Abstract
Background: The development of hand osteoarthritis (HOA) and its progression into the erosive subset are unclear, but inflammation is suspected to be the main source. To verify the involvement of inflammation in HOA pathogenesis, we evaluate serum inflammatory mediators and their association with HOA-related clinical features in patients. Methods: 153 participants (50 non-erosive HOA patients, 54 erosive HOA patients, and 49 healthy control subjects) were included in this study. All patients underwent clinical examination, which included assessment of tender and swollen small hand joints, ultrasound (US) examination, and self-reported measures (e.g., AUSCAN or algofunctional indexes). Serum inflammatory mediators were quantified using human cytokine 27-plex immunoassay. We employed linear modelling, correlation analysis, and resampling statistics to evaluate the association of these mediators to HOA. Results: We identified increased levels of nine inflammatory mediators (e.g., eotaxin, monocyte chemoattractant protein 1, interleukin-8, and tumour necrosis factor) in HOA patients compared to healthy controls. Increased mediators correlated with ultrasound findings as well as with clinically tender and swollen joint counts in patients with erosive HOA. However, none of the mediators distinguished between erosive and non-erosive HOA subtypes. Conclusion: Our findings support the hypothesis on the involvement of inflammation in HOA.
1. Introduction
Hand osteoarthritis (HOA) is the most common articular disease that causes pain and functional impairment [1,2]. The symptoms of HOA occur in approximately 6–8% of adults, with increasing prevalence during ageing and in females, and radiographic signs are found in up to two-thirds of females over 55 years of age [3]. The progression of HOA is slow but consistently leads to the loss of hyaline cartilage, changes in the subchondral bone, and osteophyte formation. Although HOA is considered a mild condition, a transient flare of the disease is often associated with inflammatory irritation of synovial tissue and pain [4].
Erosive HOA is a subset of HOA associated with more severe clinical symptoms than the non-erosive subtype [5]. The process leading to erosive changes in HOA is still unknown; however, increasing evidence indicates that inflammation is one of the substantial driving forces [6]. In fact, ultrasound (US) and magnetic resonance imaging (MRI) frequently detect synovial inflammation with structural progression and erosion development in patients with erosive HOA [7,8]. Moreover, synovial inflammation is more frequent in patients with erosive than in non-erosive HOA, which has been observed not only in erosive but also in non-erosive joints [9]. All these findings have led to the assumption that OA development and erosive evolution are the results of low-grade inflammation [10].
In general, chemoattraction and regulation of recruited immune cells, mediated by chemokines, cytokines, and growth factors, play a crucial role in OA pathophysiology and disease progression [11]. However, only a few studies have addressed the correlation between serum cytokines and clinical measures of OA. For example, interleukin (IL)-6, macrophage colony-stimulating factor (MCSF)-1, fibroblast growth factor (FGF)-21, and tumour necrosis factor-related weak inducer of apoptosis (TWEAK) have been independently associated with pain intensity in patients with knee OA [12]. In addition, plasma levels of CCL-3 and CCL-4 chemokines were correlated with the severity of radiographic damage in patients with knee OA [13]. In patients with erosive HOA, the levels of C-reactive protein (CRP) and myeloperoxidase were higher compared to those with non-erosive HOA, but the levels of CRP could not distinguish HOA patients from healthy subjects, nor did they correlate with erosive disease, structural damage, or inflammation [14,15].
However, as far as we know, no published study has shown the cytokine profile and its correlation with clinical assessments of disease severity in patients with HOA. Therefore, the present study aims to explore the serum levels of systemic mediators in patients with HOA and their association with OA-related clinical features and to elucidate the potential difference between erosive and non-erosive disease compared to healthy controls using a serum 27-plex assay of human cytokines, chemokines, and growth factors.
2. Materials and Methods
2.1. Patients
Altogether, this study included 153 subjects who met selection criteria; their sera were stored in the biobank of the Institute of Rheumatology (Prague, CZ). One hundred and four patients met the American College of Rheumatology (ACR) classification criteria for HOA [16], and they consecutively attended the outpatient department at the Institute of Rheumatology in Prague. Out of them, 50 patients were diagnosed with the non-erosive form and 54 with the erosive form. We did not apply any sampling method to balanced the sample size between subgroups. As healthy controls, 49 subjects from the biobank were selected. Control subjects were age- and sex-matched to both HOA groups, and they were without joint pain or clinical signs of HOA. CRP levels and inflammatory mediators were also evaluated in healthy control subjects. The exclusion criteria for both HOA patients and controls were the presence of systemic inflammatory disease or cancer.
Clinical examinations were performed by qualified rheumatologists. The number of clinically tender and swollen joints was recorded. Pain, stiffness, and function were assessed by the Australian/Canadian (AUSCAN) hand osteoarthritis index [17]. Hand disability was evaluated based on the algofunctional index [18]. The visual analogue scale (VAS) and the health assessment questionnaire (HAQ) were used for the assessment of pain and function/disability. Written informed consent from each subject was obtained prior to enrolment, and the study was approved by the local ethics committee at the Institute of Rheumatology in Prague, Czechia (approval number 5675/2015).
2.2. Radiographs
Radiographs of both hands were examined by an experienced radiologist who was blinded to the patients’ clinical examination and laboratory results. Erosive HOA was defined by at least one interphalangeal joint with radiographic signs of erosion. Radiographs of knees and hips were performed to screen the presence of OA in other locations using the Kellgren–Lawrence and Kallman scoring systems for all patients [19,20].
2.3. Ultrasound
Ultrasound of all joints of both hands for the assessment of power Doppler (PD) and greyscale (GS) synovitis was performed by two ultrasonographers using Esaote Mylab 60 equipment (Esaote S.p.A., Genova, Italy) and a linear transducer with an 18 MHz frequency, as described elsewhere [21]. Synovitis in GS and PD were scored semiquantitatively (0–3). The ultrasonographers were blinded to the patients’ clinical examination and laboratory results. Inter- and intraobserver reliabilities have recently been published, with moderate to very good results; Cohen’s kappas for inter- and intraobserver reliabilities ranged from 0.527 to 0.963 [22].
2.4. Laboratory Measurements
Peripheral blood samples were collected from all subjects, processed to isolate blood serum, and stored at −80 °C for Luminex-based analysis. CRP levels were quantified turbidimetrically using the Beckman Coulter AU system (Beckman Coulter, Brea, CA, USA).
2.5. Luminex Technology
For all measurements, fluorescent-bead-based instrument Bio-Plex 200 (Bio-Rad, Hercules, CA) was used according to the manufacturer’s protocol. Data were acquired on a validated and calibrated Bio-Plex 200 system (Bio-Rad) and analysed with Bio-Plex Manager 6.1 software (Bio-Rad), with a detection target of 50 beads per region, low RP1 target for CAL2 calibration, and recommended doublet discriminator (DD) gates of 5000 (Low) and 25,000 (High).
The serum levels of cytokines, chemokines, and growth factors were determined using a Bio-Plex Pro™ Human Cytokine 27-plex Assay, Group I (Bio-Rad). The kit measures the concentration of FGF basic, eotaxin, granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), interferon-γ (IFN-γ), IL-1β, IL-1RA, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12 (p70), IL-13, IL-15, IL-17A, interferon gamma-induced protein (IP)-10, monocyte chemoattractant protein (MCP)-1 (MCAF), macrophage inflammatory proteins (MIP)-1α, MIP-1β, RANTES, tumour necrosis factor (TNF), and vascular endothelial growth factor (VEGF). All reagents were applied and prepared according to the manufacturer’s protocol (#10014905). Measurements were done with the selected region of specific beads. Samples were measured in duplicate and the mean value of the duplicates was taken. Cytokine and chemokine concentrations were calculated by reference to the standard curve. All samples were measured in one run (without batch effect).
2.6. Statistical Analyses
Statistical analyses were performed using the R programme [23] and the extended R packages [24,25,26,27]. Non-normally distributed data of mediators were log10-transformed (log-transformed) for multiple linear modelling (LM) analyses. Group comparisons with regard to healthy controls, both erosive and non-erosive HOA, additional joint OA, and disease-related clinical scales were analysed using multiple LM analysis or logistic regression, including age, gender, CRP, and BMI as confounders. Multiple comparisons were adjusted by Bonferroni’s correction. The effect size was calculated as partial omega. ANOVA was used to assess the difference and p-value >0.05 suggested no difference between/among groups. Permutation and bootstrapped tests utilised nontransformed data to calculate p-values and medians of mediator concentrations with 95% confidence intervals (CIs), respectively, in 50,000 simulations (resampling). Mediators without overlapping 95% CIs and p-values < 0.05 were considered different between the two diagnostic groups.
Correlation analyses of inflammatory mediators and clinical manifestations were performed on non-erosive and erosive HOA patients separately. Since the majority of mediator concentrations and clinical manifestations revealed non-normal distribution and no transformation improved the normality, we employed Kendall’s tau coefficients. p-value <0.05 and rτ <-0.3 or >0.3 suggest an association between the mediator and the clinical manifestation.
3. Results
3.1. Characteristics of the Study Population
The demographic and clinical characteristics of all the individuals are summarised in Table 1. Altogether, our study included 153 subjects, out of whom 54 were patients with erosive HOA, 50 were patients with the non-erosive disease, and 49 were healthy controls. In agreement with the gender proportion of the disease in the population, our cohort included significantly more female subjects (χ2 = 80.52, p < 0.001; 87%), but the test of proportion did not suggest any difference among groups (χ2 = 0.04, p = 0.979). Patients with HOA were older than controls (χ2 = 4.0, p = 0.045), but age was likely insignificant (p > 0.05) among non-erosive, erosive and control groups after pairwise comparison with Bonferroni’s correction. The difference in BMI was negligible among groups. Most of the patients (89%) were taking symptomatic slow-acting drugs (SYSADOA) twice a year, nonsteroidal anti-inflammatory drugs (NSAIDs), or analgesics on demand.

Table 1.
Baseline characteristics of patients with hand osteoarthritis and control subjects. The data are presented as mean (the standard deviation; SD) for normal distribution or median (interquartile range; IQR) for deviated distribution. Logistic regression was applied to assess the difference in clinical manifestations between non-erosive and erosive disease. Gender, age, CRP, and BMI served as confounders in the model. p-values were computed using ANOVA. *** p < 0.001; * p < 0.05.
Clinical assessment parameters of hand stiffness defined by AUSCAN stiffness (χ2 = 0.45, p = 0. 505), functional limitation defined by AUSCAN function (χ2 = 2.54, p = 0.111), and the sum of AUSCAN indexes defined by the AUSCAN total (χ2 = 3.61, p = 0.058) were without difference between patients with erosive and non-erosive HOA. In addition, the number of clinically tender (χ2 =2.91, p = 0.088) and swollen (χ2 =1.77, p = 0.183) joints, VAS score (χ2 = 0.62, p = 0.433), and HAQ index (χ2 = 0.091, p = 0.763) were comparable between both subgroups of HOA in our cohort. On the contrary, hand pain defined by AUSCAN pain (χ2 = 3.89, p = 0.049) and the algofunctional index (χ2 = 5.33, p = 0.028) were probably different between non-erosive and erosive disease. US-detected pathologies such as grey scale (GS) synovitis (χ2 = 28.67, p < 0.001) and the intensity of the power Doppler (PD) signal (χ2 = 18.93, p < 0.001) were significantly more pronounced in patients with erosive compared with non-erosive disease.
3.2. Inflammatory Mediators are Elevated in Patients with Hand Osteoarthritis
The applied human cytokine assay for Luminex is predesigned to detect and quantify 27 cytokines, chemokines, and growth factors. We chose this method for the simultaneous detection of multiple cytokines, simple interpretation of results, and minimal sample volume for analysis. However, 12 molecules were undetectable due to their low serum concentrations in both patients and healthy controls.
Since the raw data of mediator levels in sera had non-normal distribution, we log-transformed data before multiple LM analyses with confounders, which identified 11 mediators with significantly higher serum concentrations between patients with HOA and healthy controls (Table 2A). Out of these significantly different mediators, eotaxin (F = 33.48, p < 0.001), IL-8 (F = 28.63, p < 0.001), IP-10 (F = 51.65, p < 0.001), MIP-1α (F = 41.74, p < 0.001), MIP-1β (F = 67.71, p < 0.001), and TNF (F = 67.93, p < 0.001) had an effect size over 0.14, indicating substantial differences between HOA patients and healthy controls (Figure 1). Other significantly elevated mediators in patients included MCP-1 (F = 18.17, p < 0.001), PDGF-bb (F = 7.33, p < 0.001), IL-1RA (F = 4.65, p < 0.001), and IL-1β (F = 4.57, p = 0.026), but they had an effect size below 0.14, suggesting a small association between the groups. IL-17 was significantly downregulated in HOA patients (F = 7.48, p = 0.007), with an effect size of 0.049, indicating negligible difference.

Table 2.
Serum levels of mediators in patients with hand osteoarthritis and healthy controls. The difference was studied between (A) HOA patients and healthy controls and (B) patients with erosive and non-erosive HOA. ANOVA was performed for both subgroups. Data were described as p-value, effect size (computed as partial omega) with 95% CI, and fold-change (calculated as the proportion of medians followed by log2-transformation; positive values indicate upregulation in HOA patients and patients with the erosive disease, respectively). Mediators are sorted in ascending order based on p-values.
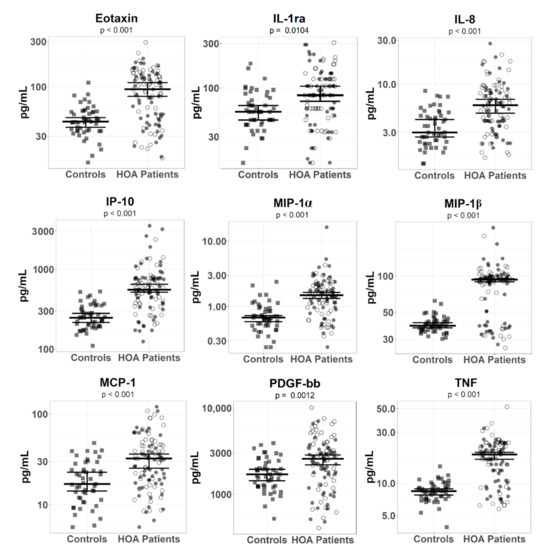
Figure 1.
Serum levels of inflammatory mediators in patients with hand osteoarthritis and in healthy controls. The error bars represent 95% confidence intervals with the median calculated by a bootstrapped method with 50,000 simulations for healthy controls and HOA patients. p-values (depicted below mediators’ names) show statistical significance between healthy controls and HOA patients and were computed by the permutation test with 50,000 simulations. Healthy controls and erosive and non-erosive HOA patients are depicted as filled squares and filled and unfilled circles, respectively.
In order to validate the output of the statistical analysis with log-transformed data, we employed a different statistical approach—resampling statistics (permutation and bootstrapped tests; Table S1A)—that belongs to nonparametric tests and does not require the assumption of normal distributions. This additional analysis revealed 11 mediators with p < 0.05, but IL-17 had overlapping CIs between HOA patients and healthy controls, indicating no statistical difference between groups, and was discarded in the subsequent selection. To sum up, this analysis confirmed the significant difference of the nine mediators (eotaxin, IL-1RA, IL-8, IP-10, MCP-1, MIP-1α, MIP-1β, PDGF-bb, and TNF) detected in the previous statistical analysis.
3.3. Inflammatory Mediators in Non-Erosive and Erosive Subgroups of Hand Osteoarthritis
In order to identify mediators that are different between erosive and non-erosive HOA, we subclassified patients into erosive and non-erosive HOA subgroups. This analysis revealed no significant difference between mediators in both HOA subtypes with p < 0.05 (Table 2B).
The permutation and bootstrapped tests revealed that medians of MCP-1 and RANTES were different between erosive and non-erosive HOA with p-value < 0.05, but their overlapping 95% CIs indicated no difference (Table S1B). No other mediator demonstrated differences between erosive and non-erosive HOA.
3.4. The Correlation between Inflammatory Mediators and Clinical Manifestations
Kendall’s correlation analysis detects the strength and direction of the association between mediators and clinical symptoms of HOA, and we considered correlations with p < 0.05 and rτ <−0.3 or >0.3 significant. This analysis revealed that US pathologies and clinically swollen and tender joint counts had the highest association to studied mediators (Table 3). All analysed correlations between mediators and clinical manifestations are shown in Table S2, including correlation coefficients and p-values.

Table 3.
The correlation analyses between the levels of inflammatory mediators and clinical features of hand osteoarthritis. The correlation analysis was performed using Kendall’s correlation on (A) patients with non-erosive HOA and (B) patients with erosive HOA. Both tables contain only clinically tender and swollen joints and US pathologies and depict correlation coefficients. Significant correlation coefficients ≤ −0.3 or ≥ 0.3 and p-values <0.05 are in bold with asterisk (* p < 0.05).
In patients with erosive HOA, we found close correlations between mediators and clinical manifestations (Table 3B). In brief, eotaxin, IL-1RA, IL-8, IP-10, MCP-1, MIP-1α, MIP-1β, PDGF bb, RANTES, and TNF were positively correlated with GS synovitis and the number of GS-positive joints, respectively. Out of these mediators, eotaxin, MCP-1, MIP-1β, and TNF were also correlated with clinically tender and swollen joint counts. On the contrary, IL-17 was negatively correlated with GS synovitis and the number of PD-positive joints, with p < 0.05, but correlation coefficients were −0.22 and −0.24, respectively (Table 3B).
The correlation analysis between mediators and clinical features in non-erosive HOA patients suggested, however, a negative correlation of MCP-1 with GS synovitis (rτ = −0.36, p < 0.001) and the number of GS-positive joints (rτ = −0.34, p = 0.001). Moreover, this analysis indicated a negative correlation of eotaxin, MIP-1α, RANTES, and PDGF-bb with GS synovitis and the number of GS-positive joints, but their correlation coefficients were between −0.3 and −0.25 (Table S2A). CRP weakly correlated only with IP-10 (rτ = 0.26 p = 0.007). Our analysis of non-erosive HOA disease did not suggest any correlation of mediators with clinically tender or swollen joint counts, which are considered predominant symptoms of HOA.
3.5. Osteoarthritis at Additional Joint Sites and Its Influence on the Levels of Inflammatory Mediators
Since the presence of OA at other sites might influence systemic cytokine levels in HOA patients, we additionally divided HOA patients based on the presence of hip and knee OA into (a) patients with hip OA, (b) patients with knee OA, (c) patients with both hip and knee OA, and (d) patients without hip or knee OA. This comparison analysis did not reveal any statistical differences between patients with and without OA at other sites (Table S3).
4. Discussion
In this study, we provide an extensive profile analysis of serum mediators and their association with OA-related clinical features in patients with HOA. We found a significant increase of nine mediators in patients with HOA compared to healthy controls. Additionally, we observed considerable correlations between these mediators, clinically tender and swollen joints, as well as US-detected pathologies in patients with erosive HOA.
Erosive HOA is considered to be more severe than the non-erosive subset; it mostly manifests worse clinical symptoms such as pain, functional impairment, and synovial inflammation [28]. In agreement, we found a significantly worse algofunctional index and worse US-detected pathologies, reflecting the role of inflammation in erosive HOA; however, probably due to the low number of patients, the number of tender and swollen joints did not significantly differ between the erosive and non-erosive subsets. Although increased serum CRP levels were previously found in erosive HOA [29], recent studies [14,30] and our results did not confirm CRP elevation in erosive compared to non-erosive HOA patients. In addition, CRP levels were not different between HOA patients and healthy controls.
However, to our knowledge, this is the first study showing the elevation of systemic chemokines and cytokines in HOA patients. Using the human cytokine assay, we profiled 27 mediators in sera of HOA patients and employed two statistical approaches to determine their potential clinical relevance. Based on the criteria of (a) p-value <0.05 and (b) nonoverlapping CI, we found nine serum mediators (eotaxin, IL-1RA, IL-8, IP-10, MIP-1α, MIP-1β, MCP-1, PDGF-bb and TNF) that were significantly elevated in HOA patients compared to healthy controls. Our findings are consistent with other studies showing that increased production of proinflammatory and chemoattractive mediators is associated with the process of OA pathology [31,32,33,34]. The majority of the elevated chemokines in patients’ sera (e.g., IL-8, MCP-1, MIP-1α, or eotaxin) play a pivotal role in the chondrocyte activation and amplification of the inflammatory process in OA [11,35,36]. These chemokines and cytokines recruit immune cells [37,38,39,40] into the affected joints and can diffuse into the bloodstream; they might also assist in better understanding of the biological background of the disease process.
Oliviero et al. [41] recently found higher levels of cytokines IL-1, IL-6, and IL-8, as well as matrix-degrading enzymes matrix metalloproteinase (MMP)-1 and MMP-3, in the synovial fluid of patients with knee OA and concomitant erosive HOA compared to those with the non-erosive disease. Although synovial fluid is an ultrafiltrate of plasma and systemic elevation of inflammatory markers could be expected, the levels of mediators were similar between both HOA subsets in our study. This inconsistency between synovial fluid and plasma could be explained by the site-specific function of mediators, or, in addition to inflammatory cytokines, other mechanisms could be responsible for the development of erosive changes. On the other hand, we were able to demonstrate a positive correlation of the nine aforementioned elevated mediators and RANTES with GS synovitis and the number of GS-positive joints, particularly in patients with erosive HOA. Altogether, the elevated levels of mediators and their association with clinical features of HOA support the hypothesis on the involvement of inflammation in the process of HOA and the potential progression into the erosive disease [11,35,36,42].
All nine increased mediators of inflammation have been previously validated as key players in modulating the immune response of various rheumatic diseases, such as rheumatoid arthritis (RA), spondyloarthritis, and systemic lupus erythematosus [43,44,45]. Therefore, based on the role of inflammation in the pathogenesis of OA [10], our results support the contribution of inflammation to the development and progression of HOA.
Two of the nine dysregulated mediators found in this study have been previously targeted by monoclonal antibodies in patients with OA to assess their efficacy. Anti-TNF targeting agents are successfully used in the treatment of inflammatory rheumatic diseases, such as RA and spondyloarthritides (review in Tylor et al.) [46]. However, the disease modification effect of adalimumab (anti-TNF monoclonal antibody) has not been proven in patients with hand OA [47,48]. Similarly, the inhibition of the important proinflammatory mediator IL-1 by lutikizumab did not improve pain or imaging outcomes in patients with erosive HOA compared with placebo [49]. In addition, diacerhein, which also reduces IL-1, was not effective in controlling the symptoms of hand OA [50]. Altogether, since targeting TNF and IL-1β have not been successful in hand OA, other dysregulated mediators in this study may represent potential therapeutic targets for OA treatment.
Interestingly, we found weak negative correlations of eotaxin, IP-10, MCP-1, MIP-1α, PDGF-bb, RANTES, and TNF with GS synovitis in patients with non-erosive HOA. We cannot explain these opposite correlations and only speculate that the immune system in these patients dampens the mediators’ activities with increasing HOA manifestation. In contrast, the immune system of patients with erosive HOA mistakenly amplifies the mediators’ activities, which probably leads to further joint damage or additional proinflammatory mechanisms to costimulate the mediators’ secretions. However, this hypothesis has to be validated in further studies.
However, there are some limitations to this study. First, the number of analysed samples was relatively small, and it was difficult to balance all confounding factors or apply advanced biostatistical approaches. Second, the applied human cytokine 27-plex assay did not reach the ELISA`s sensitivity and undetected inflammatory mediators might still play a role, although they were below the detection threshold. Third, the presence of erosive HOA was examined using the Kellgren–Lawrence and Kallman scoring systems; other scoring systems might result in different patient categorisation. Finally, this study is descriptive, and our results have to be validated by additional research.
5. Conclusions
We found systemic elevation of nine mediators in both erosive and non-erosive HOA patients compared to healthy subjects. Additionally, these mediators positively correlated with clinical and imaging findings of patients with the erosive subset of HOA. These findings support the active role of inflammation in the process of HOA. However, further studies validating our findings and longitudinal data are needed.
Supplementary Materials
The following are available online at https://www.mdpi.com/2218-273X/11/1/4/s1. Table S1: Serum levels of cytokines and chemokines in patients with hand osteoarthritis (HOA) and healthy controls. The bootstrapped method computed medians with 95% CIs and the permutation method computed p-values between (A) HOA patients and healthy controls and (B) patients with erosive and non-erosive HOA. Values represent medians and 95% CIs in brackets. Mediators are sorted in ascending order based on p-values. Table S2: Correlation analyses between the levels of inflammatory mediators and clinical factors. Kendall’s correlation analysis was performed on subjects divided into two subgroups: (A) non-erosive HOA and (B) erosive HOA patients. Both tables depict correlation coefficients and p-values. Both coefficients <−0.3 or >0.3 and p-values <0.05 for a correlation of a mediator and clinical measurement are in bold. Table S3: Osteoarthritis at additional joint sites and its influence on the levels of inflammatory mediators. The effect of OA at additional joint sites was measured in HOA patients, who were divided into (a) patients with hip OA, (b) patients with knee OA, (c) patients with both hip and knee OA, and (d) patient without hip or knee OA. The LM analysis included age, gender, BMI, and CRP as confounders. p-values were computed using ANOVA and effect size was calculated as partial omega with 95% CI.
Author Contributions
L.Š. conceived and coordinated the study, data acquisition and interpretation and critically revising the manuscript. J.B. is primarily responsible for study design, acquisition and assembly of data, analysis and interpretation of data, drafting of the article, and critically revising the manuscript. T.K. and H.H. are responsible for plasma sample collection, biomarker measurements, and critically revising the manuscript. M.T. is responsible for sample collection and critically revising the manuscript. O.R., O.Š., J.G., J.V., and K.P. are responsible for data acquisition and interpretation and critically revising the manuscript. All authors have read and approved the final manuscript.
Funding
This work was supported by the project MHCR NV18-01-00542, a project of the MHCR for conceptual research development No. 023728, and research project SVV 260 523.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the local ethics committee at the Institute of Rheuma-tology in Prague, Czechia (approval number 5675/2015).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data is contained within the article or supplementary material.
Acknowledgments
The authors thank Zdeňka Leopoldová for technical and administrative assistance and Xiao Fu for proofreading the manuscript.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Kloppenburg, M.; Berenbaum, F. Osteoarthritis year in review 2019: Epidemiology and therapy. Osteoarthr. Cartil. 2020, 28, 242–248. [Google Scholar] [CrossRef]
- James, S.L.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef]
- Kalichman, L.; Hernández-Molina, G. Hand osteoarthritis: An epidemiological perspective. Semin. Arthritis Rheum. 2010, 39, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Mathiessen, A.; Conaghan, P.G. Synovitis in osteoarthritis: Current understanding with therapeutic implications. Arthritis Res. 2017, 19, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gazeley, D.J.; Yeturi, S.; Patel, P.J.; Rosenthal, A.K. Erosive osteoarthritis: A systematic analysis of definitions used in the literature. Semin. Arthritis Rheum. 2016, 46, 395–403. [Google Scholar] [CrossRef]
- Hurnakova, J.; Hulejová, H.; Zavada, J.; Hánová, P.; Komarc, M.; Mann, H.; Klein, M.; Šléglová, O.; Olejárová, M.; Forejtova, S.; et al. Relationship between serum calprotectin (S100A8/9) and clinical, laboratory and ultrasound parameters of disease activity in rheumatoid arthritis: A large cohort study. PLoS ONE 2017, 12, e0183420. [Google Scholar] [CrossRef]
- Haugen, I.K.; Slatkowsky-Christensen, B.; Bøyesen, P.; Sesseng, S.; Van Der Heijde, D.; Kvien, T.K. MRI findings predict radiographic progression and development of erosions in hand osteoarthritis. Ann. Rheum. Dis. 2016, 75, 117–123. [Google Scholar] [CrossRef]
- Haugen, I.K.; Mathiessen, A.; Slatkowskychristensen, B.; Magnusson, K.; Bøyesen, P.; Sesseng, S.; Van Der Heijde, D.; Kvien, T.K.; Hammer, H.B. Synovitis and radiographic progression in non-erosive and erosive hand osteoarthritis: Is erosive hand osteoarthritis a separate inflammatory phenotype? Osteoarthr. Cartil. 2016, 24, 647–654. [Google Scholar] [CrossRef]
- Kortekaas, M.; Kwok, W.Y.; Reijnierse, M.; Huizinga, T.W.J.; Kloppenburg, M. In erosive hand osteoarthritis more inflammatory signs on ultrasound are found than in the rest of hand osteoarthritis. Ann. Rheum. Dis. 2012, 72, 930–934. [Google Scholar] [CrossRef]
- Chow, Y.Y.; Chin, K.-Y. The Role of inflammation in the pathogenesis of osteoarthritis. Mediat. Inflamm. 2020, 2020, 1–19. [Google Scholar] [CrossRef]
- Scanzello, C.R. Chemokines and inflammation in osteoarthritis: Insights from patients and animal models. J. Orthop. Res. 2017, 35, 735–739. [Google Scholar] [CrossRef] [PubMed]
- Giordano, R.; Petersen, K.K.; Andersen, H.H.; Simonsen, O.; Arendt-Nielsen, L. Serum inflammatory markers in patients with knee osteoarthritis. Clin. J. Pain 2020, 36, 229–237. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, Z.; Zhang, Z.; Kang, Y.; Huang, G.; Wang, S.; Huang, H.; Liao, W.; Zhang, Z. CCL3 serves as a potential plasma biomarker in knee degeneration (osteoarthritis). Osteoarthr. Cartil. 2015, 23, 1405–1411. [Google Scholar] [CrossRef]
- Funck-Brentano, T.; Nethander, M.; Movérare-Skrtic, S.; Richette, P.; Ohlsson, C. Causal factors for knee, hip, and hand osteoarthritis: A Mendelian randomization study in the UK biobank. Arthritis Rheumatol. 2019, 71, 1634–1641. [Google Scholar] [CrossRef]
- Van Delft, M.A.M.; Van Beest, S.; Kloppenburg, M.; Trouw, L.A.; Ioan-Facsinay, A. Presence of autoantibodies in erosive hand osteoarthritis and association with clinical presentation. J. Rheumatol. 2018, 46, 101–105. [Google Scholar] [CrossRef]
- Altman, R.; Alarcon, G.; Appelrouth, D.; Bloch, D.; Borenstein, D.; Brandt, K.; Brown, C.; Cooke, T.D.; Daniel, W.; Gray, R.; et al. The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hand. Arthritis Rheum. 1990, 33, 1601–1610. [Google Scholar] [CrossRef] [PubMed]
- Bellamy, N.; Campbell, J.; Haraoui, B.; Gerecz-Simon, E.; Buchbinder, R.; Hobby, K.; MacDermid, J. Clinimetric properties of the AUSCAN Osteoarthritis Hand Index: An evaluation of reliability, validity and responsiveness. Osteoarthr. Cartil. 2002, 10, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Dreiser, R.L.; Maheu, E.; Guillou, G.B.; Caspard, H.; Grouin, J.M. Validation of an algofunctional index for osteoarthritis of the hand. Rev. Rhum. Engl. Ed. 1995, 62, 43S–53S. [Google Scholar] [PubMed]
- Kellgren, J.H.; Lawrence, J.S. Radiological assessment of osteo-arthrosis. Ann. Rheum. Dis. 2008, 16, 494–502. [Google Scholar] [CrossRef]
- Kallman, D.A.; Wigley, F.M.; Scott, W.W.; Hochberg, M.C.; Tobin, J.D. New radiographic grading scales for osteoarthritis of the hand. Reliability for determining prevalence and progression. Arthritis Rheum. 1989, 32, 1584–1591. [Google Scholar] [CrossRef]
- Kropáčková, T.; Šléglová, O.; Růžičková, O.; Vencovský, J.; Pavelka, K.; Šenolt, L. Lower serum clusterin levels in patients with erosive hand osteoarthritis are associated with more pain. BMC Musculoskelet. Disord. 2018, 19, 264. [Google Scholar] [CrossRef] [PubMed]
- Hurnakova, J.; Zavada, J.; Hánová, P.; Hulejová, H.; Klein, M.; Mann, H.; Šléglová, O.; Olejárová, M.; Forejtova, S.; Růžičková, O.; et al. Serum calprotectin (S100A8/9): An independent predictor of ultrasound synovitis in patients with rheumatoid arthritis. Arthritis Res. 2015, 17, 252. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Grömping, U. Inference with linear equality and inequality constraints UsingR: The Packageic.infer. J. Stat. Softw. 2010, 33, 1–31. [Google Scholar] [CrossRef]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.D.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S-Plus; Statistics and Computing; Springer New York: New York, NY, USA, 2002; ISBN 978-1-4419-3008-8. [Google Scholar]
- Wickham, H. ggplot2; Springer New York: New York, NY, USA, 2009; ISBN 978-0-387-98140-6. [Google Scholar]
- Wittoek, R.; Carron, P.; Verbruggen, G. Structural and inflammatory sonographic findings in erosive and non-erosive osteoarthritis of the interphalangeal finger joints. Ann. Rheum. Dis. 2010, 69, 2173–2176. [Google Scholar] [CrossRef]
- Punzi, L.; Ramonda, R.; Oliviero, F.; Sfriso, P.; Mussap, M.; Plebani, M.; Podswiadek, M.; Todesco, S. Value of C reactive protein in the assessment of erosive osteoarthritis of the hand. Ann. Rheum. Dis. 2005, 64, 955–957. [Google Scholar] [CrossRef]
- Dolzani, P.; Assirelli, E.; Pulsatelli, L.; Addimanda, O.; Mancarella, L.; Peri, G.; Mantovani, A.; Facchini, A.; Meliconi, R. Systemic inflammation and antibodies to citrullinated peptides in hand osteoarthritis. Clin. Exp. Rheumatol. 2011, 29, 1006–1009. [Google Scholar]
- Hulejová, H.; Barešová, V.; Klézl, Z.; Polanská, M.; Adam, M.; Šenolt, L. Increased level of cytokines and matrix metalloproteinases in osteoarthritic subchondral bone. Cytokine 2007, 38, 151–156. [Google Scholar] [CrossRef]
- Nees, T.A.; Rosshirt, N.; Zhang, J.A.; Reiner, T.; Sorbi, R.; Tripel, E.; Walker, T.; Schiltenwolf, M.; Hagmann, S.; Moradi, B. Synovial cytokines significantly correlate with osteoarthritis-related knee pain and disability: Inflammatory mediators of potential clinical relevance. J. Clin. Med. 2019, 8, 1343. [Google Scholar] [CrossRef]
- Snelling, S.J.B.; Bas, S.; Puskas, G.J.; Dakin, S.G.; Suva, D.; Finckh, A.; Gabay, C.; Hoffmeyer, P.; Carr, A.J.; Lubbeke-Wolff, A. Presence of IL-17 in synovial fluid identifies a potential inflammatory osteoarthritic phenotype. PLoS ONE 2017, 12, e0175109. [Google Scholar] [CrossRef]
- Liu, S.-C.; Hsu, C.-J.; Fong, Y.-C.; Chuang, S.-M.; Tang, C. CTGF induces monocyte chemoattractant protein-1 expression to enhance monocyte migration in human synovial fibroblasts. Biochim. Biophys. Acta (BBA)-Bioenerg. 2013, 1833, 1114–1124. [Google Scholar] [CrossRef] [PubMed]
- Houard, X.; Goldring, M.B.; Berenbaum, F. Homeostatic mechanisms in articular cartilage and role of inflammation in osteoarthritis. Curr. Rheumatol. Rep. 2013, 15, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Berenbaum, F.; Griffin, T.M.; Liu-Bryan, R. Review: Metabolic regulation of inflammation in osteoarthritis. Arthritis Rheumatol. 2017, 69, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.-H.; Hsieh, M.-S.; Liang, Y.-C.; Li, C.-Y.; Sheu, M.-T.; Chou, D.-T.; Chen, T.-F.; Chen, C.-H. Production of the chemokine eotaxin-1 in osteoarthritis and its role in cartilage degradation. J. Cell. Biochem. 2004, 93, 929–939. [Google Scholar] [CrossRef] [PubMed]
- Baba, T.; Mukaida, N. Role of macrophage inflammatory protein (MIP)-1α/CCL3 in leukemogenesis. Mol. Cell. Oncol. 2014, 1, e29899. [Google Scholar] [CrossRef]
- Ibi, M.; Horie, S.; Kyakumoto, S.; Chosa, N.; Yoshida, M.; Kamo, M.; Ohtsuka, M.; Ishisaki, A. Cell-cell interactions between monocytes/macrophages and synoviocyte-like cells promote inflammatory cell infiltration mediated by augmentation of MCP-1 production in temporomandibular joint. Biosci. Rep. 2018, 38. [Google Scholar] [CrossRef]
- He, Y.; Liang, X.; Wu, X.; Meng, C.; Wu, B.; Fu, D.; Jin, S.; Yang, S.; Wang, H. Association between interleukin 8−251 A/T and +781 C/T polymorphisms and osteoarthritis risk. Immunol. Lett. 2014, 162, 207–211. [Google Scholar] [CrossRef]
- Oliviero, F.; Ramonda, R.; Scanu, A.; Galozzi, P.; Favero, M.; Punzi, L. Levels of inflammatory cytokines and metalloproteinases are increased in knee synovial fluid of patients with concomitant erosive hand osteoarthritis. Clin. Exp. Rheumatol 2020, 38, 800. [Google Scholar]
- Meliconi, R.; Pulsatelli, L. Are mechanisms of inflammation joint-specific in osteoarthritis? Rheumatology 2018, 58, 743–745. [Google Scholar] [CrossRef]
- Miyabe, Y.; Lian, J.; Miyabe, C.; Luster, A.D. Chemokines in rheumatic diseases: Pathogenic role and therapeutic implications. Nat. Rev. Rheumatol. 2019, 15, 731–746. [Google Scholar] [CrossRef]
- Baraliakos, X.; Gensler, L.S.; D’Angelo, S.; Iannone, F.; Favalli, E.G.; De Peyrecave, N.; Auteri, S.E.; Caporali, R. Biologic therapy and spinal radiographic progression in patients with axial spondyloarthritis: A structured literature review. Ther. Adv. Musculoskelet. Dis. 2020, 12, 1759720x2090604. [Google Scholar] [CrossRef] [PubMed]
- Schmid, A.S.; Neri, D. Advances in antibody engineering for rheumatic diseases. Nat. Rev. Rheumatol. 2019, 15, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P. Pharmacology of TNF blockade in rheumatoid arthritis and other chronic inflammatory diseases. Curr. Opin. Pharmacol. 2010, 10, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Aitken, D.; Laslett, L.L.; Pan, F.; Haugen, I.K.; Otahal, P.; Bellamy, N.; Bird, P.; Jones, G. A randomised double-blind placebo-controlled crossover trial of HUMira (adalimumab) for erosive hand OsteoaRthritis-the HUMOR trial. Osteoarthr. Cartil. 2018, 26, 880–887. [Google Scholar] [CrossRef] [PubMed]
- Philp, A.M.; Davis, E.T.; Jones, S.W. Developing anti-inflammatory therapeutics for patients with osteoarthritis. Rheumatology 2016, 56, kew278. [Google Scholar] [CrossRef] [PubMed]
- Kloppenburg, M.; Peterfy, C.; Haugen, I.K.; Kroon, F.; Chen, S.; Wang, L.; Liu, W.; Levy, G.; Fleischmann, R.M.; Berenbaum, F.; et al. Phase IIa, placebo-controlled, randomised study of lutikizumab, an anti-interleukin-1α and anti-interleukin-1β dual variable domain immunoglobulin, in patients with erosive hand osteoarthritis. Ann. Rheum. Dis. 2019, 78, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.; Kim, J.W.; Moon, K.W.; Yang, J.A.; Lee, E.Y.; Song, Y.W.; Lee, E.B. The efficacy of Diacerein in hand osteoarthritis: A double-blind, randomized, placebo-controlled study. Clin. Ther. 2013, 35, 431–439. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).