Evaluation of the Adverse Effects of Chronic Exposure to Donepezil (An Acetylcholinesterase Inhibitor) in Adult Zebrafish by Behavioral and Biochemical Assessments
Abstract
:1. Introduction
2. Experimental Methodology
2.1. Animal Husbandry
2.2. Preparation and Exposure to Donepezil HCl
2.3. T-maze Test (Short-Term Memory Test)
2.4. A Set of Behavioral Tests
2.5. Circadian Locomotor Activity Test
2.6. Color Preferences Assay
2.7. Estimation of Neurotransmitter Activity, Oxidative Stress Level, Antioxidant Enzyme Stress Hormone, and Neuropeptides in Brain Tissue
2.8. Determination of ATP, Oxidative Stress Level and Antioxidant Enzyme Activity in Muscle Tissues
2.9. Statistical Analysis
3. Results
3.1. Effect of DPZ Exposure in Short-Term Memory in Zebrafish
3.2. Effects of DPZ in Novel Tank Test for Zebrafish
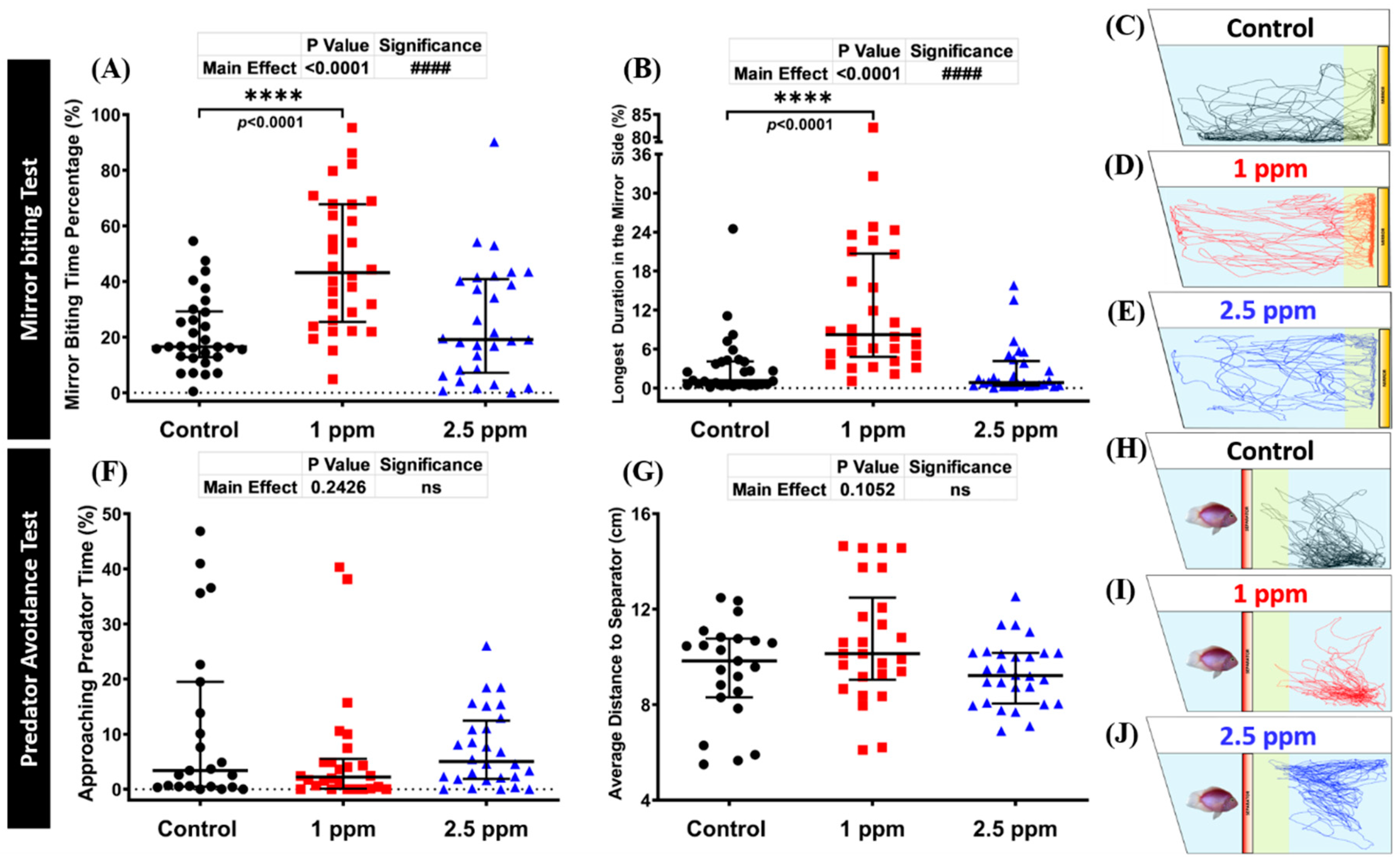
3.3. Effects of DPZ in Aggressive Behavior of Zebrafish
3.4. Effects of DPZ in Predator Avoidance Behavior of Zebrafish
3.5. Effects of DPZ Exposure in Social Interaction of Zebrafish
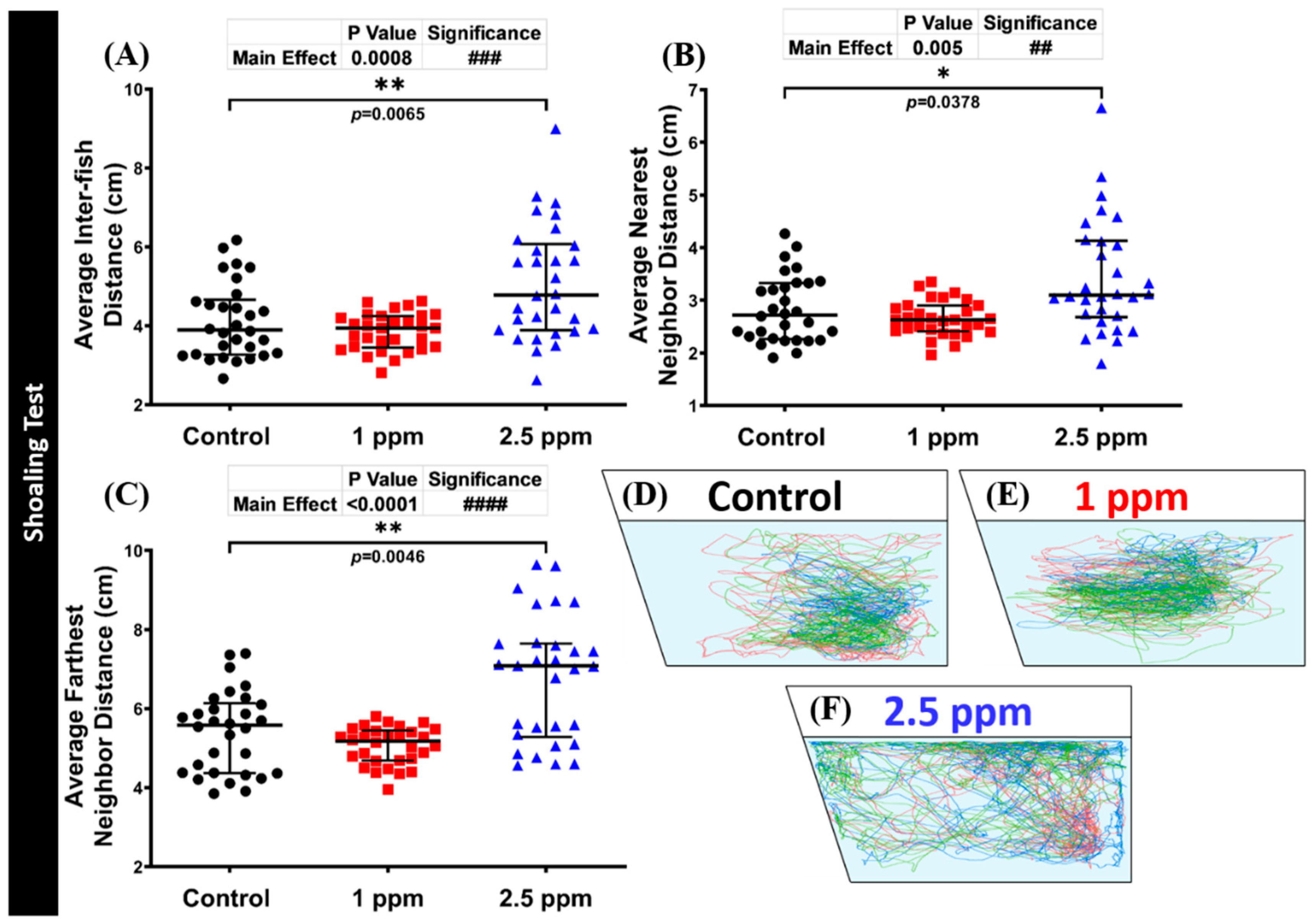
3.6. Effects of DPZ Exposure in Shoaling Formation of Zebrafish
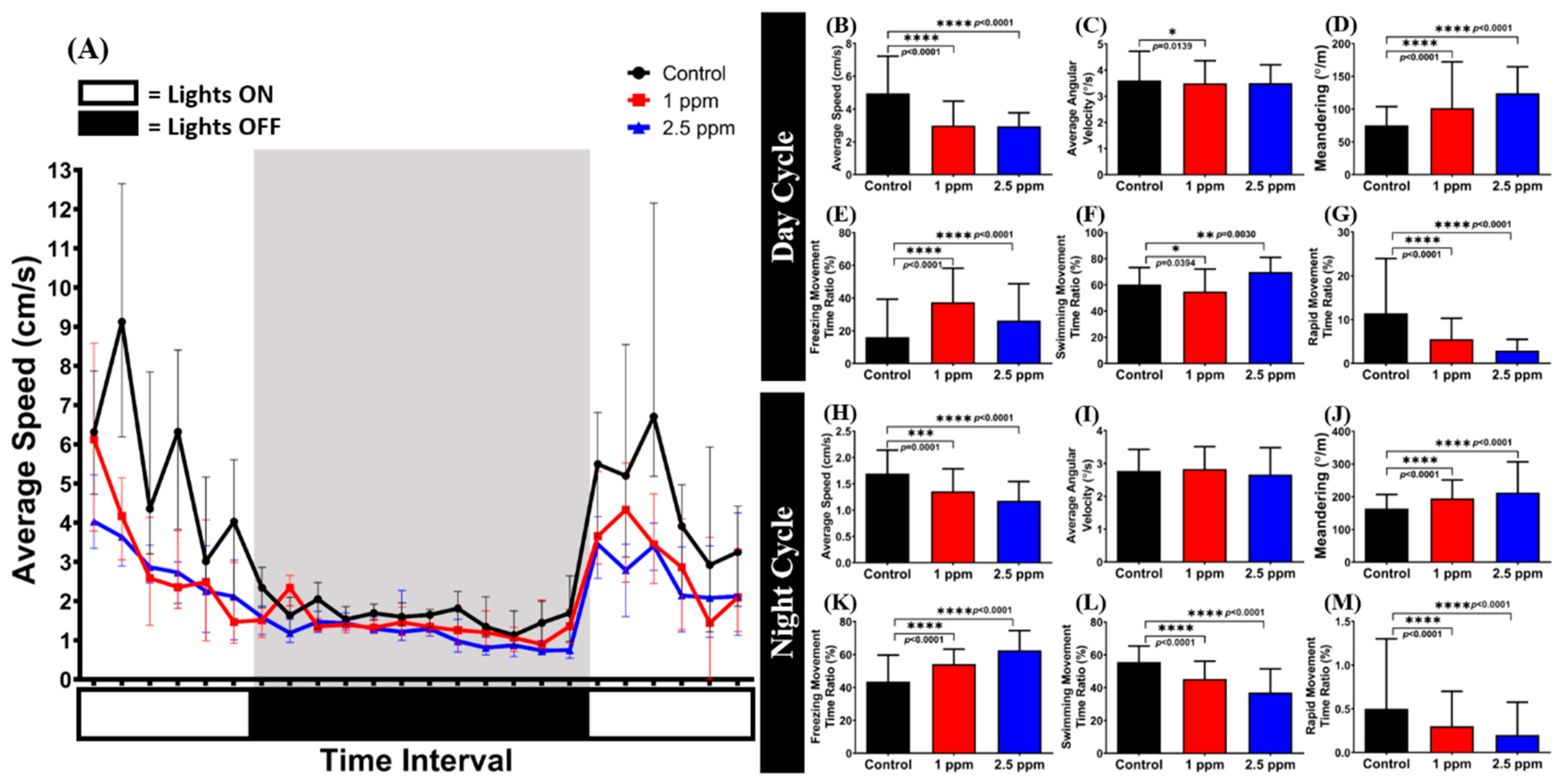
3.7. Effects of Donepezil Exposure in Circadian Locomotor Activity of Zebrafish
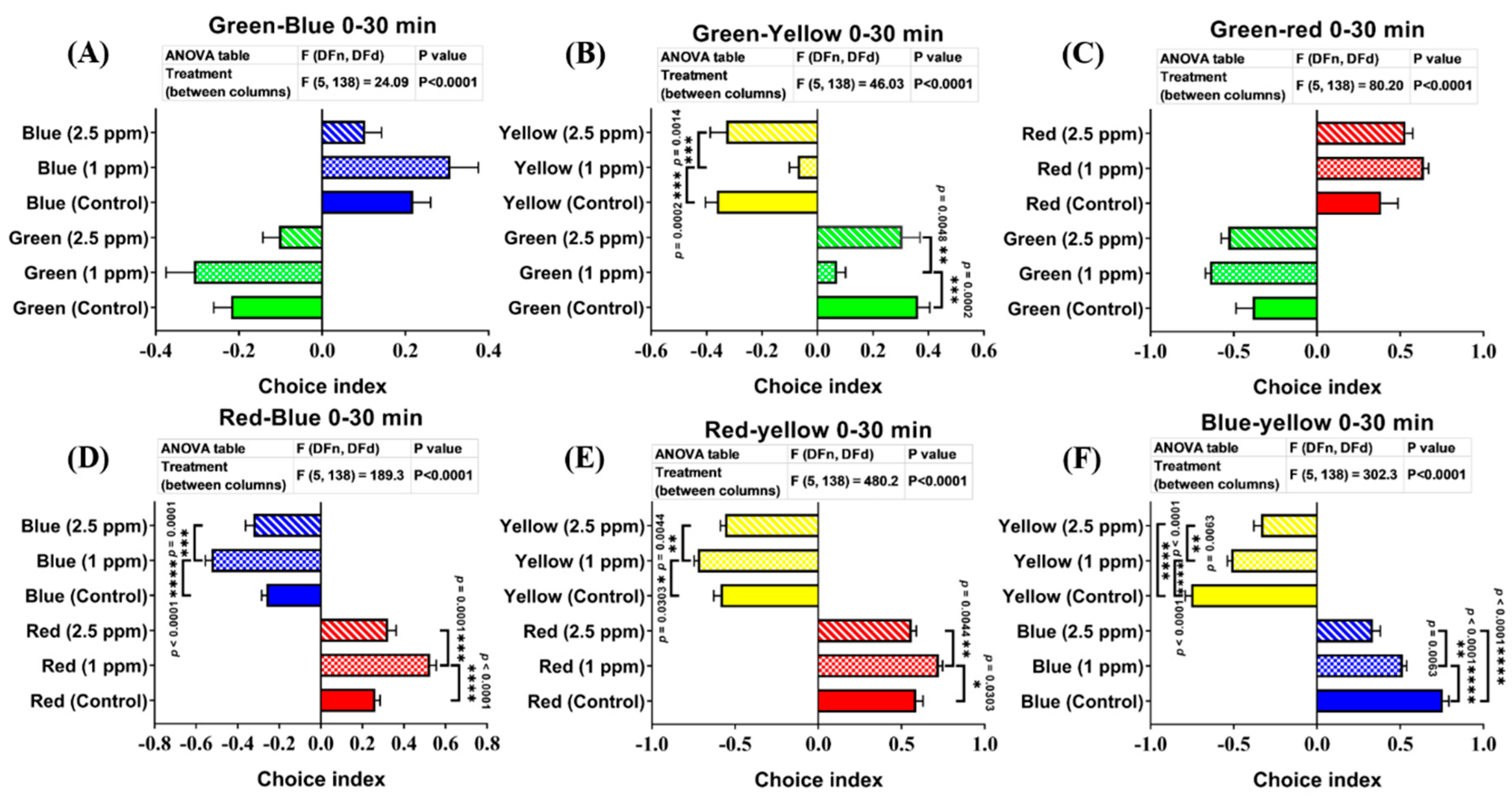
3.8. Effects of DPZ Exposure in Color Preference of Zebrafish
3.9. Effect of DPZ Exposure on Biomarker Expression in Zebrafish
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Risk reduction of cognitive decline and dementia: WHO guidelines. In Risk Reduction of Cognitive Decline and Dementia: WHO Guidelines; World Health Organization: Geneva, Switzerland, 2019; Available online: https://apps.who.int/iris/bitstream/handle/10665/312180/9789241550543-eng.pdf?ua=1&ua=1&dom=prime&src=syn (accessed on 28 August 2020).
- Cummings, J.; Lai, T.J.; Hemrungrojn, S.; Mohandas, E.; Yun Kim, S.; Nair, G.; Dash, A. Role of donepezil in the management of neuropsychiatric symptoms in Alzheimer’s disease and dementia with lewy bodies. CNS Neurosci. Ther. 2016, 22, 159–166. [Google Scholar] [CrossRef]
- Carrasco, M.M.; Agüera, L.; Gil, P.; Moríñigo, A.; Leon, T. Safety and effectiveness of donepezil on behavioral symptoms in patients with Alzheimer disease. Alzheimer Dis. Assoc. Disord. 2011, 25, 333–340. [Google Scholar] [CrossRef]
- Schredl, M.; Weber, B.; Leins, M.-L.; Heuser, I. Donepezil-induced REM sleep augmentation enhances memory performance in elderly, healthy persons. Exp. Gerontol. 2001, 36, 353–361. [Google Scholar] [CrossRef]
- Cummings, J.L.; Donohue, J.A.; Brooks, R.L. The relationship between donepezil and behavioral disturbances in patients with Alzheimer’s disease. Am. J. Geriatr. Psychiatry 2000, 8, 134–140. [Google Scholar] [CrossRef]
- Burt, T. Donepezil and related cholinesterase inhibitors as mood and behavioral controlling agents. Curr. Psychiatry Rep. 2000, 2, 473–478. [Google Scholar] [CrossRef] [PubMed]
- De Leon-Casasola, O.A. Cancer Pain: Pharmacologic, Interventional, and Palliative Approaches; WB Saunders Company: Philadelphia, PA, USA, 2006. [Google Scholar]
- Kauffman, T.L.; Barr, J.O.; Moran, M.L. Geriatric Rehabilitation Manual; Elsevier Health Sciences: Shanghai, China, 2007. [Google Scholar]
- Jackson, S.; Ham, R.J.; Wilkinson, D. The safety and tolerability of donepezil in patients with Alzheimer’s disease. Br. J. Clin. Pharmacol. 2004, 58, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cutuli, D.; De Bartolo, P.; Caporali, P.; Tartaglione, A.M.; Oddi, D.; D’Amato, F.R.; Nobili, A.; D’Amelio, M.; Petrosini, L. Neuroprotective effects of donepezil against cholinergic depletion. Alzheimer’s Res. Ther. 2013, 5, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimura, M.; Akasofu, S.; Ogura, H.; Sawada, K. Protective effect of donepezil against Aβ (1–40) neurotoxicity in rat septal neurons. Brain Res. 2005, 1047, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Martín-Jiménez, R.; Campanella, M.; Russell, C. New zebrafish models of neurodegeneration. Curr. Neurol. Neurosci. Rep. 2015, 15, 33. [Google Scholar] [CrossRef] [Green Version]
- Kopp, R.; Legler, J.; Legradi, J. Alterations in locomotor activity of feeding zebrafish larvae as a consequence of exposure to different environmental factors. Environ. Sci. Pollut. Res. 2018, 25, 4085–4093. [Google Scholar] [CrossRef] [Green Version]
- Best, J.D.; Berghmans, S.; Hunt, J.J.; Clarke, S.C.; Fleming, A.; Goldsmith, P.; Roach, A.G. Non-associative learning in larval zebrafish. Neuropsychopharmacology 2008, 33, 1206–1215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faria, M.; Prats, E.; Novoa-Luna, K.A.; Bedrossiantz, J.; Gómez-Canela, C.; Gómez-Oliván, L.M.; Raldúa, D. Development of a vibrational startle response assay for screening environmental pollutants and drugs impairing predator avoidance. Sci. Total. Environ. 2019, 650, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Westerfield, M. A Guide for the Laboratory Use of Zebrafish (Danio rerio); University of Oregon Press: Eugene, OR, USA, 2000. [Google Scholar]
- De Abreu, M.S.; Giacomini, A.C.; dos Santos, B.E.; Genario, R.; Marchiori, N.I.; da Rosa, L.G.; Kalueff, A.V. Effects of lidocaine on adult zebrafish behavior and brain acetylcholinesterase following peripheral and systemic administration. Neurosci. Lett. 2019, 692, 181–186. [Google Scholar] [CrossRef]
- Kim, J.; Shin, W. How to do random allocation (randomization). Clin. Orthop. Surg. 2014, 6, 103–109. [Google Scholar] [CrossRef] [Green Version]
- Kotani, S.; Yamauchi, T.; Teramoto, T.; Ogura, H. Donepezil, an acetylcholinesterase inhibitor, enhances adult hippocampal neurogenesis. Chem.-Biol. Interact. 2008, 175, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Cutuli, D.; Foti, F.; Mandolesi, L.; De Bartolo, P.; Gelfo, F.; Federico, F.; Petrosini, L. Cognitive performances of cholinergically depleted rats following chronic donepezil administration. J. Alzheimer’s Dis. 2009, 17, 161–176. [Google Scholar] [CrossRef]
- Lim, I.; Joung, H.-Y.; Yu, A.R.; Shim, I.; Kim, J.S. PET evidence of the effect of donepezil on cognitive performance in an animal model of chemobrain. BioMed Res. Int. 2016, 2016. [Google Scholar] [CrossRef] [Green Version]
- Creeley, C.E.; Wozniak, D.F.; Nardi, A.; Farber, N.B.; Olney, J.W. Donepezil markedly potentiates memantine neurotoxicity in the adult rat brain. Neurobiol. Aging 2008, 29, 153–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, L.; Wang, Y.; Su, L.; Ren, H.; Wang, C.; Chen, J.; Fu, X. Donepezil attenuates obesity-associated oxidative stress and central inflammation and improves memory deficit in mice fed a high-fat diet. Dement.Geriatr. Cogn. Disord. 2019, 48, 154–163. [Google Scholar] [CrossRef]
- Sullivan, L. Power and sample size determination. Available online: https://sphweb.bumc.bu.edu/otlt/mph-modules/bs/bs704_power/bs704_power_print.html (accessed on 28 August 2020).
- Rabbane, M.G.; Rahman, M.M.; Hossain, M.A. Effects of stocking density on growth of zebrafish (Danio rerio, Hamilton, 1822). Bangladesh J. Zool. 2016, 44, 209–218. [Google Scholar] [CrossRef] [Green Version]
- Lidster, K.; Readman, G.D.; Prescott, M.J.; Owen, S.F. International survey on the use and welfare of zebrafish Danio rerio in research. J. Fish Biol. 2017, 90, 1891–1905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aleström, P.; D’Angelo, L.; Midtlyng, P.J.; Schorderet, D.F.; Schulte-Merker, S.; Sohm, F.; Warner, S. Zebrafish: Housing and husbandry recommendations. Lab. Anim. 2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castranova, D.; Lawton, A.; Lawrence, C.; Baumann, D.P.; Best, J.; Coscolla, J.; Doherty, A.; Ramos, J.; Hakkesteeg, J.; Wang, C. The effect of stocking densities on reproductive performance in laboratory zebrafish (Danio rerio). Zebrafish 2011, 8, 141–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ngoc Hieu, B.T.; Ngoc Anh, N.T.; Audira, G.; Juniardi, S.; Liman, R.A.D.; Villaflores, O.B.; Lai, Y.-H.; Chen, J.-R.; Liang, S.-T.; Huang, J.-C. Development of a modified three-day t-maze protocol for evaluating learning and memory capacity of adult zebrafish. Int. J. Mol. Sci. 2020, 21, 1464. [Google Scholar] [CrossRef] [Green Version]
- Aoki, R.; Tsuboi, T.; Okamoto, H. Y-maze avoidance: An automated and rapid associative learning paradigm in zebrafish. Neurosci. Res. 2015, 91, 69–72. [Google Scholar] [CrossRef] [Green Version]
- Colwill, R.M.; Raymond, M.P.; Ferreira, L.; Escudero, H. Visual discrimination learning in zebrafish (Danio rerio). Behav. Process. 2005, 70, 19–31. [Google Scholar] [CrossRef]
- Mueller, K.P.; Neuhauss, S.C. Automated visual choice discrimination learning in zebrafish (Danio rerio). J. Integr. Neurosci. 2012, 11, 73–85. [Google Scholar] [CrossRef] [Green Version]
- Audira, G.; Sarasamma, S.; Chen, J.-R.; Juniardi, S.; Sampurna, B.; Liang, S.-T.; Lai, Y.-H.; Lin, G.-M.; Hsieh, M.-C.; Hsiao, C.-D. Zebrafish mutants carrying leptin a (lepa) gene deficiency display obesity, anxiety, less aggression and fear, and circadian rhythm and color preference dysregulation. Int. J. Mol. Sci. 2018, 19, 4038. [Google Scholar] [CrossRef] [Green Version]
- Braida, D.; Ponzoni, L.; Martucci, R.; Sparatore, F.; Gotti, C.; Sala, M. Role of neuronal nicotinic acetylcholine receptors (nAChRs) on learning and memory in zebrafish. Psychopharmacology 2014, 231, 1975–1985. [Google Scholar] [CrossRef]
- Audira, G.; Sampurna, B.; Juniardi, S.; Liang, S.-T.; Lai, Y.-H.; Hsiao, C.-D. A versatile setup for measuring multiple behavior endpoints in zebrafish. Inventions 2018, 3, 75. [Google Scholar] [CrossRef] [Green Version]
- Audira, G.; Sampurna, B.P.; Juniardi, S.; Liang, S.-T.; Lai, Y.-H.; Han, L.; Hsiao, C.-D. Establishing simple image-based methods and a cost-effective instrument for toxicity assessment on circadian rhythm dysregulation in fish. Biol. Open 2019, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, D.C.; Anthony, C.D. Using randomization techniques to analyse behavioural data. Anim. Behav. 1996, 51, 733–738. [Google Scholar] [CrossRef] [Green Version]
- Audira, G.; Sampurna, B.P.; Juniardi, S.; Liang, S.-T.; Lai, Y.-H.; Hsiao, C.-D. A simple setup to perform 3D locomotion tracking in zebrafish by using a single camera. Inventions 2018, 3, 11. [Google Scholar] [CrossRef] [Green Version]
- Itoh, J.; Nabeshima, T.; Kameyama, T. Utility of an elevated plus-maze for the evaluation of memory in mice: Effects of nootropics, scopolamine and electroconvulsive shock. Psychopharmacology 1990, 101, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Dudchenko, P.A. An overview of the tasks used to test working memory in rodents. Neurosci. Biobehav. Rev. 2004, 28, 699–709. [Google Scholar] [CrossRef] [PubMed]
- Deacon, R.M.; Rawlins, J.N.P. T-maze alternation in the rodent. Nat. Protoc. 2006, 1, 7. [Google Scholar] [CrossRef] [PubMed]
- Williams, F.E.; White, D.; Messer, W.S., Jr. A simple spatial alternation task for assessing memory function in zebrafish. Behav. Process. 2002, 58, 125–132. [Google Scholar] [CrossRef]
- Darland, T.; Dowling, J.E. Behavioral screening for cocaine sensitivity in mutagenized zebrafish. Proc. Natl. Acad. Sci. USA 2001, 98, 11691–11696. [Google Scholar] [CrossRef] [Green Version]
- Levin, E.D.; Pizarro, K.; Pang, W.G.; Harrison, J.; Ramsdell, J.S. Persisting behavioral consequences of prenatal domoic acid exposure in rats. Neurotoxicology Teratol. 2005, 27, 719–725. [Google Scholar] [CrossRef]
- Mathangi, D.; Namasivayam, A. Effect of chronic cyanide intoxication on memory in albino rats. Food Chem. Toxicol. 2000, 38, 51–55. [Google Scholar] [CrossRef]
- McGaugh, J.L.; Roozendaal, B. Drug enhancement of memory consolidation: Historical perspective and neurobiological implications. Psychopharmacology 2009, 202, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, R.N.; Pamplona, F.A.; Fernandes, M.S. The cannabinoid antagonist SR141716A facilitates memory acquisition and consolidation in the mouse elevated T-maze. Neurosci. Lett. 2005, 380, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Graeff, F.; Viana, M.; Tomaz, C. The elevated T maze, a new experimental model of anxiety and memory: Effect of diazepam. Braz. J. Med Biol. Res. 1993, 26, 67–70. [Google Scholar] [PubMed]
- Egan, R.J.; Bergner, C.L.; Hart, P.C.; Cachat, J.M.; Canavello, P.R.; Elegante, M.F.; Elkhayat, S.I.; Bartels, B.K.; Tien, A.K.; Tien, D.H. Understanding behavioral and physiological phenotypes of stress and anxiety in zebrafish. Behav. Brain Res. 2009, 205, 38–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pham, M.; Raymond, J.; Hester, J.; Kyzar, E.; Gaikwad, S.; Bruce, I.; Fryar, C.; Chanin, S.; Enriquez, J.; Bagawandoss, S. Assessing social behavior phenotypes in adult zebrafish: Shoaling, social preference, and mirror biting tests. In Zebrafish Protocols for Neurobehavioral Research; Springer: Berlin/Heidelberg, Germany, 2012; pp. 231–246. [Google Scholar]
- Liu, Z.-H.; Li, Y.-W.; Hu, W.; Chen, Q.-L.; Shen, Y.-J. Mechanisms involved in tributyltin-enhanced aggressive behaviors and fear responses in male zebrafish. Aquat. Toxicol. 2020, 220, 105408. [Google Scholar] [CrossRef]
- Cachat, J.; Kyzar, E.J.; Collins, C.; Gaikwad, S.; Green, J.; Roth, A.; El-Ounsi, M.; Davis, A.; Pham, M.; Landsman, S. Unique and potent effects of acute ibogaine on zebrafish: The developing utility of novel aquatic models for hallucinogenic drug research. Behav. Brain Res. 2013, 236, 258–269. [Google Scholar] [CrossRef] [PubMed]
- Buske, C. Zebrafish Shoaling Behavior: Its Development, Quantification, Neuro-Chemical Correlates, and Application in a Disease Model; University of Toronto: Toronto, ON, Canada, 2013. [Google Scholar]
- Mizuno, S.; Kameda, A.; Inagaki, T.; Horiguchi, J. Effects of donepezil on Alzheimer’s disease: The relationship between cognitive function and rapid eye movement sleep. Psychiatry Clin. Neurosci. 2004, 58, 660–665. [Google Scholar] [CrossRef]
- Wolter, M.E.; Svoboda, K.R. Doing the locomotion: Insights and potential pitfalls associated with using locomotor activity as a readout of the circadian rhythm in larval zebrafish. J. Neurosci. Methods 2020, 330, 108465. [Google Scholar] [CrossRef]
- Del Pozo, A.; Sánchez-Férez, J.A.; Sánchez-Vázquez, F.J. Circadian rhythms of self-feeding and locomotor activity in zebrafish (Danio rerio). Chronobiol. Int. 2011, 28, 39–47. [Google Scholar] [CrossRef]
- Blaser, R.E.; Rosemberg, D.B. Measures of anxiety in zebrafish (Danio rerio): Dissociation of black/white preference and novel tank test. PLoS ONE 2012, 7, e36931. [Google Scholar] [CrossRef] [Green Version]
- Burns, A.; Rossor, M.; Hecker, J.; Gauthier, S.; Petit, H.; Möller, H.-J.; Rogers, S.; Friedhoff, L. The Effects of Donepezil in Alzheimer’s Disease–Results from a Multinational Trial1. Dement. Geriatr. Cogn. Disord. 1999, 10, 237–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winblad, B.; Engedal, K.; Soininen, H.; Verhey, F.; Waldemar, G.; Wimo, A.; Wetterholm, A.-L.; Zhang, R.; Haglund, A.; Subbiah, P. A 1-year, randomized, placebo-controlled study of donepezil in patients with mild to moderate AD. Neurology 2001, 57, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Karvat, G.; Kimchi, T. Acetylcholine elevation relieves cognitive rigidity and social deficiency in a mouse model of autism. Neuropsychopharmacology 2014, 39, 831. [Google Scholar] [CrossRef] [PubMed]
- Bryson, H.M.; Benfield, P. Donepezil. Drugs Aging 1997, 10, 234–239; discussion 240–231. [Google Scholar] [CrossRef]
- Kosasa, T.; Kuriya, Y.; Matsui, K.; Yamanishi, Y. Effect of donepezil hydrochloride (E2020) on basal concentration of extracellular acetylcholine in the hippocampus of rats. Eur. J. Pharmacol. 1999, 380, 101–107. [Google Scholar] [CrossRef]
- Hawkins, C.L.; Davies, M.J. Generation and propagation of radical reactions on proteins. Biochim. Biophys. Acta (BBA)-Bioenerg. 2001, 1504, 196–219. [Google Scholar] [CrossRef] [Green Version]
- Keller, J.; Schmitt, F.; Scheff, S.; Ding, Q.; Chen, Q.; Butterfield, D.; Markesbery, W. Evidence of increased oxidative damage in subjects with mild cognitive impairment. Neurology 2005, 64, 1152–1156. [Google Scholar] [CrossRef]
- Behl, C.; Skutella, T.; Frank, L.H.; Post, A.; Widmann, M.; Newton, C.J.; Holsboer, F. Neuroprotection against oxidative stress by estrogens: Structure-activity relationship. Mol. Pharmacol. 1997, 51, 535–541. [Google Scholar] [CrossRef]
- Ighodaro, O.; Akinloye, O. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef] [Green Version]
- Saxena, G.; Singh, S.P.; Agrawal, R.; Nath, C. Effect of donepezil and tacrine on oxidative stress in intracerebral streptozotocin-induced model of dementia in mice. Eur. J. Pharmacol. 2008, 581, 283–289. [Google Scholar] [CrossRef]
- Munishamappa, V.; Vijayakumar, A.; Rajathilagam, T. Evaluation of the antioxidant activity of donepezil-in vitro study. Natl. J. Physiol. Pharm. Pharmacol. 2019, 9, 108–110. [Google Scholar] [CrossRef]
- Folkesson, A.; Honoré, P.H.; Andersen, L.M.; Kristensen, P.; Bjerrum, O.J. Low dose of donepezil improves gabapentin analgesia in the rat spared nerve injury model of neuropathic pain: Single and multiple dosing studies. J. Neural Transm. 2010, 117, 1377–1385. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, J.E. Clinical limitations of acetylcholinesterase antagonists. J. Crit. Care 2009, 24, 21–28. [Google Scholar] [CrossRef]
- Dunn, N.; Pearce, G.; Shakir, S. Adverse effects associated with the use of donepezil in general practice in England. J. Psychopharmacol. 2000, 14, 406–408. [Google Scholar] [CrossRef] [PubMed]
- Bouman, W.P.; Pinner, G. Violent behavior associated with donepezil. Am. J. Psychiatry 1998, 155, 1626a–1626. [Google Scholar] [CrossRef]
- Hamilton, T.J.; Morrill, A.; Lucas, K.; Gallup, J.; Harris, M.; Healey, M.; Pitman, T.; Schalomon, M.; Digweed, S.; Tresguerres, M. Establishing zebrafish as a model to study the anxiolytic effects of scopolamine. Sci. Rep. 2017, 7, 15081. [Google Scholar] [CrossRef] [Green Version]
- Miller, N.; Gerlai, R. From schooling to shoaling: Patterns of collective motion in zebrafish (Danio rerio). PLoS ONE 2012, 7, e48865. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.-W.; Liang, C.-S.; Wan, F.-J. Adjunctive Donepezil for anxiety symptoms with poor response to paroxetine in a patient with probable mild cognitive impairment. Clin. Neuropharmacol. 2018, 41, 236–237. [Google Scholar] [CrossRef]
- Alsop, D.; Vijayan, M. The zebrafish stress axis: Molecular fallout from the teleost-specific genome duplication event. Gen. Comp. Endocrinol. 2009, 161, 62–66. [Google Scholar] [CrossRef]
- Barcellos, L.J.G.; Ritter, F.; Kreutz, L.C.; Quevedo, R.M.; da Silva, L.B.; Bedin, A.C.; Finco, J.; Cericato, L. Whole-body cortisol increases after direct and visual contact with a predator in zebrafish, Danio rerio. Aquaculture 2007, 272, 774–778. [Google Scholar] [CrossRef]
- Brody, S.; Preut, R.; Schommer, K.; Schürmeyer, T.H. A randomized controlled trial of high dose ascorbic acid for reduction of blood pressure, cortisol, and subjective responses to psychological stress. Psychopharmacology 2002, 159, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Harris, T.; Borsanyi, S.; Messari, S.; Stanford, K.; Cleary, S.; Shiers, H.; Brown, G.; Herbert, J. Morning cortisol as a risk factor for subsequent major depressive disorder in adult women. Br. J. Psychiatry 2000, 177, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Herbert, J. Cortisol and depression: Three questions for psychiatry. Psychol. Med. 2013, 43, 449–469. [Google Scholar] [CrossRef] [PubMed]
- Aboulafia-Brakha, T.; Suchecki, D.; Gouveia-Paulino, F.; Nitrini, R.; Ptak, R. Cognitive–behavioural group therapy improves a psychophysiological marker of stress in caregivers of patients with Alzheimer’s disease. Aging Ment. Health 2014, 18, 801–808. [Google Scholar] [CrossRef] [Green Version]
- Field, T.; Hernandez-Reif, M.; Diego, M.; Schanberg, S.; Kuhn, C. Cortisol decreases and serotonin and dopamine increase following massage therapy. Int. J. Neurosci. 2005, 115, 1397–1413. [Google Scholar] [CrossRef]
- Kaimal, G.; Ray, K.; Muniz, J. Reduction of cortisol levels and participants’ responses following art making. Art Ther. 2016, 33, 74–80. [Google Scholar] [CrossRef] [Green Version]
- Olsen, R.H.; Johnson, L.A.; Zuloaga, D.G.; Limoli, C.L.; Raber, J. Enhanced hippocampus-dependent memory and reduced anxiety in mice over-expressing human catalase in mitochondria. J. Neurochem. 2013, 125, 303–313. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Salam, O.M.; Youness, E.R.; Morsy, F.A.; Mahfouz, M.M.; Kenawy, S.A. Study of the effect of antidepressant drugs and donepezil on aluminum-induced memory impairment and biochemical alterations in rats. Comp. Clin. Pathol. 2015, 24, 847–860. [Google Scholar] [CrossRef]
- Riedel, G.; Kang, S.; Choi, D.; Platt, B. Scopolamine-induced deficits in social memory in mice: Reversal by donepezil. Behav. Brain Res. 2009, 204, 217–225. [Google Scholar] [CrossRef]
- Boada-Rovira, M.; Brodaty, H.; Cras, P.; Baloyannis, S.; Emre, M.; Zhang, R.; Bahra, R. Efficacy and safety of donepezil in patients with Alzheimer’s disease. Drugs Aging 2004, 21, 43–53. [Google Scholar] [CrossRef]
- Rockwood, K.; Graham, J.; Fay, S. Goal setting and attainment in Alzheimer’s disease patients treated with donepezil. J. Neurol., Neurosurg. Psychiatry 2002, 73, 500–507. [Google Scholar] [CrossRef] [PubMed]
- López-Olmeda, J.F.; Madrid, J.A.; Sánchez-Vázquez, F.J. Light and temperature cycles as zeitgebers of zebrafish (Danio rerio) circadian activity rhythms. Chronobiol. Int. 2006, 23, 537–550. [Google Scholar] [CrossRef] [PubMed]
- Hurd, M.W.; Debruyne, J.; Straume, M.; Cahill, G.M. Circadian rhythms of locomotor activity in zebrafish. Physiol. Behav. 1998, 65, 465–472. [Google Scholar] [CrossRef]
- Inglis, F. The tolerability and safety of cholinesterase inhibitors in the treatment of dementia. Int. J. Clin. Pract. Suppl. 2002, 127, 45–63. [Google Scholar]
- Mimica, N.; Presečki, P. Side effects of approved antidementives. Psychiatr. Danub. 2009, 21, 108–113. [Google Scholar] [PubMed]
- Klein, D.C. Arylalkylamine N-acetyltransferase:“The Timezyme”. J. Biol. Chem. 2007, 282, 4233–4237. [Google Scholar] [CrossRef] [Green Version]
- Zhdanova, I.V.; Wang, S.Y.; Leclair, O.U.; Danilova, N.P. Melatonin promotes sleep-like state in zebrafish. Brain Res. 2001, 903, 263–268. [Google Scholar] [CrossRef]
- Gandhi, A.V.; Mosser, E.A.; Oikonomou, G.; Prober, D.A. Melatonin is required for the circadian regulation of sleep. Neuron 2015, 85, 1193–1199. [Google Scholar] [CrossRef] [Green Version]
- Ziv, L.; Muto, A.; Schoonheim, P.J.; Meijsing, S.H.; Strasser, D.; Ingraham, H.A.; Schaaf, M.J.; Yamamoto, K.R.; Baier, H. An affective disorder in zebrafish with mutation of the glucocorticoid receptor. Mol. Psychiatry 2013, 18, 681. [Google Scholar] [CrossRef]
- Leproult, R.; Copinschi, G.; Buxton, O.; Van Cauter, E. Sleep loss results in an elevation of cortisol levels the next evening. Sleep 1997, 20, 865–870. [Google Scholar]
- Agboton, C.; Mahdavian, S.; Singh, A.; Ghazvini, P.; Hill, A.; Sweet, R. Impact of nighttime donepezil administration on sleep in the older adult population: A retrospective study. Ment. Health Clin. 2014, 4, 257–259. [Google Scholar] [CrossRef]
- Avdesh, A.; Martin-Iverson, M.T.; Mondal, A.; Chen, M.; Askraba, S.; Morgan, N.; Lardelli, M.; Groth, D.M.; Verdile, G.; Martins, R.N. Evaluation of color preference in zebrafish for learning and memory. J. Alzheimer’s Dis. 2012, 28, 459–469. [Google Scholar] [CrossRef]
- Panula, P.; Sallinen, V.; Sundvik, M.; Kolehmainen, J.; Torkko, V.; Tiittula, A.; Moshnyakov, M.; Podlasz, P. Modulatory neurotransmitter systems and behavior: Towards zebrafish models of neurodegenerative diseases. Zebrafish 2006, 3, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Da Rocha, A.; Kist, L.; Almeida, E.; Silva, D.; Bonan, C.; Altenhofen, S.; Kaufmann Jr, C.; Bogo, M.; Barros, D.; Oliveira, S. Neurotoxicity in zebrafish exposed to carbon nanotubes: Effects on neurotransmitters levels and antioxidant system. Comp. Biochem. Physiol. Part C: Toxicol. Pharmacol. 2019, 218, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Rico, E.; Rosemberg, D.; Seibt, K.; Capiotti, K.; Da Silva, R.; Bonan, C. Zebrafish neurotransmitter systems as potential pharmacological and toxicological targets. Neurotoxicology Teratol. 2011, 33, 608–617. [Google Scholar] [CrossRef] [PubMed]
- Parsey, R.V. Serotonin receptor imaging: Clinically useful? J. Nucl. Med. 2010, 51, 1495–1498. [Google Scholar] [CrossRef] [Green Version]
- Brancaccio, P.; Lippi, G.; Maffulli, N. Biochemical markers of muscular damage. Clin. Chem. Lab. Med. 2010, 48, 757–767. [Google Scholar] [CrossRef] [PubMed]
- Dell’Osso, B.; Buoli, M.; Baldwin, D.S.; Altamura, A.C. Serotonin norepinephrine reuptake inhibitors (SNRIs) in anxiety disorders: A comprehensive review of their clinical efficacy. Hum. Psychopharmacol. Clin. Exp. 2010, 25, 17–29. [Google Scholar] [CrossRef]
- Comninos, A.N.; Dhillo, W.S. Emerging roles of kisspeptin in sexual and emotional brain processing. Neuroendocrinology 2018, 106, 195–202. [Google Scholar] [CrossRef]
- Lindeyer, C.M.; Langen, E.M.; Swaney, W.T.; Reader, S.M. Nonapeptide influences on social behaviour: Effects of vasotocin and isotocin on shoaling and interaction in zebrafish. Behaviour 2015, 152, 897–915. [Google Scholar] [CrossRef]
- Zimmermann, F.F.; Gaspary, K.V.; Siebel, A.M.; Bonan, C.D. Oxytocin reversed MK-801-induced social interaction and aggression deficits in zebrafish. Behav. Brain Res. 2016, 311, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Giacomini, A.; Bueno, B.; Marcon, L.; Scolari, N.; Genario, R.; Demin, K.; Kolesnikova, T.; Kalueff, A.; Abreu, M. An Acetylcholinesterase (Ache) Inhibitor Donepezil Increases Anxiety and Cortisol Levels in Adult Zebrafish. J. Psychopharmacol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Shearman, E.; Rossi, S.; Szasz, B.; Juranyi, Z.; Fallon, S.; Pomara, N.; Sershen, H.; Lajtha, A. Changes in cerebral neurotransmitters and metabolites induced by acute donepezil and memantine administrations: A microdialysis study. Brain Res. Bull. 2006, 69, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Courtney, C.; Farrell, D.; Gray, R.; Hills, R.; Lynch, L.; Sellwood, E.; Edwards, S.; Hardyman, W.; Raftery, J.; Crome, P. Long-term donepezil treatment in 565 patients with Alzheimer’s disease (AD2000): Randomised double-blind trial. Lancet (London, England) 2004, 363, 2105–2115. [Google Scholar]



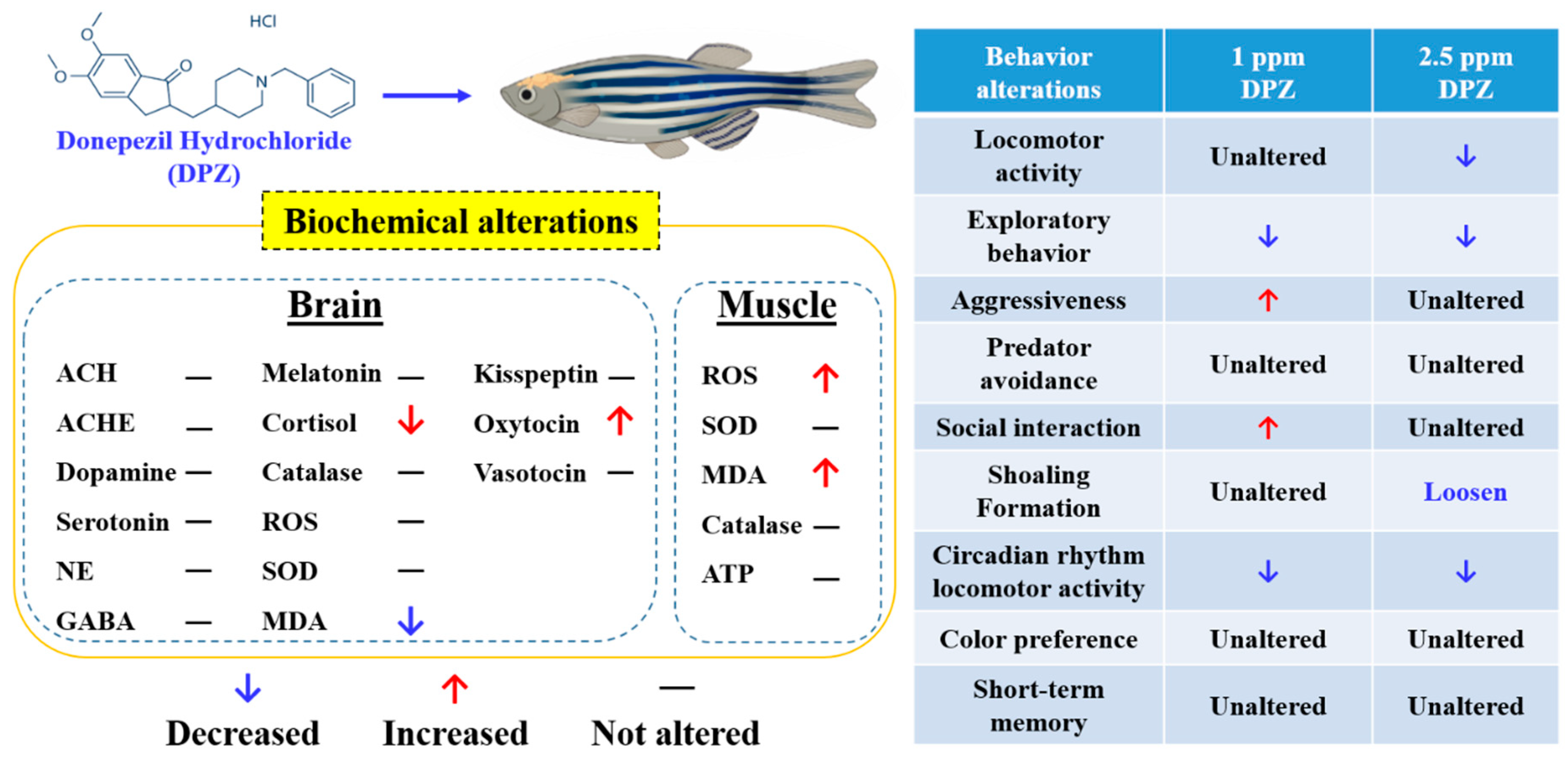
| Biomarker | Control | 1 ppm | 2.5 ppm | Unit | Significance | p-Value |
|---|---|---|---|---|---|---|
| Brain | ||||||
| Reactive Oxygen Species (ROS) | 1.478 ± 0.220 | 1.696 ± 0.133 | 1.437 ± 0.108 | U/μg of total protein | NO | 0.812 |
| Superoxide Dismutase (SOD) | 0.324 ± 0.023 | 0.315 ± 0.028 | 0.294 ± 0.013 | U/μg of total protein | NO | 0.6533 |
| Malondialdehyde (MDA) | 0.010 ± 0.0003 | 0.009 ± 0.0004 | 0.008 ± 0.0002 (**) | nmol/μg of total protein | YES | 0.0055 |
| Cortisol | 2.900 ± 0.190 | 2.485 ± 0.081 | 2.021 ± 0.168 (*) | pg/μg of total protein | YES | 0.0193 |
| Acetylcholine (ACh) | 0.0315 ± 0.003 | 0.0398 ± 0.002 | 0.0372 ± 0.002 | ng/μg of total protein | NO | 0.0936 |
| Acetylcholine esterase (AChE) | 0.173 ± 0.021 | 0.183 ± 0.014 | 0.183 ± 0.005 | nmol/μg of total protein | NO | 0.5106 |
| Serotonin (5-HT) | 0.363 ± 0.012 | 0.372 ± 0.012 | 0.334 ± 0.008 | ng/μg of total protein | NO | 0.1437 |
| Norepinephrine | 0.011 ± 0.0006 | 0.010 ± 0.0004 | 0.011 ± 0.0006 | ng/μg of total protein | NO | 0.4131 |
| Dopamine | 0.175 ± 0.001 | 0.210 ± 0.011 | 0.199 ± 0.008 | U/μg of total protein | NO | 0.0716 |
| γ-aminobutyric acid (GABA) | 1.05 × 10−5 ± 1.7 × 10−7 | 1.09 × 10−5 ± 6.6 × 10−7 | 9.90 × 10−6 ± 4.9 × 10−7 | μmol/μg of total protein | NO | 0.4104 |
| Melatonin | 0.129 ± 0.005 | 0.133 ± 0.003 | 0.130 ± 0.009 | pg/μg of total protein | NO | 0.8793 |
| Kisspeptin | 0.798 ± 0.043 | 0.736 ± 0.010 | 0.746 ± 0.034 | ng/μg of total protein | NO | 0.4026 |
| Oxytocin | 0.045 ± 0.001 | 0.060 ± 0.002 (*) | 0.048 ± 0.004 | pg/μg of total protein | YES | 0.0184 |
| Vasotocin | 2.603 ± 0.118 | 2.234 ± 0.146 | 2.216 ± 0.056 | pg/μg of total protein | NO | 0.0891 |
| Muscle | ||||||
| ROS | 1.368 ± 0.141 | 1.479 ± 0.096 | 1.893 ± 0.120 (*) | U/μg of total protein | YES | 0.0478 |
| SOD | 0.241 ± 0.022 | 0.237 ± 0.013 | 0.288 ± 0.012 | U/μg of total protein | NO | 0.1191 |
| MDA | 0.005 ± 0.0003 | 0.006 ± 0.0002 | 0.007 ± 0.0003 (**) | nmol/μg of total protein | YES | 0.0086 |
| Catalase | 0.062 ± 0.003 | 0.061 ± 0.002 | 0.070 ± 0.004 | U/μg of total protein | NO | 0.1821 |
| ATP | 6.104 ± 0.476 | 6.045 ± 0.575 | 7.239 ± 0.224 | nmol/μg of total protein | NO | 0.1882 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Audira, G.; Ngoc Anh, N.T.; Ngoc Hieu, B.T.; Malhotra, N.; Siregar, P.; Villalobos, O.; Villaflores, O.B.; Ger, T.-R.; Huang, J.-C.; Chen, K.H.-C.; et al. Evaluation of the Adverse Effects of Chronic Exposure to Donepezil (An Acetylcholinesterase Inhibitor) in Adult Zebrafish by Behavioral and Biochemical Assessments. Biomolecules 2020, 10, 1340. https://doi.org/10.3390/biom10091340
Audira G, Ngoc Anh NT, Ngoc Hieu BT, Malhotra N, Siregar P, Villalobos O, Villaflores OB, Ger T-R, Huang J-C, Chen KH-C, et al. Evaluation of the Adverse Effects of Chronic Exposure to Donepezil (An Acetylcholinesterase Inhibitor) in Adult Zebrafish by Behavioral and Biochemical Assessments. Biomolecules. 2020; 10(9):1340. https://doi.org/10.3390/biom10091340
Chicago/Turabian StyleAudira, Gilbert, Nguyen Thi Ngoc Anh, Bui Thi Ngoc Hieu, Nemi Malhotra, Petrus Siregar, Omar Villalobos, Oliver B. Villaflores, Tzong-Rong Ger, Jong-Chin Huang, Kelvin H.-C. Chen, and et al. 2020. "Evaluation of the Adverse Effects of Chronic Exposure to Donepezil (An Acetylcholinesterase Inhibitor) in Adult Zebrafish by Behavioral and Biochemical Assessments" Biomolecules 10, no. 9: 1340. https://doi.org/10.3390/biom10091340
APA StyleAudira, G., Ngoc Anh, N. T., Ngoc Hieu, B. T., Malhotra, N., Siregar, P., Villalobos, O., Villaflores, O. B., Ger, T.-R., Huang, J.-C., Chen, K. H.-C., & Hsiao, C.-D. (2020). Evaluation of the Adverse Effects of Chronic Exposure to Donepezil (An Acetylcholinesterase Inhibitor) in Adult Zebrafish by Behavioral and Biochemical Assessments. Biomolecules, 10(9), 1340. https://doi.org/10.3390/biom10091340









