Pompe Disease: New Developments in an Old Lysosomal Storage Disorder
Abstract
1. Introduction
2. An Expanded Set of Clinical Characteristics
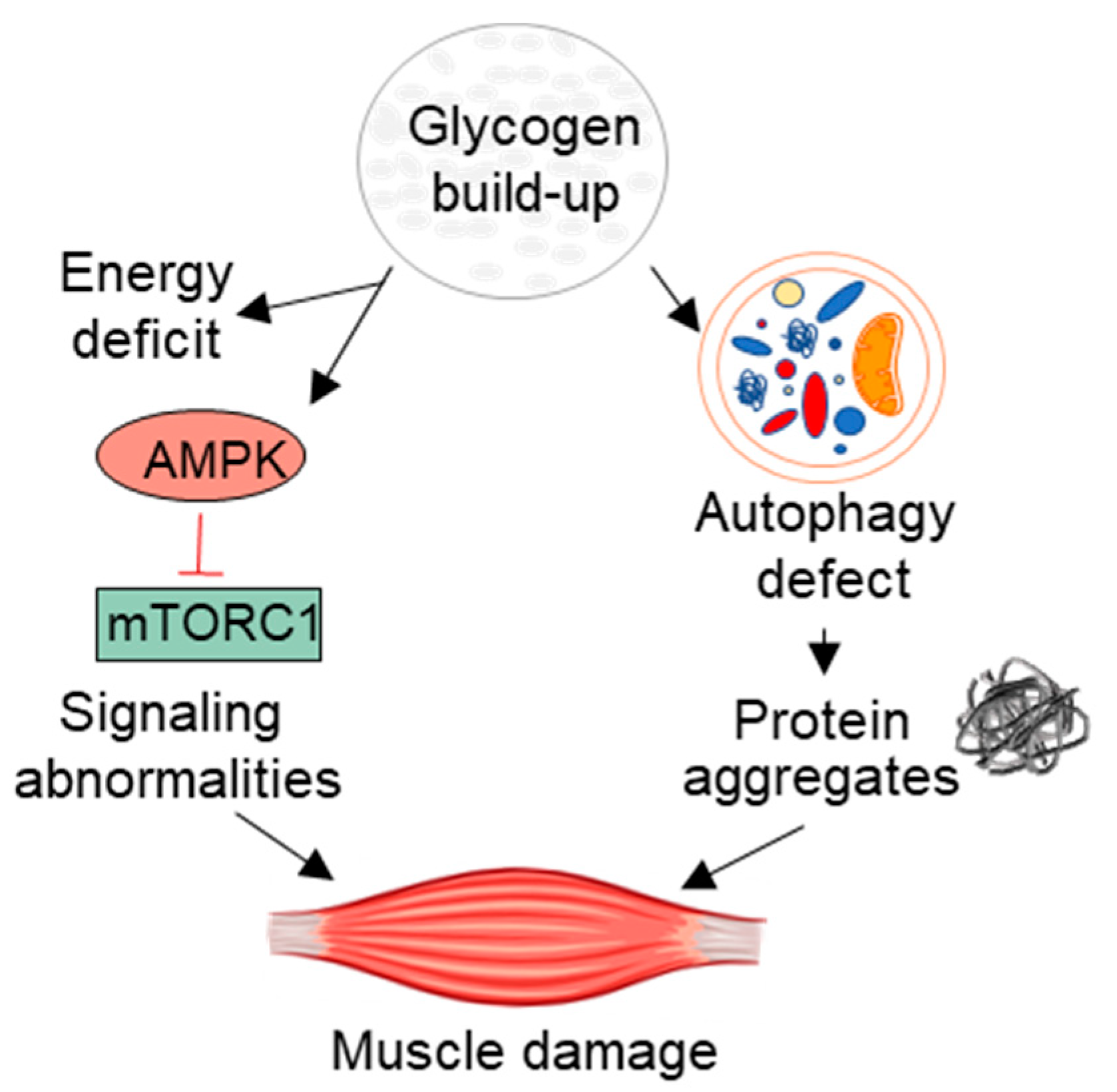
3. Beyond the Lysosome: Pathogenic Cascade and Muscle Regeneration
4. Evolution of Therapy
4.1. Next-Generation ERT
4.2. Gene Therapy
Author Contributions
Funding
Conflicts of Interest
References
- Martina, J.A.; Raben, N.; Puertollano, R. SnapShot: Lysosomal Storage Diseases. Cell 2020, 180, 602. [Google Scholar] [CrossRef]
- Pompe, J.C. Over idiopatische hypertrophie van het hart. Ned. Tijdschr. Geneeskd. 1932, 76, 304. [Google Scholar]
- Hers, H.G. Alpha-glucosidase deficiency in generalize glycogen storage disease (Pompe’s disease). Biochem. J. 1963, 86, 11. [Google Scholar] [CrossRef] [PubMed]
- Martiniuk, F.; Chen, A.; Mack, A.; Arvanitopoulos, E.; Chen, Y.; Rom, W.N.; Codd, W.J.; Hanna, B.; Alcabes, P.; Raben, N.; et al. Carrier frequency for glycogen storage disease type II in New York and estimates of affected individuals born with the disease. Am. J. Med Genet. 1998, 79, 69–72. [Google Scholar] [CrossRef]
- Bodamer, O.; Scott, C.R.; Giugliani, R.; Pompe Disease Newborn Screening Working Group; on behalf of the Pompe Disease Newborn Screening Working Group. Newborn Screening for Pompe Disease. Pediatrics 2017, 140, S4–S13. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Feuchtbaum, L.; Sciortino, S.; Matteson, J.; Mathur, D.; Bishop, T.; Olney, R.S. The First Year Experience of Newborn Screening for Pompe Disease in California. Int. J. Neonatal Screen 2020, 6, 9. [Google Scholar] [CrossRef]
- Chien, Y.H.; Chiang, S.-C.; Zhang, X.K.; Keutzer, J.; Lee, N.-C.; Huang, A.-C.; Chen, C.-A.; Wu, M.-H.; Huang, P.-H.; Tsai, F.-J.; et al. Early Detection of Pompe Disease by Newborn Screening Is Feasible: Results From the Taiwan Screening Program. Pediatrics 2008, 122, e39–e45. [Google Scholar] [CrossRef]
- Wisselaar, H.A.; Kroos, M.A.; Hermans, M.M.; Van Beeumen, J.; Reuser, A.J. Structural and functional changes of lysosomal acid alpha-glucosidase during intracellular transport and maturation. J. Boil. Chem. 1993, 268, 2223–2231. [Google Scholar]
- Moreland, R.J.; Jin, X.; Zhang, X.K.; Decker, R.W.; Albee, K.L.; Lee, K.L.; Cauthron, R.D.; Brewer, K.; Edmunds, T.; Canfield, W.M. Lysosomal Acid α-Glucosidase Consists of Four Different Peptides Processed from a Single Chain Precursor. J. Boil. Chem. 2004, 280, 6780–6791. [Google Scholar] [CrossRef]
- Dahms, N.M.; Lobel, P.; Kornfeld, S. Mannose 6-phosphate receptors and lysosomal enzyme targeting. J. Boil. Chem. 1989, 264. [Google Scholar]
- Ghosh, P.; Dahms, N.M.; Kornfeld, S. Mannose 6-phosphate receptors: New twists in the tale. Nat. Rev. Mol. Cell Boil. 2003, 4, 202–213. [Google Scholar] [CrossRef]
- Roig-Zamboni, V.; Cobucci-Ponzano, B.; Iacono, R.; Ferrara, M.C.; Germany, S.; Bourne, Y.; Parenti, G.; Moracci, M.; Sulzenbacher, G. Structure of human lysosomal acid α-glucosidase–a guide for the treatment of Pompe disease. Nat. Commun. 2017, 8, 1111. [Google Scholar] [CrossRef]
- Hirschhorn, R.; Reuser, A.J. Glycogen Storage Disease Type II: Acid Alphaglucosidase (Acid Maltase) Deficiency. The Metabolic and Molecular Basis of Inherited Disease; McGraw-Hill: New York, NY, USA, 2001. [Google Scholar]
- Güngör, D.; Reuser, A.J. How to describe the clinical spectrum in Pompe disease? Am. J. Med Genet. Part A 2013, 161, 399–400. [Google Scholar] [CrossRef] [PubMed]
- Peruzzo, P.; Pavan, E.; Dardis, A. Molecular genetics of Pompe disease: A comprehensive overview. Ann. Transl. Med. 2019, 7, 278. [Google Scholar] [CrossRef] [PubMed]
- Huie, M.L.; Chen, A.S.; Tsujino, S.; Shanske, S.; DiMauro, S.; Engel, A.G.; Hirschhorn, R. Aberrant splicing in adult onset glycogen storage disease type II (GSDII): Molecular identification of an IVS1 (-13T-->G) mutation in a majority of patients and a novel IVS10 (+1GT-->CT) mutation. Hum. Mol. Genet. 1994, 3, 1. [Google Scholar]
- Boerkoel, C.F.; Exelbert, R.; Nicastri, C.; Nichols, R.C.; Miller, F.W.; Plotz, P.H.; Raben, N. Leaky splicing mutation in the acid maltase gene is associated with delayed onset of glycogenosis type II. Am. J. Hum. Genet. 1995, 56, 887–897. [Google Scholar] [PubMed]
- Raben, N.; Nichols, R.C.; Martiniuk, F.; Plotz, P.H. A model of mRNA splicing in adult lysosomal storage disease (glycogenosis type II). Hum. Mol. Genet. 1996, 5, 995–1000. [Google Scholar] [CrossRef] [PubMed]
- Dardis, A.; Zanin, I.; Zampieri, S.; Stuani, C.; Pianta, A.; Romanello, M.; Baralle, F.E.; Bembi, B.; Buratti, E. Functional characterization of the common c.-32-13T>G mutation of GAA gene: Identification of potential therapeutic agents. Nucleic Acids Res. 2013, 42, 1291–1302. [Google Scholar] [CrossRef] [PubMed]
- Bergsma, A.J.; In’t Groen, S.L.; van den Dorpel, J.J.; van den Hout, H.J.; van der Beek, N.A.; Schoser, B.; Toscano, A.; Musumeci, O.; Bembi, B.; Dardis, A.; et al. A genetic modifier of symptom onset in Pompe disease. EBioMedicine 2019, 43, 553–561. [Google Scholar] [CrossRef]
- In’t Groen, S.L.; de Faria, D.O.; Iuliano, A.; van den Hout, J.M.; Douben, H.; Dijkhuizen, T.; Cassiman, D.; Witters, P.; Romero, M.Á.B.; de Klein, A.; et al. Novel GAA Variants and Mosaicism in Pompe Disease Identified by Extended Analyses of Patients with an Incomplete DNA Diagnosis. Mol. Ther. Methods Clin. Dev. 2020, 17, 337–348. [Google Scholar] [CrossRef]
- Raben, N.; Danon, M.; Gilbert, A.; Dwivedi, S.; Collins, B.; Thurberg, B.; Mattaliano, R.; Nagaraju, K.; Plotz, P. Enzyme replacement therapy in the mouse model of Pompe disease. Mol. Genet. Metab. 2003, 80, 159–169. [Google Scholar] [CrossRef] [PubMed]
- McVie-Wylie, A.; Lee, K.; Qiu, H.; Jin, X.; Do, H.; Gotschall, R.; Thurberg, B.; Rogers, C.; Raben, N.; O’Callaghan, M.; et al. Biochemical and pharmacological characterization of different recombinant acid α-glucosidase preparations evaluated for the treatment of Pompe disease. Mol. Genet. Metab. 2008, 94, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Kishnani, P.S.; Corzo, D.; Nicolino, M.; Byrne, B.; Mandel, H.; Hwu, W.L.; Leslie, N.; Levine, J.; Spenser, C.; McDonald, M.; et al. Recombinant human acid [alpha]-glucosidase: Major clinical benefits in infantile-onset Pompe disease. Neurology 2007, 68, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Nicolino, M.; Byrne, B.; Wraith, J.E.; Leslie, N.; Mandel, H.; Freyer, D.R.; Arnold, G.L.; Pivnick, E.K.; Ottinger, C.J.; Robinson, P.H.; et al. Clinical outcomes after long-term treatment with alglucosidase alfa in infants and children with advanced Pompe disease. Genet. Med. 2009, 11, 210–219. [Google Scholar] [CrossRef]
- Slonim, A.E.; Bulone, L.; Ritz, S.; Goldberg, T.; Chen, A.; Martiniuk, F. Identification of two subtypes of infantile acid maltase deficiency. J. Pediatr. 2000, 137, 283–285. [Google Scholar] [CrossRef]
- Kishnani, P.S.; Hwu, W.-L.; Mandel, H.; Nicolino, M.; Yong, F.; Corzo, D. A retrospective, multinational, multicenter study on the natural history of infantile-onset Pompe disease. J. Pediatr. 2006, 148, 671–676. [Google Scholar] [CrossRef]
- Prater, S.N.; Banugaria, S.G.; DeArmey, S.M.; Botha, E.G.; Stege, E.M.; E Case, L.; Jones, H.N.; Phornphutkul, C.; Wang, R.Y.; Young, S.P.; et al. The emerging phenotype of long-term survivors with infantile Pompe disease. Genet. Med. 2012, 14, 800–810. [Google Scholar] [CrossRef]
- DeRuisseau, L.R.; Fuller, D.D.; Qiu, K.; DeRuisseau, K.C.; Donnelly, W.H.; Mah, C.; Reier, P.J.; Byrne, B.J. Neural deficits contribute to respiratory insufficiency in Pompe disease. Proc. Natl. Acad. Sci. USA 2009, 106, 9419–9424. [Google Scholar] [CrossRef]
- Hahn, A.; Schänzer, A. Long-term outcome and unmet needs in infantile-onset Pompe disease. Ann. Transl. Med. 2019, 7, 283. [Google Scholar] [CrossRef]
- Pena, L.D.M.; Proia, A.D.; Kishnani, P.S.; Zschocke, J. Postmortem Findings and Clinical Correlates in Individuals with Infantile-Onset Pompe Disease. JIMD Rep. 2015, 23, 45–54. [Google Scholar] [CrossRef]
- McIntosh, P.T.; Hobson-Webb, L.D.; Kazi, Z.B.; Prater, S.N.; Banugaria, S.G.; Austin, S.; Wang, R.; Enterline, D.S.; Frush, D.P.; Kishnani, P.S. Neuroimaging findings in infantile Pompe patients treated with enzyme replacement therapy. Mol. Genet. Metab. 2018, 123, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Ebbink, B.J.; Poelman, E.; Aarsen, F.K.; Hoogenboom-Plug, I.; Régal, L.; Muentjes, C.; Beek, N.A.M.E.V.D.; Lequin, M.H.; Van Der Ploeg, A.T.; Hout, J.M. Classic infantile Pompe patients approaching adulthood: A cohort study on consequences for the brain. Dev. Med. Child Neurol. 2018, 60, 579–586. [Google Scholar] [CrossRef]
- Kishnani, P.S.; Goldenberg, P.C.; DeArmey, S.L.; Heller, J.; Benjamin, D.; Young, S.; Bali, D.; Smith, S.A.; Li, J.S.; Mandel, H.; et al. Cross-reactive immunologic material status affects treatment outcomes in Pompe disease infants. Mol. Genet. Metab. 2010, 99, 26–33. [Google Scholar] [CrossRef]
- Chien, Y.H.; Hwu, W.-L.; Lee, N.-C. Pompe Disease: Early Diagnosis and Early Treatment Make a Difference. Pediatr. Neonatol. 2013, 54, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Niu, D.; Tai, S.; Wang, T.; Su, H.; Huang, L.; Soong, W. Airway abnormalities in very early treated infantile-onset Pompe disease: A large-scale survey by flexible bronchoscopy. Am. J. Med Genet. Part A 2020, 182, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Güngör, D.; de Vries, J.M.; Brusse, E.; Kruijshaar, M.E.; Hop, W.C.; Murawska, M.; van den Berg, L.E.; Reuser, A.J.; van Doorn, P.A.; Hagemans, M.L.; et al. Enzyme replacement therapy and fatigue in adults with Pompe disease. Mol. Genet. Metab. 2013, 109, 174–178. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Güngör, D.; Schober, A.; Kruijshaar, M.; Plug, I.; Karabul, N.; Deschauer, M.; Van Doorn, P.; Van Der Ploeg, A.; Schoser, B.; Hanisch, F. Pain in adult patients with Pompe disease. Mol. Genet. Metab. 2013, 109, 371–376. [Google Scholar] [CrossRef]
- Keeler, A.M.; Liu, D.; Zieger, M.; Xiong, L.; Salemi, J.; Bellvé, K.; Byrne, B.J.; Fuller, D.D.; ZhuGe, R.; ElMallah, M.K. Airway smooth muscle dysfunction in Pompe (GAA-/-) mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2017, 312, L873–L881. [Google Scholar] [CrossRef]
- Chan, J.; Desai, A.K.; Kazi, Z.B.; Corey, K.; Austin, S.; Webb, L.D.H.; E Case, L.; Jones, H.N.; Kishnani, P.S. The emerging phenotype of late-onset Pompe disease: A systematic literature review. Mol. Genet. Metab. 2017, 120, 163–172. [Google Scholar] [CrossRef]
- Toscano, A.; Rodolico, C.; Musumeci, O. Multisystem late onset Pompe disease (LOPD): An update on clinical aspects. Ann. Transl. Med. 2019, 7, 284. [Google Scholar] [CrossRef]
- Alandy-Dy, J.; Wencel, M.; Hall, K.; Simon, J.; Chen, Y.; Valenti, E.; Yang, J.; Bali, D.; Lakatos, A.; Goyal, N.; et al. Variable clinical features and genotype-phenotype correlations in 18 patients with late-onset Pompe disease. Ann. Transl. Med. 2019, 7, 276. [Google Scholar] [CrossRef] [PubMed]
- Anderson, L.J.; Henley, W.; Wyatt, K.M.; Nikolaou, V.; Waldek, S.; Hughes, D.; Lachmann, R.H.; Logan, S. Effectiveness of enzyme replacement therapy in adults with late-onset Pompe disease: Results from the NCS-LSD cohort study. J. Inherit. Metab. Dis. 2014, 37, 945–952. [Google Scholar] [CrossRef]
- Schoser, B.; Stewart, A.; Kanters, S.; Hamed, A.; Jansen, J.; Chan, K.; Karamouzian, M.; Toscano, A. Survival and long-term outcomes in late-onset Pompe disease following alglucosidase alfa treatment: A systematic review and meta-analysis. J. Neurol. 2016, 264, 621–630. [Google Scholar] [CrossRef]
- McCall, A.L.; Salemi, J.; Bhanap, P.; Strickland, L.M.; Elmallah, M.K. The impact of Pompe disease on smooth muscle: A review. J. Smooth Muscle Res. 2018, 54, 100–118. [Google Scholar] [CrossRef] [PubMed]
- Stepien, K.M.; Hendriksz, C.J.; Roberts, M.; Sharma, R. Observational clinical study of 22 adult-onset Pompe disease patients undergoing enzyme replacement therapy over 5years. Mol. Genet. Metab. 2016, 117, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Harlaar, L.; Hogrel, J.-Y.; Perniconi, B.; Kruijshaar, M.E.; Rizopoulos, D.; Taouagh, N.; Canal, A.; Brusse, E.; Van Doorn, P.A.; Van Der Ploeg, A.T.; et al. Large variation in effects during 10 years of enzyme therapy in adults with Pompe disease. Neurology 2019, 93, e1756–e1767. [Google Scholar] [CrossRef]
- Kulessa, M.; Weyer-Menkhoff, I.; Viergutz, L.; Kornblum, C.; Claeys, K.G.; Schneider, I.; Plöckinger, U.; Young, P.; Boentert, M.; Vielhaber, S.; et al. An integrative correlation of myopathology, phenotype and genotype in late onset Pompe disease. Neuropathol. Appl. Neurobiol. 2019, 46, 359–374. [Google Scholar] [CrossRef]
- Van Der Ploeg, A.T.; Kruijshaar, M.E.; Toscano, A.; Laforet, P.; Angelini, C.; Lachmann, R.H.; Pascual, S.I.P.; Roberts, M.; Rösler, K.; Stulnig, T.M.; et al. European consensus for starting and stopping enzyme replacement therapy in adult patients with Pompe disease: A 10-year experience. Eur. J. Neurol. 2017, 24, 768-e31. [Google Scholar] [CrossRef]
- Van Kooten, H.; Harlaar, L.; Van Der Beek, N.; Van Doorn, P.; Van Der Ploeg, A.; Brusse, E.; Chair, W.V.D.P.; Ditters, I.; Hoogendijk-Boon, M.; Huidekoper, H.; et al. Discontinuation of enzyme replacement therapy in adults with Pompe disease: Evaluating the European POmpe Consortium stop criteria. Neuromuscul. Disord. 2020, 30, 59–66. [Google Scholar] [CrossRef]
- Rairikar, M.V.; Case, L.E.; Bailey, L.A.; Kazi, Z.B.; Desai, A.K.; Berrier, K.L.; Coats, J.; Gandy, R.; Quinones, R.; Kishnani, P.S. Insight into the phenotype of infants with Pompe disease identified by newborn screening with the common c.-32-13T>G “late-onset” GAA variant. Mol. Genet. Metab. 2017, 122, 99–107. [Google Scholar] [CrossRef]
- Tarallo, A.; Carissimo, A.; Gatto, F.; Nusco, E.; Toscano, A.; Musumeci, O.; Coletta, M.; Karali, M.; Acampora, E.; Damiano, C.; et al. microRNAs as biomarkers in Pompe disease. Genet. Med. 2018, 21, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Carrasco-Rozas, A.; Fernández-Simón, E.; Lleixà, M.C.; Belmonte, I.; Pedrosa-Hernandez, I.; Montiel-Morillo, E.; Nuñez-Peralta, C.; Llauger Rossello, J.; Segovia, S.; De Luna, N.; et al. Identification of serum microRNAs as potential biomarkers in Pompe disease. Ann. Clin. Transl. Neurol. 2019, 6, 1214–1224. [Google Scholar] [CrossRef]
- Taverna, S.; Cammarata, G.; Colomba, P.; Sciarrino, S.; Zizzo, C.; Francofonte, D.; Zora, M.; Scalia, S.; Brando, C.; Curto, A.L.; et al. Pompe disease: Pathogenesis, molecular genetics and diagnosis. Aging 2020, 12. [Google Scholar] [CrossRef] [PubMed]
- Thurberg, B.; Maloney, C.L.; Vaccaro, C.; Afonso, K.; Tsai, A.C.-H.; Bossen, E.; Kishnani, P.S.; O’Callaghan, M. Characterization of pre- and post-treatment pathology after enzyme replacement therapy for pompe disease. Lab. Investig. 2006, 86, 1208–1220. [Google Scholar] [CrossRef]
- Raben, N.; Nagaraju, K.; Lee, E.; Kessler, P.; Byrne, B.; Lee, L.; Lamarca, M.; King, C.; Ward, J.; Sauer, B.; et al. Targeted Disruption of the Acid α-Glucosidase Gene in Mice Causes an Illness with Critical Features of Both Infantile and Adult Human Glycogen Storage Disease Type II. J. Boil. Chem. 1998, 273, 19086–19092. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N.; Levine, B.; Cuervo, A.M.; Klionsky, D.J. Autophagy fights disease through cellular self-digestion. Nature 2008, 451, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N.; Yamamoto, A.; Matsui, M.; Yoshimori, T.; Ohsumi, Y. In Vivo Analysis of Autophagy in Response to Nutrient Starvation Using Transgenic Mice Expressing a Fluorescent Autophagosome Marker. Mol. Boil. Cell 2004, 15, 1101–1111. [Google Scholar] [CrossRef] [PubMed]
- Onodera, J.; Ohsumi, Y. Autophagy Is Required for Maintenance of Amino Acid Levels and Protein Synthesis under Nitrogen Starvation. J. Boil. Chem. 2005, 280, 31582–31586. [Google Scholar] [CrossRef]
- Gundersen, K. Muscle memory and a new cellular model for muscle atrophy and hypertrophy. J. Exp. Boil. 2016, 219, 235–242. [Google Scholar] [CrossRef]
- Masiero, E.; Agatea, L.; Mammucari, C.; Blaauw, B.; Loro, E.; Komatsu, M.; Metzger, D.; Reggiani, C.; Schiaffino, S.; Sandri, M. Autophagy Is Required to Maintain Muscle Mass. Cell Metab. 2009, 10, 507–515. [Google Scholar] [CrossRef]
- Masiero, E.; Sandri, M. Autophagy inhibition induces atrophy and myopathy in adult skeletal muscles. Autophagy 2010, 6, 307–309. [Google Scholar] [CrossRef]
- Nishino, I. Autophagic Vacuolar Myopathy. Semin. Pediatr. Neurol. 2006, 13, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Galperin, M.; Melvin, G.; Horowits, R.; Raben, N.; Plotz, P.; Yu, L. Impaired organization and function of myofilaments in single muscle fibers from a mouse model of Pompe disease. J. Appl. Physiol. 2010, 108, 1383–1388. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nascimbeni, A.C.; Fanin, M.; Angelini, C.; Sandri, M. Autophagy dysregulation in Danon disease. Cell Death Dis. 2017, 8, e2565. [Google Scholar] [CrossRef] [PubMed]
- Meena, N.K.; Ralston, E.; Raben, N.; Puertollano, R. Enzyme Replacement Therapy Can Reverse Pathogenic Cascade in Pompe Disease. Mol. Ther. Methods Clin. Dev. 2020, 18, 199–214. [Google Scholar] [CrossRef]
- Spampanato, C.; Feeney, E.; Li, L.; Cardone, M.; Lim, J.-A.; Annunziata, F.; Zare, H.; Polishchuk, R.; Puertollano, R.; Parenti, G.; et al. Transcription factor EB (TFEB) is a new therapeutic target for Pompe disease. EMBO Mol. Med. 2013, 5, 691–706. [Google Scholar] [CrossRef]
- Fukuda, T.; Ewan, L.; Bauer, M.; Mattaliano, R.J.; Zaal, K.; Ralston, E.; Plotz, P.H.; Raben, N. Dysfunction of endocytic and autophagic pathways in a lysosomal storage disease. Ann. Neurol. 2006, 59, 700–708. [Google Scholar] [CrossRef]
- Nascimbeni, A.C.; Fanin, M.; Tasca, E.; Angelini, C.; Sandri, M. Impaired autophagy affects acid α-glucosidase processing and enzyme replacement therapy efficacy in late-onset glycogen storage disease type II. Neuropathol. Appl. Neurobiol. 2015, 41, 672–675. [Google Scholar] [CrossRef]
- Shea, L.; Raben, N. Autophagy in skeletal muscle: Implications for Pompe disease. Int. J. Clin. Pharmacol. Ther. 2009, 47, S42–S47. [Google Scholar] [CrossRef]
- Fukuda, T.; Ahearn, M.; Roberts, A.; Mattaliano, R.J.; Zaal, K.; Ralston, E.; Plotz, P.H.; Raben, N. Autophagy and Mistargeting of Therapeutic Enzyme in Skeletal Muscle in Pompe Disease. Mol. Ther. 2006, 14, 831–839. [Google Scholar] [CrossRef]
- Inoki, K.; Kim, J.; Guan, K.-L. AMPK and mTOR in Cellular Energy Homeostasis and Drug Targets. Annu. Rev. Pharmacol. Toxicol. 2012, 52, 381–400. [Google Scholar] [CrossRef] [PubMed]
- Sancak, Y.; Bar-Peled, L.; Zoncu, R.; Markhard, A.L.; Nada, S.; Sabatini, D.M. Ragulator-Rag Complex Targets mTORC1 to the Lysosomal Surface and Is Necessary for Its Activation by Amino Acids. Cell 2010, 141, 290–303. [Google Scholar] [CrossRef] [PubMed]
- Zoncu, R.; Bar-Peled, L.; Efeyan, A.; Wang, S.; Sancak, Y.; Sabatini, D.M. mTORC1 Senses Lysosomal Amino Acids Through an Inside-Out Mechanism That Requires the Vacuolar H+-ATPase. Science 2011, 334, 678–683. [Google Scholar] [CrossRef]
- Bar-Peled, L.; Sabatini, D.M. Regulation of mTORC1 by amino acids. Trends Cell Boil. 2014, 24, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Inoki, K.; Li, Y.; Xu, T.; Guan, K.-L. Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Dev. 2003, 17, 1829–1834. [Google Scholar] [CrossRef]
- Demetriades, C.; Plescher, M.; Teleman, A.A. Lysosomal recruitment of TSC2 is a universal response to cellular stress. Nat. Commun. 2016, 7, 10662. [Google Scholar] [CrossRef]
- Menon, S.; Dibble, C.C.; Talbott, G.; Hoxhaj, G.; Valvezan, A.J.; Takahashi, H.; Cantley, L.C.; Manning, B.D. Spatial control of the TSC complex integrates insulin and nutrient regulation of mTORC1 at the lysosome. Cell 2014, 156, 771–785. [Google Scholar] [CrossRef]
- Sakamoto, K.; Zarrinpashneh, E.; Budas, G.R.; Pouleur, A.-C.; Dutta, A.; Prescott, A.R.; Vanoverschelde, J.-L.J.; Ashworth, A.; Jovanović, A.; Alessi, D.R.; et al. Deficiency of LKB1 in heart prevents ischemia-mediated activation of AMPKalpha2 but not AMPKalpha1. Am. J. Physiol. Metab. 2005, 290, E780–E788. [Google Scholar] [CrossRef]
- Kim, J.; Kundu, M.; Viollet, B.; Guan, K.-L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 2011, 13, 132–141. [Google Scholar] [CrossRef]
- Egan, D.F.; Kim, J.; Shaw, R.J.; Guan, K.-L. The autophagy initiating kinase ULK1 is regulated via opposing phosphorylation by AMPK and mTOR. Autophagy 2011, 7, 645–646. [Google Scholar] [CrossRef]
- Bentzinger, C.F.; Lin, S.; Romanino, K.; Castets, P.; Guridi, M.; Summermatter, S.; Handschin, C.; Tintignac, L.; Hall, M.N.; Rüegg, M.A. Differential response of skeletal muscles to mTORC1 signaling during atrophy and hypertrophy. Skelet. Muscle 2013, 3, 6. [Google Scholar] [CrossRef] [PubMed]
- Grumati, P.; Coletto, L.; Sabatelli, P.; Cescon, M.; Angelin, A.; Bertaggia, E.; Blaauw, B.; Urciuolo, A.; Tiepolo, T.; Merlini, L.; et al. Autophagy is defective in collagen VI muscular dystrophies, and its reactivation rescues myofiber degeneration. Nat. Med. 2010, 16, 1313–1320. [Google Scholar] [CrossRef]
- Sandri, M.; Coletto, L.; Grumati, P.; Bonaldo, P. Misregulation of autophagy and protein degradation systems in myopathies and muscular dystrophies. J. Cell Sci. 2013, 126, 5325–5333. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Li, L.; Shirihai, O.S.; Trudeau, K.M.; Puertollano, R.; Raben, N. Modulation of mTOR signaling as a strategy for the treatment of Pompe disease. EMBO Mol. Med. 2017, 9, 353–370. [Google Scholar] [CrossRef]
- Lim, J.-A.; Sun, B.; Puertollano, R.; Raben, N. Therapeutic Benefit of Autophagy Modulation in Pompe Disease. Mol. Ther. 2018, 26, 1783–1796. [Google Scholar] [CrossRef]
- Shemesh, A.; Wang, Y.; Yang, Y.; Yang, G.S.; Johnson, D.E.; Backer, J.M.; Pessin, J.E.; Zong, H. Suppression of mTOR1 Activation in Acid- alpha -Glucosidase Deficient Cells and Mice is Ameliorated by Leucine Supplementation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014. [Google Scholar] [CrossRef]
- Yoshida, T.; Awaya, T.; Jonouchi, T.; Kimura, R.; Kimura, S.; Era, T.; Heike, T.; Sakurai, H. A Skeletal Muscle Model of Infantile-onset Pompe Disease with Patient-specific iPS Cells. Sci. Rep. 2017, 7, 13473. [Google Scholar] [CrossRef] [PubMed]
- Yoon, M.-S. mTOR as a Key Regulator in Maintaining Skeletal Muscle Mass. Front. Physiol. 2017, 8. [Google Scholar] [CrossRef]
- Ching, J.K.; Elizabeth, S.V.; Ju, J.-S.; Lusk, C.; Pittman, S.K.; Weihl, C.C. mTOR dysfunction contributes to vacuolar pathology and weakness in valosin-containing protein associated inclusion body myopathy. Hum. Mol. Genet. 2012, 22, 1167–1179. [Google Scholar] [CrossRef]
- Hintze, S.; Limmer, S.; Dabrowska-Schlepp, P.; Berg, B.; Krieghoff, N.; Busch, A.; Schaaf, A.; Meinke, P.; Schoser, B. Moss-Derived Human Recombinant GAA Provides an Optimized Enzyme Uptake in Differentiated Human Muscle Cells of Pompe Disease. Int. J. Mol. Sci. 2020, 21, 2642. [Google Scholar] [CrossRef]
- Xu, S.; Lun, Y.; Frascella, M.; Garcia, A.; Soska, R.; Nair, A.; Ponery, A.S.; Schilling, A.; Feng, J.; Tuske, S.; et al. Improved efficacy of a next-generation ERT in murine Pompe disease. JCI Insight 2019, 4, 125358. [Google Scholar] [CrossRef]
- Schoser, B. Pompe disease: What are we missing? Ann. Transl. Med. 2019, 7, 292. [Google Scholar] [CrossRef]
- Yin, H.; Price, F.; Rudnicki, M.A. Satellite Cells and the Muscle Stem Cell Niche. Physiol. Rev. 2013, 93, 23–67. [Google Scholar] [CrossRef]
- Lepper, C.; Partridge, T.A.; Fan, C.-M. An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration. Development 2011, 138, 3639–3646. [Google Scholar] [CrossRef] [PubMed]
- Rudnicki, M.A.; Le Grand, F.; McKinnell, I.; Kuang, S. The Molecular Regulation of Muscle Stem Cell Function. Cold Spring Harb. Symp. Quant. Boil. 2008, 73, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Schaaf, G.; Van Gestel, T.J.M.; Brusse, E.; Verdijk, R.M.; De Coo, I.F.M.; Van Doorn, P.A.; Van Der Ploeg, A.T.; Pijnappel, W.P. Lack of robust satellite cell activation and muscle regeneration during the progression of Pompe disease. Acta Neuropathol. Commun. 2015, 3, 65. [Google Scholar] [CrossRef] [PubMed]
- Schaaf, G.; Van Gestel, T.J.M.; In’t Groen, S.L.; De Jong, B.; Boomaars, B.; Tarallo, A.; Cardone, M.; Parenti, G.; Van Der Ploeg, A.T.; Pijnappel, W.P. Satellite cells maintain regenerative capacity but fail to repair disease-associated muscle damage in mice with Pompe disease. Acta Neuropathol. Commun. 2018, 6, 119. [Google Scholar] [CrossRef] [PubMed]
- Lagalice, L.; Pichon, J.; Gougeon, E.; Soussi, S.; Deniaud, J.; Ledevin, M.; Maurier, V.; Leroux, I.; Durand, S.; Ciron, C.; et al. Satellite cells fail to contribute to muscle repair but are functional in Pompe disease (glycogenosis type II). Acta Neuropathol. Commun. 2018, 6, 116. [Google Scholar] [CrossRef] [PubMed]
- Tang, A.H.; Rando, T.A. Induction of autophagy supports the bioenergetic demands of quiescent muscle stem cell activation. EMBO J. 2014, 33, 2782–2797. [Google Scholar] [CrossRef]
- Lim, J.-A.; Li, L.; Raben, N. Pompe disease: From pathophysiology to therapy and back again. Front. Aging Neurosci. 2014, 6. [Google Scholar] [CrossRef]
- Kohler, L.; Puertollano, R.; Raben, N. Pompe Disease: From Basic Science to Therapy. Neurotherapeutics 2018, 15, 928–942. [Google Scholar] [CrossRef]
- Parenti, G.; Andria, G.; Valenzano, K.J. Pharmacological Chaperone Therapy: Preclinical Development, Clinical Translation, and Prospects for the Treatment of Lysosomal Storage Disorders. Mol. Ther. 2015, 23, 1138–1148. [Google Scholar] [CrossRef] [PubMed]
- Do, H.V.; Khanna, R.; Gotschall, R. Challenges in treating Pompe disease: An industry perspective. Ann. Transl. Med. 2019, 7, 291. [Google Scholar] [CrossRef] [PubMed]
- Wenk, J.; Hille, A.; Von Figura, K. Quantitation of Mr 46000 and Mr 300000 mannose 6-phosphate receptors in human cells and tissues. Biochem. Int. 1991, 23. [Google Scholar]
- Funk, B.; Kessler, U.; Eisenmenger, W.; Hansmann, A.; Kolb, H.J.; Kiess, W. Expression of the insulin-like growth factor-II/mannose-6-phosphate receptor in multiple human tissues during fetal life and early infancy. J. Clin. Endocrinol. Metab. 1992, 75, 424–431. [Google Scholar]
- Zhu, Y.; Li, X.; McVie-Wylie, A.; Jiang, C.; Thurberg, B.L.; Raben, N.; Mattaliano, R.J.; Cheng, S.H. Carbohydrate-remodelled acid α-glucosidase with higher affinity for the cation-independent mannose 6-phosphate receptor demonstrates improved delivery to muscles of Pompe mice. Biochem. J. 2005, 389, 619–628. [Google Scholar] [CrossRef]
- Zhu, Y.; Jiang, J.-L.; Gumlaw, N.K.; Zhang, J.; Bercury, S.D.; Ziegler, R.J.; Lee, K.; Kudo, M.; Canfield, W.M.; Edmunds, T.; et al. Glycoengineered Acid α-Glucosidase with Improved Efficacy at Correcting the Metabolic Aberrations and Motor Function Deficits in a Mouse Model of Pompe Disease. Mol. Ther. 2009, 17, 954–963. [Google Scholar] [CrossRef]
- Pena, L.D.M.; Barohn, R.J.; Byrne, B.J.; Desnuelle, C.; Goker-Alpan, O.; Ladha, S.; Laforêt, P.; Mengel, K.E.; Pestronk, A.; Pouget, J.; et al. Safety, tolerability, pharmacokinetics, pharmacodynamics, and exploratory efficacy of the novel enzyme replacement therapy avalglucosidase alfa (neoGAA) in treatment-naïve and alglucosidase alfa-treated patients with late-onset Pompe disease: A phase 1, open-label, multicenter, multinational, ascending dose study. Neuromuscul. Disord. 2019, 29, 167–186. [Google Scholar] [CrossRef]
- Porto, C.; Cardone, M.; Fontana, F.; Rossi, B.; Tuzzi, M.R.; Tarallo, A.; Barone, M.V.; Andria, G.; Parenti, G. The Pharmacological Chaperone N-butyldeoxynojirimycin Enhances Enzyme Replacement Therapy in Pompe Disease Fibroblasts. Mol. Ther. 2009, 17, 964–971. [Google Scholar] [CrossRef]
- Khanna, R.; Flanagan, J.J.; Feng, J.; Soska, R.; Frascella, M.; Pellegrino, L.J.; Lun, Y.; Guillen, D.; Lockhart, D.J.; Valenzano, K.J. The Pharmacological Chaperone AT2220 Increases Recombinant Human Acid α-Glucosidase Uptake and Glycogen Reduction in a Mouse Model of Pompe Disease. PLoS ONE 2012, 7, e40776. [Google Scholar] [CrossRef]
- Parenti, G.; Fecarotta, S.; La Marca, G.; Rossi, B.; Ascione, S.; Donati, M.A.; Morandi, L.O.; Ravaglia, S.; Pichiecchio, A.; Ombrone, D.; et al. A Chaperone Enhances Blood α-Glucosidase Activity in Pompe Disease Patients Treated with Enzyme Replacement Therapy. Mol. Ther. 2014, 22, 2004–2012. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.E.; Tse, C.-M.; Chan, G.; Heinze, E.R.; Nishimura, R.N.; Weisbart, R.H. Intranuclear Protein Transduction through a Nucleoside Salvage Pathway. J. Boil. Chem. 2007, 282, 20790–20793. [Google Scholar] [CrossRef] [PubMed]
- Weisbart, R.H.; Gera, J.; Chan, G.; Hansen, J.E.; Li, E.; Cloninger, C.; Levine, A.J.; Nishimura, R.N. A Cell-Penetrating Bispecific Antibody for Therapeutic Regulation of Intracellular Targets. Mol. Cancer Ther. 2012, 11, 2169–2173. [Google Scholar] [CrossRef] [PubMed]
- Weisbart, R.H.; Chan, G.; Jordaan, G.; Noble, P.W.; Liu, Y.; Glazer, P.M.; Nishimura, R.N.; Hansen, J.E. DNA-dependent targeting of cell nuclei by a lupus autoantibody. Sci. Rep. 2015, 5, 12022. [Google Scholar] [CrossRef]
- Yi, H.; Sun, T.; Armstrong, D.; Borneman, S.; Yang, C.; Austin, S.; Kishnani, P.S.; Sun, B. Antibody-mediated enzyme replacement therapy targeting both lysosomal and cytoplasmic glycogen in Pompe disease. J. Mol. Med. 2017, 95, 513–521. [Google Scholar] [CrossRef]
- Kishnani, P.; Lachmann, R.; Mozaffar, T.; Walters, C.; Case, L.; Appleby, M.; Libri, V.; Kak, M.; Wencel, M.; Landy, H. Safety and efficacy of VAL-1221, a novel fusion protein targeting cytoplasmic glycogen, in patients with late-onset Pompe disease. Mol. Genet. Metab. 2019, 126, S85–S86. [Google Scholar] [CrossRef]
- Koeberl, D.; Luo, X.; Sun, B.; McVie-Wylie, A.; Dai, J.; Li, S.; Banugaria, S.G.; Chen, Y.-T.; Bali, D.S. Enhanced efficacy of enzyme replacement therapy in Pompe disease through mannose-6-phosphate receptor expression in skeletal muscle. Mol. Genet. Metab. 2011, 103, 107–112. [Google Scholar] [CrossRef]
- Koeberl, D.D.; Li, S.; Dai, J.; Thurberg, B.L.; Bali, D.; Kishnani, P.S. Beta2 Agonists enhance the efficacy of simultaneous enzyme replacement therapy in murine Pompe disease. Mol. Genet. Metab. 2012, 105, 221–227. [Google Scholar] [CrossRef]
- Koeberl, D.; Austin, S.; E Case, L.; Smith, E.C.; Buckley, A.F.; Young, S.P.; Bali, D.; Kishnani, P.S. Adjunctive albuterol enhances the response to enzyme replacement therapy in late-onset Pompe disease. FASEB J. 2014, 28, 2171–2176. [Google Scholar] [CrossRef]
- Koeberl, D.; E Case, L.; Smith, E.C.; Walters, C.; Han, S.-O.; Li, Y.; Chen, W.; Hornik, C.P.; Huffman, K.M.; Kraus, W.E.; et al. Correction of Biochemical Abnormalities and Improved Muscle Function in a Phase I/II Clinical Trial of Clenbuterol in Pompe Disease. Mol. Ther. 2018, 26, 2304–2314. [Google Scholar] [CrossRef]
- Doerfler, P.A.; Nayak, S.; Corti, M.; Morel, L.; Herzog, R.W.; Byrne, B.J. Targeted approaches to induce immune tolerance for Pompe disease therapy. Mol. Ther. Methods Clin. Dev. 2016, 3, 15053. [Google Scholar] [CrossRef]
- Ronzitti, G.; Collaud, F.; Laforet, P.; Mingozzi, F. Progress and challenges of gene therapy for Pompe disease. Ann. Transl. Med. 2019, 7, 287. [Google Scholar] [CrossRef]
- Byrne, B.J.; Fuller, D.D.; Smith, B.K.; Clement, N.; Coleman, K.; Cleaver, B.; Vaught, L.; Falk, D.J.; McCall, A.; Corti, M. Pompe disease gene therapy: Neural manifestations require consideration of CNS directed therapy. Ann. Transl. Med. 2019, 7, 290. [Google Scholar] [CrossRef]
- Salabarria, S.; Nair, J.; Clement, N.; Smith, B.; Raben, N.; Fuller, D.; Byrne, B.J.; Corti, M. Advancements in AAV-mediated Gene Therapy for Pompe Disease. J. Neuromuscul. Dis. 2020, 7, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Mingozzi, F.; High, K.A. Overcoming the Host Immune Response to Adeno-Associated Virus Gene Delivery Vectors: The Race Between Clearance, Tolerance, Neutralization, and Escape. Annu. Rev. Virol. 2017, 4, 511–534. [Google Scholar] [CrossRef] [PubMed]
- Mah, C.S.; Pacak, C.A.; Cresawn, K.O.; DeRuisseau, L.R.; Germain, S.; Lewis, M.A.; Cloutier, D.A.; Fuller, D.D.; Byrne, B.J. Physiological Correction of Pompe Disease by Systemic Delivery of Adeno-associated Virus Serotype 1 Vectors. Mol. Ther. 2007, 15, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Mah, C.S.; Falk, D.J.; Germain, S.A.; Kelley, J.S.; Lewis, M.A.; A Cloutier, D.; DeRuisseau, L.R.; Conlon, T.J.; Cresawn, K.O.; Fraites, T.J., Jr.; et al. Gel-mediated Delivery of AAV1 Vectors Corrects Ventilatory Function in Pompe Mice with Established Disease. Mol. Ther. 2010, 18, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Elmallah, M.K.; Falk, D.J.; Nayak, S.; Federico, R.A.; Sandhu, M.S.; Poirier, A.; Byrne, B.J.; Fuller, D.D. Sustained Correction of Motoneuron Histopathology Following Intramuscular Delivery of AAV in Pompe Mice. Mol. Ther. 2014, 22, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.K.; Collins, S.W.; Conlon, T.J.; Mah, C.S.; Lawson, L.A.; Martin, A.D.; Fuller, D.D.; Cleaver, B.D.; Clement, N.; Phillips, D.; et al. Phase I/II Trial of Adeno-Associated Virus–Mediated Alpha-Glucosidase Gene Therapy to the Diaphragm for Chronic Respiratory Failure in Pompe Disease: Initial Safety and Ventilatory Outcomes. Hum. Gene Ther. 2013, 24, 630–640. [Google Scholar] [CrossRef] [PubMed]
- Byrne, P.I.; Collins, S.; Mah, C.C.; Smith, B.; Conlon, T.; Martin, S.D. Phase I/II trial of diaphragm delivery of recombinant adeno-associated virus acid alpha-glucosidase (rAAaV1-CMV-GAA) gene vector in patients with Pompe disease. Hum. Gene Ther. Clin. Dev. 2014, 25, 134–163. [Google Scholar] [CrossRef]
- Corti, M.; Liberati, C.; Smith, B.K.; Lawson, L.A.; Tuna, I.S.; Conlon, T.J.; Coleman, K.E.; Islam, S.; Herzog, R.W.; Fuller, D.D.; et al. Safety of Intradiaphragmatic Delivery of Adeno-Associated Virus-Mediated Alpha-Glucosidase (rAAV1-CMV-hGAA) Gene Therapy in Children Affected by Pompe Disease. Hum. Gene Ther. Clin. Dev. 2017, 28, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Amalfitano, A.; McVie-Wylie, A.J.; Hu, H.; Dawson, T.L.; Raben, N.; Plotz, P.; Chen, Y.T. Systemic correction of the muscle disorder glycogen storage disease type II after hepatic targeting of a modified adenovirus vector encoding human acid-α-glucosidase. Proc. Natl. Acad. Sci. USA 1999, 96, 8861–8866. [Google Scholar] [CrossRef]
- Kiang, A.; Hartman, Z.C.; Liao, S.; Xu, F.; Serra, D.; Palmer, D.J.; Ng, P.; Amalfitano, A.; Kiang, Z.C.H.A. Fully Deleted Adenovirus Persistently Expressing GAA Accomplishes Long-Term Skeletal Muscle Glycogen Correction in Tolerant and Nontolerant GSD-II Mice. Mol. Ther. 2006, 13, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Rastall, D.P.W.; Seregin, S.S.; Aldhamen, Y.A.; Kaiser, L.; Mullins, C.; Liou, A.; Ing, F.; Pereria-Hicks, C.; Godbehere-Roosa, S.; Palmer, D.; et al. Long-term, high-level hepatic secretion of acid α-glucosidase for Pompe disease achieved in non-human primates using helper-dependent adenovirus. Gene Ther. 2016, 23, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Mingozzi, F.; High, K.A. Therapeutic in vivo gene transfer for genetic disease using AAV: Progress and challenges. Nat. Rev. Genet. 2011, 12, 341–355. [Google Scholar] [CrossRef] [PubMed]
- Franco, L.M.; Sun, B.; Yang, X.; Bird, A.; Zhang, H.; Schneider, A.; Brown, T.; Young, S.P.; Clay, T.M.; Amalfitano, A.; et al. Evasion of Immune Responses to Introduced Human Acid α-Glucosidase by Liver-Restricted Expression in Glycogen Storage Disease Type II. Mol. Ther. 2005, 12, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Bird, A.; Young, S.P.; Kishnani, P.S.; Chen, Y.-T.; Koeberl, D. Enhanced Response to Enzyme Replacement Therapy in Pompe Disease after the Induction of Immune Tolerance. Am. J. Hum. Genet. 2007, 81, 1042–1049. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Kulis, M.D.; Young, S.P.; Hobeika, A.C.; Li, S.; Bird, A.; Zhang, H.; Li, Y.; Clay, T.M.; Burks, W.; et al. Immunomodulatory Gene Therapy Prevents Antibody Formation and Lethal Hypersensitivity Reactions in Murine Pompe Disease. Mol. Ther. 2010, 18, 353–360. [Google Scholar] [CrossRef]
- Bond, J.; Kishnani, P.; Koeberl, D. Immunomodulatory, liver depot gene therapy for Pompe disease. Cell. Immunol. 2019, 342, 103737. [Google Scholar] [CrossRef]
- Kishnani, P.S.; Koeberl, D. Liver depot gene therapy for Pompe disease. Ann. Transl. Med. 2019, 7, 288. [Google Scholar] [CrossRef]
- Han, S.-O.; Ronzitti, G.; Arnson, B.; Leborgne, C.; Li, S.; Mingozzi, F.; Koeberl, D. Low-Dose Liver-Targeted Gene Therapy for Pompe Disease Enhances Therapeutic Efficacy of ERT via Immune Tolerance Induction. Mol. Ther. Methods Clin. Dev. 2017, 4, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Puzzo, F.; Colella, P.; Biferi, M.G.; Bali, D.; Paulk, N.K.; Vidal, P.; Collaud, F.; Simon-Sola, M.; Charles, S.; Hardet, R.; et al. Rescue of Pompe disease in mice by AAV-mediated liver delivery of secretable acid α-glucosidase. Sci. Transl. Med. 2017, 9, eaam6375. [Google Scholar] [CrossRef] [PubMed]
- Cagin, U.; Puzzo, F.; Gomez, M.J.; Moya-Nilges, M.; Sellier, P.; Abad, C.; Van Wittenberghe, L.; Daniele, N.; Guerchet, N.; Gjata, B.; et al. Rescue of Advanced Pompe Disease in Mice with Hepatic Expression of Secretable Acid α-Glucosidase. Mol. Ther. 2020, 28. [Google Scholar] [CrossRef] [PubMed]
| Intervention/Treatment | Characteristics/Delivery Method | Company/Institution | Clinical Trial Phase/Identifier | References |
|---|---|---|---|---|
| ERT | ||||
| neo-GAA | Glycoengineered recombinant GAA with increased bis-M6P levels (avalglucosidase alfa) | Genzyme, a Sanofi Company | Completed/NCT01898364 Phase 3/NCT02782741 | Zhu et al. [108] Pena et al. [109] |
| AT-GAA (ATB200/AT2221) | rhGAA bearing high bis-M6P with a pharmacological chaperone (miglustat) | Amicus Therapeutics | Phase 3/NCT03729362 Phase 3/NCT03911505 | Khanna et al. [111] Xu et al. [92] Meena et al. [66] |
| VAL-1221 | Fusion protein containing antibody 3E10 and rhGAA | Valerion Therapeutics | Terminated/NCT02898753 | Weisbart et al. [114,115] Yi et al. [116] Kishnani et al. [117] |
| ERT + Clenbuterol | Alglucosidase alfa with β2-adrenergic agonist clenbuterol | Duke University | Completed/NCT01942590 | Koeberl et al. [118,119,120,121] |
| Gene Therapy | ||||
| rAAV2/1-CMV-hGAA | Intramuscular injection into the diaphragm | University of Florida | Completed/NCT00976352 | Smith et al. [130] Byrne et al. [131] Corti et al. [132] |
| rAAV9-DES-hGAA | Intramuscular re-administration | Lacerta Therapeutics/University of Florida | Phase 1/2/NCT02240407 | Salabarria et al. [125] |
| AAV2/8-LSPhGAA | Screening for eligibility Ascending dose intravenous administration | Duke University Asklepios Biopharmaceutical/Duke University | Completed/NCT03285126 Phase 1/2/ NCT03533673 | Kishnani et al. [141] Han et al. [142] |
| SPK-3006 (AAV liver directed secretable GAA) | Intravenous administration | Spark Therapeutics | Phase 1/2/ NCT04093349 | Puzzo et al. [143] Cagin et al. [144] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meena, N.K.; Raben, N. Pompe Disease: New Developments in an Old Lysosomal Storage Disorder. Biomolecules 2020, 10, 1339. https://doi.org/10.3390/biom10091339
Meena NK, Raben N. Pompe Disease: New Developments in an Old Lysosomal Storage Disorder. Biomolecules. 2020; 10(9):1339. https://doi.org/10.3390/biom10091339
Chicago/Turabian StyleMeena, Naresh K., and Nina Raben. 2020. "Pompe Disease: New Developments in an Old Lysosomal Storage Disorder" Biomolecules 10, no. 9: 1339. https://doi.org/10.3390/biom10091339
APA StyleMeena, N. K., & Raben, N. (2020). Pompe Disease: New Developments in an Old Lysosomal Storage Disorder. Biomolecules, 10(9), 1339. https://doi.org/10.3390/biom10091339




