The Impacts of Fish Oil and/or Probiotic Intervention on Low-Grade Inflammation, IGFBP-1 and MMP-8 in Pregnancy: A Randomized, Placebo-Controlled, Double-Blind Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
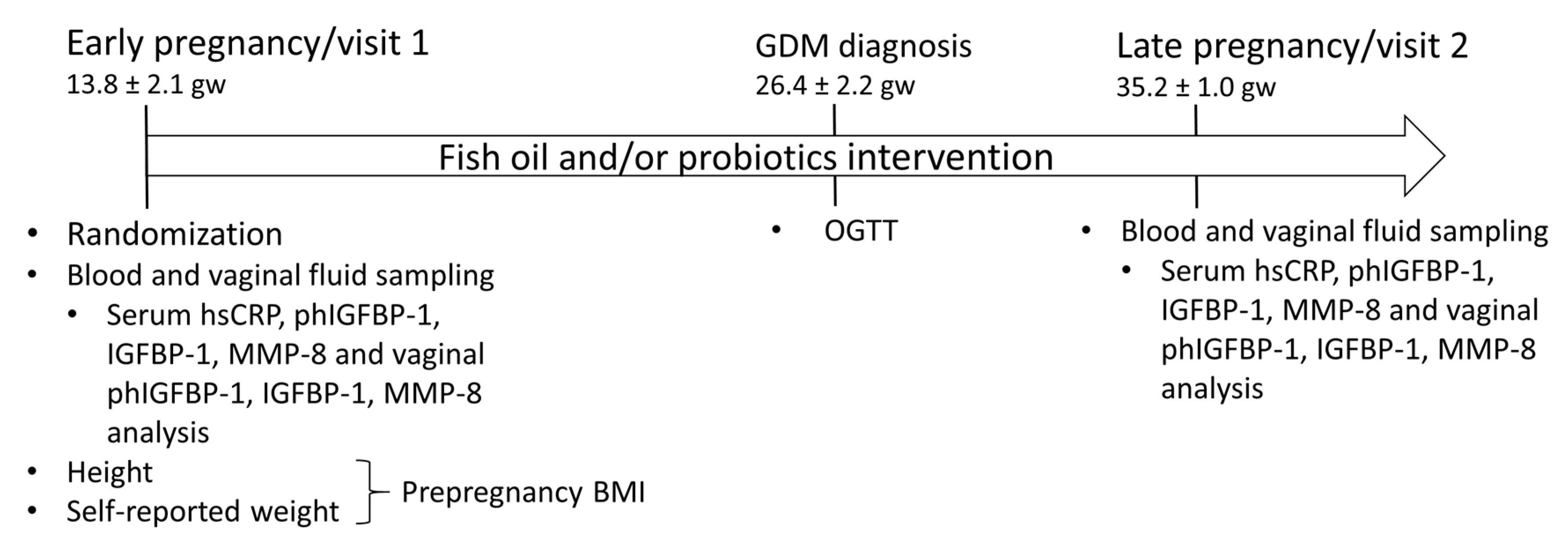
2.1. Study Design and Subjects
2.2. Sampling and Analyses
2.3. Statistics
3. Results
3.1. Clinical Characteristics
3.2. The Impact of the Dietary Intervention on hsCRP, IGFBP-1 and MMP-8
3.3. HsCRP, IGFBP-1 and MMP-8 as Predictors of GDM
3.4. Evolution of Low-Grade Inflammation, IGFBP-1 and MMP-8 over Pregnancy
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Plows, J.F.; Stanley, J.L.; Baker, P.N.; Reynolds, C.M.; Vickers, M.H. The Pathophysiology of Gestational Diabetes Mellitus. Int. J. Mol. Sci. 2018, 19, 3342. [Google Scholar] [CrossRef] [PubMed]
- Rajpathak, S.N.; Gunter, M.J.; Wylie-Rosett, J.; Ho, G.Y.F.; Kaplan, R.C.; Muzumdar, R.; Rohan, T.E.; Strickler, H.D. The role of insulin-like growth factor-I and its binding proteins in glucose homeostasis and type 2 diabetes. Diabetes Metab. Res. Rev. 2009, 25, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Kallioniemi, H.; Rahkonen, L.; Heikinheimo, O.; Paavonen, J. Early pregnancy vaginal fluid phosphorylated insulin-like growth factor binding protein-1 predicts preterm delivery. Prenat. Diagn. 2013, 33, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Conde-Agudelo, A.; Romero, R. Cervical phosphorylated insulin-like growth factor binding protein-1 test for the prediction of preterm birth: A systematic review and metaanalysis. Am. J. Obstet. Gynecol. 2016, 214, 57–73. [Google Scholar] [CrossRef] [PubMed]
- Myntti, T.; Rahkonen, L.; Pätäri-Sampo, A.; Tikkanen, M.; Sorsa, T.; Juhila, J.; Helve, O.; Andersson, S.; Paavonen, J.; Stefanovic, V. Comparison of amniotic fluid matrix metalloproteinase-8 and cathelicidin in the diagnosis of intra-amniotic infection. J. Perinatol. 2016, 36, 1049–1054. [Google Scholar] [CrossRef] [PubMed]
- Myntti, T.; Rahkonen, L.; Nupponen, I.; Pätäri-Sampo, A.; Tikkanen, M.; Sorsa, T.; Juhila, J.; Andersson, S.; Paavonen, J.; Stefanovic, V. Amniotic Fluid Infection in Preterm Pregnancies with Intact Membranes. Dis. Markers 2017, 2017, 1–9. [Google Scholar] [CrossRef]
- Mokkala, K.; Juhila, J.; Houttu, N.; Sorsa, T.; Laitinen, K. Early pregnancy serum IGFBP-1 relates to lipid profile in overweight and obese women. Heliyon 2020, 6, e04788. [Google Scholar] [CrossRef]
- Vilmi-Kerälä, T.; Lauhio, A.; Tervahartiala, T.; Palomäki, O.; Uotila, J.; Sorsa, T.; Palomäki, A. Subclinical inflammation associated with prolonged TIMP-1 upregulation and arterial stiffness after gestational diabetes mellitus: A hospital-based cohort study. Cardiovasc. Diabetol. 2017, 16, 49. [Google Scholar] [CrossRef]
- Frystyk, J. Free insulin-like growth factors—Measurements and relationships to growth hormone secretion and glucose homeostasis. Growth Horm. IGF Res. 2004, 14, 337–375. [Google Scholar] [CrossRef]
- Lauhio, A.; Färkkilä, E.; Pietiläinen, K.H.; Åström, P.; Winkelmann, A.; Tervahartiala, T.; Pirilä, E.; Rissanen, A.; Kaprio, J.; Sorsa, T.; et al. Association of MMP-8 with obesity, smoking and insulin resistance. Eur. J. Clin. Investig. 2016, 46, 757–765. [Google Scholar] [CrossRef]
- Karamali, M.; Nasiri, N.; Shavazi, N.T.; Jamilian, M.; Bahmani, F.; Tajabadi-Ebrahimi, M.; Asemi, Z. The Effects of Synbiotic Supplementation on Pregnancy Outcomes in Gestational Diabetes. Probiotics Antimicrob. Proteins 2017, 10, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, A.; Soltani, S.; Ghorabi, S.; Keshtkar, A.; Daneshzad, E.; Nasri, F.; Mazloomi, S.M. Effect of probiotic and synbiotic supplementation on inflammatory markers in health and disease status: A systematic review and meta-analysis of clinical trials. Clin. Nutr. 2020, 39, 789–819. [Google Scholar] [CrossRef] [PubMed]
- Jamilian, M.; Samimi, M.; Kolahdooz, F.; Khalaji, F.; Razavi, M.; Asemi, Z. Omega-3 fatty acid supplementation affects pregnancy outcomes in gestational diabetes: A randomized, double-blind, placebo-controlled trial. J. Matern. Neonatal Med. 2016, 29, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Jamilian, M.; Samimi, M.; Mirhosseini, N.; Ebrahimi, F.A.; Aghadavod, E.; Taghizadeh, M.; Asemi, Z. A Randomized Double-Blinded, Placebo-Controlled Trial Investigating the Effect of Fish Oil Supplementation on Gene Expression Related to Insulin Action, Blood Lipids, and Inflammation in Gestational Diabetes Mellitus-Fish Oil Supplementation and Gestational Diabetes. Nutrients 2018, 10, 163. [Google Scholar]
- Haghiac, M.; Yang, X.-H.; Presley, L.; Smith, S.; Dettelback, S.; Minium, J.; Belury, M.A.; Catalano, P.M.; De Mouzon, S.H. Dietary Omega-3 Fatty Acid Supplementation Reduces Inflammation in Obese Pregnant Women: A Randomized Double-Blind Controlled Clinical Trial. PLoS ONE 2015, 10, e0137309. [Google Scholar] [CrossRef]
- Mirmasoumi, G.; Fazilati, M.; Foroozanfard, F.; Vahedpoor, Z.; Mahmoodi, S.; Taghizadeh, M.; Esfeh, N.K.; Mohseni, M.; Karbassizadeh, H.; Asemi, Z. The Effects of Flaxseed Oil Omega-3 Fatty Acids Supplementation on Metabolic Status of Patients with Polycystic Ovary Syndrome: A Randomized, Double-Blind, Placebo-Controlled Trial. Exp. Clin. Endocrinol. Diabetes 2017, 126, 222–228. [Google Scholar] [CrossRef]
- Chan, D.C.; Watts, G.F.; Barrett, P.H.R.; Beilin, L.J.; A Mori, T. Effect of Atorvastatin and Fish Oil on Plasma High-Sensitivity C-Reactive Protein Concentrations in Individuals with Visceral Obesity. Clin. Chem. 2002, 48, 877–883. [Google Scholar] [CrossRef]
- Taghizadeh, M.; Asemi, Z. Effects of synbiotic food consumption on glycemic status and serum hs-CRP in pregnant women: A randomized controlled clinical trial. Hormones 2002, 13, 398–406. [Google Scholar] [CrossRef]
- Abribat, T.; Nedelec, B.; Jobin, N.; Garrel, D.R. Decreased serum insulin-like growth factor-I in burn patients: Relationship with serum insulin-like growth factor binding protein-3 proteolysis and the influence of lipid composition in nutritional support. Crit. Care Med. 2000, 28, 2366–2372. [Google Scholar] [CrossRef]
- GholamHosseini, S.; Nematipour, E.; Djazayeri, A.; Javanbakht, M.H.; Koohdani, F.; Zareei, M.; Djalali, M. ω-3 fatty acid differentially modulated serum levels of IGF1 and IGFBP3 in men with CVD: A randomized, double-blind placebo-controlled study. Nutrients 2015, 31, 480. [Google Scholar] [CrossRef]
- Young, L.R.; Kurzer, M.S.; Thomas, W.; Redmon, J.B.; Raatz, S.K. Low-fat diet with omega-3 fatty acids increases plasma insulin-like growth factor concentration in healthy postmenopausal women. Nutr. Res. 2013, 33, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Moya-Pérez, A.; Romo-Vaquero, M.; Tomás-Barberán, F.; Sanz, Y.; García-Conesa, M.-T. Hepatic molecular responses to Bifidobacterium pseudocatenulatum CECT 7765 in a mouse model of diet-induced obesity. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Jung, S.-R.; Lee, S.-Y.; Lee, N.-K.; Paik, H.-D.; Lim, S.-I. Lactobacillus plantarum Strain Ln4 Attenuates Diet-Induced Obesity, Insulin Resistance, and Changes in Hepatic mRNA Levels Associated with Glucose and Lipid Metabolism. Nutrients 2018, 10, 643. [Google Scholar] [CrossRef] [PubMed]
- Jäsberg, H.; Tervahartiala, T.; Sorsa, T.; Söderling, E.; Haukioja, A. Probiotic intervention influences the salivary levels of Matrix Metalloproteinase (MMP)-9 and Tissue Inhibitor of metalloproteinases (TIMP)-1 in healthy adults. Arch. Oral Biol. 2018, 85, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Ince, G.; Gursoy, H.; Ipçi, Ş.D.; Cakar, G.; Emekli-Alturfan, E.; Yilmaz, S. Clinical and Biochemical Evaluation of Lozenges Containing Lactobacillus reuteri as an Adjunct to Non-Surgical Periodontal Therapy in Chronic Periodontitis. J. Periodontol. 2015, 86, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Pellonperä, O.; Mokkala, K.; Houttu, N.; Vahlberg, T.; Koivuniemi, E.; Tertti, K.; Rönnemaa, T.; Laitinen, K. Efficacy of Fish Oil and/or Probiotic Intervention on the Incidence of Gestational Diabetes Mellitus in an At-Risk Group of Overweight and Obese Women: A Randomized, Placebo-Controlled, Double-Blind Clinical Trial. Diabetes Care 2019, 42, 1009–1017. [Google Scholar]
- Dekker, J.; Collett, M.; Prasad, J.; Gopal, P. Functionality of Probiotics—Potential for Product Development. Nutr. Probl. Elder. 2007, 60, 196–208. [Google Scholar] [CrossRef]
- Amar, J.; Chabo, C.; Waget, A.; Klopp, P.; Vachoux, C.; Bermúdez-Humarán, L.G.; Smirnova, N.; Bergé, M.; Sulpice, T.; Lahtinen, S.; et al. Intestinal mucosal adherence and translocation of commensal bacteria at the early onset of type 2 diabetes: Molecular mechanisms and probiotic treatment. EMBO Mol. Med. 2011, 3, 559–572. [Google Scholar] [CrossRef]
- Klein, A.; Friedrich, U.; Vogelsang, H.; Jahreis, G. Lactobacillus acidophilus 74-2 and Bifidobacterium animalis subsp lactis DGCC 420 modulate unspecific cellular immune response in healthy adults. Eur. J. Clin. Nutr. 2007, 62, 584–593. [Google Scholar] [CrossRef]
- Klein, A.; Friedrich, U.; Vogelsang, H.; Bauer, A.; Kaatz, M.; Hipler, U.C.; Schmidt, I.; Jahreis, G. The immune system in healthy adults and patients with atopic dermatitis seems to be affected differently by a probiotic intervention. Clin. Exp. Allergy 2007, 38, 93–102. [Google Scholar]
- Calder, P.C. Long-chain fatty acids and inflammation. Proc. Nutr. Soc. 2012, 71, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Larqué, E.; Gil-Sánchez, A.; Prieto-Sanchez, M.T.; Koletzko, B. Omega 3 fatty acids, gestation and pregnancy outcomes. Br. J. Nutr. 2012, 107, S77–S84. [Google Scholar] [CrossRef] [PubMed]
- Lalia, A.Z.; Lanza, I.R. Insulin-Sensitizing Effects of Omega-3 Fatty Acids: Lost in Translation? Nutrients 2016, 8, 329. [Google Scholar] [CrossRef] [PubMed]
- Working Group Established by the Finnish Medical Society Duodecim; The Medical Advisory Board of the Finnish Diabetes Association; The Finnish Gynecological Association. Gestational Diabetes: Current Care Guidelines; The Finnish Medical Society Duodecim: Helsinki, Finland, 2013; Available online: www.kaypahoito.fi (accessed on 1 October 2018).
- Nuutila, M. Phosphorylated isoforms of insulin-like growth factor binding protein-1 in the cervix as a predictor of cervical ripeness. Obstet. Gynecol. 1999, 94, 243–249. [Google Scholar] [PubMed]
- Kruit, H.; Heikinheimo, O.; Sorsa, T.; Juhila, J.; Paavonen, J.; Rahkonen, L. Cervical biomarkers as predictors of successful induction of labour by Foley catheter. J. Obstet. Gynaecol. 2018, 38, 927–932. [Google Scholar] [CrossRef] [PubMed]
- Tuomainen, A.M.; Nyyssönen, K.; Laukkanen, J.A.; Tervahartiala, T.; Tuomainen, T.-P.; Salonen, J.T.; Sorsa, T.; Pussinen, P. Serum Matrix Metalloproteinase-8 Concentrations Are Associated with Cardiovascular Outcome in Men. Arter. Thromb. Vasc. Biol. 2007, 27, 2722–2728. [Google Scholar] [CrossRef]
- Krauss-Etschmann, S.; Hartl, D.; Rzehak, P.; Heinrich, J.; Shadid, R.; Ramírez-Tortosa, M.D.C.; Campoy, C.; Pardillo, S.; Schendel, D.J.; Decsi, T.; et al. Decreased cord blood IL-4, IL-13, and CCR4 and increased TGF-β levels after fish oil supplementation of pregnant women. J. Allergy Clin. Immunol. 2008, 121, 464–470.e6. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Omega-3 Fatty Acids in Inflammation and Autoimmune Diseases. J. Am. Coll. Nutr. 2002, 21, 495–505. [Google Scholar] [CrossRef]
- Singer, P.; Shapiro, H.; Theilla, M.; Anbar, R.; Singer, J.; Cohen, J. Anti-inflammatory properties of omega-3 fatty acids in critical illness: Novel mechanisms and an integrative perspective. Intensive Care Med. 2008, 34, 1580–1592. [Google Scholar] [CrossRef]
- Warstedt, K.; Furuhjelm, C.; Duchen, K.; Fälth-Magnusson, K.; Fagerås, M. The Effects of Omega-3 Fatty Acid Supplementation in Pregnancy on Maternal Eicosanoid, Cytokine, and Chemokine Secretion. Pediatr. Res. 2009, 66, 212–217. [Google Scholar] [CrossRef]
- Asemi, Z.; Jazayeri, S.; Najafi, M.; Samimi, M.; Mofid, V.; Shidfar, F.; Foroushani, A.R.; Shahaboddin, M.E. Effects of daily consumption of probiotic yoghurt on inflammatory factors in pregnant women: A randomized controlled trial. Pak. J. Biol. Sci. 2011, 14, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Samimi, M.; Jamilian, M.; Asemi, Z.; Esmaillzadeh, A. Effects of omega-3 fatty acid supplementation on insulin metabolism and lipid profiles in gestational diabetes: Randomized, double-blind, placebo-controlled trial. Clin. Nutr. 2015, 34, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Badehnoosh, B.; Karamali, M.; Zarrati, M.; Jamilian, M.; Bahmani, F.; Tajabadi-Ebrahimi, M.; Jafari, P.; Rahmani, E.; Asemi, Z. The effects of probiotic supplementation on biomarkers of inflammation, oxidative stress and pregnancy outcomes in gestational diabetes. J. Matern. Neonatal Med. 2018, 31, 1128–1136. [Google Scholar] [CrossRef] [PubMed]
- Hajifaraji, M.; Jahanjou, F.; Abbasalizadeh, F.; Aghamohammadzadeh, N.; Abbasi, M.M.; Dolatkhah, N. Effect of probiotic supplements in women with gestational diabetes mellitus on inflammation and oxidative stress biomarkers: A randomized clinical trial. Asia Pac. J. Clin. Nutr. 2018, 27, 581–591. [Google Scholar]
- Stenman, L.K.; Lehtinen, M.J.; Meland, N.; Christensen, J.E.; Yeung, N.; Saarinen, M.T.; Courtney, M.; Burcelin, R.; Lähdeaho, M.-L.; Linros, J.; et al. Probiotic With or Without Fiber Controls Body Fat Mass, Associated With Serum Zonulin, in Overweight and Obese Adults—Randomized Controlled Trial. EBioMedicine 2016, 13, 190–200. [Google Scholar] [CrossRef]
- Milajerdi, A.; Mousavi, S.M.; Sadeghi, A.; Salari-Moghaddam, A.; Parohan, M.; Larijani, B.; Larijani, B. The effect of probiotics on inflammatory biomarkers: A meta-analysis of randomized clinical trials. Eur. J. Nutr. 2020, 59, 633–649. [Google Scholar] [CrossRef]
- Naser, W.; Adam, I.; Rayis, D.A.; Ahmed, M.A.; Hamdan, H.Z. Serum magnesium and high-sensitivity C-reactive protein as a predictor for gestational diabetes mellitus in Sudanese pregnant women. BMC Pregnancy Childbirth 2019, 19, 1–5. [Google Scholar] [CrossRef]
- Alyas, S.; Roohi, N.; Ashraf, S.; Ilyas, S.; Ilyas, A. Early pregnancy biochemical markers of placentation for screening of gestational diabetes mellitus (GDM). Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 2353–2356. [Google Scholar] [CrossRef]
- Campos, S.P.; Baumann, H. Insulin is a prominent modulator of the cytokine-stimulated expression of acute-phase plasma protein genes. Mol. Cell. Biol. 1992, 12, 1789–1797. [Google Scholar] [CrossRef][Green Version]
- Pantham, P.; Aye, I.L.M.H.; Powell, T.L. Inflammation in maternal obesity and gestational diabetes mellitus. Placenta 2015, 36, 709–715. [Google Scholar] [CrossRef]
- Qiu, C.; Vadachkoria, S.; Meryman, L.; Frederick, I.O.; Williams, M.A. Maternal plasma concentrations of IGF-1, IGFBP-1, and C-peptide in early pregnancy and subsequent risk of gestational diabetes mellitus. Am. J. Obstet. Gynecol. 2005, 193, 1691–1697. [Google Scholar] [CrossRef] [PubMed]
- Loukovaara, M.; Leinonen, P.; Teramo, K.; Nurminen, E.; Andersson, S.; Rutanen, E.-M. Effect of maternal diabetes on phosphorylation of insulin-like growth factor binding protein-1 in cord serum. Diabet. Med. 2005, 22, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Vickers, M.H.; Taylor, R.S.; Fraser, M.; McCowan, L.M.E.; Baker, P.; Perry, J.K. Maternal serum placental growth hormone, insulin-like growth factors and their binding proteins at 20 weeks’ gestation in pregnancies complicated by gestational diabetes mellitus. Hormones 2017, 16, 282–290. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ramirez, V.I.; Miller, E.; Meireles, C.L.; Gelfond, J.; A Krummel, D.; Powell, T. Adiponectin and IGFBP-1 in the development of gestational diabetes in obese mothers. BMJ Open Diabetes Res. Care 2014, 2, e000010. [Google Scholar] [CrossRef]
- Perez, M.A.; Hansen, R.A.; Harris, M.A.; Allen, K.G. Dietary docosahexaenoic acid alters pregnant rat reproductive tissue prostaglandin and matrix metalloproteinase production. J. Nutr. Biochem. 2006, 17, 446–453. [Google Scholar] [CrossRef]
- Yan, J.; Charles, J.F. Gut Microbiota and IGF-1. Calcif. Tissue Int. 2018, 102, 406–414. [Google Scholar] [CrossRef]
- Similä, M.E.; Kontto, J.; Virtamo, J.; Hätönen, K.A.; Valsta, L.M.; Sundvall, J.; Männistö, S. Insulin-like growth factor I, binding proteins -1 and -3, risk of type 2 diabetes and macronutrient intakes in men. Br. J. Nutr. 2019, 121, 938–944. [Google Scholar] [CrossRef]
- Smith, W.J.; E Underwood, L.; Clemmons, D.R. Effects of caloric or protein restriction on insulin-like growth factor-I (IGF-I) and IGF-binding proteins in children and adults. J. Clin. Endocrinol. Metab. 1995, 80, 443–449. [Google Scholar] [CrossRef]
- Christian, L.M.; Porter, K. Longitudinal changes in serum proinflammatory markers across pregnancy and postpartum: Effects of maternal body mass index. Cytokine 2014, 70, 134–140. [Google Scholar] [CrossRef]
- Giudice, L.C.; Farrell, E.M.; Pham, H.; Lamson, G.; Rosenfeld, R.G. Insulin-Like Growth Factor Binding Proteins in Maternal Serum Throughout Gestation and in the Puerperium: Effects of a Pregnancy-Associated Serum Protease Activity. J. Clin. Endocrinol. Metab. 1990, 71, 806–816. [Google Scholar] [CrossRef]
- Skjærbæk, C.; Frystyk, J.; Ørskov, H.; Flyvbjerg, A. Free IGF-I, IGFBP-1, and the Binary Complex of IGFBP-1 and IGF-I Are Increased during Human Pregnancy. Horm. Res. Paediatr. 2004, 62, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Stewart, F.M.; Freeman, D.J.; E Ramsay, J.; Greer, I.A.; Caslake, M.; Ferrell, W.R. Longitudinal Assessment of Maternal Endothelial Function and Markers of Inflammation and Placental Function throughout Pregnancy in Lean and Obese Mothers. J. Clin. Endocrinol. Metab. 2007, 92, 969–975. [Google Scholar] [CrossRef] [PubMed]
- Yu, N.; Cui, H.; Chen, X.; Chang, Y. Changes of serum pentraxin-3 and hypersensitive CRP levels during pregnancy and their relationship with gestational diabetes mellitus. PLoS ONE 2019, 14, e0224739. [Google Scholar] [CrossRef] [PubMed]
- Ashford, K.B.; Chavan, N.R.; Wiggins, A.T.; Sayre, M.M.; McCubbin, A.; Critchfield, A.S.; O’Brien, J. Comparison of Serum and Cervical Cytokine Levels throughout Pregnancy between Preterm and Term Births. Am. J. Perinatol. Rep. 2018, 8, e113–e120. [Google Scholar] [CrossRef] [PubMed]
- Rajpathak, S.N.; McGinn, A.P.; Strickler, H.D.; Rohan, T.E.; Pollak, M.; Cappola, A.R.; Kuller, L.; Xue, X.; Newman, A.B.; Strotmeyer, E.S.; et al. Insulin-like growth factor-(IGF)-axis, inflammation, and glucose intolerance among older adults. Growth Horm. IGF Res. 2008, 18, 166–173. [Google Scholar] [CrossRef]
- Rahkonen, L.; Rutanen, E.-M.; Unkila-Kallio, L.; Nuutila, M.; Nieminen, P.; Sorsa, T.; Paavonen, J. Factors affecting matrix metalloproteinase-8 levels in the vaginal and cervical fluids in the first and second trimester of pregnancy. Hum. Reprod. 2009, 24, 2693–2702. [Google Scholar] [CrossRef]
- Olsen, S.; Rensen, J.D.S.O.; Secher, N.; Hedegaard, M.; Henriksen, T.B.; Hansen, H.; Grant, A. Randomised controlled trial of effect of fish-oil supplementation on pregnancy duration. Lancet 1992, 339, 1003–1007. [Google Scholar] [CrossRef]
- Products, N.A.A.; EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the Tolerable Upper Intake Level of eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA) and docosapentaenoic acid (DPA). EFSA J. 2012, 10, 2815. [Google Scholar] [CrossRef]
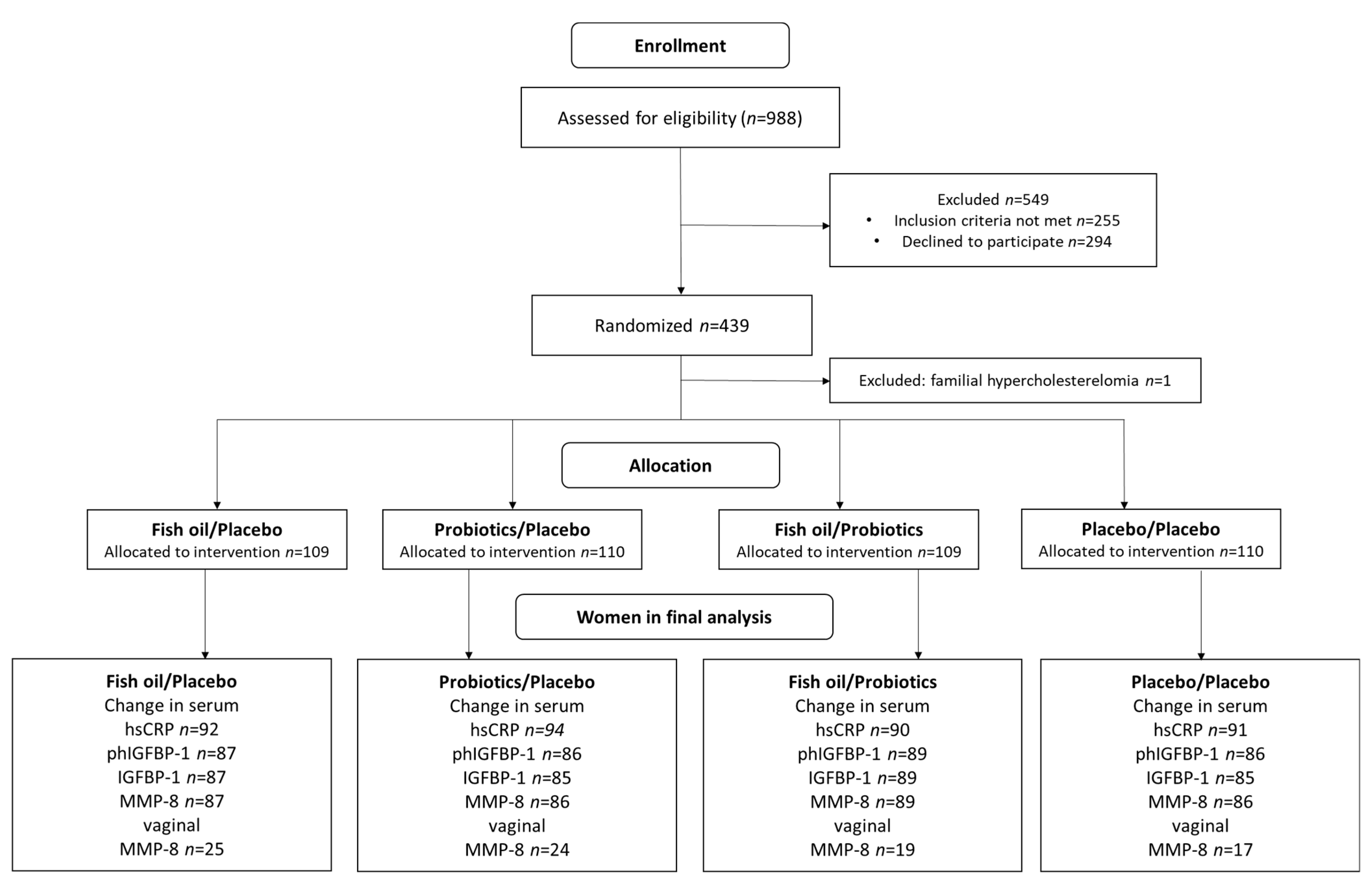
| Fish Oil + Placebo | Probiotics + Placebo | Fish Oil + Probiotics | Placebo + Placebo | All | |||
|---|---|---|---|---|---|---|---|
| Serum Markers | Subjects (n) | p Value a | |||||
| hsCRP (mg/L) b | |||||||
| Early pregnancy | 1.68 ± 0.76 | 1.63 ± 0.79 | 1.58 ± 0.74 | 1.57 ± 0.80 | 1.61 ± 0.77 | 108/109/108/109/434 | |
| Late pregnancy | 1.39 ± 0.85 | 1.32 ± 0.80 | 1.29 ± 0.78 | 1.26 ± 0.74 | 1.32 ± 0.79 | 93/94/90/92/369 | 0.72 |
| Mean change | −0.29 | −0.33 | −0.32 | −0.30 | −0.31 c | 92/94/90/91/367 | 0.97 |
| 95%CI | −0.43–(−0.15) | −0.46–(−0.20) | −0.45–(−0.18) | −0.44–(−0.16) | −0.38–(−0.24) c | ||
| MMP-8 (ng/mL) b | |||||||
| Early pregnancy | 3.04 ± 0.66 | 2.90 ± 0.64 | 2.89 ± 0.60 | 2.87 ± 0.58 | 2.93 ± 0.62 | 107/106/106/108/427 | |
| Late pregnancy | 2.87 ± 0.73 | 2.79 ± 0.64 | 2.75 ± 0.71 | 2.83 ± 0.70 | 2.81 ± 0.70 | 89/89/90/87/355 | 0.68 |
| Mean change | −0.15 | −0.11 | −0.13 | −0.06 | −0.11 c | 87/86/89/86/348 | 0.89 |
| 95%CI | −0.30–0.10 | −0.28–0.06 | −0.27–0.02 | −0.22–0.09 | −0.19–(−0.03) c | ||
| phIGFBP-1 (ng/mL) | |||||||
| Early pregnancy | 708.53 ± 343.32 | 701.59 ± 340.79 | 702.04 ± 309.79 | 696.04 ± 355.21 | 702.04 ± 336.64 | 107/107/106/108/428 | |
| Late pregnancy | 1160.02 ± 509.38 | 1236.36 ± 489.00 | 1179.84 ± 422.35 | 1153.10 ± 380.46 | 1182.34 ± 452.68 | 89/88/90/87/354 | 0.61 |
| Mean change | 452.97 | 490.88 | 466.85 | 467.59 | 469.57 c | 87/86/89/86/348 | 0.93 |
| 95%CI | 377.67–528.26 | 404.56–577.21 | 379.56–554.15 | 404.64–530.54 | 430.62–508.53 c | ||
| IGFBP-1 (ng/mL) b | |||||||
| Early pregnancy | 3.85 ± 0.74 | 3.84 ± 0.78 | 3.90 ± 0.73 | 3.90 ± 0.70 | 3.87 ± 0.74 | 107/106/105/108/427 | |
| Late pregnancy | 4.16 ± 0.63 | 4.26 ± 0.56 | 4.23 ± 0.56 | 4.21 ± 0.60 | 4.21 ± 0.58 | 89/88/90/86/353 | 0.72 |
| Mean change | 0.28 | 0.35 | 0.33 | 0.34 | 0.33 c | 87/85/89/85/348 | 0.83 |
| 95%CI | 0.17–0.39 | 0.24–0.46 | 0.21–0.44 | 0.22–0.46 | 0.27–0.38 c | ||
| Vaginal markers | |||||||
| MMP-8 (ng/mL) b | |||||||
| Early pregnancy | 3.45 ± 1.62 | 3.22 ± 1.46 | 3.06 ± 1.95 | 3.46 ± 1.59 | 3.29 ± 1.65 | 28/29/29/28/115 | |
| Late pregnancy | 3.44 ± 1.34 | 3.96 ± 1.48 | 3.33 ± 1.67 | 3.64 ± 1.21 | 3.61 ± 1.44 | 35/35/27/27/124 | 0.31 |
| Mean change | 0.04 | 0.55 | 0.26 | −0.04 | 0.21 c | 25/24/19/17/85 | 0.48 |
| 95%CI | −0.55−0.63 | 0.16–0.93 | −0.41–0.98 | −0.88–0.79 | −0.09–0.50 c |
| Overweight Pregnant Women | Obese Pregnant Women | n | p Value a | |
|---|---|---|---|---|
| Serum MMP-8 (ng/mL) b | ||||
| Fish oil + Placebo | −0.17 (−0.38–0.05) | −0.12 (−0.35–0.12) | 47/40 | 0.74 |
| Probiotics + Placebo | 0.33 (−0.17–0.23) | −0.40 (−0.70–(-0.11)) | 58/28 | 0.02 |
| Fish oil + Probiotics | −0.005 (−0.17–0.16) | −0.33 (−0.62–(-0.04)) | 56/33 | 0.03 |
| Placebo + Placebo | −0.12 (−0.32–0.08) | 0.02 (−0.24–0.28) | 51/35 | 0.40 |
| Serum IGFBP-1 (ng/mL) b | ||||
| Fish oil + Placebo | 0.32 (0.18–0.47) | 0.23 (0.05–0.42) | 47/40 | 0.44 |
| Probiotics + Placebo | 0.32 (0.18–0.45) | 0.42 (0.23–0.62) | 57/28 | 0.36 |
| Fish oil + Probiotics | 0.20 (0.07–0.33) | 0.54 (0.33–0.76) | 56/33 | 0.008 |
| Placebo + Placebo | 0.35 (0.20–0.51) | 0.32 (0.14–0.51) | 51/34 | 0.81 |
| Women Remaining Healthy | Women Who Developed GDM | ||||
|---|---|---|---|---|---|
| Serum Markers | n | n | p Value a,c | ||
| hsCRP (mg/L) b | 1.59 ± 0.79 | 276 | 1.71 ± 0.61 | 83 | 0.22 |
| phIGFBP-1 (ng/mL) | 753.24 ± 335.11 | 271 | 635.85 ± 315.29 | 82 | 0.005 |
| IGFBP-1 (ng/mL) b | 3.96 ± 0.69 | 269 | 3.78 ± 0.72 | 82 | 0.042 |
| MMP-8 (ng/mL) b | 2.92 ± 0.62 | 270 | 2.89 ± 0.61 | 82 | 0.77 |
| Vaginal markers | |||||
| MMP-8 (ng/mL) b | 3.42 ± 1.53 | 69 | 2.97 ± 1.52 | 29 | 0.19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Houttu, N.; Mokkala, K.; Koivuniemi, E.; Pellonperä, O.; Juhila, J.; Sorsa, T.; Laitinen, K. The Impacts of Fish Oil and/or Probiotic Intervention on Low-Grade Inflammation, IGFBP-1 and MMP-8 in Pregnancy: A Randomized, Placebo-Controlled, Double-Blind Clinical Trial. Biomolecules 2021, 11, 5. https://doi.org/10.3390/biom11010005
Houttu N, Mokkala K, Koivuniemi E, Pellonperä O, Juhila J, Sorsa T, Laitinen K. The Impacts of Fish Oil and/or Probiotic Intervention on Low-Grade Inflammation, IGFBP-1 and MMP-8 in Pregnancy: A Randomized, Placebo-Controlled, Double-Blind Clinical Trial. Biomolecules. 2021; 11(1):5. https://doi.org/10.3390/biom11010005
Chicago/Turabian StyleHouttu, Noora, Kati Mokkala, Ella Koivuniemi, Outi Pellonperä, Juuso Juhila, Timo Sorsa, and Kirsi Laitinen. 2021. "The Impacts of Fish Oil and/or Probiotic Intervention on Low-Grade Inflammation, IGFBP-1 and MMP-8 in Pregnancy: A Randomized, Placebo-Controlled, Double-Blind Clinical Trial" Biomolecules 11, no. 1: 5. https://doi.org/10.3390/biom11010005
APA StyleHouttu, N., Mokkala, K., Koivuniemi, E., Pellonperä, O., Juhila, J., Sorsa, T., & Laitinen, K. (2021). The Impacts of Fish Oil and/or Probiotic Intervention on Low-Grade Inflammation, IGFBP-1 and MMP-8 in Pregnancy: A Randomized, Placebo-Controlled, Double-Blind Clinical Trial. Biomolecules, 11(1), 5. https://doi.org/10.3390/biom11010005






