The Vasoactive Role of Perivascular Adipose Tissue and the Sulfide Signaling Pathway in a Nonobese Model of Metabolic Syndrome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Guide for the Use and Care of Laboratory Animals
2.2. Experimental Animals and Basic Parameters
2.3. Functional Study
2.4. Total NO-Synthase Activity
2.5. Superoxide Levels
2.6. Western Blotting
2.7. Morphological Study
2.8. Statistical Analysis
2.9. Drugs
3. Results
3.1. General Characteristics of Experimental Animals
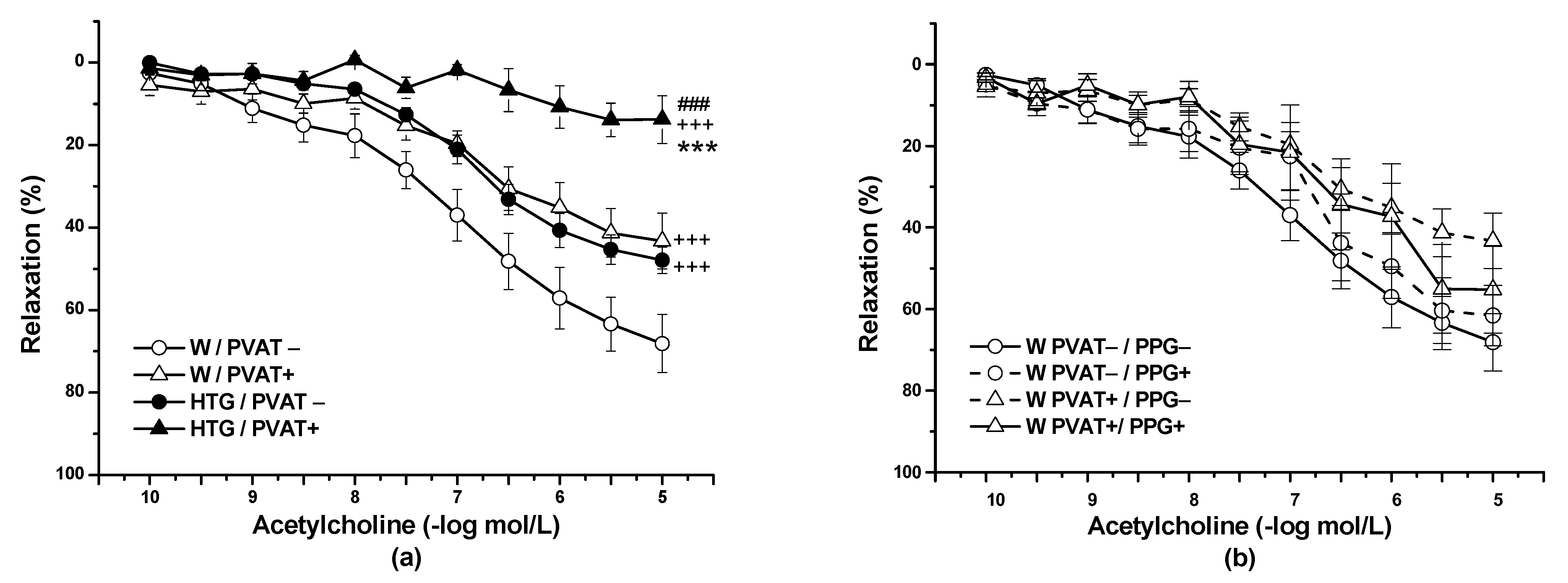
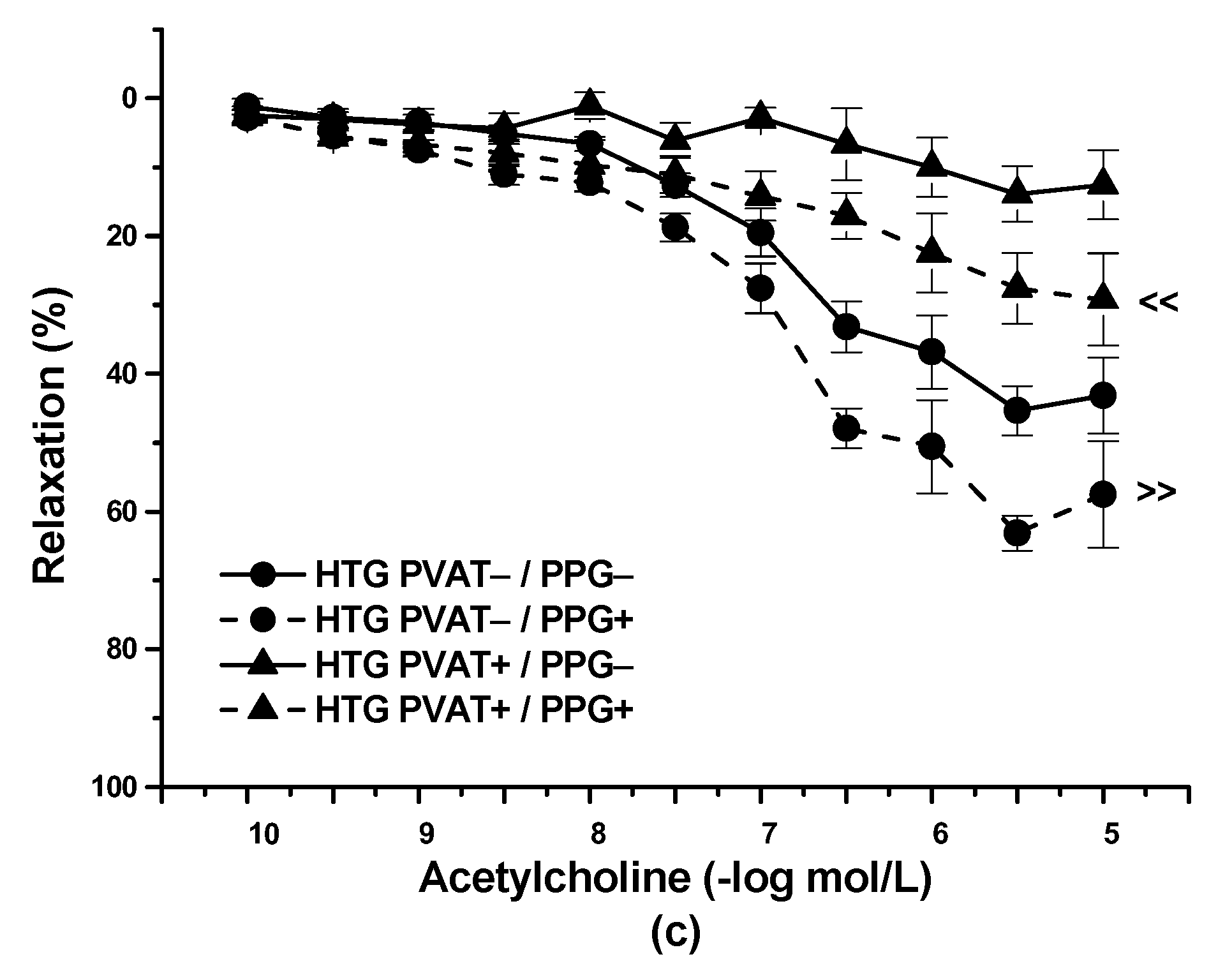
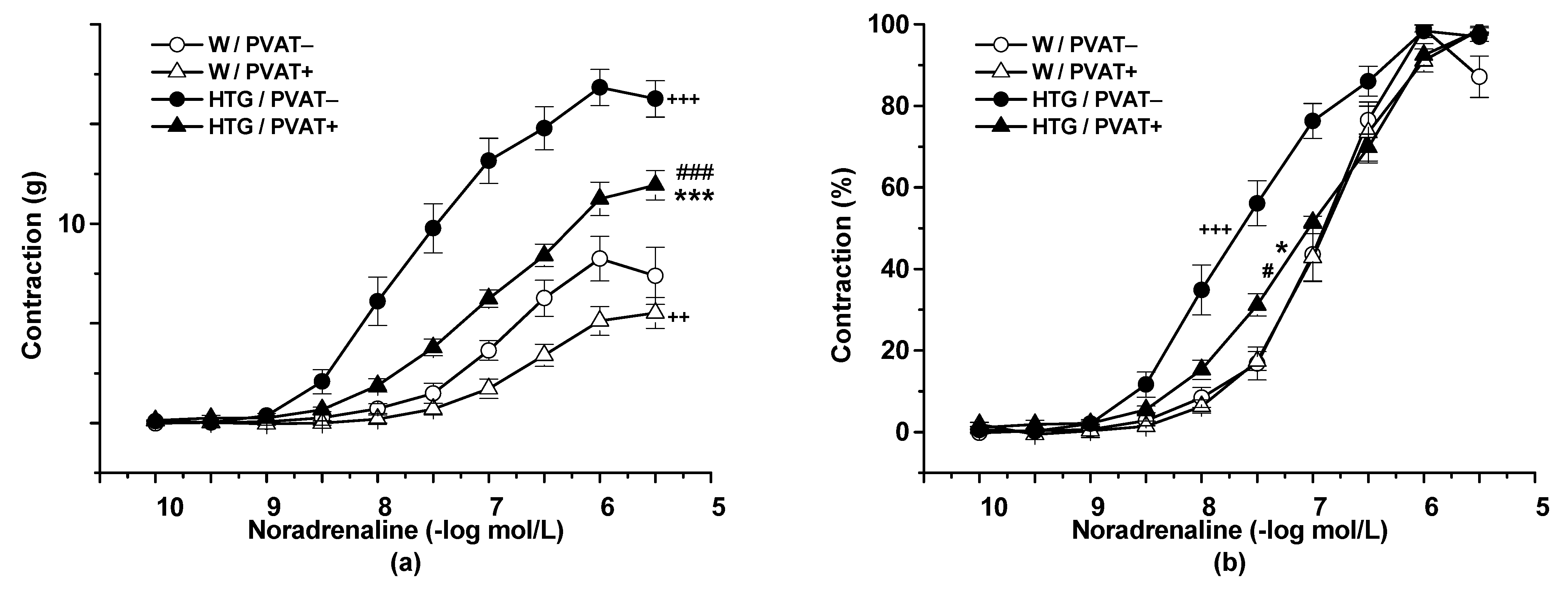
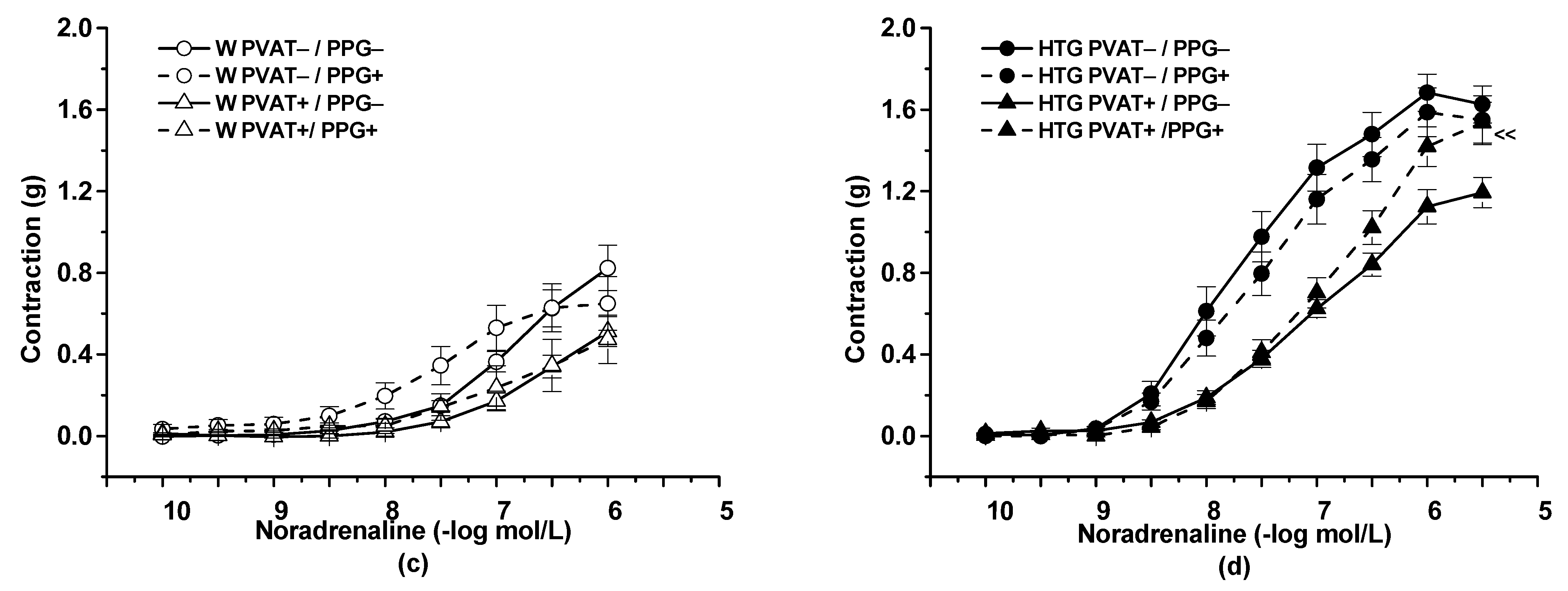
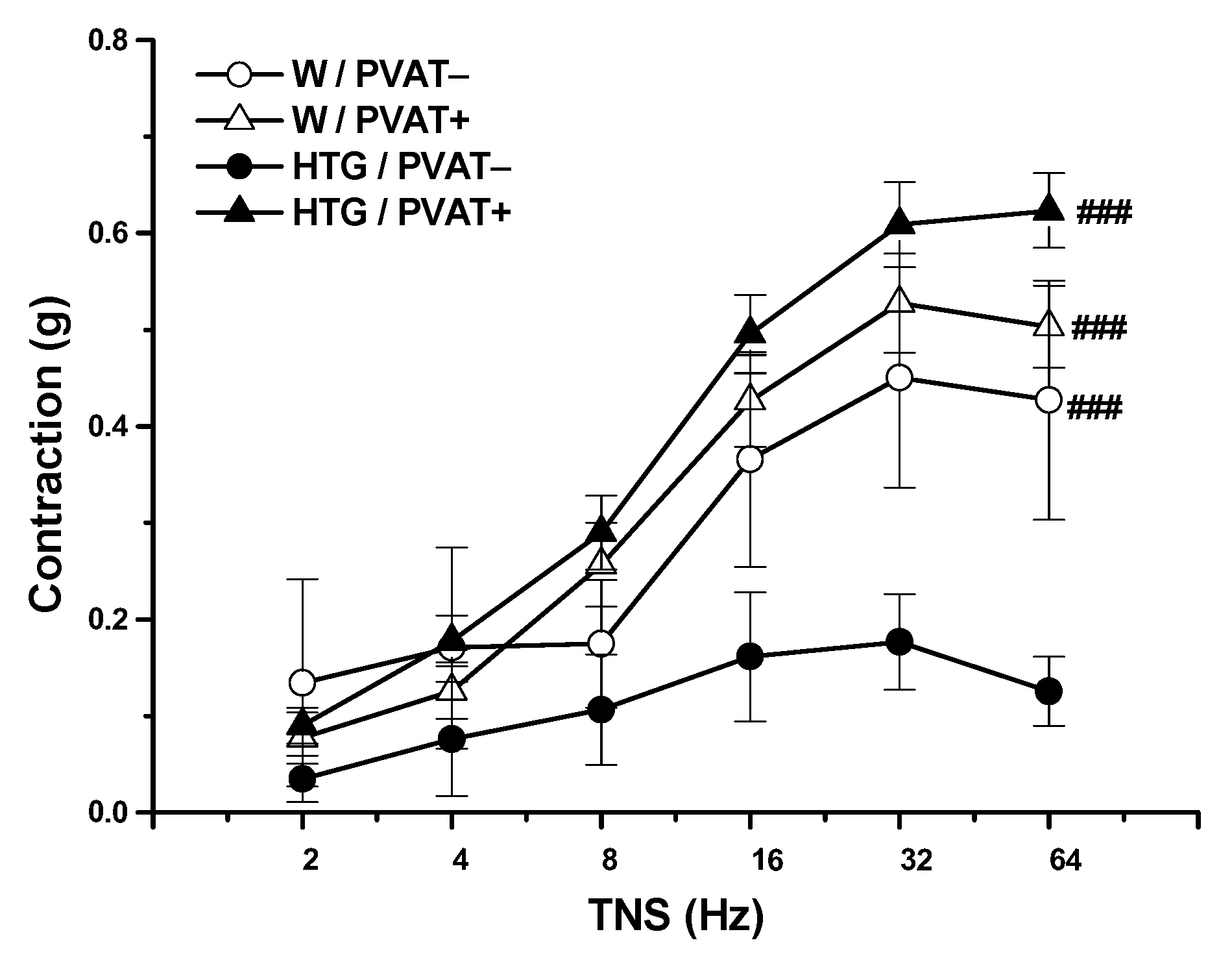
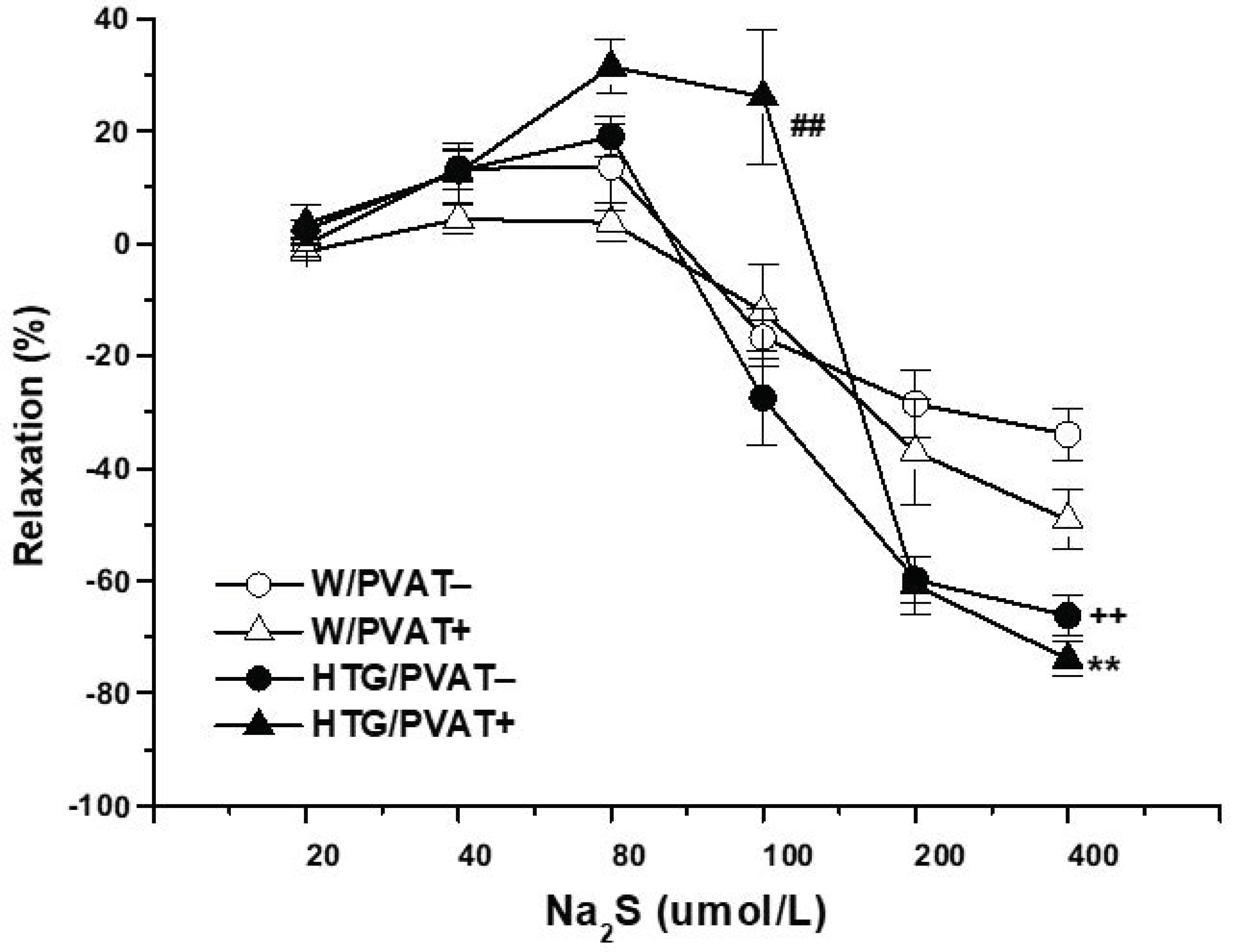
3.2. Functional Study
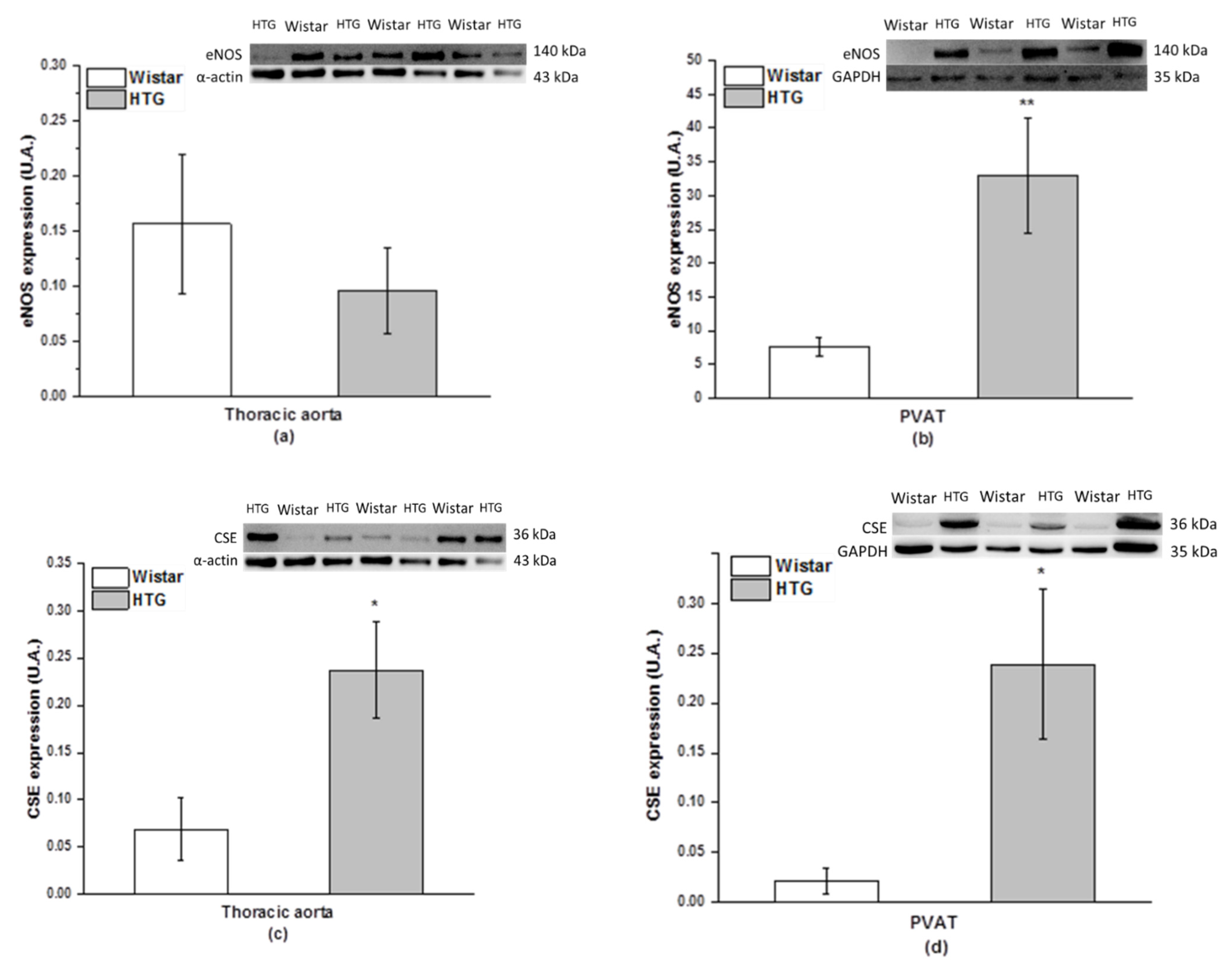
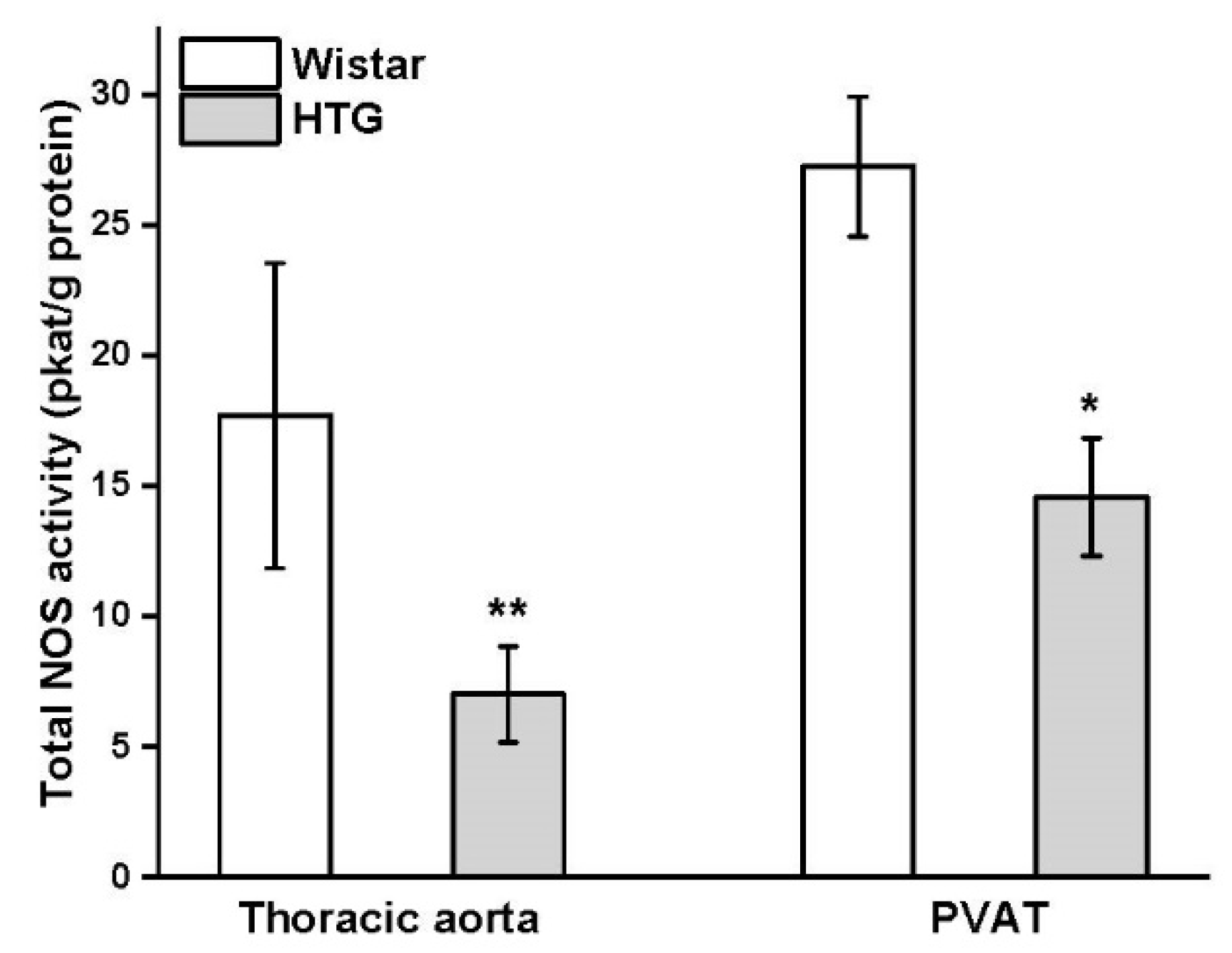
3.3. Protein Expression of eNOS and CSE and Total Activity of NOS
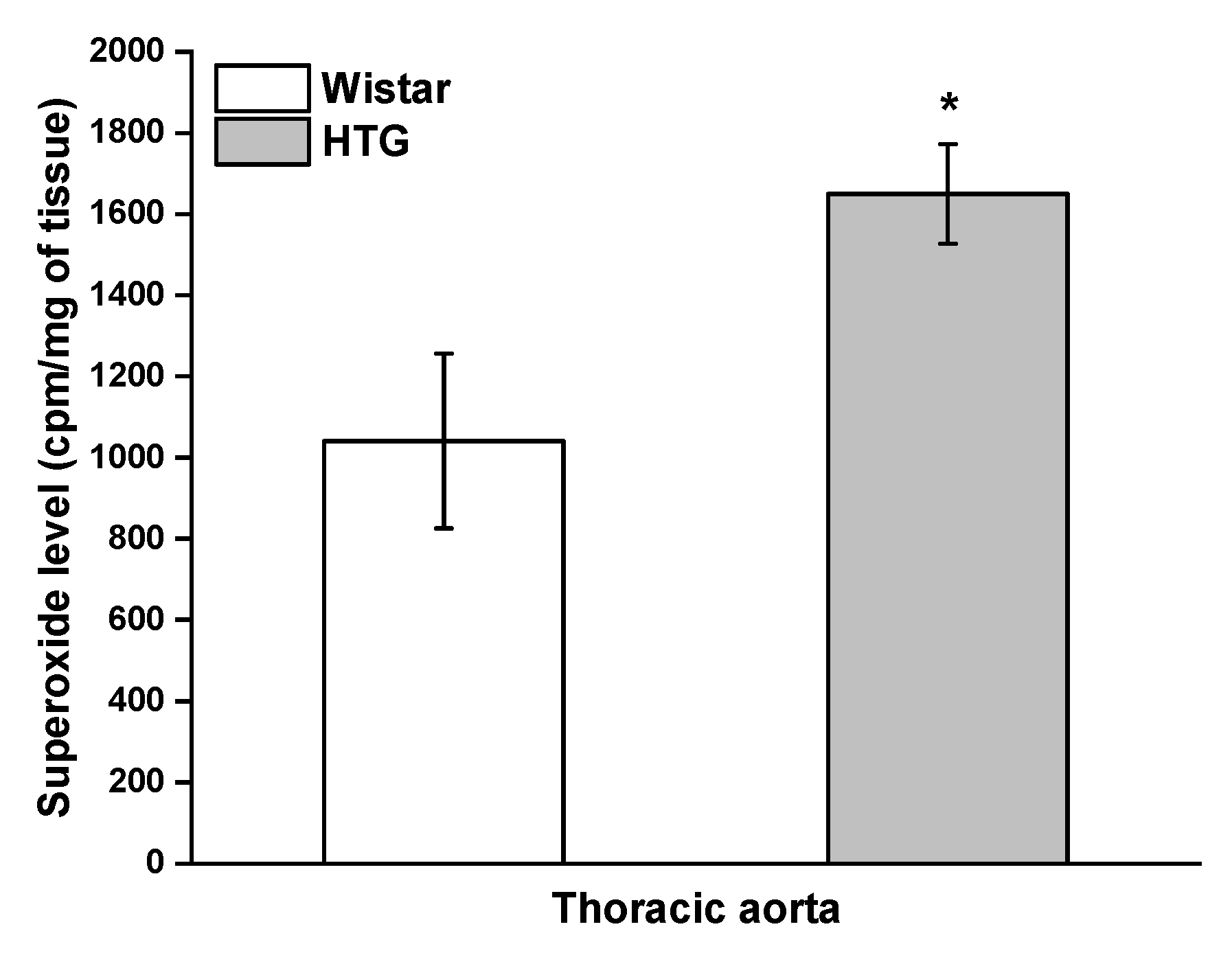
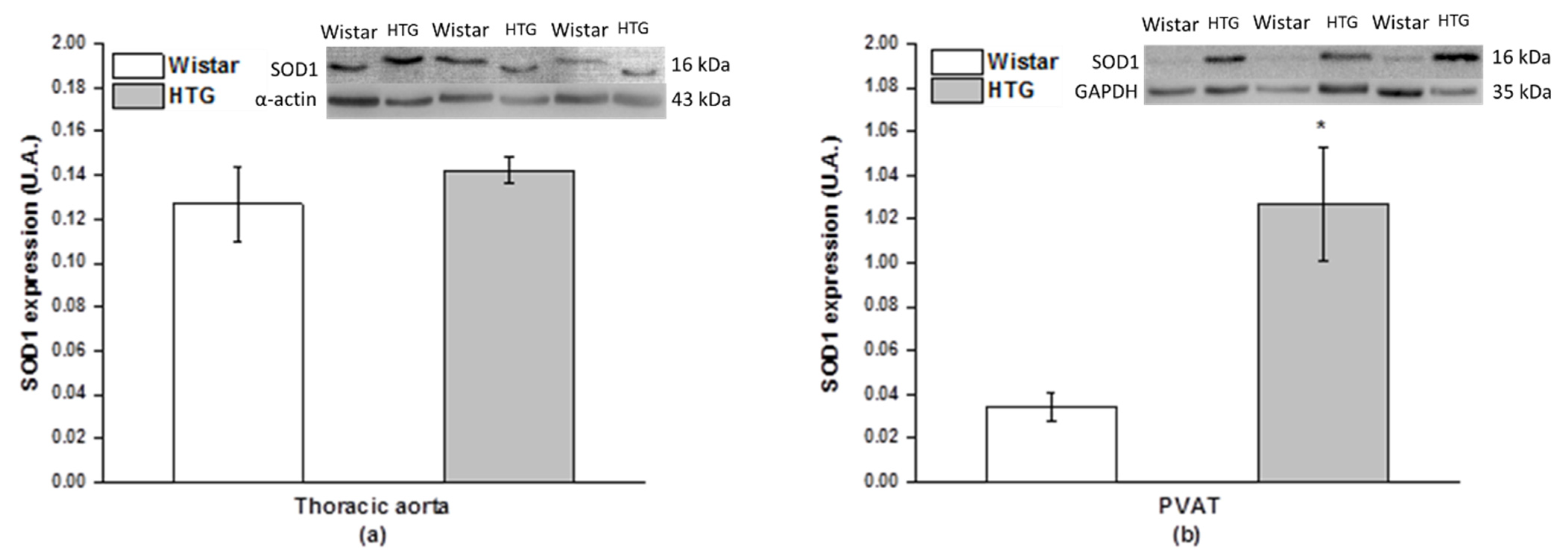
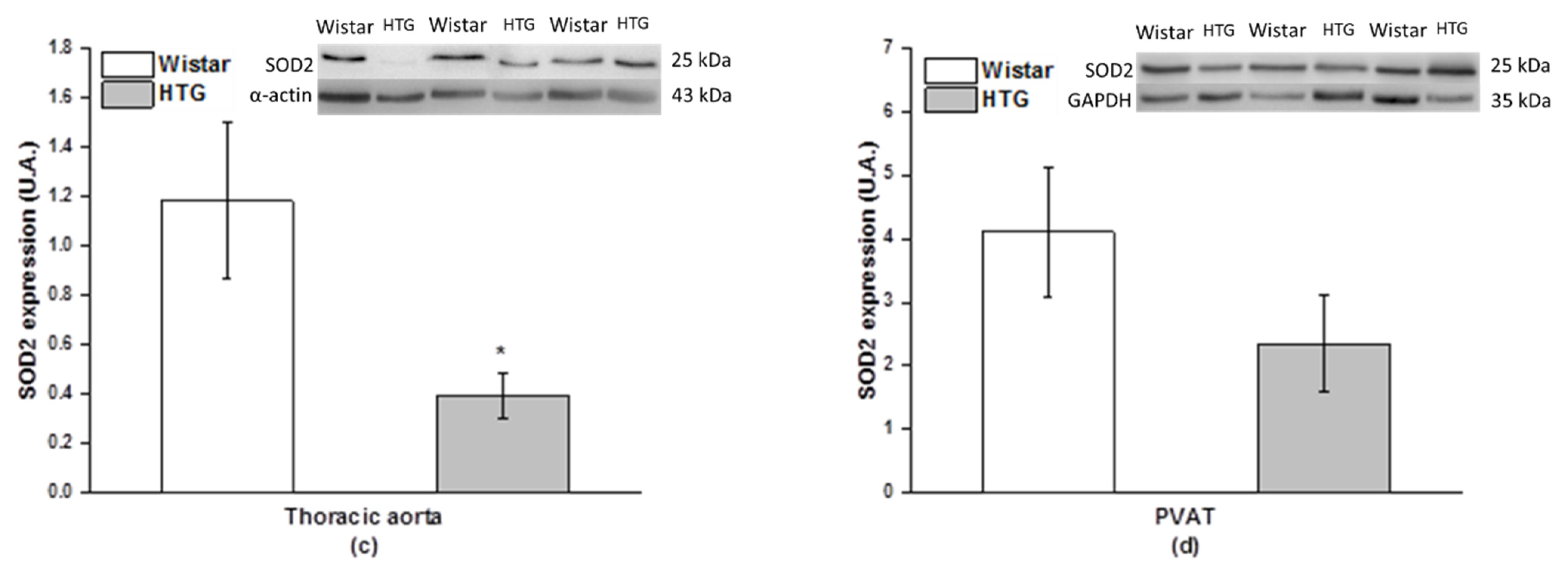
3.4. Redox State in the Vascular System
3.5. Morphological Study
4. Discussion
4.1. Procontractile vs. Anticontractile Actions of PVAT
4.2. Endothelial Function and PVAT
4.3. PVAT–H2S Interaction
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schleifenbaum, J.; Kohn, C.; Voblova, N.; Dubrovska, G.; Zavarirskaya, O.; Gloe, T.; Crean, C.S.; Luft, F.C.; Huang, Y.; Schubert, R.; et al. Systemic peripheral artery relaxation by KCNQ channel openers and hydrogen sulfide. J. Hypertens. 2010, 28, 1875–1882. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Zhao, J.; Chen, Y.; Ma, T.; Xu, G.; Tang, C.; Liu, X.; Geng, B. Hydrogen sulfide derived from periadventitial adipose tissue is a vasodilator. J. Hypertens. 2009, 27, 2174–2185. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Zhang, J.; Lu, Y.; Wang, R. The vasorelaxant effect of H(2)S as a novel endogenous gaseous K(ATP) channel opener. EMBO J. 2001, 20, 6008–6016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mustafa, A.K.; Sikka, G.; Gazi, S.K.; Steppan, J.; Jung, S.M.; Bhunia, A.K.; Barodka, V.M.; Gazi, F.K.; Barrow, R.K.; Wang, R.; et al. Hydrogen sulfide as endothelium-derived hyperpolarizing factor sulfhydrates potassium channels. Circ. Res. 2011, 109, 1259–1268. [Google Scholar] [CrossRef]
- Liu, Y.H.; Lu, M.; Hu, L.F.; Wong, P.T.-H.; Webb, G.D.; Bian, J.-S. Hydrogen sulfide in the mammalian cardiovascular system. Antioxid. Redox. Signal. 2012, 17, 141–185. [Google Scholar] [CrossRef] [PubMed]
- Coletta, C.; Papapetropoulos, A.; Erdelyi, K.; Olah, G.; Modis, K.; Panopoulos, P.; Asimakopoulou, A.; Gero, D.; Sharina, I.; Martin, E.; et al. Hydrogen sulfide and nitric oxide are mutually dependent in the regulation of angiogenesis and endothelium-dependent vasorelaxation. Proc. Natl. Acad. Sci. USA 2012, 109, 9161–9166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kubo, S.; Doe, I.; Kurokawa, Y.; Nishikawa, H.; Kawabata, A. Direct inhibition of endothelial nitric oxide synthase by hydrogen sulfide: Contribution to dual modulation of vascular tension. Toxicology 2007, 232, 138–146. [Google Scholar] [CrossRef]
- Xie, Z.Z.; Liu, Y.; Bian, J.S. Hydrogen Sulfide and Cellular Redox Homeostasis. Oxid. Med. Cell. Longev. 2016, 2016, 6043038. [Google Scholar] [CrossRef] [Green Version]
- Cacanyiova, S.; Majzunova, M.; Golas, S.; Berenyiova, A. The role of perivascular adipose tissue and endogenous hydrogen sulfide in vasoactive responses of isolated mesenteric arteries in normotensive and spontaneously hypertensive rats. J. Physiol. Pharmacol. 2019, 70. [Google Scholar] [CrossRef]
- Beltowski, J.; Jamroz-Wisniewska, A. Hydrogen Sulfide in the Adipose Tissue-Physiology, Pathology and a Target for Pharmacotherapy. Molecules 2017, 22, 63. [Google Scholar] [CrossRef] [Green Version]
- Beltowski, J. Endogenous hydrogen sulfide in perivascular adipose tissue: Role in the regulation of vascular tone in physiology and pathology. Can. J. Physiol. Pharmacol. 2013, 91, 889–898. [Google Scholar] [CrossRef] [PubMed]
- Vrana, A.; Kazdova, L. The hereditary hypertriglyceridemic nonobese rat: An experimental model of human hypertriglyceridemia. Transplant. Proc. 1990, 22, 2579. [Google Scholar] [PubMed]
- Zicha, J.; Pechanova, O.; Cacanyiova, S.; Cebova, M.; Kristek, F.; Torok, J.; Simko, F.; Dobesova, Z.; Kunes, J. Hereditary hypertriglyceridemic rat: A suitable model of cardiovascular disease and metabolic syndrome? Physiol. Res. 2006, 55 (Suppl. 1), S49–S63. [Google Scholar] [PubMed]
- Skottova, N.; Kazdova, L.; Oliyarnyk, O.; Vecera, R.; Sobolova, L.; Ulrichova, J. Phenolics-rich extracts from Silybum marianum and Prunella vulgaris reduce a high-sucrose diet induced oxidative stress in hereditary hypertriglyceridemic rats. Pharmacol. Res. 2004, 50, 123–130. [Google Scholar] [CrossRef]
- Cacanyiova, S.; Cebova, M.; Kunes, J.; Kristek, F. Comparison of vascular function and structure of iliac artery in spontaneously hypertensive and hereditary hypertriglyceridemic rats. Physiol. Res. 2006, 55 (Suppl. 1), S73–S80. [Google Scholar]
- Pechanova, O.; Bernatova, I.; Pelouch, V.; Simko, F. Protein remodelling of the heart in NO-deficient hypertension: The effect of captopril. J. Mol. Cell. Cardiol. 1997, 29, 3365–3374. [Google Scholar] [CrossRef]
- Skop, V.C.; Malinska, H.; Trnovska, J.; Huttl, M.; Cahova, M.; Blachnio-Zabielska, A.; Baranowski, M.; Burian, M.; Oliyarnyk, O.; Kazdova, L. Positive effects of voluntary running on metabolic syndrome-related disorders in non-obese hereditary hypertriacylglycerolemic rats. PLoS ONE 2015, 10, e0122768. [Google Scholar] [CrossRef] [Green Version]
- Markova, I.; Miklankova, D.; Huttl, M.; Kacer, P.; Skibova, J.; Kucera, J.; Sedlacek, R.; Kacerova, T.; Kazdova, L.; Malinska, H. The Effect of Lipotoxicity on Renal Dysfunction in a Nonobese Rat Model of Metabolic Syndrome: A Urinary Proteomic Approach. J. Diabetes Res. 2019, 2019, 8712979. [Google Scholar] [CrossRef]
- Liu, K.; Ren, X.M.; You, Q.S.; Gu, M.M.; Wang, F.; Wang, S.; Ma, C.H.; Li, W.N.; Ye, Q. Ameliorative Effect of Dangguibuxue Decoction against Cyclophosphamide- Induced Heart Injury in Mice. Biomed. Res. Int. 2018, 2018, 8503109. [Google Scholar] [CrossRef] [Green Version]
- Trovato, F.M.; Castrogiovanni, P.; Szychlinska, M.A.; Purrello, F.; Musumeci, G. Impact of Western and Mediterranean Diets and Vitamin D on Muscle Fibers of Sedentary Rats. Nutrients 2018, 10, 231. [Google Scholar] [CrossRef] [Green Version]
- Zicha, J.; Kunes, J.; Devynck, M.A. Abnormalities of membrane function and lipid metabolism in hypertension: A review. Am. J. Hypertens. 1999, 12, 315–331. [Google Scholar] [CrossRef]
- Emilova, R.; Dimitrova, D.; Mladenov, M.; Daneva, T.; Schubert, R.; Gagov, H. Cystathionine gamma-lyase of perivascular adipose tissue with reversed regulatory effect in diabetic rat artery. Biotechnol. Biotechnol. Equip. 2015, 29, 147–151. [Google Scholar] [CrossRef] [Green Version]
- Kaprinay, B.; Liptak, B.; Slovak, L.; Svik, K.; Knezl, V.; Sotnikova, R.; Gasparova, Z. Hypertriglyceridemic rats fed high fat diet as a model of metabolic syndrome. Physiol. Res. 2016, 65, S515–S518. [Google Scholar] [CrossRef] [PubMed]
- Torok, J.; Koprdova, R.; Cebova, M.; Kunes, J.; Kristek, F. Functional and structural pattern of arterial responses in hereditary hypertriglyceridemic and spontaneously hypertensive rats in early stage of experimental hypertension. Physiol. Res. 2006, 55 (Suppl. 1), S65–S71. [Google Scholar]
- Lu, C.; Su, L.Y.; Lee, R.M.K.W.; Gao, Y.J. Mechanisms for perivascular adipose tissue-mediated potentiation of vascular contraction to perivascular neuronal stimulation: The role of adipocyte-derived angiotensin II. Eur. J. Pharmacol. 2010, 634, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.J.; Takemori, K.; Su, L.Y.; An, W.S.; Lu, C.; Sharma, A.M.; Lee, R.M.K.W. Perivascular adipose tissue promotes vasoconstriction: The role of superoxide anion. Cardiovasc. Res. 2006, 71, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Knapp, L.T.; Klann, E. Potentiation of hippocampal synaptic transmission by superoxide requires the oxidative activation of protein kinase C. J. Neurosci. 2002, 22, 674–683. [Google Scholar] [CrossRef] [Green Version]
- Lewis, T.V.; Dart, A.M.; Chin-Dusting, J.P. Endothelium-dependent relaxation by acetylcholine is impaired in hypertriglyceridemic humans with normal levels of plasma LDL cholesterol. J. Am. Coll. Cardiol. 1999, 33, 805–812. [Google Scholar] [CrossRef] [Green Version]
- Gustafson, B.; Hammarstedt, A.; Andersson, C.X.; Smith, U. Inflamed adipose tissue: A culprit underlying the metabolic syndrome and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2276–2283. [Google Scholar] [CrossRef]
- Rizvi, A.A. Cytokine biomarkers, endothelial inflammation, and atherosclerosis in the metabolic syndrome: Emerging concepts. Am. J. Med. Sci. 2009, 338, 310–318. [Google Scholar] [CrossRef]
- Malinska, H.; Skop, V.; Trnovska, J.; Markova, I.; Svoboda, P.; Kazdova, L.; Haluzik, M. Metformin attenuates myocardium dicarbonyl stress induced by chronic hypertriglyceridemia. Physiol. Res. 2018, 67, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Kristek, F.; Edelsteinova, S.; Sebokova, E.; Kyselovic, J.; Klimes, I. Structural changes in the aorta of the hereditary hypertriglyceridemic rat. Ann. N. Y. Acad. Sci. 1997, 827, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Simko, F.; Pelouch, V.; Torok, J.; Luptak, I.; Matuskova, J.; Pechanova, O.; Babal, P. Protein remodeling of the heart ventricles in hereditary hypertriglyceridemic rat: Effect of ACE-inhibition. J. Biomed. Sci. 2005, 12, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Banos, G.; Carvajal, K.; Cardoso, G.; Zamora, J.; Franco, M. Vascular reactivity and effect of serum in a rat model of hypertriglyceridemia and hypertension. Am. J. Hypertens. 1997, 10, 379–388. [Google Scholar] [CrossRef] [Green Version]
- Kusterer, K.; Pohl, T.; Fortmeyer, H.P.; Marz, W.; Scharnagl, H.; Oldenburg, A.; Angermuller, S.; Fleming, I.; Usadel, K.H.; Busse, R. Chronic selective hypertriglyceridemia impairs endothelium-dependent vasodilatation in rats. Cardiovasc. Res. 1999, 42, 783–793. [Google Scholar] [CrossRef] [Green Version]
- Bartus, M.; Lomnicka, M.; Lorkowska, B.; Franczyk, M.; Kostogrys, R.B.; Pisulewski, P.M.; Chlopicki, S. Hypertriglyceridemia but not hypercholesterolemia induces endothelial dysfunction in the rat. Pharmacol. Rep. 2005, 57, 127–137. [Google Scholar]
- Pechanova, O.; Bernatová, I.; Babal, P.; Martinez, C.M.; Kysela, S.; Stvrtina, S.; Andriantsitohaina, R. Red wine polyphenols prevent cardiovascular alterations in L-NAME-induced hypertension. J. Hypertens. 2004, 22, 1551–1559. [Google Scholar] [CrossRef]
- Chait, A.; Brazg, R.L.; Tribble, D.L.; Krauss, R.M. Susceptibility of small, dense, low-density lipoproteins to oxidative modification in subjects with the atherogenic lipoprotein phenotype, pattern B. Am. J. Med. 1993, 94, 350–356. [Google Scholar] [CrossRef]
- Payne, G.A.; Bohlen, H.G.; Dincer, U.D.; Borbouse, L.; Tune, J.D. Periadventitial adipose tissue impairs coronary endothelial function via PKC-beta-dependent phosphorylation of nitric oxide synthase. Am. J. Physiol. Heart. Circ. Physiol. 2009, 297, H460–H465. [Google Scholar] [CrossRef] [Green Version]
- Ketonen, J.; Shi, J.; Martonen, E.; Mervaala, E. Periadventitial adipose tissue promotes endothelial dysfunction via oxidative stress in diet-induced obese C57Bl/6 mice. Circ. J. 2010, 74, 1479–1487. [Google Scholar] [CrossRef] [Green Version]
- Kwaifa, I.K.; Bahari, H.; Yong, Y.K.; Noor, S.M. Endothelial Dysfunction in Obesity-Induced Inflammation: Molecular Mechanisms and Clinical Implications. Biomolecules 2020, 10, 291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brancaleone, V.; Roviezzo, F.; Vellecco, V.; De Gruttola, L.; Bucci, M.; Cirino, G. Biosynthesis of H2S is impaired in non-obese diabetic (NOD) mice. Br. J. Pharmacol. 2008, 155, 673–680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaorska, E.; Tomasova, L.; Koszelewski, D.; Ostaszewski, R.; Ufnal, M. Hydrogen Sulfide in Pharmacotherapy, Beyond the Hydrogen Sulfide-Donors. Biomolecules 2020, 10, 323. [Google Scholar] [CrossRef] [Green Version]
- Torok, J.; Babal, P.; Matuskova, J.; Luptak, I.; Klimes, I.; Simko, F. Impaired endothelial function of thoracic aorta in hereditary hypertriglyceridemic rats. Ann. N. Y. Acad. Sci. 2002, 967, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Cacanyiova, S.; Berenyiova, A.; Kristek, F.; Drobna, M.; Ondrias, K.; Grman, M. The adaptive role of nitric oxide and hydrogen sulphide in vasoactive responses of thoracic aorta is triggered already in young spontaneously hypertensive rats. J. Physiol. Pharmacol. 2016, 67, 501–512. [Google Scholar]
- Berenyiova, A.; Drobna, M.; Cebova, M.; Kristek, F.; Cacanyiova, S. Changes in the vasoactive effects of nitric oxide, hydrogen sulfide and the structure of the rat thoracic aorta: The role of age and essential hypertension. J. Physiol. Pharmacol. 2018, 69. [Google Scholar] [CrossRef]
- Szijarto, I.A.; Marko, L.; Filipovic, M.R.; Miljkovic, J.L.; Tabeling, C.; Tsvetkov, D.; Wang, N.; Rabelo, L.A.; Witzenrath, M.; Diedrich, A.; et al. Cystathionine gamma-Lyase-Produced Hydrogen Sulfide Controls Endothelial NO Bioavailability and Blood Pressure. Hypertension 2018, 71, 1210–1217. [Google Scholar] [CrossRef]
- Geng, B.; Cui, Y.; Zhao, J.; Yu, F.; Zhu, Y.; Xu, G.; Zhang, Z.; Tang, C.; Du, J. Hydrogen sulfide downregulates the aortic L-arginine/nitric oxide pathway in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, R1608–R1618. [Google Scholar] [CrossRef]
| Parameter | Wistar | HTG |
|---|---|---|
| SBP (mmHg) | 118.48 ± 1.76 | 135.67 ± 2.14 * |
| BW (g) | 425 ± 11 | 388 ± 10 * |
| HW (mg) | 1.23 ± 0.29 | 1.06 ± 0.23 |
| TL (mm) | 39.48 ± 0.51 | 41.71 ± 0.38 |
| HW/BW (mg/g) | 2.93 ± 0.05 | 2.57 ± 0.09 * |
| HW/TL (mg/mm) | 31.42 ± 0.66 | 25.47 ± 0.57 * |
| RFW (mg) | 26.8 ± 0.28 | 45.0 ± 0.31 * |
| RFW/TL (mg/mm) | 68.34 ± 6.98 | 108.16 ± 7.23 ** |
| GLU (mmol/L) | 4.8 ± 0.2 | 4.9 ± 0.2 |
| OGTT AUC (mmol/L/2 h) | 790 ± 58 | 907 ± 26 ** |
| TAG (mmol/L) | 1.57 ± 0.14 | 5.26 ± 0.45 *** |
| Chol (mg/dL) | 1.76 ± 0.09 | 1.58 ± 0.05 |
| HDL-C (mmol/L) | 1.32 ± 0.07 | 0.57 ± 0.05 ** |
| NEFA (mmol/L) | 0.212 ± 0.012 | 0.410 ± 0.034 ** |
| TAG in heart (μmol/g) | 1.98 ± 0.23 | 2.42 ± 0.20 |
| TAG in kidney (μmol/g) | 3.45 ± 0.56 | 4.10 ± 0.44 |
| MCP-1 (pg/mL) | 170.0 ± 14.0 | 277.6 ± 30.8 * |
| TNFα (pg/mL) | 1.67 ± 0.24 | 1.84 ± 0.15 |
| IL-6 (pg/mL) | 13.26 ± 1.78 | 15.49 ± 2.67 |
| Leptin (ng/mL) | 2.71 ± 0.13 | 3.56 ± 0.11 ** |
| hsCRP (μg/mL) | 220.9 ± 7.8 | 230.2 ± 16.7 |
| ALT (U/L) | 19.30 ± 2.67 | 33.12 ± 0.93 *** |
| Crea (μmol/L) | 27.38 ± 0.68 | 31.56 ± 1.36 * |
| Urea (mmol/L) | 5.90 ± 0.12 | 7.73 ± 0.19 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cacanyiova, S.; Golas, S.; Zemancikova, A.; Majzunova, M.; Cebova, M.; Malinska, H.; Hüttl, M.; Markova, I.; Berenyiova, A. The Vasoactive Role of Perivascular Adipose Tissue and the Sulfide Signaling Pathway in a Nonobese Model of Metabolic Syndrome. Biomolecules 2021, 11, 108. https://doi.org/10.3390/biom11010108
Cacanyiova S, Golas S, Zemancikova A, Majzunova M, Cebova M, Malinska H, Hüttl M, Markova I, Berenyiova A. The Vasoactive Role of Perivascular Adipose Tissue and the Sulfide Signaling Pathway in a Nonobese Model of Metabolic Syndrome. Biomolecules. 2021; 11(1):108. https://doi.org/10.3390/biom11010108
Chicago/Turabian StyleCacanyiova, Sona, Samuel Golas, Anna Zemancikova, Miroslava Majzunova, Martina Cebova, Hana Malinska, Martina Hüttl, Irena Markova, and Andrea Berenyiova. 2021. "The Vasoactive Role of Perivascular Adipose Tissue and the Sulfide Signaling Pathway in a Nonobese Model of Metabolic Syndrome" Biomolecules 11, no. 1: 108. https://doi.org/10.3390/biom11010108
APA StyleCacanyiova, S., Golas, S., Zemancikova, A., Majzunova, M., Cebova, M., Malinska, H., Hüttl, M., Markova, I., & Berenyiova, A. (2021). The Vasoactive Role of Perivascular Adipose Tissue and the Sulfide Signaling Pathway in a Nonobese Model of Metabolic Syndrome. Biomolecules, 11(1), 108. https://doi.org/10.3390/biom11010108






