Inflammation in Obesity-Related Complications in Children: The Protective Effect of Diet and Its Potential Role as a Therapeutic Agent
Abstract
1. Introduction
2. Methods
3. Adipose Tissue-Associated Inflammation
4. Pediatric Obesity and Related Inflammatory Complications
4.1. Endocrine System
4.2. Cardiovascular System
4.3. Pulmonary System
4.4. Hepatobiliary System
4.5. Neurological System
4.6. Musculoskeletal System
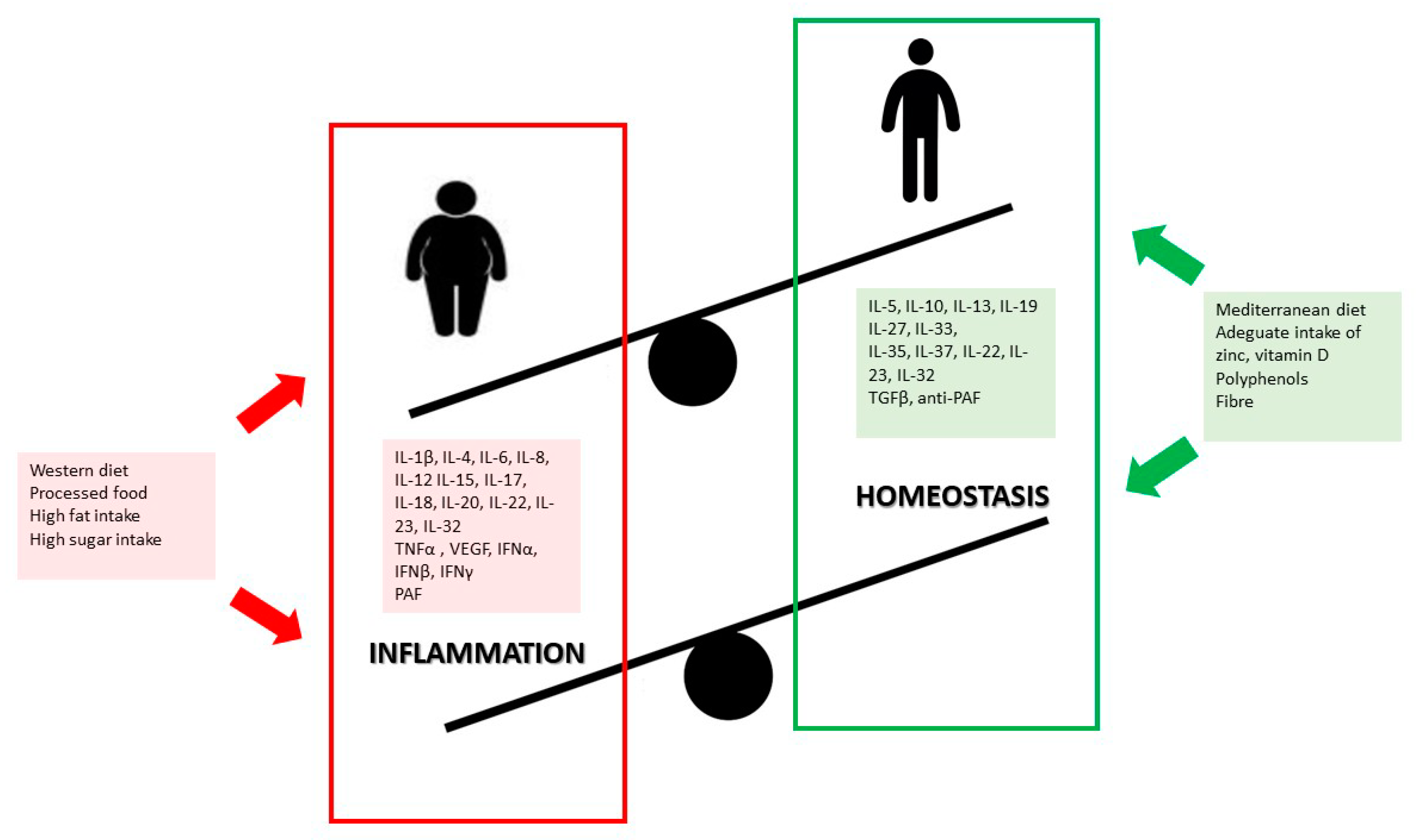
5. Diet and Lifestyle
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization Home Page. Available online: https://www.who.int/childgrowth/en/ (accessed on 15 September 2020).
- Hans, H.P.K. 25–70% of Adults in Europe Are Overweight; World Health Organization, 2010; Available online: https://www.euro.who.int/en/about-us/regional-director/news/news/2010/12/2570-of-adults-in-europe-are-overweight (accessed on 15 September 2020).
- Ogden, C.L.; Carroll, M.D.; Kit, B.K.; Flegal, K.M. Prevalence of Childhood and Adult Obesity in the United States, 2011–2012. JAMA 2014, 311, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Mărginean, C.O.; Meliţ, L.E.; Ghiga, D.V.; Mărginean, M.O. Early Inflammatory Status Related to Pediatric Obesity. Front. Pediatr. 2019, 7, 241. [Google Scholar] [CrossRef]
- Ferrante, A.W. Obesity-induced inflammation: A metabolic dialogue in the language of inflammation. J. Intern. Med. 2007, 262, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, W.M.; Nobre, M.R.; Jatene, F.B. Evidence-based clinical practice. Part II—Searching evidence databases. Rev. Assoc. Med. Bras. 2004, 50, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Pearson, T.A.; Mensah, G.A.; Alexander, R.W. Markers of inflammation and cardiovascular disease: Application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 2003, 107, 499–511. [Google Scholar] [CrossRef]
- Dahlman, I.; Elsen, M.; Tennagels, N.; Korn, M.; Brockmann, B.; Sell, H.; Eckel, J.; Arner, P. Functional annotation of the human fat cell secretome. Arch. Physiol. Biochem. 2012, 118, 84–91. [Google Scholar] [CrossRef]
- Kojta, I.; Chacińska, M.; Błachnio-Zabielska, A. Obesity, Bioactive Lipids, and Adipose Tissue Inflammation in Insulin Resistance. Nutrients 2020, 12, 1305. [Google Scholar] [CrossRef]
- Liu, Z.W.; Ma, Y.P.; Cui, Q.W.; Xu, J.; Tang, Z.G.; Wang, Y.; He, C.H.; Wang, X. Toll-like receptor 4 plays a key role in advanced glycation end products-induced M1 macrophage polarization. Biochem. Biophys. Res. Commun. 2020, 531, 602–608. [Google Scholar] [CrossRef]
- Kerr, J.F.R.; Wyllie, A.H.; Currie, A.R. Apoptosis: A Basic Biological Phenomenon with Wideranging Implications in Tissue Kinetics. Br. J. Cancer 1972, 26, 239–257. [Google Scholar] [CrossRef]
- Murano, I.; Barbatelli, G.; Parisani, V.; Latini, C.; Muzzonigro, G.; Castellucci, M.; Cinti, S. Dead adipocytes, detected as crown-like structures, are prevalent in visceral fat depots of genetically obese mice. J. Lipid Res. 2008, 49, 1562–1568. [Google Scholar] [CrossRef]
- Winer, S.; Chan, J.; Paltser, G.; Truong, D.; Tsui, H.; Bahrami, J.; Dorfman, R.; Wang, J.; Zielenski, J.; Mastronardi, F.; et al. Normalization of Obesity-Associated Insulin Resistance through Immunotherapy: CD4+ T Cells Control Glucose Homeostasis. Nat. Med. 2009, 15, 921–929. [Google Scholar] [CrossRef]
- Yang, H.; Youm, Y.H.; Vandanmagsar, B.; Ravussin, A.; Gimble, J.M.; Greenway, F.; Stephens, J.M.; Mynatt, R.L.; Dixit, V.D. Obesity increases the production of proinflammatory mediators from adipose tissue T cells and compromises TCR repertoire diversity: Implications for systemic inflammation and insulin resistance. J. Immunol. 2010, 185, 1836–1845. [Google Scholar] [CrossRef] [PubMed]
- Whicher, J.T.; Westacott, C.I. The acute phase response. In Biochemistry of Inflammation; Whicher, J.T., Evans, S.W., Eds.; Kluwer Academic: London, UK, 1992; pp. 243–271. [Google Scholar]
- Galgani, M.; Procaccini, C.; De Rosa, V.; Carbone, F.; Chieffi, P.; La Cava, A. Leptin modulates the survival of autoreactive CD4+ T cells through the nutrient/energy-sensing mammalian target of rapamycin signaling pathway. J. Immunol. 2010, 185, 7474–7479. [Google Scholar] [CrossRef] [PubMed]
- Thoudaml, T.; Jeon, J.H.; Ha, C.M.; Lee, I.K. Role of Mitochondria-Associated Endoplasmic Reticulum Membrane in Inflammation-Mediated Metabolic Diseases. Mediat. Inflamm. 2016. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Wang, J.P.; Jiang, Y.Y.; Li, H.; Hu, Y.H.; Lee, K.O.; Li, G.W. Fasting Plasma Insulin at 5 Years of Age Predicted Subsequent Weight Increase in Early Childhood over a 5-Year Period—The Da Qing Children Cohort Study. PLoS ONE 2015, 10, e0127389. [Google Scholar] [CrossRef]
- Olshansky, S.J.; Passaro, D.J.; Hershow, R.C.; Layden, J.; Carnes, B.A.; Brody, J. A potential decline in life expectancy in the United States in the 21st century. N. Engl. J. Med. 2005, 352, 1138–1145. [Google Scholar] [CrossRef] [PubMed]
- Kanakadurga, S.; Carey, N.L. The initiation of metabolic inflammation in childhood obesity. J. Clin. Investig. 2017, 127, 65–73. [Google Scholar]
- Calle, M.C.; Fernandez, M.L. Inflammation and type 2 diabetes. Diabe. Metab. 2012, 38, 183–191. [Google Scholar] [CrossRef]
- Baptiste, G.B.; Battista, M.C.; Trottier, A.; Baillargeon, J.P. Insulin and hyperandrogenism in women with polycystic ovary syndrome. J. Steroid Biochem. Mol. Biol. 2010, 122, 42–52. [Google Scholar] [CrossRef]
- Laitinen, J.; Taponen, S.; Martikainen, H.; Pouta, A.; Millwood, I.; Hartikainen, A.L.; Ruokonen, A.; Sovio, U.; McCarthy, M.I.; Franks, S.; et al. Body Size From Birth to Adulthood as a Predictor of Self-Reported Polycystic Ovary Syndrome Symptoms. Int. J. Obes. Relat. Metab. Disord. 2003, 27, 710–715. [Google Scholar] [CrossRef]
- Snider, A.P.; Wood, J.R. Obesity Induces Ovarian Inflammation and Reduces Oocyte Quality. Reproduction 2019, 158, R79–R90. [Google Scholar] [CrossRef]
- Skaznik-Wikiel, M.E.; Swindle, D.C.; Allshouse, A.A.; Polotsky, A.J.; McManaman, J.L. High-Fat Diet Causes Subfertility and Compromised Ovarian Function Independent of Obesity in mice. Biol. Reprod. 2016, 94, 108. [Google Scholar]
- Nteeba, J.; Ganesan, S.; Keating, A.F. Progressive obesity alters ovarian folliculogenesis with impacts on pro-inflammatory and steroidogenic signaling in female mice. Biol. Reprod. 2014, 91, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Anderson, C.L.; Timme, K.R.; Kurz, S.G.; Fernando, S.C.; Wood, J.R. 2016 Obesity-dependent increases in oocyte mRNAs are associated with increases in proinflammatory signaling and gut microbial abundance of Lachnospiraceae in female mice. Endocrinology 2016, 157, 1630–1643. [Google Scholar] [CrossRef]
- Ruebel, M.; Shankar, K.; Gaddy, D.; Lindsey, F.; Badger, T.; Andres, A. Maternal obesity is associated with ovarian inflammation and upregulation of early growth response factor 1. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E269–E277. [Google Scholar] [CrossRef]
- Igosheva, N.; Abramov, A.Y.; Poston, L.; Eckert, J.J.; Fleming, T.P.; Duchen, M.R.; McConnell, J. Maternal diet-induced obesity alters mitochondrial activity and redox status in mouse oocytes and zygotes. PLoS ONE 2010, 5, e10074. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.M. Atherosclerotic Cardiovascular Disease Beginning in Childhood Korean. Circ. J. 2010, 40, 1–9. [Google Scholar]
- Berenson, G.S.; Srinivasan, S.R.; Bao, W. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa Heart Study. N. Engl. J. Med. 1998, 338, 1650. [Google Scholar] [CrossRef] [PubMed]
- McGill, H.C.; McMahon, C.A. Determinants of atherosclerosis in the young. Pathobiological Determinants of Atherosclerosis in Youth (PDAY) Research Group. Am. J. Cardiol. 1993, 13, 1291–1298. [Google Scholar]
- Bridger, B. Childhood obesity and cardiovascular disease. Paediatr. Child Health 2009, 14, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Rosner, B.; Prineas, R.; Daniels, S.R.; Loggie, J. Blood pressure differences between blacks and whites in relation to body size among US children and adolescents. Am. J. Epidemiol. 2000, 151, 1007–1019. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, L.T.; Burns, T.L.; Stanford, W.; Thompson, B.H.; Witt, J.D.; Rost, C.A. Coronary risk factors measured in childhood and young adult life are associated with coronary artery calcification in young adults: The Muscatine Study. J. Am. Coll. Cardiol. 1996, 27, 277–284. [Google Scholar] [CrossRef]
- Wang, Z.; Nakayama, T. Inflammation: A Link between Obesity and Cardiovascular Disease Mediators Inflamm. Mediat. Inflamm. 2010. [Google Scholar] [CrossRef] [PubMed]
- Stumpf, C.; Sheriff, A.; Zimmermann, S.; Schaefauer, L.; Schlundt, C.; Raaz, D.; Garlichs, C.D.; Achenbach, S. C-reactive protein levels predict systolic heart failure and outcome in patients with first ST-elevation myocardial infarction treated with coronary angioplasty. Arch. Med. Sci. 2017, 13, 1086–1093. [Google Scholar] [CrossRef] [PubMed]
- DeBoer, M.D. Obesity, systemic inflammation, and increased risk for cardiovascular disease and diabetes among adolescents: A need for screening tools to target interventions. Nutrition 2013, 29, 379–386. [Google Scholar] [CrossRef]
- Cvejoska-Cholakovska, V.; Kocova, M.; Velikj-Stefanovska, V.; Vlashki, E. The Association between Asthma and Obesity in Children—Inflammatory and Mechanical Factors. J. Med. Sci. 2019, 7, 1314–1319. [Google Scholar] [CrossRef]
- Chen, Z.; Salam, M.T.; Alderete, T.L.; Habre, R.; Bastain, T.M.; Berhane, K. Effects of childhood asthma on the development of obesity among school-aged children. Am. J. Respir. Crit. Care Med. 2017, 195, 1181–1188. [Google Scholar] [CrossRef]
- Lang, J.E.; Hossain, J.; Holbrook, J.T.; Teague, W.G.; Gold, B.D.; Wise, R.A. Gastrooesophageal reflux and worse asthma control in obese children: A case of symptom misattribution? Thorax 2016, 71, 238–246. [Google Scholar] [CrossRef]
- Lang, J.E.; Hossain, M.J.; Lima, J.J. Overweight children report qualitatively distinct asthma symptoms: Analysis of validated symptom measures. J. Allergy Clin. Immunol. 2015, 135, 886–893. [Google Scholar] [CrossRef]
- Lang, J.E.; Hossain, J.; Dixon, A.E.; Shade, D.; Wise, R.A.; Peters, S.P. Does age impact the obese asthma phenotype? Longitudinal asthma control, airway function, and airflow perception among mild persistent asthmatics. Chest 2011, 140, 1524–1533. [Google Scholar] [CrossRef]
- Wood, L.G. Metabolic dysregulation. Driving the obese asthma phenotype in adolescents? Am. J. Respir. Crit. Care Med. 2015, 191, 121–122. [Google Scholar] [CrossRef] [PubMed]
- Ahmadizar, F.; Vijverberg, S.J.; Arets, H.G.; de Boer, A.; Lang, J.E.; Kattan, M. Childhood obesity in relation to poor asthma control and exacerbation: A metaanalysis. Eur. Respir. J. 2016, 48, 1063–1073. [Google Scholar] [CrossRef] [PubMed]
- Borrell, L.N.; Nguyen, E.A.; Roth, L.A.; Oh, S.S.; Tcheurekdjian, H.; Sen, S. Childhood obesity and asthma control in the GALA II and SAGE II studies. Am. J. Respir. Crit. Care Med. 2013, 187, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Holguin, F.; Bleecker, E.R.; Busse, W.W.; Calhoun, W.J.; Castro, M.; Erzurum, S.C. Obesity and asthma: An association modified by age of asthma onset. J. Allergy Clin. Immunol. 2011, 127, 1486–1493. [Google Scholar] [CrossRef]
- Rastogi, D.; Fraser, S.; Oh, J.; Huber, A.M.; Schulman, Y.; Bhagtani, R.H. Inflammation, metabolic dysregulation, and pulmonary function among obese urban adolescents with asthma. Am. J. Respir. Crit. Care Med. 2015, 191, 149–160. [Google Scholar] [CrossRef]
- Desai, D.; Newby, C.; Symon, F.A.; Haldar, P.; Shah, S.; Gupta, S. Elevated sputum interleukin-5 and submucosal eosinophilia in obese individuals with severe asthma. Am. J. Respir. Crit. Care Med. 2013, 188, 657–663. [Google Scholar] [CrossRef]
- Pascal, M.; Perez-Gordo, M.; Caballero, T.; Escribese, M.M.; Natividad Lopez Longo, M.; Luengo, O.; Victor, L.M. Microbiome and Allergic Diseases. Front. Immunol. 2018, 9, 1584. [Google Scholar] [CrossRef]
- Peters, U.; Dixon, A.E.; Forno, E. Obesity and Asthma. J. Allergy Clin. Immunol. 2018, 141, 1169–1179. [Google Scholar] [CrossRef]
- Lugogo, N.; Francisco, D.; Addison, K.J.; Manne, A.; Pederson, W.; Ingram, J.L. Obese asthmatic patients have decreased surfactant protein A levels: Mechanisms and implications. J. Allergy Clin. Immunol. 2017, 141, 918–926. [Google Scholar] [CrossRef]
- Kattan, M.; Kumar, R.; Bloomberg, G.R.; Mitchell, H.E.; Calatroni, A.; Gergen, P.J. Asthma control, adiposity, and adipokines among inner-city adolescents. J. Allergy Clin. Immunol. 2010, 125, 584–592. [Google Scholar] [CrossRef]
- Sood, A.; Shore, S.A. Adiponectin, leptin, and resistin in asthma: Basic mechanisms through population studies. J. Allergy 2013. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Del-Rio-Navarro, B.E.; Torres-Alcantara, S.; Perez-Ontiveros, J.A.; Ruiz-Bedolla, E.; Saucedo-Ramirez, O.J. Adipokines, asymmetrical dimethylarginine, and pulmonary function in adolescents with asthma and obesity. J. Asthma 2017, 54, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Baek, H.S.; Kim, Y.D.; Shin, J.H.; Kim, J.H.; Oh, J.W.; Lee, H.B. Serum leptin and adiponectin levels correlate with exercise-induced bronchoconstriction in children with asthma. Ann. Allergy Asthma Immunol. 2011, 107, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Sideleva, O.; Suratt, B.T.; Black, K.E.; Tharp, W.G.; Pratley, R.E.; Forgione, P. Obesity and asthma: An inflammatory disease of adipose tissue not the airway. Am. J. Respir. Crit. Care Med. 2012, 186, 598–605. [Google Scholar] [CrossRef]
- Arias-Loste, M.T.; Iruzubieta, P.; Crespo, J. Paediatric non-alcoholic fatty liver disease: A more complex disease than in the adulthood? Hepatobiliary Surg. Nutr. 2019, 8, 270–273. [Google Scholar] [CrossRef]
- Wang, Y.; Viscarra, S.K.; Sook Sul, H. Transcriptional Regulation of Hepatic Lipogenesis. Nat. Rev. Mol. Cell Biol. 2015, 16, 678–689. [Google Scholar] [CrossRef]
- Ferraioli, G.; Calcaterra, V.; Lissandrin, R.; Guazzotti, M.; Maiocchi, L.; Tinelli, C.; De Silvestri, A.; Regalbuto, C.; Pelizzo, G.; Larizza, D.; et al. Noninvasive assessment of liver steatosis in children: The clinical value of controlled attenuation parameter. BMC Gastroenterol. 2017, 17, 61. [Google Scholar] [CrossRef]
- Blackwood, B.P.; Grabowski, J. Chronic cholecystitis in the pediatric population: An underappreciated disease process. Gastroenterol. Hepatol. Bed Bench 2017, 10, 125–130. [Google Scholar]
- Faz, G.; Butler, I.J.; Koenig, M.K. Incidence of papilledema and obesity in children diagnosed with idiopathic “benign” intracranial hypertension: Case series and review. J. Child Neurol. 2010, 25, 1389–1392. [Google Scholar] [CrossRef]
- Barmherzig, R.; Szperka, C.L. Pseudotumor Cerebri Syndrome in Children. Curr. Pain Headache Rep. 2019, 23, 58. [Google Scholar] [CrossRef]
- McGeeney, B.E.; Friedman, D.I. Pseudotumor cerebri pathophysiology. Headache 2014, 54, 445–458. [Google Scholar] [CrossRef] [PubMed]
- Rook, B.S.; Phillips, P.H. Pediatric pseudotumor cerebri. Curr. Opin. Ophthalmol. 2016, 27, 416–419. [Google Scholar] [CrossRef] [PubMed]
- Lampl, Y.; Eshel, Y.; Kessler, A.; Fux, A.; Gilad, R.; Boaz, M.; Matas, Z.; Sadeh, M. Serum leptin level in women with idiopathic intracranial hypertension. J. Neurol. Neurosurg. Psychiatry 2002, 72, 642–643. [Google Scholar] [CrossRef] [PubMed]
- Ostlund, R.E., Jr.; Yang, J.W.; Klein, S.; Gingerich, R. Relation betweenplasma leptin concentration and body fat, gender, diet, age, and metabolic covariates. J. Clin. Endocrinol. Metab. 1996, 81, 3909–3913. [Google Scholar] [PubMed]
- Schwartz, M.W.; Peskind, E.; Raskind, M.; Boyko, E.J.; Porte, D., Jr. Cerebrospinal fluid leptin levels: Relationship to plasma levels and to adiposity in humans. Nat. Med. 1996, 2, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Caro, J.F.; Kolaczynski, J.W.; Nyce, M.R.; Ohannesian, J.P.; Opentanova, I.; Goldman, W.H.; Lynn, R.B.; Zhang, P.L.; Sinha, M.K.; Considine, R.V. Decreased cerebrospinal-fluid/serum leptin ratio in obesity: A possible mechanism for leptin resistance. Lancet 1996, 348, 159–161. [Google Scholar] [CrossRef]
- Van Heek, M.; Compton, D.S.; France, C.F.; Tedesco, R.P.; Fawzi, A.B.; Graziano, M.P.; Sybertz, E.J.; Strader, C.D.; Davis, H.R., Jr. Diet-induced obese mice develop peripheral, but not central, resistance to leptin. J. Clin. Investig. 1997, 99, 385–390. [Google Scholar] [CrossRef]
- Howard, J.K.; Flier, J.S. Attenuation of leptin and insulin signaling by SOCS proteins Trends. Endocrinol. Metab. 2006, 17, 365–371. [Google Scholar]
- Markey, K.A.; Uldall, M.; Botfield, H.; Cato, L.D.; Miah, M.A.; Hassan-Smith, G.L.; Jensen, R.H.; Gonzalez, A.M.; Sinclair, A.J. Idiopathic intracranial hypertension hormones and 11β-hydroxysteroid dehydrogenases. J. Pain Res. 2016, 9, 223–232. [Google Scholar]
- Galvin, J.A.; Van Stavern, G.P. Clinical characterization of idiopathic intracranial hypertension at the Detroit Medical Center. J. Neurol. Sci. 2004, 223, 157–160. [Google Scholar] [CrossRef]
- Tomlinson, J.W.; Walker, E.A.; Bujalska, I.J.; Draper, N.; Lavery, G.G.; Cooper, M.S.; Hewison, M.; Stewart, P.M. 11beta-hydroxysteroid dehydrogenase type 1: A tissue-specific regulator of glucocorticoid response. Endocr. Rev. 2004, 25, 831–866. [Google Scholar] [CrossRef] [PubMed]
- Rask, E.; Olsson, T.; Soderberg, S.; Andrew, R.; Livingstone, D.E.; Johnson, O.; Walker, B.R. Tissue-specific dysregulation of cortisol metabolism in human obesity. J. Clin. Endocrinol. Metab. 2001, 86, 1418–1421. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.M.; Boulton, A.; Kumar, S.; Clark, P.M.S.; Shackleton, C.H.L. Cortisol metabolism in human obesity: Impaired cortisone→cortisol conversion in subjects with central adiposity. J. Clin. Endocrinol. Metab. 1999, 84, 1022–1027. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lindquist, S.; Chen, R.; Myrnas, T.; Angsten, G.; Olsson, T.; Hernell, O. Depot-specific messenger RNA expression of 11 beta-hydroxysteroid dehydrogenase type 1 and leptin in adipose tissue of children and adults. Int. J. Obes. (Lond.) 2007, 31, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Paulmyer-Lacroix, O.; Boullu, S.; Oliver, C.; Alessi, M.C.; Grinol, M. Expression of the mRNA coding for 11beta-hydroxysteroid dehydrogenase type 1 in adipose tissue from obese patients: An in situ hybridization study. J. Clin. Endocrinol. Metab. 2002, 87, 2701–2705. [Google Scholar] [PubMed]
- Tomlinson, J.W.; Moore, J.; Cooper, M.S.; Bujalska, I.; Shahmanesh, M.; Burt, C.; Strain, A.; Hewison, M.; Stewart, P.M. Regulation of expression of 11beta-hydroxysteroid dehydrogenase type 1 in adipose tissue: Tissue specific induction by cytokines. Endocrinology 2001, 142, 1982–1989. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, A.J.; Ball, M.K.; Burdon, M.A.; Clarke, C.E.; Stewart, P.M.; Curnow, J.; Rauz, S. Exploring the pathogenesis of IIH: An inflammatory perspective. J. Neuroimmunol. 2008, 201, 212–220. [Google Scholar] [CrossRef] [PubMed]
- VanHerpen, N.A.; Schrauwen-Hinderling, V.B. Lipid accumulation in non adipose tissue and lipotoxicity. Physiol. Behav. 2008, 94, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Boden, G.; Shulman, G.I. Free fatty acids in obesity and type2 dia- betes: Defining their role in the development of insulin resistance and beta-cell dysfunction. Eur. J. Clin. Investig. 2002, 32, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Mittendorfer, B.; Magkos, F.; Fabbrini, E.; Mohammed, B.S.; Klein, S. Relationship between body fat mass and free fatty acid kinetics in men and women. Obesity (Silver Spring) 2009, 17, 1872–1877. [Google Scholar] [CrossRef] [PubMed]
- Goodpaster, B.H.; Theriault, R.; Watkins, S.C.; Kelley, D.E. Intramuscular lipid content is increased in obesity and decreased by weight loss. Metabolism 2000, 49, 467–472. [Google Scholar] [CrossRef]
- Bonen, A.; Parolin, M.L.; Steinberg, G.R.; Calles-Escandon, J.; Tandon, N.N.; Glatz, J.F. Triacylglycerol accumulation in human obesity and type 2 diabetes is associated with increased rates of skeletal lmuscle fatty acid transport and increased sarcolemmal FAT/CD36. FASEB J. 2004, 18, 1144–1146. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.; Dufour, S.; Petersen, K.F.; Lebon, V.; Enoksson, S.; Ma, Y.Z. Assessment of skeletal muscle triglyceride content by(1) nuclear magnetic resonance spectroscopy in lean and obese adolescents: Relationships to insulin sensitivity, total body fat, and central adiposity. Diabetes 2002, 51, 1022–1027. [Google Scholar] [CrossRef]
- Boesch, C.; Machann, J.; Vermathen, P.; Schick, F. Role of proton MR for the study of muscle lipid metabolism. NMR Biomed. 2002, 19, 968–988. [Google Scholar] [CrossRef]
- Fujimoto, T.; Ohsaki, Y.; Cheng, J.; Suzuki, M.; Shinohara, Y. Lipid droplets: A classic organelle with new outfits. Histochem. Cell Biol. 2008, 130, 263–279. [Google Scholar] [CrossRef]
- Thiele, C.; Spandl, J. Cell biology of lipid droplets. Curr. Opin. Cell Biol. 2008, 20, 378–385. [Google Scholar] [CrossRef]
- Simoneau, J.A.; Colberg, S.R.; Thaete, F.L.; Kelley, D.E. Skeletal muscle glycolytic and oxidative enzyme capacities are determinants of insulin sensitivity and muscle composition in obese women. FASEB J. 1995, 9, 273–278. [Google Scholar] [CrossRef]
- Malenfant, P.; Joanisse, D.R.; Theriault, R.; Goodpaster, B.H.; Kelley, D.E.; Simoneau, J.A. Fat content in individual muscle fibers of lean and obese subjects. Int. J. Obes. Relat. Metab. Disord. 2001, 25, 1316–1321. [Google Scholar] [CrossRef]
- Charge, S.B.; Rudnicki, M.A. Cellular and molecular regulation of muscle regeneration. Physiol. Rev. 2004, 84, 209–238. [Google Scholar] [CrossRef]
- Hu, Z.; Wang, H.; Lee, I.H.; Modi, S.; Wang, X.; Du, J. PTEN inhibition improves muscle regeneration in mice fed a high-fat diet. Diabetes 2010, 59, 1312–1320. [Google Scholar] [CrossRef]
- Woo, M.; Isganaitis, E.; Cerletti, M.; Fitzpatrick, C.; Wagers, A.J.; Jimenez Chillaron, J. Early life nutrition modulates muscle stem cell number: Implications for muscle mass and repair. Stem Cells Dev. 2011, 20, 1763–1769. [Google Scholar] [CrossRef]
- Mann, C.J.; Perdiguero, E.; Kharraz, Y.; Aguilar, S.; Pessina, P.; Serrano, A.L. Aberrant repair and fibrosis development in skeletal muscle. Skelet. Muscle 2011, 1, 21. [Google Scholar] [CrossRef] [PubMed]
- Kharraz, Y.; Guerra, J.; Mann, C.J.; Serrano, A.L.; Munoz-Canoves, P. Macrophage plasticity and the role of inflammation in skeletal muscle repair. Mediat. Inflamm. 2013, 491, 497. [Google Scholar] [CrossRef]
- Arnold, L.; Henry, A.; Poron, F.; Baba-Amer, Y.; VanRooijen, N.; Plonquet, A. Inflammatory monocytes recruited after skeletal muscle injurys witch into anti-inflammatory macrophages to support myogenesis. J. Exp. Med. 2014, 204, 1057–1069. [Google Scholar] [CrossRef] [PubMed]
- Warren, G.L.; Hulderman, T.; Mishra, D.; Gao, X.; Millecchia, L.; O’Farrell, L. Chemokinereceptor CCR2 involvement in skeletal muscle regeneration. FASEB J. 2005, 19, 413–415. [Google Scholar] [CrossRef] [PubMed]
- Akhmedov, D.; Berdeaux, R. The effects of obesity on skeletal muscle regeneration. Front. Physiol. 2013, 4, 371. [Google Scholar] [CrossRef] [PubMed]
- Gregory, J.W. Prevention of Obesity and Metabolic Syndrome in Children. Front. Endocrinol. (Lausanne) 2019, 10, 669. [Google Scholar] [CrossRef]
- Marcellini, F.; Giuli, C.; Papa, R. Obesity and body mass index (BMI) in relation to life-style and psycho-social aspects. Archiv. Gerontol. Geriatr. 2009, 49, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Sedibe, M.H.; Pisa, P.T.; Feeley, A.B. Dietary habits and eating practices and their association with overweight and obesity in rural and urban black South African adolescents. Nutrients 2018, 10, 145. [Google Scholar] [CrossRef]
- Allegri, C.; Turconi, G.; Cena, H. Dietary attitudes and diseases of comfort. Eat. Weight Disord. 2011, 16, e226–e235. [Google Scholar] [CrossRef]
- De Giuseppe, R.; Di Napoli, I.; Porri, D.; Cena, H. Pediatric Obesity and Eating Disorders Symptoms: The Role of the Multidisciplinary Treatment. A Systematic Review. Front. Pediatr. 2019, 7, 123. [Google Scholar] [CrossRef] [PubMed]
- Danford, C.A.; Schultz, C.; Marvicsin, D. Parental roles in the development of obesity in children: Challenges and opportunities. Res. Rep. Biol. 2015, 6, 39–46. [Google Scholar] [CrossRef]
- Hardy, L.L.; Bell, J.; Bauman, A. Association between adolescents’ consumption of total and different types of sugar-sweetened beverages with oral health impacts and weight status. Aust. N. Z. J. Public Health 2018, 42, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Hooper, L.; Abdelhamid, A.; Bunn, D. Effects of total fat intake on body weight. Cochrane Database Syst. Rev. 2015, 8. [Google Scholar] [CrossRef]
- You, J.; Choo, J. Adolescent overweight and obesity: Links to socioeconomic status and fruit and vegetable intakes. Int. J. Environ. Res. Public Health 2016, 13, 307. [Google Scholar] [CrossRef]
- Christ, A.; Latz, E. The Western lifestyle has lasting effects on metaflammation. Nat. Rev. Immunol. 2019, 19, 267–268. [Google Scholar] [CrossRef]
- Aguayo-Patrón, S.V.; Calderón de la Barca, A.N. Old Fashioned vs. Ultra-Processed-Based Current Diets: Possible Implication in the Increased Susceptibility to Type 1 Diabetes and Celiac Disease in Childhood. Foods 2017, 6, 100. [Google Scholar] [CrossRef]
- Müller, D.N.; Wilck, N.; Haase, S.; Kleinewietfeld, M.; Linker, R.A. Sodium in the Microenvironment Regulates Immune Responses and Tissue Homeostasis. Nat. Rev. Immunol. 2019, 19, 243–254. [Google Scholar] [CrossRef]
- Falkner, B. Monitoring and management of hypertension with obesity in adolescents. Integr. Blood Press Control 2017, 10, 33–39. [Google Scholar] [CrossRef]
- Funtikova, A.N.; Navarro, E.; Bawaked, R.A.; Fíto, M.; Schröder, H. Impact of diet on cardiometabolic health in children and adolescents. Nutr. J. 2015, 14, 118. [Google Scholar] [CrossRef]
- Probst, Y.C.; Guan, V.X.; Kent, K. Dietary phytochemical intake from foods and health outcomes: A systematic review protocol and preliminary scoping. BMJ Open 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Magrone, T.; Jirillo, E. Childhood obesity: Immune response and nutritional approaches. Front. Immunol. 2015, 6, 76. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lim, H. Nutritional Management in Childhood Obesity. J. Obes. Metab. Syndr. 2019, 28, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Powell, S.R. The Antioxidant Properties of Zinc. J. Nutr. 2000, 130, 1447S–1454S. [Google Scholar] [CrossRef]
- Liebert, M.A. Effect of Zinc Supplementation on Markers of Insulin Resistance, Oxidative Stress, and Inflammation among Prepubescent Children with Metabolic Syndrome. Metab. Syndr. Relat. Disord. 2010, 8, 505–510. [Google Scholar]
- Bonaventura, P.; Benedetti, G.; Albarede, F.; Miossec, P. Zinc and its role in immunity and infammation. Autoimmun. Rev. 2015, 14, 277–285. [Google Scholar] [CrossRef]
- Khorsandi, H.; Nikpayam, O.; Yousefi, R.; Parandoosh, M.; Hosseinzadeh, N.; Saidpour, A.; Ghorbani, A. Zinc supplementation improves body weight management, infammatory biomarkers and insulin resistance in individuals with obesity: A randomized, placebo-controlled, double-blind trial. Diabetol. Metab. Syndr. 2019, 11, 101. [Google Scholar] [CrossRef]
- Vimaleswaran, K.S.; Berry, D.J.; Lu, C.; Tikkanen, E.; Pilz, S.; Hiraki, L.T. Causal relationship between obesity and vitamin D status: Bi-directional Mendelian randomization analysis of multiple cohorts. PLoS Med. 2013. [Google Scholar] [CrossRef]
- Zakharova, I.; Klimov, L.; Kuryaninova, V.; Nikitina, I.; Malyavskaya, S.; Svetlana Dolbnya, S.; Kasyanova, A.; Atanesyan, R.; Stoyan, M.; Todieva, A.; et al. Vitamin D Insufficiency in Overweight and Obese Children and Adolescents. Front. Endocrinol. (Lausanne) 2019, 10, 103. [Google Scholar] [CrossRef]
- Tagliaferri, S.; Porri, D.; De Giuseppe, R.; Manuelli, M.; Alessio, F.; Cena, H. The Controversial Role of Vitamin D as an Antioxidant: Results from Randomised Controlled Trials. Nutr. Res. Rev. 2019, 32, 99–105. [Google Scholar] [CrossRef]
- Christakos, S.; Dhawan, P.; Verstuyf, A.; Verlinden, L.; Carmeliet, G. Vitamin D: Metabolism, Molecular Mechanism of Action, and Pleiotropic Effects. Physiol. Rev. 2016, 96, 365–408. [Google Scholar] [CrossRef] [PubMed]
- Reyman, M.; Verrijn Stuart, A.A.; van Summeren, M.; Rakhshandehroo, M.; Nuboer, R.; de Boer, F.K.; van den Ham, H.J.; Kalkhoven, E.; Prakken, B.; Schipper, H.S. Vitamin D Deficiency in Childhood Obesity Is Associated With High Levels of Circulating Inflammatory Mediators, and Low Insulin Sensitivity. Int. J. Obes. (Lond.) 2014, 38, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Mousa, A.; Naderpoor, N.; Teede, H.; Scragg, R.; de Courten, B. Vitamin D supplementation for improvement of chronic low-grade inflammation in patients with type 2 diabetes: A systematic review and meta-analysis of randomized controlled trials. Nutr. Rev. 2018, 76, 380–394. [Google Scholar] [CrossRef]
- Pisani, D.F.; Amri, E.Z.; Ailhaud, G. Disequilibrium of polyunsaturated fatty acids status and its dual effect in modulating adipose tissue development and functions. OCL 2015, 22, D405. [Google Scholar] [CrossRef]
- Del-Río-Navarro, B.E.; Miranda-Lora, A.L.; Huang, F.; Hall-Mondragon, M.S.; Leija-Martínez, J.J. Effect of supplementation with omega-3 fatty acids on hypertriglyceridemia in pediatric patients with obesity. Pediatr. Endocrinol. Metab. 2019, 32, 811–819. [Google Scholar] [CrossRef]
- Pischon, T.; Hankinson, S.E.; Hotamisligil, G.S.; Rifai, N.; Willett, W.C.; Rimm, E.B. Habitual dietary intake of n-3 and n-6 fatty acids in relation to inflammatory markers among us men and women. Circulation 2003, 108, 155–160. [Google Scholar] [CrossRef]
- Lopez-Garcia, E.; Schulze, M.B.; Manson, J.E.; Meigs, J.B.; Albert, C.M.; Rifai, N.; Willett, W.C.; Hu, F.B. Consumption of (n-3) fatty acids is related to plasma biomarkers of inflammation and endothelial activation in women. J. Nutr. 2004, 134, 1806–1811. [Google Scholar] [CrossRef]
- Ferrucci, L.; Cherubini, A.; Bandinelli, S.; Bartali, B.; Corsi, A.; Lauretani, F. Relationship of plasma polyunsaturated fatty acids to circulating inflammatory markers. J. Clin. Endocr. Metab. 2006, 91, 439–446. [Google Scholar] [CrossRef]
- Chaves Curioni, C.; Roig Alves, N.N.; Zago, L. Omega-3 supplementation in the treatment of overweight and obese children and adolescents: A systematic review. J. Funct. Foods 2019, 52, 340–347. [Google Scholar] [CrossRef]
- Connaughton, R.M. Impact of anti-inflammatory nutrients on obesity-associated metabolic-inflammation from childhood through to adulthood. In Proceedings of the Nutrition Society Irish Section Meeting, Conference on ‘Nutrition at Key Life Stages: New Findings, New Approaches’ Symposium 1: Nutritional Issues in Adolescence and Adulthood, University College Cork, Cork, UK, 16–19 June 2015. [Google Scholar]
- Cena, H.; Calder, P.C. Defining a Healthy Diet: Evidence for the Role of Contemporary Dietary Patterns in Health and Disease. Nutrients 2020, 12, 334. [Google Scholar] [CrossRef]
- Wang, Y.F.; Xue, H. American Cancer Society Guideline for Diet and Physical Activity for Cancer Prevention. Pediatric Obes. 2019. [Google Scholar] [CrossRef]
- Sureda, A.; Bibiloni, M.D.M.; Julibert, A.; Bouzas, C.; Argelich, E.; Llompart, I.; Pons, A.; Tur, J.A. Adherence to the Mediterranean Diet and Inflammatory Markers. Nutrients 2018, 10, 62. [Google Scholar] [CrossRef]
- Goodpaster, B.H.; Sparks, L.M. Metabolic Flexibility in Health and Disease. Cell Metab. 2017, 25, 1027–1036. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Martínez-González, M.A.; Tong, T.Y.N.; Forouhi, N.G.; Khandelwal, S.; Prabhakaran, D.; Mozaffarian, D.; de Lorgeril, M. Definitions and potential health benefits of the Mediterranean diet: Views from experts around the world. BMC Med. 2014, 12, 112. [Google Scholar] [CrossRef]
- Shan, Z.; Li, Y.; Baden, M.Y.; Bhupathiraju, S.N.; Wang, D.D.; Sun, Q.; Rexrode, K.M.; Rimm, E.B.; Qi, L.; Willett, W.C.; et al. Association Between Healthy Eating Patterns and Risk of Cardiovascular Disease. JAMA Intern. Med. 2020, 180, 1090–1100. [Google Scholar] [CrossRef]
- Riccioni, G.; Speranza, L.; Pesce, M.; Cusenza, S.; D’Orazio, N.; Glade, M.J. Novel phytonutrient contributors to antioxidant protection against cardiovascular disease. Nutrition 2012, 28, 605–610. [Google Scholar] [CrossRef]
- Sari, I.; Baltaci, Y.; Bagci, C.; Davutoglu, V.; Erel, O.; Celik, H.; Ozer, O.; Aksoy, N.; Aksoy, M. Effect of pistachio diet on lipid parameters, endothelial function, inflammation, and oxidative status: A prospective study. Nutrition 2010, 26, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Wisnuwardan, R.W. Polyphenol intake and metabolic syndrome risk in European adolescents: The HELENA study. Eur. J. Nutr. 2020, 59, 801–812. [Google Scholar] [CrossRef]
- Siriwardhana, N.; Kalupahana, N.S.; Cekanova, M.; LeMieux, M.; Greer, B.; Moustaid-Moussa, N. Modulation of adipose tissue inflammation by bioactive food compounds. J. Nutr. Biochem. 2013, 24, 613–623. [Google Scholar] [CrossRef]
- Abeysekara, P.; Turchi, R.; O’Neil, M. Obesity and children with special healthcare needs: Special considerations for a special population. Curr. Opin. Pediatr. 2014, 26, 508–515. [Google Scholar] [CrossRef]
- Wang, Y.; Jia, P.; Cheng, X.; Xue, H. Improvement in food environments may help prevent childhood obesity: Evidence from a 9-year cohort study. Pediatr. Obes. 2019. [Google Scholar] [CrossRef]
- Sirico, F.; Bianco, A.; D’Alicandro, G.; Castaldo, C.; Montagnani, S.; Spera, R.; Di Meglio, F.; Nurzynska, D. Effects of Physical Exercise on Adiponectin, Leptin, and Inflammatory Markers in Childhood Obesity: Systematic Review and Meta-Analysis. Child Obes. 2018, 14, 207–217. [Google Scholar] [CrossRef]
- McMurray, R.G.; Ondrak, K.S. Cardiometabolic risk factors in children: The importance of physical activity. Am. J. Lifestyle Med. 2013, 7, 292–303. [Google Scholar] [CrossRef]
- Jurdana, M.; Jenko-Praznikar, Z.; Mohorko, N.; Petelin, A.; Jakus, T.; Simunic, B.; Pisot, R. Impact of 14-day bed rest on serum adipokines and low-grade inflammation in younger and older adults. Age (Dordr.) 2015, 37, 116. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calcaterra, V.; Regalbuto, C.; Porri, D.; Pelizzo, G.; Mazzon, E.; Vinci, F.; Zuccotti, G.; Fabiano, V.; Cena, H. Inflammation in Obesity-Related Complications in Children: The Protective Effect of Diet and Its Potential Role as a Therapeutic Agent. Biomolecules 2020, 10, 1324. https://doi.org/10.3390/biom10091324
Calcaterra V, Regalbuto C, Porri D, Pelizzo G, Mazzon E, Vinci F, Zuccotti G, Fabiano V, Cena H. Inflammation in Obesity-Related Complications in Children: The Protective Effect of Diet and Its Potential Role as a Therapeutic Agent. Biomolecules. 2020; 10(9):1324. https://doi.org/10.3390/biom10091324
Chicago/Turabian StyleCalcaterra, Valeria, Corrado Regalbuto, Debora Porri, Gloria Pelizzo, Emanuela Mazzon, Federica Vinci, Gianvincenzo Zuccotti, Valentina Fabiano, and Hellas Cena. 2020. "Inflammation in Obesity-Related Complications in Children: The Protective Effect of Diet and Its Potential Role as a Therapeutic Agent" Biomolecules 10, no. 9: 1324. https://doi.org/10.3390/biom10091324
APA StyleCalcaterra, V., Regalbuto, C., Porri, D., Pelizzo, G., Mazzon, E., Vinci, F., Zuccotti, G., Fabiano, V., & Cena, H. (2020). Inflammation in Obesity-Related Complications in Children: The Protective Effect of Diet and Its Potential Role as a Therapeutic Agent. Biomolecules, 10(9), 1324. https://doi.org/10.3390/biom10091324








