essential for differentiation and survival of vertebrate sensory neurons. Neuron 2004, 42, 703–716. [Google Scholar] [CrossRef]
- Krock, B.L.; Perkins, B.D. The intraflagellar transport protein IFT57 is required for cilia maintenance and regulates IFT-particle-kinesin-II dissociation in vertebrate photoreceptors. J. Cell Sci. 2008, 121, 1907–1915. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Wallingford, J.B.; Gross, J.M. Cluap1 is essential for ciliogenesis and photoreceptor maintenance in the vertebrate eye. Investig. Ophthalmol. Vis. Sci. 2014, 55, 4585–4592. [Google Scholar] [CrossRef] [PubMed]
- Gross, J.M.; Perkins, B.D.; Amsterdam, A.; Egaña, A.; Darland, T.; Matsui, J.I.; Sciascia, S.; Hopkins, N.; Dowling, J.E. Identification of zebrafish insertional mutants with defects in visual system development and function. Genetics 2005, 170, 245–261. [Google Scholar] [CrossRef] [PubMed]
- Boubakri, M.; Chaya, T.; Hirata, H.; Kajimura, N.; Kuwahara, R.; Ueno, A.; Malicki, J.; Furukawa, T.; Omori, Y. Loss of ift122, a retrograde intraflagellar transport (IFT) complex component, leads to slow, progressive photoreceptor degeneration due to inefficient opsin transport. J. Biol. Chem. 2016, 291, 24465–24474. [Google Scholar] [CrossRef] [PubMed]
- Pooranachandran, N.; Malicki, J.J. Unexpected roles for ciliary kinesins and intraflagellar transport proteins. Genetics 2016, 203, 771–785. [Google Scholar] [CrossRef] [PubMed]
- Raghupathy, R.K.; Zhang, X.; Alhasani, R.H.; Zhou, X.; Mullin, M.; Reilly, J.; Li, W.; Liu, M.; Shu, X. Abnormal photoreceptor outer segment development and early retinal degeneration in kif3a mutant zebrafish. Cell Biochem. Funct. 2016, 34, 429–440. [Google Scholar] [CrossRef]
- Burgalossi, A.; Jung, S.; Meyer, G.; Jockusch, W.J.; Jahn, O.; Taschenberger, H.; O’Connor, V.M.; Nishiki, T.-i.; Takahashi, M.; Brose, N.; et al. SNARE protein recycling by αSNAP and βSNAP supports synaptic vesicle priming. Neuron 2010, 68, 473–487. [Google Scholar] [CrossRef]
- Nishiwaki, Y.; Yoshizawa, A.; Kojima, Y.; Oguri, E.; Nakamura, S.; Suzuki, S.; Yuasa-Kawada, J.; Kinoshita-Kawada, M.; Mochizuki, T.; Masai, I. The BH3-only SNARE BNip1 mediates photoreceptor apoptosis in response to vesicular fusion defects. Dev. Cell 2013, 25, 374–387. [Google Scholar] [CrossRef]
- Nishiwaki, Y.; Komori, A.; Sagara, H.; Suzuki, E.; Manabe, T.; Hosoya, T.; Nojima, Y.; Wada, H.; Tanaka, H.; Okamoto, H.; et al. Mutation of cGMP phosphodiesterase 6α′-subunit gene causes progressive degeneration of cone photoreceptors in zebrafish. Mech. Dev. 2008, 125, 932–946. [Google Scholar] [CrossRef]
- Bujakowska, K.M.; Zhang, Q.; Siemiatkowska, A.M.; Liu, Q.; Place, E.; Falk, M.J.; Consugar, M.; Lancelot, M.E.; Antonio, A.; Lonjou, C.; et al. Mutations in IFT172 cause isolated retinal degeneration and Bardet-Biedl syndrome. Hum. Mol. Genet. 2015, 24, 230–242. [Google Scholar] [CrossRef] [PubMed]
- Bachmann-Gagescu, R.; Phelps, I.G.; Dempsey, J.C.; Sharma, V.A.; Ishak, G.E.; Boyle, E.A.; Wilson, M.; Marques lourenço, C.; Arslan, M.; Shendure, J.; et al. KIAA0586 is mutated in Joubert syndrome. Hum. Mutat. 2015, 36, 831–835. [Google Scholar] [CrossRef] [PubMed]
- Naharros, I.O.; Cristian, F.B.; Zang, J.; Gesemann, M.; Ingham, P.W.; Neuhauss, S.C.F.; Bachmann-Gagescu, R. The ciliopathy protein TALPID3/KIAA0586 acts upstream of Rab8 activation in zebrafish photoreceptor outer segment formation and maintenance. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef]
- Edvardson, S.; Shaag, A.; Zenvirt, S.; Erlich, Y.; Hannon, G.J.; Shanske, A.L.; Gomori, J.M.; Ekstein, J.; Elpeleg, O. Joubert syndrome 2 (JBTS2) in Ashkenazi Jews is associated with a TMEM216 mutation. Am. J. Hum. Genet. 2010, 86, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Valente, E.M.; Logan, C.V.; Mougou-Zerelli, S.; Lee, J.H.; Silhavy, J.L.; Brancati, F.; Iannicelli, M.; Travaglini, L.; Romani, S.; Illi, B.; et al. Mutations in TMEM216 perturb ciliogenesis and cause Joubert, Meckel and related syndromes. Nat. Genet. 2010, 42, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cao, S.; Yu, M.; Hu, H. TMEM216 deletion causes mislocalization of cone opsin and rhodopsin and photoreceptor degeneration in zebrafish. Investig. Ophthalmol. Vis. Sci. 2020, 61, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Asai-Coakwell, M.; March, L.; Dai, X.H.; DuVal, M.; Lopez, I.; French, C.R.; Famulski, J.; De Baere, E.; Francis, P.J.; Sundaresan, P.; et al. Contribution of growth differentiation factor 6-dependent cell survival to early-onset retinal dystrophies. Hum. Mol. Genet. 2013, 22, 1432–1442. [Google Scholar] [CrossRef]
- Nadolski, N.J.; Balay, S.D.; Wong, C.X.L.; Waskiewicz, A.J.; Hocking, J.C. Abnormal cone and rod photoreceptor morphogenesis in gdf6a mutant zebrafish. Investig. Ophthalmol. Vis. Sci. 2020, 61. [Google Scholar] [CrossRef]
- Noor, A.; Windpassinger, C.; Patel, M.; Stachowiak, B.; Mikhailov, A.; Azam, M.; Irfan, M.; Siddiqui, Z.K.; Naeem, F.; Paterson, A.D.; et al. CC2D2A, encoding a coiled-coil and C2 domain protein, causes autosomal-recessive mental retardation with retinitis pigmentosa. Am. J. Hum. Genet. 2008, 82, 1011–1018. [Google Scholar] [CrossRef]
- Ferland, R.J.; Eyaid, W.; Collura, R.V.; Tully, L.D.; Hill, R.S.; Al-Nouri, D.; Al-Rumayyan, A.; Topcu, M.; Gascon, G.; Bodell, A.; et al. Abnormal cerebellar development and axonal decussation due to mutations in AHI1 in Joubert syndrome. Nat. Genet. 2004, 36, 1008–1013. [Google Scholar] [CrossRef]
- Cantagrel, V.; Silhavy, J.L.; Bielas, S.L.; Swistun, D.; Marsh, S.E.; Bertrand, J.Y.; Audollent, S.; Attié-Bitach, T.; Holden, K.R.; Dobyns, W.B.; et al. Mutations in the cilia gene ARL13B lead to the classical form of Joubert syndrome. Am. J. Hum. Genet. 2008, 83, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Bachmann-Gagescu, R.; Phelps, I.G.; Stearns, G.; Link, B.A.; Brockerhoff, S.E.; Moens, C.B.; Doherty, D. The ciliopathy gene cc2d2a controls zebrafish photoreceptor outer segment development through a role in Rab8-dependent vesicle trafficking. Hum. Mol. Genet. 2011, 20, 4041–4055. [Google Scholar] [CrossRef] [PubMed]
- Lessieur, E.M.; Fogerty, J.; Gaivin, R.J.; Song, P.; Perkins, B.D. The ciliopathy gene ahi1 is required for zebrafish cone photoreceptor outer segment morphogenesis and survival. Investig. Ophthalmol. Vis. Sci. 2017, 58, 448–460. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Song, P.; Dudinsky, L.; Fogerty, J.; Gaivin, R.; Perkins, B.D. Arl13b interacts with vangl2 to regulate cilia and photoreceptor outer segment length in zebrafish. Investig. Ophthalmol. Vis. Sci. 2016, 57, 4517–4526. [Google Scholar] [CrossRef] [PubMed]
- Den Hollander, A.I.; Koenekoop, R.K.; Mohamed, M.D.; Arts, H.H.; Boldt, K.; Towns, K.V.; Sedmak, T.; Beer, M.; Nagel-Wolfrum, K.; McKibbin, M.; et al. Mutations in LCA5, encoding the ciliary protein lebercilin, cause Leber congenital amaurosis. Nat. Genet. 2007, 39, 889–895. [Google Scholar] [CrossRef]
- Qu, Z.; Yimer, T.A.; Xie, S.; Wong, F.; Yu, S.; Liu, X.; Han, S.; Ma, J.; Lu, Z.; Hu, X.; et al. Knocking out lca5 in zebrafish causes cone-rod dystrophy due to impaired outer segment protein trafficking. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 2694–2705. [Google Scholar] [CrossRef]
- Nishimura, D.Y.; Baye, L.M.; Perveen, R.; Searby, C.C.; Avila-Fernandez, A.; Pereiro, I.; Ayuso, C.; Valverde, D.; Bishop, P.N.; Manson, F.D.C.; et al. Discovery and functional analysis of a retinitis pigmentosa gene, C2ORF71. Am. J. Hum. Genet. 2010, 86, 686–695. [Google Scholar] [CrossRef]
- Corral-Serrano, J.C.; Messchaert, M.; Dona, M.; Peters, T.A.; Kamminga, L.M.; Van Wijk, E.; Collin, R.W.J. C2orf71a/pcare1 is important for photoreceptor outer segment morphogenesis and visual function in zebrafish. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Abd El-Aziz, M.M.; Barragan, I.; O’Driscoll, C.A.; Goodstadt, L.; Prigmore, E.; Borrego, S.; Mena, M.; Pieras, J.I.; El-Ashry, M.F.; Safieh, L.A.; et al. EYS, encoding an ortholog of Drosophila spacemaker, is mutated in autosomal recessive retinitis pigmentosa. Nat. Genet. 2008, 40, 1285–1287. [Google Scholar] [CrossRef]
- Collin, R.W.J.; Littink, K.W.; Klevering, B.J.; van den Born, L.I.; Koenekoop, R.K.; Zonneveld, M.N.; Blokland, E.A.W.; Strom, T.M.; Hoyng, C.B.; den Hollander, A.I.; et al. Identification of a 2 Mb human ortholog of Drosophila eyes shut/spacemaker that Is mutated in patients with retinitis pigmentosa. Am. J. Hum. Genet. 2008, 83, 594–603. [Google Scholar] [CrossRef]
- Katagiri, S.; Akahori, M.; Hayashi, T.; Yoshitake, K.; Gekka, T.; Ikeo, K.; Tsuneoka, H.; Iwata, T. Autosomal recessive cone-rod dystrophy associated with compound heterozygous mutations in the EYS gene. Doc. Ophthalmol. 2014, 128, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Alfano, G.; Kruczek, P.M.; Shah, A.Z.; Kramarz, B.; Jeffery, G.; Zelhof, A.C.; Bhattacharya, S.S. EYS is a protein associated with the ciliary axoneme in rods and cones. PLoS ONE 2016, 11, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Liu, Y.; Li, J.; Natale, B.N.; Cao, S.; Wang, D.; Amack, J.D.; Hu, H. Eyes shut homolog is required for maintaining the ciliary pocket and survival of photoreceptors in zebrafish. Biol. Open 2016, 5, 1662–1673. [Google Scholar] [CrossRef] [PubMed]
- Messchaert, M.; Dona, M.; Broekman, S.; Peters, T.A.; Corral-Serrano, J.C.; Slijkerman, R.W.N.; van Wijk, E.; Collin, R.W.J. Eyes shut homolog is important for the maintenance of photoreceptor morphology and visual function in zebrafish. PLoS ONE 2018, 13, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Hu, X.; Liu, F.; Soares, D.C.; Liu, X.; Yu, S.; Gao, M.; Han, S.; Qin, Y.; Li, C.; et al. Ablation of EYS in zebrafish causes mislocalisation of outer segment proteins, F-actin disruption and cone-rod dystrophy. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Nishimura, D.Y.; Searby, C.C.; Carmi, R.; Elbedour, K.; Van Maldergem, L.; Fulton, A.B.; Lam, B.L.; Powell, B.R.; Swiderski, R.E.; Bugge, K.E.; et al. Positional cloning of a novel gene on chromosome 16q causing Bardet-Biedl syndrome (BBS2). Hum. Mol. Genet. 2001, 10, 865–874. [Google Scholar] [CrossRef]
- Shevach, E.; Ali, M.; Mizrahi-Meissonnier, L.; McKibbin, M.; El-Asrag, M.; Watson, C.M.; Inglehearn, C.F.; Ben-Yosef, T.; Blumenfeld, A.; Jalas, C.; et al. Association between missense mutations in the BBS2 gene and nonsyndromic retinitis pigmentosa. Jama Ophthalmol. 2015, 133, 312–318. [Google Scholar] [CrossRef]
- Song, P.; Fogerty, J.; Cianciolo, L.T.; Stupay, R.; Perkins, B.D. Cone photoreceptor degeneration and neuroinflammation in the zebrafish Bardet-Biedl syndrome 2 (bbs2) mutant does not lead to retinal regeneration. Front. Cell Dev. Biol. 2020, 8, 1–13. [Google Scholar] [CrossRef]
- Sergouniotis, P.I.; Davidson, A.E.; Mackay, D.S.; Li, Z.; Yang, X.; Plagnol, V.; Moore, A.T.; Webster, A.R. Recessive mutations in KCNJ13, encoding an inwardly rectifying potassium channel subunit, cause leber congenital amaurosis. Am. J. Hum. Genet. 2011, 89, 183–190. [Google Scholar] [CrossRef]
- Toms, M.; Burgoyne, T.; Tracey-White, D.; Richardson, R.; Dubis, A.M.; Webster, A.R.; Futter, C.; Moosajee, M. Phagosomal and mitochondrial alterations in RPE may contribute to KCNJ13 retinopathy. Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef]
- Xu, M.; Yamada, T.; Sun, Z.; Eblimit, A.; Lopez, I.; Wang, F.; Manya, H.; Xu, S.; Zhao, L.; Li, Y.; et al. Mutations in POMGNT1 cause non-syndromic retinitis pigmentosa. Hum. Mol. Genet. 2016, 25, 1479–1488. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yu, M.; Shang, X.; Nguyen, M.H.H.; Balakrishnan, S.; Sager, R.; Hu, H. Eyes shut homolog (EYS) interacts with matriglycan of O-mannosyl glycans whose deficiency results in EYS mislocalization and degeneration of photoreceptors. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Han, S.; Qu, Z.; Liu, F.; Li, J.; Yu, S.; Reilly, J.; Tu, J.; Liu, X.; Lu, Z.; et al. Knockout of Nr2e3 prevents rod photoreceptor differentiation and leads to selective L-/M-cone photoreceptor degeneration in zebrafish. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 1273–1283. [Google Scholar] [CrossRef] [PubMed]
- Oel, A.P.; Neil, G.N.; Dong, E.M.; Balay, S.D.; Collett, K.; Allison, W.T. Nrl is dispensable for specification of rod photoreceptors in adult zebrafish contrasting a deeply conserved requirement earlier in ontogeny. iScience 2020, 23. [Google Scholar] [CrossRef]
- Maw, M.A.; Corbeil, D.; Koch, J.; Hellwig, A.; Wilson-Wheeler, J.C.; Bridges, R.J.; Kumaramanickavel, G.; John, S.; Nancarrow, D.; Röper, K.; et al. A frameshift mutation in prominin (mouse)-like 1 causes human retinal degeneration. Hum. Mol. Genet. 2000, 9, 27–34. [Google Scholar] [CrossRef]
- Zhang, Q.; Zulfiqar, F.; Xiao, X.; Riazuddin, S.A.; Ahmad, Z.; Caruso, R.; MacDonald, I.; Sieving, P.; Riazuddin, S.A.; Hejtmancik, J.F. Severe retinitis pigmentosa mapped to 4p15 and associated with a novel mutation in the PROM1 gene. Hum. Genet. 2007, 122, 293–299. [Google Scholar] [CrossRef]
- Yang, Z.; Chen, Y.; Lillo, C.; Chien, J.; Yu, Z.; Michaelides, M.; Klein, M.; Howes, K.A.; Li, Y.; Kaminoh, Y.; et al. Mutant prominin 1 found in patients with macular degeneration disrupts photoreceptor disk morphogenesis in mice. J. Clin. Investig. 2008, 118, 2908–2916. [Google Scholar] [CrossRef]
- Lu, Z.; Hu, X.; Reilly, J.; Jia, D.; Liu, F.; Yu, S.; Liu, X.; Xie, S.; Qu, Z.; Qin, Y.; et al. Deletion of the transmembrane protein Prom1b in zebrafish disrupts outer-segment morphogenesis and causes photoreceptor degeneration. J. Biol. Chem. 2019, 294, 13953–13963. [Google Scholar] [CrossRef]
- Lessieur, E.M.; Song, P.; Nivar, G.C.; Piccillo, E.M.; Fogerty, J.; Rozic, R.; Perkins, B.D. Ciliary genes arl13b, ahi1 and cc2d2a differentially modify expression of visual acuity phenotypes but do not enhance retinal degeneration due to mutation of cep290 in zebrafish. PLoS ONE 2019, 14, 1–26. [Google Scholar] [CrossRef]
- Thiadens, A.A.H.J.; den Hollander, A.I.; Roosing, S.; Nabuurs, S.B.; Zekveld-Vroon, R.C.; Collin, R.W.J.; De Baere, E.; Koenekoop, R.K.; van Schooneveld, M.J.; Strom, T.M.; et al. Homozygosity mapping reveals PDE6C mutations in patients with early-onset cone photoreceptor disorders. Am. J. Hum. Genet. 2009, 85, 240–247. [Google Scholar] [CrossRef]
- Sohocki, M.M.; Bowne, S.J.; Sullivan, L.S.; Blackshaw, S.; Cepko, C.L.; Payne, A.M.; Bhattacharya, S.S.; Khaliq, S.; Mehdi, S.Q.; Birch, D.G.; et al. Mutations in a new photoreceptor-pineal gene on 17p cause Leber congenital amaurosis. Nat. Genet. 2000, 24, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Sohocki, M.M.; Perrault, I.; Leroy, B.P.; Payne, A.M.; Dharmaraj, S.; Bhattacharya, S.S.; Kaplan, J.; Maumenee, I.H.; Koenekoop, R.; Meire, F.M.; et al. Prevalence of AIPL1 mutations in inherited retinal degenerative disease. Mol. Genet. Metab. 2000, 70, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Iribarne, M.; Nishiwaki, Y.; Nakamura, S.; Araragi, M.; Oguri, E.; Masai, I. Aipl1 is required for cone photoreceptor function and survival through the stability of Pde6c and Gc3 in zebrafish. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wycisk, K.A.; Zeitz, C.; Feil, S.; Wittmer, M.; Forster, U.; Neidhardt, J.; Wissinger, B.; Zrenner, E.; Wilke, R.; Kohl, S.; et al. Mutation in the auxiliary calcium-channel subunit CACNA2D4 causes autosomal recessive cone dystrophy. Am. J. Hum. Genet. 2006, 79, 973–977. [Google Scholar] [CrossRef] [PubMed]
- Daly, C.; Shine, L.; Heffernan, T.; Deeti, S.; Reynolds, A.L.; O’Connor, J.J.; Dillon, E.T.; Duffy, D.J.; Kolch, W.; Cagney, G.; et al. A brain-derived neurotrophic factor mimetic is sufficient to restore cone photoreceptor visual function in an inherited blindness model. Sci. Rep. 2017, 7, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Vilardi, F.; Stephan, M.; Clancy, A.; Janshoff, A.; Schwappach, B. WRB and CAML are necessary and sufficient to mediate tail-anchored protein targeting to the ER membrane. PLoS ONE 2014, 9. [Google Scholar] [CrossRef]
- Daniele, L.L.; Emran, F.; Lobo, G.P.; Gaivin, R.J.; Perkins, B.D. Mutation of wrb, a component of the guided entry of Tail-Anchored protein pathway, disrupts photoreceptor synapse structure and function. Investig. Ophthalmol. Vis. Sci. 2016, 57, 2942–2954. [Google Scholar] [CrossRef]
- Lin, S.Y.; Vollrath, M.A.; Mangosing, S.; Shen, J.; Cardenas, E.; Corey, D.P. The zebrafish pinball wizard gene encodes WRB, a tail-anchored-protein receptor essential for inner-ear hair cells and retinal photoreceptors. J. Physiol. 2016, 594, 895–914. [Google Scholar] [CrossRef]
- Kohl, S.; Baumann, B.; Rosenberg, T.; Kellner, U.; Lorenz, B.; Vadalà, M.; Jacobson, S.G.; Wissinger, B. Mutations in the cone photoreceptor G-protein α-subunit gene GNAT2 in patients with achromatopsia. Am. J. Hum. Genet. 2002, 71, 422–425. [Google Scholar] [CrossRef]
- Brockerhoff, S.E.; Rieke, F.; Matthews, H.R.; Taylor, M.R.; Kennedy, B.; Ankoudinova, I.; Niemi, G.A.; Tucker, C.L.; Xiao, M.; Cilluffo, M.C.; et al. Light stimulates a transducin-independent increase of cytoplasmic Ca2+ and suppression of current in cones from the zebrafish mutant nof. J. Neurosci. 2003, 23, 470–480. [Google Scholar] [CrossRef]
- Bech-Hansen, N.T.; Naylor, M.J.; Maybaum, T.A.; Pearce, W.G.; Koop, B.; Fishman, G.A.; Mets, M.; Musarella, M.A.; Boycott, K.M. Loss-of-function mutations in a calcium-channel α1-subunit gene in Xp11.23 cause incomplete X-linked congenital stationary night blindness. Nat. Genet. 1998, 19, 264–267. [Google Scholar] [CrossRef]
- Strom, T.M.; Nyakatura, G.; Apfelstedt-Sylla, E.; Hellebrand, H.; Lorenz, B.; Weber, B.H.F.; Wutz, K.; Gutwillinger, N.; Rüther, K.; Drescher, B.; et al. An L-type calcium-channel gene mutated in incomplete X-linked congenital stationary night blindness. Nat. Genet. 1998, 19, 260–263. [Google Scholar] [CrossRef] [PubMed]
- Jalkanen, R.; Mänlyjärvi, M.; Tobias, R.; Isosomppi, J.; Sankila, E.M.; Alitalo, T.; Bech-Hansen, N.T. X linked cone-rod dystrophy, CORDX3, is caused by a mutation in the CACNA1F gene. J. Med. Genet. 2006, 43, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Baumann, L.; Gerstner, A.; Zong, X.; Biel, M.; Wahl-Schott, C. Functional characterization of the L-type Ca2+ channel Cav1.4α1 from mouse retina. Investig. Ophthalmol. Vis. Sci. 2004, 45, 708–713. [Google Scholar] [CrossRef] [PubMed]
- Van Epps, H.A.; Hayashi, M.; Lucast, L.; Stearns, G.W.; Hurley, J.B.; De Camilli, P.; Brockerhoff, S.E. The zebrafish nrc mutant reveals a role for the polyphosphoinositide phosphatase synaptojanin 1 in cone photoreceptor ribbon anchoring. J. Neurosci. 2004, 24, 8641–8650. [Google Scholar] [CrossRef]
- Allwardt, B.A.; Lall, A.B.; Brockerhoff, S.E.; Dowling, J.E. Synapse formation is arrested in retinal photoreceptors of the zebrafish nrc mutant. J. Neurosci. 2001, 21, 2330–2342. [Google Scholar] [CrossRef]
- Holzhausen, L.C.; Lewis, A.A.; Cheong, K.K.; Brockerhoff, S.E. Differential role for synaptojanin 1 in rod and cone photoreceptors. J. Comp. Neurol. 2009, 517, 633–644. [Google Scholar] [CrossRef]
- Huang, D.F.; Wang, M.Y.; Yin, W.U.; Ma, Y.Q.; Wang, H.A.N.; Xue, T.; Ren, D.L.; Hu, B. Zebrafish lacking circadian gene per2 exhibit visual function deficiency. Front. Behav. Neurosci. 2018, 12, 1–10. [Google Scholar] [CrossRef]
- Van Rooijen, E.; Voest, E.E.; Logister, I.; Bussmann, J.; Korving, J.; Van Eeden, F.J.; Giles, R.H.; Schulte-Merker, S. Von Hippel-Lindau tumor suppressor mutants faithfully model pathological hypoxia-driven angiogenesis and vascular retinopathies in zebrafish. Dmm Dis. Model. Mech. 2010, 3, 343–353. [Google Scholar] [CrossRef]
- Schori, C.; Agbaga, M.P.; Brush, R.S.; Ayyagari, R.; Grimm, C.; Samardzija, M. Elovl4 5-bp deletion does not accelerate cone photoreceptor degeneration in an all-cone mouse. PLoS ONE 2018, 13, 1–16. [Google Scholar] [CrossRef]
- Zhao, L.; Zabel, M.K.; Wang, X.; Ma, W.; Shah, P.; Fariss, R.N.; Qian, H.; Parkhurst, C.N.; Gan, W.; Wong, W.T. Microglial phagocytosis of living photoreceptors contributes to inherited retinal degeneration. Embo Mol. Med. 2015, 7, 1179–1197. [Google Scholar] [CrossRef] [PubMed]
- Sancho-Pelluz, J.; Alavi, M.V.; Sahaboglu, A.; Kustermann, S.; Farinelli, P.; Azadi, S.; Van Veen, T.; Romero, F.J.; Paquet-Durand, F.; Ekström, P. Excessive HDAC activation is critical for neurodegeneration in the rd1 mouse. Cell Death Dis. 2010, 1, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Trifunović, D.; Arango-Gonzalez, B.; Comitato, A.; Barth, M.; del Amo, E.M.; Kulkarni, M.; Sahaboglu, A.; Hauck, S.M.; Urtti, A.; Arsenijevic, Y.; et al. HDAC inhibition in the cpfl1 mouse protects degenerating cone photoreceptors in vivo. Hum. Mol. Genet. 2016, 25, 4462–4472. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.I.; Leman, L.J.; Ku, S.; Vickers, C.J.; Olsen, C.A.; Montero, A.; Ghadiri, M.R.; Gottesfeld, J.M. Cyclic tetrapeptide HDAC inhibitors as potential therapeutics for spinal muscular atrophy: Screening with iPSC-derived neuronal cells. Bioorganic Med. Chem. Lett. 2017, 27, 3289–3293. [Google Scholar] [CrossRef]
- Janczura, K.J.; Volmar, C.H.; Sartor, G.C.; Rao, S.J.; Ricciardi, N.R.; Lambert, G.; Brothers, S.P.; Wahlestedt, C. Inhibition of HDAC3 reverses Alzheimer’s disease-related pathologies in vitro and in the 3xTg-AD mouse model. Proc. Natl. Acad. Sci. USA 2018, 115, E11148–E11157. [Google Scholar] [CrossRef]
- Kawase, R.; Nishimura, Y.; Ashikawa, Y.; Sasagawa, S.; Murakami, S.; Yuge, M.; Okabe, S.; Kawaguchi, K.; Yamamoto, H.; Moriyuki, K.; et al. EP300 protects from light-induced retinopathy in zebrafish. Front. Pharm. 2016, 7, 1–13. [Google Scholar] [CrossRef]
- D’Ydewalle, C.; Krishnan, J.; Chiheb, D.M.; Van Damme, P.; Irobi, J.; Kozikowski, A.P.; Berghe, P.V.; Timmerman, V.; Robberecht, W.; Van Den Bosch, L. HDAC6 inhibitors reverse axonal loss in a mouse model of mutant HSPB1-induced Charcot-Marie-Tooth disease. Nat. Med. 2011, 17, 968–974. [Google Scholar] [CrossRef]
- Sundaramurthi, H.; Roche, S.L.; Grice, G.L.; Moran, A.; Dillion, E.T.; Campiani, G.; Nathan, J.A.; Kennedy, B.N. Selective histone deacetylase 6 inhibitors restore cone photoreceptor vision or outer segment morphology in zebrafish and mouse models of retinal blindness. Front. Cell Dev. Biol. 2020, 8. [Google Scholar] [CrossRef]
- Leyk, J.; Daly, C.; Janssen-Bienhold, U.; Kennedy, B.N.; Richter-Landsberg, C. HDAC6 inhibition by tubastatin A is protective against oxidative stress in a photoreceptor cell line and restores visual function in a zebrafish model of inherited blindness. Cell Death Dis. 2017, 8, e3028. [Google Scholar] [CrossRef]
- Kokel, D.; Bryan, J.; Laggner, C.; White, R.; Cheung, C.Y.J.; Mateus, R.; Healey, D.; Kim, S.; Werdich, A.A.; Haggarty, S.J.; et al. Rapid behavior-based identification of neuroactive small molecules in the zebrafish. Nat. Chem. Biol. 2010, 6, 231–237. [Google Scholar] [CrossRef]
- Rihel, J.; Prober, D.A.; Arvanites, A.; Lam, K.; Zimmerman, S.; Jang, S.; Haggarty, S.J.; Kokel, D.; Rubin, L.L.; Peterson, R.T.; et al. Zebrafish behavioral profiling links drugs to biological targets and rest/wake regulation. Science 2010, 327, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Ganzen, L.; Ko, M.; Zhang, M.; Xie, R.; Chen, Y.; Zhang, L.; James, R.; Mumm, J.; van Rijn, R.; Zhong, W.; et al. Drug screening with zebrafish visual behavior identifies carvedilol as a potential treatment for retinitis pigmentosa. bioRxiv 2020. [Google Scholar] [CrossRef]
- Viringipurampeer, I.A.; Shan, X.; Gregory-Evans, K.; Zhang, J.P.; Mohammadi, Z.; Gregory-Evans, C.Y. Rip3 knockdown rescues photoreceptor cell death in blind pde6c zebrafish. Cell Death Differ. 2014, 21, 665–675. [Google Scholar] [CrossRef][Green Version]
- Giridharan, V.V.; Thandavarayan, R.A.; Sato, S.; Ko, K.M.; Konishi, T. Prevention of scopolamine-induced memory deficits by schisandrin B, an antioxidant lignan from Schisandra chinensis in mice. Free Radic. Res. 2011, 45, 950–958. [Google Scholar] [CrossRef] [PubMed]
- Ko, K.M.; Lam, B.Y.H. Schisandrin B protects against tert-butylhydroperoxide induced cerebral toxicity by enhancing glutathione antioxidant status in mouse brain. Mol. Cell. Biochem. 2002, 238, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xiang, L.; Liu, Y.; Venkatraman, P.; Chong, L.; Cho, J.; Bonilla, S.; Jin, Z.B.; Pang, C.P.; Ko, K.M.; et al. A naturally-derived compound schisandrin B enhanced light sensation in the pde6c zebrafish model of retinal degeneration. PLoS ONE 2016, 11, 1–19. [Google Scholar] [CrossRef][Green Version]
- Chen, N.; Chiu, P.Y.; Ko, K.M. Schisandrin B enhances cerebral mitochondrial antioxidant status and structural integrity, and protects against cerebral ischemia/reperfusion injury in rats. Biol. Pharm. Bull. 2008, 31, 1387–1391. [Google Scholar] [CrossRef]
- Ji, X.; Shen, Y.; Guo, X. Isolation, structures, and bioactivities of the polysaccharides from Gynostemma pentaphyllum (Thunb.) Makino: A review. Biomed. Res. Int. 2018, 2018. [Google Scholar] [CrossRef]
- Alhasani, R.H.; Zhou, X.; Biswas, L.; Li, X.; Reilly, J.; Zeng, Z.; Shu, X. Gypenosides attenuate retinal degeneration in a zebrafish retinitis pigmentosa model. Exp. Eye Res. 2020, 201, 108291. [Google Scholar] [CrossRef]
- Deeti, S.; O’Farrell, S.; Kennedy, B.N. Early safety assessment of human oculotoxic drugs using the zebrafish visualmotor response. J. Pharm. Toxicol. Methods 2014, 69, 1–8. [Google Scholar] [CrossRef]
- Vaché, C.; Besnard, T.; le Berre, P.; García-García, G.; Baux, D.; Larrieu, L.; Abadie, C.; Blanchet, C.; Bolz, H.J.; Millan, J.; et al. Usher syndrome type 2 caused by activation of an USH2A pseudoexon: Implications for diagnosis and therapy. Hum. Mutat. 2012, 33, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Slijkerman, R.; Goloborodko, A.; Broekman, S.; de Vrieze, E.; Hetterschijt, L.; Peters, T.; Gerits, M.; Kremer, H.; van Wijk, E. Poor splice-site recognition in a humanized zebrafish knockin model for the recurrent deep-intronic c.7595-2144A>G mutation in USH2A. Zebrafish 2018, 15, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Slijkerman, R.; van Diepen, H.; Albert, S.; Dona, M.; Venselaar, H.; Zang, J.; Neuhauss, S.; Peters, T.; Broekman, S.; Pennings, R.; et al. Antisense oligonucleotide-based treatment of retinitis pigmentosa caused by mutations in USH2A exon 13. bioRxiv 2020. [Google Scholar] [CrossRef]
- Baux, D.; Blanchet, C.; Hamel, C.; Meunier, I.; Larrieu, L.; Faugère, V.; Vaché, C.; Castorina, P.; Puech, B.; Bonneau, D.; et al. Enrichment of LOVD-USHbases with 152 USH2A genotypes defines an extensive mutational spectrum and highlights missense hotspots. Hum. Mutat. 2014, 35, 1179–1186. [Google Scholar] [CrossRef]
- Cameron, D.A. Cellular proliferation and neurogenesis in the injured retina of adult zebrafish. Vis. Neurosci. 2000, 17, 789–797. [Google Scholar] [CrossRef]
- Wan, Y.; Almeida, A.D.; Rulands, S.; Chalour, N.; Muresan, L.; Wu, Y.; Simons, B.D.; He, J.; Harris, W.A. The ciliary marginal zone of the zebrafish retina: Clonal and time-lapse analysis of a continuously growing tissue. Development 2016, 143, 1099–1107. [Google Scholar] [CrossRef]
- Yurco, P.; Cameron, D.A. Responses of Muller glia to retinal injury in adult zebrafish. Vis. Res. 2005, 45, 991–1002. [Google Scholar] [CrossRef]
- Marcucci, F.; Murcia-Belmonte, V.; Wang, Q.; Coca, Y.; Ferreiro-Galve, S.; Kuwajima, T.; Khalid, S.; Ross, M.E.; Mason, C.; Herrera, E. The ciliary margin zone of the mammalian retina generates retinal ganglion cells. Cell Rep. 2016, 17, 3153–3164. [Google Scholar] [CrossRef]
- Bhatia, B.; Jayaram, H.; Singhal, S.; Jones, M.F.; Limb, G.A. Differences between the neurogenic and proliferative abilities of Müller glia with stem cell characteristics and the ciliary epithelium from the adult human eye. Exp. Eye Res. 2011, 93, 852–861. [Google Scholar] [CrossRef]
- Ahmad, I.; Tang, L.; Pham, H. Identification of neural progenitors in the adult mammalian eye. Biochem. Biophys. Res. Commun. 2000, 270, 517–521. [Google Scholar] [CrossRef]
- Bhatia, B.; Singhal, S.; Lawrence, J.M.; Khaw, P.T.; Limb, G.A. Distribution of Müller stem cells within the neural retina: Evidence for the existence of a ciliary margin-like zone in the adult human eye. Exp. Eye Res. 2009, 89, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Fleisch, V.C.; Fraser, B.; Allison, W.T. Investigating regeneration and functional integration of CNS neurons: Lessons from zebrafish genetics and other fish species. Biochim. Biophys. Acta Mol. Basis Dis. 2011, 1812, 364–380. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, M.; Barthel, L.K.; Raymond, P.A. A self-renewing division of zebrafish Muller glial cells generates neuronal progenitors that require N-cadherin to regenerate retinal neurons. Development 2013, 140, 4510–4521. [Google Scholar] [CrossRef]
- Fausett, B.V.; Goldman, D. A role for alpha1 tubulin-expressing Muller glia in regeneration of the injured zebrafish retina. J. Neurosci. 2006, 26, 6303–6313. [Google Scholar] [CrossRef] [PubMed]
- Otteson, D.C.; Hitchcock, P.F. Stem cells in the teleost retina: Persistent neurogenesis and injury-induced regeneration. Vis. Res. 2003, 43, 927–936. [Google Scholar] [CrossRef]
- Ramachandran, R.; Fausett, B.V.; Goldman, D. Ascl1a regulates Müller glia dedifferentiation and retinal regeneration through a Lin-28-dependent, let-7 microRNA signalling pathway. Nat. Cell Biol. 2010, 12, 1101–1107. [Google Scholar] [CrossRef]
- Nelson, C.M.; Ackerman, K.M.; O’Hayer, P.; Bailey, T.J.; Gorsuch, R.A.; Hyde, D.R. Tumor necrosis factor-alpha is produced by dying retinal neurons and is required for Müller glia proliferation during zebrafish retinal regeneration. J. Neurosci. 2013, 33, 6524–6539. [Google Scholar] [CrossRef]
- Lawrence, J.M.; Singhal, S.; Bhatia, B.; Keegan, D.J.; Reh, T.A.; Luthert, P.J.; Khaw, P.T.; Limb, G.A. MIO-M1 cells and similar muller glial cell lines derived from adult human retina exhibit neural stem cell characteristics. Stem Cells 2007, 25, 2033–2043. [Google Scholar] [CrossRef]
- Bringmann, A.; Pannicke, T.; Grosche, J.; Francke, M.; Wiedemann, P.; Skatchkov, S.N.; Osborne, N.N.; Reichenbach, A. Müller cells in the healthy and diseased retina. Prog. Retin. Eye Res. 2006, 25, 397–424. [Google Scholar] [CrossRef]
- Giannelli, S.G.; Demontis, G.C.; Pertile, G.; Rama, P.; Broccoli, V. Adult human Muller glia cells are a highly efficient source of rod photoreceptors. Stem Cells 2011, 29, 344–356. [Google Scholar] [CrossRef]
- Jayaram, H.; Jones, M.F.; Eastlake, K.; Cottrill, P.B.; Becker, S.; Wiseman, J.; Khaw, P.T.; Limb, G.A. Transplantation of photoreceptors derived from human Muller glia restore rod function in the P23H rat. Stem Cells Transl Med. 2014, 3, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Lenkowski, J.R.; Raymond, P.A. Müller glia: Stem cells for generation and regeneration of retinal neurons in teleost fish. Prog. Retin. Eye Res. 2014, 40, 94–123. [Google Scholar] [CrossRef] [PubMed]
- Qian, C.; Dong, B.; Wang, X.-Y.; Zhou, F.-Q. In vivo glial trans-differentiation for neuronal replacement and functional recovery in central nervous system. Febs J. 2020. [Google Scholar] [CrossRef] [PubMed]
- Goldman, D. Müller glial cell reprogramming and retina regeneration. Nat. Rev. Neurosci. 2014, 15, 431–442. [Google Scholar] [CrossRef]
- Wan, J.; Goldman, D. Retina regeneration in zebrafish. Curr. Opin. Genet. Dev. 2016, 40, 41–47. [Google Scholar] [CrossRef]
- Madelaine, R.; Mourrain, P. Endogenous retinal neural stem cell reprogramming for neuronal regeneration. Neural Regen. Res. 2017, 12, 1765–1767. [Google Scholar] [CrossRef]
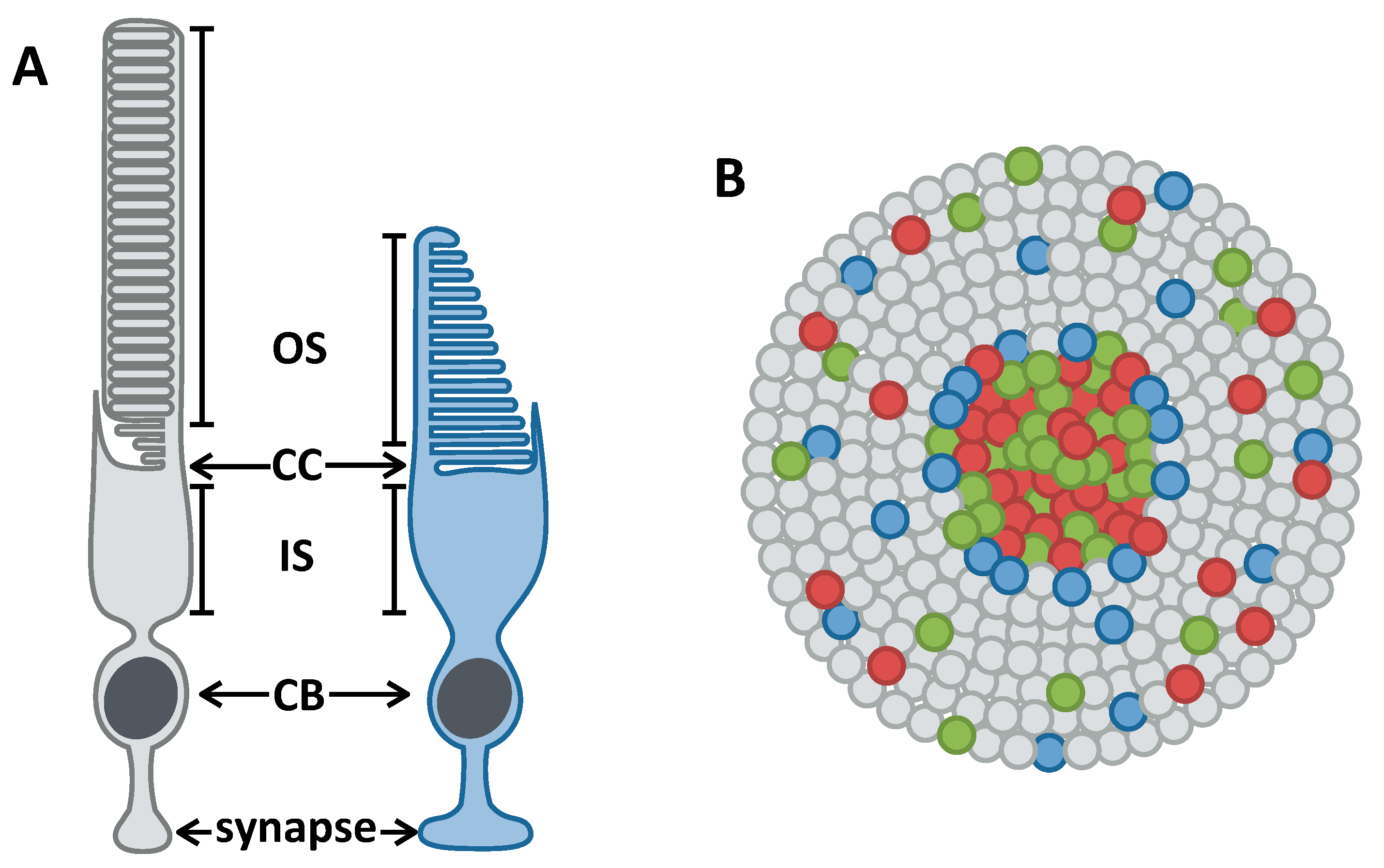
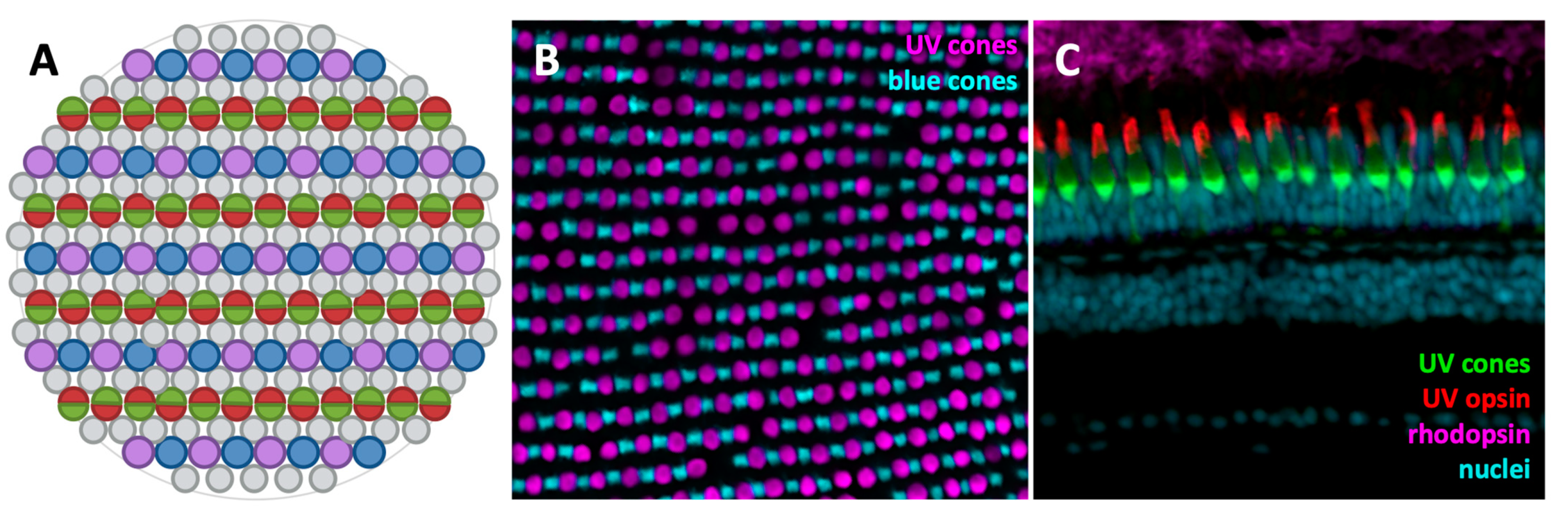
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noel, N.C.L.; MacDonald, I.M.; Allison, W.T. Zebrafish Models of Photoreceptor Dysfunction and Degeneration. Biomolecules 2021, 11, 78. https://doi.org/10.3390/biom11010078
Noel NCL, MacDonald IM, Allison WT. Zebrafish Models of Photoreceptor Dysfunction and Degeneration. Biomolecules. 2021; 11(1):78. https://doi.org/10.3390/biom11010078
Chicago/Turabian StyleNoel, Nicole C. L., Ian M. MacDonald, and W. Ted Allison. 2021. "Zebrafish Models of Photoreceptor Dysfunction and Degeneration" Biomolecules 11, no. 1: 78. https://doi.org/10.3390/biom11010078
APA StyleNoel, N. C. L., MacDonald, I. M., & Allison, W. T. (2021). Zebrafish Models of Photoreceptor Dysfunction and Degeneration. Biomolecules, 11(1), 78. https://doi.org/10.3390/biom11010078





