Characterization of In Vivo Function(s) of Members of the Plant Mitochondrial Carrier Family
Abstract
1. Introduction
2. Expression of MCF Members
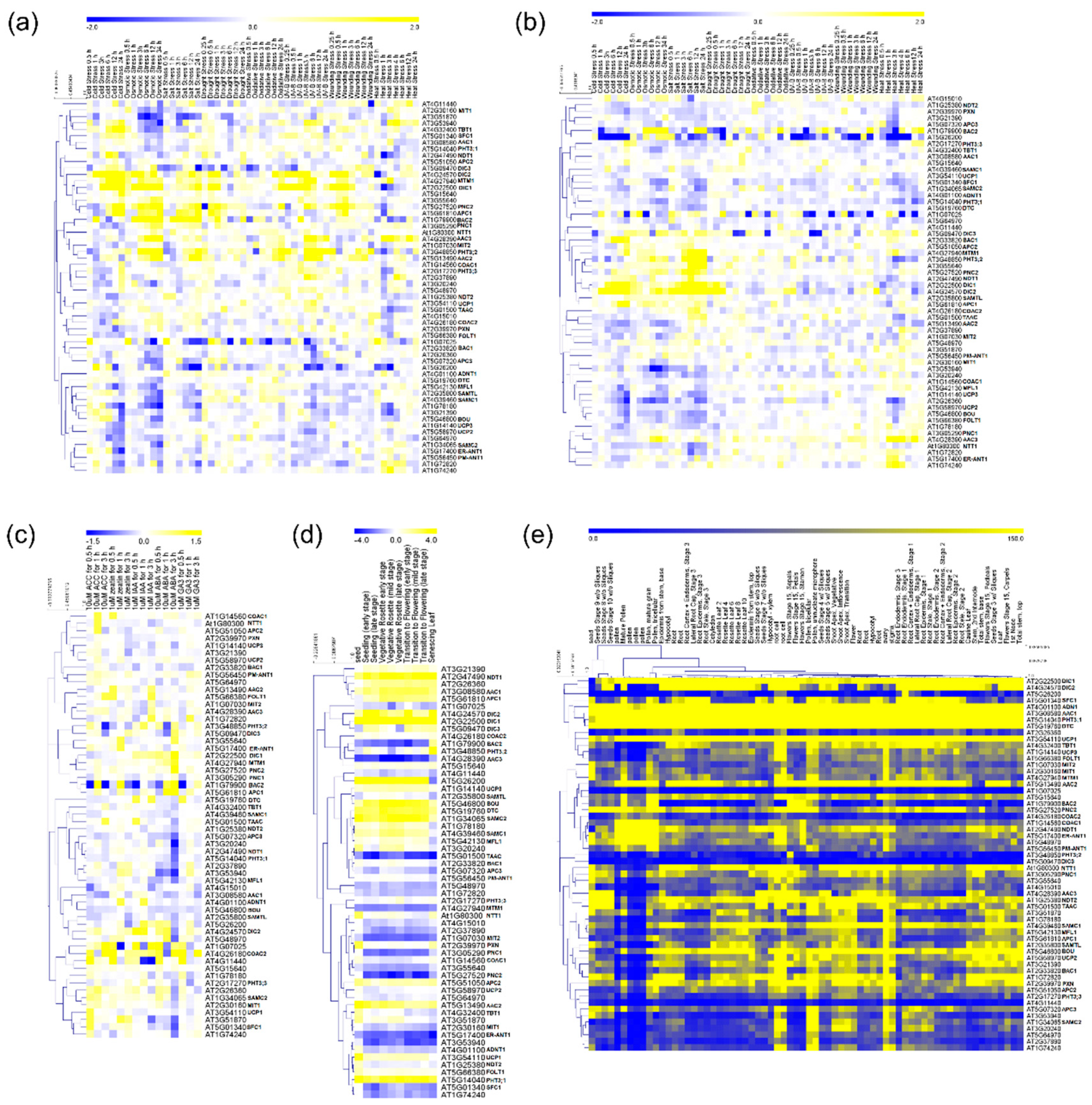
2.1. Environmental Specific Gene Expression
2.2. Hormone Treatment Gene Expression
2.3. Developmental-Specific Gene Expression
2.4. Tissue-Specific Gene Expression
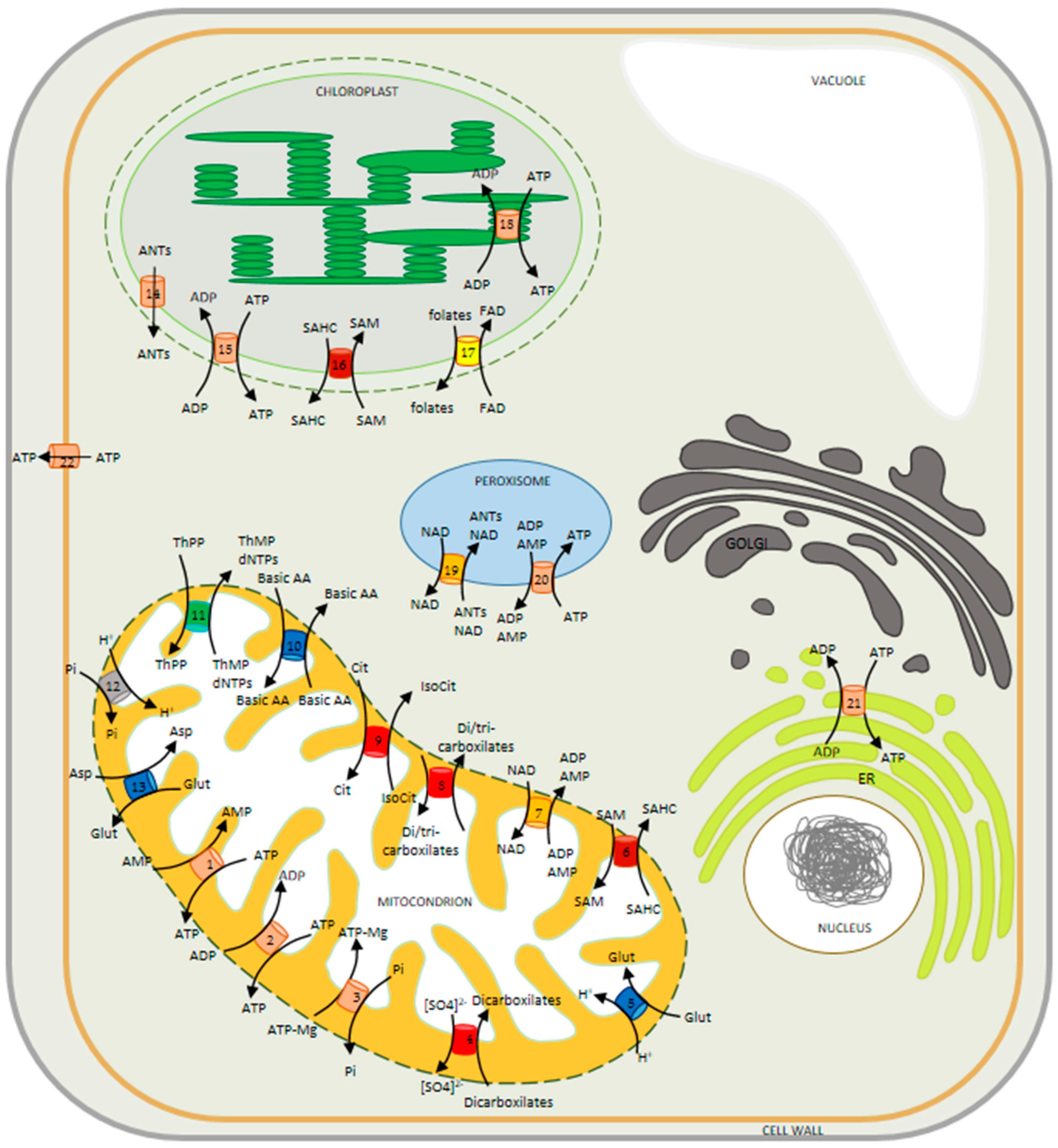
3. Subcellular Localization of MCF Members and Characterization of Lines Deficient in the Expression of the Transporters
3.1. Non-Mitochondrial MCFs
3.2. Mitochondrial MCFs
3.2.1. Coenzyme A Transporters
3.2.2. Phosphate Transporters
3.2.3. NAD Transporters
3.2.4. Uncoupling Proteins
3.2.5. Organic Acid Transporters
Dicarboxylic Acid Transporters
Dicarboxylic/Tricarboxylic Acid Transporters
Succinate/Fumarate Transporter
3.2.6. Amino Acid Transporters
3.2.7. Iron Transporters
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kunji, E.R.S.; Robinson, A.J. Coupling of proton and substrate translocation in the transport cycle of mitochondrial carriers. Curr. Opin. Struct. Biol. 2010, 20, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, F. The mitochondrial transporter family (slc25): Physiological and pathological implications. Pflug. Arch. Eur. J. Physiol. 2004, 447, 689–709. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, F. New functions for novel mitochondrial transporters. Biochim. Biophys. Acta Bioenerg. 2008, 1777, S3. [Google Scholar] [CrossRef][Green Version]
- Klingenberg, M. The ADP and ATP transport in mitochondria and its carrier. Biochim. Biophys. Acta Biomembr. 2008, 1778, 1978–2021. [Google Scholar] [CrossRef]
- Palmieri, F.; Pierri, C.L. Mitochondrial metabolite transport. Essays Biochem. 2010, 47, 37–52. [Google Scholar]
- Palmieri, F.; Pierri, C.L. Structure and function of mitochondrial carriers - role of the transmembrane helix p and g residues in the gating and transport mechanism. Febs Lett. 2010, 584, 1931–1939. [Google Scholar] [CrossRef]
- Satrustegui, J.; Pardo, B.; Del Arco, A. Mitochondrial transporters as novel targets for intracellular calcium signaling. Physiol. Rev. 2007, 87, 29–67. [Google Scholar] [CrossRef]
- Monne, M.; Miniero, D.V.; Daddabbo, L.; Palmieri, L.; Porcelli, V.; Palmieri, F. Mitochondrial transporters for ornithine and related amino acids: A review. Amino Acids 2015, 47, 1763–1777. [Google Scholar] [CrossRef]
- Monne, M.; Vozza, A.; Lasorsa, F.M.; Porcelli, V.; Palmieri, F. Mitochondrial carriers for aspartate, glutamate and other amino acids: A review. Int. J. Mol. Sci. 2019, 20, 4456. [Google Scholar] [CrossRef]
- Palmieri, F. Mitochondrial transporters of the SLC25 family and associated diseases: A review. J. Inherit. Metab. Dis. 2014, 37, 565–575. [Google Scholar] [CrossRef]
- Robinson, A.J.; Kunji, E.R.; Gross, A. Mitochondrial carrier homolog 2 (MTCH2): The recruitment and evolution of a mitochondrial carrier protein to a critical player in apoptosis. Exp. Cell Res. 2012, 318, 1316–1323. [Google Scholar] [CrossRef] [PubMed]
- Ruprecht, J.J.; Kunji, E.R.S. The SLC25 mitochondrial carrier family: Structure and mechanism. Trends Biochem. Sci. 2020, 45, 244–258. [Google Scholar] [CrossRef] [PubMed]
- Taylor, E.B. Functional properties of the mitochondrial carrier system. Trends Cell Biol. 2017, 27, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, F.; Pierri, C.L.; De Grassi, A.; Nunes-Nesi, A.; Fernie, A.R. Evolution, structure and function of mitochondrial carriers: A review with new insights. Plant J. 2011, 66, 161–181. [Google Scholar] [CrossRef] [PubMed]
- Fernie, A.R.; Cavalcanti, J.H.F.; Nunes-Nesi, A. Metabolic roles of plant mitochondrial carriers. Biomolecules 2020, 10, 1013. [Google Scholar] [CrossRef]
- Genevestigator. Available online: https://genevestigator.com/gv/ (accessed on 24 May 2020).
- Ferrari, C.; Shivhare, D.; Hansen, B.O.; Pasha, A.; Esteban, E.; Provart, N.J.; Kragler, F.; Fernie, A.R.; Tohge, T.; Mutwil, M. Expression atlas of Selaginella moellendorffii provides insights into the evolution of vasculature, secondary metabolism, and roots. Plant Cell 2020, 32, 853–870. [Google Scholar] [CrossRef]
- Sibout, R.; Proost, S.; Hansen, B.O.; Vaid, N.; Giorgi, F.M.; Ho-Yue-Kuang, S.; Legee, F.; Cezart, L.; Bouchabke-Coussa, O.; Soulhat, C.; et al. Expression atlas and comparative coexpression network analyses reveal important genes involved in the formation of lignified cell wall in brachypodium distachyon. New Phytol. 2017, 215, 1009–1025. [Google Scholar] [CrossRef]
- Schwacke, R.; Ponce-Soto, G.Y.; Krause, K.; Bolger, A.M.; Arsova, B.; Hallab, A.; Gruden, K.; Stitt, M.; Bolger, M.E.; Usadel, B. Mapman4: A refined protein classification and annotation framework applicable to multi-omics data analysis. Mol. Plant 2019, 12, 879–892. [Google Scholar] [CrossRef]
- Mutwil, M.; Klie, S.; Tohge, T.; Giorgi, F.M.; Wilkins, O.; Campbell, M.M.; Fernie, A.R.; Usadel, B.; Nikoloski, Z.; Persson, S. Planet: Combined sequence and expression comparisons across plant networks derived from seven species. Plant Cell 2011, 23, 895–910. [Google Scholar] [CrossRef]
- Ruprecht, C.; Mendrinna, A.; Tohge, T.; Sampathkumar, A.; Klie, S.; Fernie, A.R.; Nikoloski, Z.; Persson, S.; Mutwil, M. Famnet: A framework to identify multiplied modules driving pathway expansion in plants. Plant Physiol. 2016, 170, 1878–1894. [Google Scholar] [CrossRef]
- Alejandro, S.; Lee, Y.; Tohge, T.; Sudre, D.; Osorio, S.; Park, J.; Bovet, L.; Lee, Y.; Geldner, N.; Fernie, A.R.; et al. AtABCG29 is a monolignol transporter involved in lignin biosynthesis. Curr. Biol. 2012, 22, 1207–1212. [Google Scholar] [CrossRef] [PubMed]
- Da Fonseca-Pereira, P.; Neri-Silva, R.; Cavalcanti, J.H.F.; Brito, D.S.; Weber, A.P.M.; Araujo, W.L.; Nunes-Nesi, A. Data-mining bioinformatics: Connecting adenylate transport and metabolic responses to stress. Trends Plant Sci. 2018, 23, 961–974. [Google Scholar] [CrossRef] [PubMed]
- Haferkamp, I.; Schmitz-Esser, S. The plant mitochondrial carrier family: Functional and evolutionary aspects. Front. Plant Sci. 2012, 3, 2. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Z. Evolution by gene duplication: An update. Trends Ecol. Evol. 2003, 18, 292–298. [Google Scholar] [CrossRef]
- Palmieri, F.; Rieder, B.; Ventrella, A.; Blanco, E.; Do, P.T.; Nunes-Nesi, A.; Trauth, A.U.; Fiermonte, G.; Tjaden, J.; Agrimi, G.; et al. Molecular identification and functional characterization of Arabidopsis thaliana mitochondrial and chloroplastic NAD+ carrier proteins. J. Biol. Chem. 2009, 284, 31249–31259. [Google Scholar] [CrossRef]
- de Souza Chaves, I.; Feitosa-Araujo, E.; Florian, A.; Medeiros, D.B.; da Fonseca-Pereira, P.; Charton, L.; Heyneke, E.; Apfata, J.A.C.; Pires, M.V.; Mettler-Altmann, T.; et al. The mitochondrial NAD+ transporter (NDT1) plays important roles in cellular NAD+ homeostasis in Arabidopsis thaliana. Plant J. Cell Mol. Biol. 2019, 100, 487–504. [Google Scholar] [CrossRef]
- Feitosa-Araujo, E.; Chaves, I.S.; Florian, A.; da Fonseca-Pereira, P.; Apfata, J.A.C.; Heyneke, E.; Medeiros, D.B.; Pires, M.V.; Mettler-Altmann, T.; Neuhaus, H.E.; et al. Down-regulation of a mitochondrial NAD+ transporter (NDT2) alters seed production and germination in Arabidopsis. Plant Cell Physiol. 2020, 61, 897–908. [Google Scholar] [CrossRef]
- Bouvier, F.; Linka, N.; Isner, J.-C.; Mutterer, J.; Weber, A.P.M.; Camara, B. Arabidopsis SAMT1 defines a plastid transporter regulating plastid biogenesis and plant development. Plant Cell 2006, 18, 3088–3105. [Google Scholar] [CrossRef]
- Agrimi, G.; Di Noia, M.A.; Marobbio, C.M.T.; Fiermonte, G.; Lasorsa, F.M.; Palmieri, F. Identification of the human mitochondrial s-adenosylmethionine transporter: Bacterial expression, reconstitution, functional characterization and tissue distribution. Biochem. J. 2004, 379, 183–190. [Google Scholar] [CrossRef]
- Palmieri, L.; Arrigoni, R.; Blanco, E.; Carrari, F.; Zanor, M.I.; Studart-Guimaraes, C.; Fernie, A.R.; Palmieri, F. Molecular identification of an arabidopsis s-adenosylmethionine transporter. Analysis of organ distribution, bacterial expression, reconstitution into liposomes, and functional characterization. Plant Physiol. 2006, 142, 855–865. [Google Scholar] [CrossRef]
- Bahaji, A.; Jose Munoz, F.; Ovecka, M.; Baroja-Fernandez, E.; Montero, M.; Li, J.; Hidalgo, M.; Almagro, G.; Teresa Sesma, M.; Ezquer, I.; et al. Specific delivery of AtBT1 to mitochondria complements the aberrant growth and sterility phenotype of homozygous Atbt1 Arabidopsis mutants. Plant J. 2011, 68, 1115–1121. [Google Scholar] [CrossRef] [PubMed]
- Bahaji, A.; Munoz, F.J.; Segui-Simarro, J.M.; Camacho-Fernandez, C.; Rivas-Sendra, A.; Parra-Vega, V.; Ovecka, M.; Li, J.; Sanchez-Lopez, A.M.; Almagro, G.; et al. Mitochondrial Zea mays Brittle1-1 is a major determinant of the metabolic fate of incoming sucrose and mitochondrial function in developing maize endosperms. Front. Plant Sci. 2019, 10, 242. [Google Scholar] [CrossRef] [PubMed]
- Bahaji, A.; Ovecka, M.; Barany, I.; Carmen Risueno, M.; Jose Munoz, F.; Baroja-Fernandez, E.; Montero, M.; Li, J.; Hidalgo, M.; Teresa Sesma, M.; et al. Dual targeting to mitochondria and plastids of AtBT1 and ZmBT1, two members of the mitochondrial carrier family. Plant Cell Physiol. 2011, 52, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Leroch, M.; Neuhaus, H.E.; Kirchberger, S.; Zimmermann, S.; Melzer, M.; Gerhold, J.; Tjaden, J. Identification of a novel adenine nucleotide transporter in the endoplasmic reticulum of Arabidopsis. Plant Cell 2008, 20, 438–451. [Google Scholar] [CrossRef]
- Rieder, B.; Neuhaus, H.E. Identification of an arabidopsis plasma membrane-located ATP transporter important for anther development. Plant Cell 2011, 23, 1932–1944. [Google Scholar] [CrossRef]
- The Bio-Analytic Resource for Plant Biology. Available online: http://www.bar.utoronto.ca/# (accessed on 24 May 2020).
- Klepikova, A.V.; Kasianov, A.S.; Gerasimov, E.S.; Logacheva, M.D.; Penin, A.A. A high resolution map of the Arabidopsis thaliana developmental transcriptome based on RNA-seq profiling. Plant J. Cell Mol. Biol. 2016, 88, 1058–1070. [Google Scholar] [CrossRef]
- Toka, I.; Planchais, S.; Cabassa, C.; Justin, A.-M.; De Vos, D.; Richard, L.; Savoure, A.; Carol, P. Mutations in the hyperosmotic stress-responsive mitochondrial basic amino acid carrier2 enhance proline accumulation in Arabidopsis. Plant Physiol. 2010, 152, 1851–1862. [Google Scholar] [CrossRef]
- Planchais, S.; Cabassa, C.; Toka, I.; Justin, A.M.; Renou, J.P.; Savoure, A.; Carol, P. Basic amino acid carrier 2 gene expression modulates arginine and urea content and stress recovery in Arabidopsis leaves. Front. Plant Sci. 2014, 5, 330. [Google Scholar] [CrossRef]
- Figueira, T.R.; Arruda, P. Differential expression of uncoupling mitochondrial protein and alternative oxidase in the plant response to stress. J. Bioenerg. Biomembr. 2011, 43, 67–70. [Google Scholar] [CrossRef]
- Smith, A.M.; Ratcliffe, R.G.; Sweetlove, L.J. Activation and function of mitochondrial uncoupling protein in plants. J. Biol. Chem. 2004, 279, 51944–51952. [Google Scholar] [CrossRef]
- Borecky, J.; Nogueira, F.T.; de Oliveira, K.A.; Maia, I.G.; Vercesi, A.E.; Arruda, P. The plant energy-dissipating mitochondrial systems: Depicting the genomic structure and the expression profiles of the gene families of uncoupling protein and alternative oxidase in monocots and dicots. J. Exp. Bot. 2006, 57, 849–864. [Google Scholar] [CrossRef] [PubMed]
- Van Aken, O.; Zhang, B.; Carrie, C.; Uggalla, V.; Paynter, E.; Giraud, E.; Whelan, J. Defining the mitochondrial stress response in Arabidopsis thaliana. Mol. Plant 2009, 2, 1310–1324. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, D.M.; Araújo, W.L.; Fernie, A.R.; Schippers, J.H.; Mueller-Roeber, B. Translatome and metabolome effects triggered by gibberellins during rosette growth in Arabidopsis. J. Exp. Bot. 2012, 63, 2769–2786. [Google Scholar] [CrossRef] [PubMed]
- Racca, S.; Welchen, E.; Gras, D.E.; Tarkowská, D.; Turečková, V.; Maurino, V.G.; Gonzalez, D.H. Interplay between cytochrome c and gibberellins during Arabidopsis vegetative development. Plant J. Cell Mol. Biol. 2018, 94, 105–121. [Google Scholar] [CrossRef]
- Keech, O.; Pesquet, E.; Ahad, A.; Askne, A.; Nordvall, D.; Vodnala, S.M.; Tuominen, H.; Hurry, V.; Dizengremel, P.; Gardeström, P. The different fates of mitochondria and chloroplasts during dark-induced senescence in Arabidopsis leaves. Plant Cell Environ. 2007, 30, 1523–1534. [Google Scholar] [CrossRef]
- Hildebrandt, T.M.; Nunes Nesi, A.; Araújo, W.L.; Braun, H.P. Amino acid catabolism in plants. Mol. Plant 2015, 8, 1563–1579. [Google Scholar] [CrossRef]
- Chrobok, D.; Law, S.R.; Brouwer, B.; Lindén, P.; Ziolkowska, A.; Liebsch, D.; Narsai, R.; Szal, B.; Moritz, T.; Rouhier, N.; et al. Dissecting the metabolic role of mitochondria during developmental leaf senescence. Plant Physiol. 2016, 172, 2132–2153. [Google Scholar] [CrossRef]
- Gallardo, K.; Job, C.; Groot, S.P.C.; Puype, M.; Demol, H.; Vandekerckhove, J.; Job, D. Importance of methionine biosynthesis for Arabidopsis seed germination and seedling growth. Physiol. Plant 2002, 116, 238–247. [Google Scholar] [CrossRef]
- Lee, C.P.; Millar, A.H. The plant mitochondrial transportome: Balancing metabolic demands with energetic constraints. Trends Plant Sci. 2016, 21, 662–676. [Google Scholar] [CrossRef]
- Toleco, M.R.; Naake, T.; Zhang, Y.; Heazlewood, J.L.; Fernie, A.R. Plant mitochondrial carriers: Molecular gatekeepers that help to regulate plant central carbon metabolism. Plants 2020, 9, 117. [Google Scholar] [CrossRef]
- Subcellular Localization Database for Arabidopsis Proteins. Available online: https://suba.live/ (accessed on 24 May 2020).
- ARAMEMNON, Plant Membrane Protein Database. Available online: http://aramemnon.uni-koeln.de (accessed on 24 May 2020).
- Eubel, H.; Meyer, E.H.; Taylor, N.L.; Bussell, J.D.; O’Toole, N.; Heazlewood, J.L.; Castleden, I.; Small, I.D.; Smith, S.M.; Millar, A.H. Novel proteins, putative membrane transporters, and an integrated metabolic network are revealed by quantitative proteomic analysis of Arabidopsis cell culture peroxisomes. Plant Physiol. 2008, 148, 1809–1829. [Google Scholar] [CrossRef] [PubMed]
- Millar, A.H.; Heazlewood, J.L. Genomic and proteomic analysis of mitochondrial carrier proteins in Arabidopsis. Plant Physiol. 2003, 131, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Linka, N.; Theodoulou, F.L.; Haslam, R.P.; Linka, M.; Napier, J.A.; Neuhaus, H.E.; Weber, A.P.M. Peroxisomal ATP import is essential for seedling development in Arabidopsis thaliana. Plant Cell 2008, 20, 3241–3257. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fukao, Y.; Hayashi, Y.; Mano, S.; Hayashi, M.; Nishimura, M. Developmental analysis of a putative ATP/ADP carrier protein localized on glyoxysomal membranes during the peroxisome transition in pumpkin cotyledons. Plant Cell Physiol. 2001, 42, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Arai, Y.; Hayashi, M.; Nishimura, M. Proteomic identification and characterization of a novel peroxisomal adenine nucleotide transporter supplying ATP for fatty acid beta-oxidation in soybean and Arabidopsis. Plant Cell 2008, 20, 3227–3240. [Google Scholar] [CrossRef]
- Bedhomme, M.; Hoffmann, M.; McCarthy, E.A.; Gambonnet, B.; Moran, R.G.; Rebeille, F.; Ravanel, S. Folate metabolism in plants—An Arabidopsis homolog of the mammalian mitochondrial folate transporter mediates folate import into chloroplasts. J. Biol. Chem. 2005, 280, 34823–34831. [Google Scholar] [CrossRef]
- Kirchberger, S.; Tjaden, J.; Neuhaus, H.E. Characterization of the Arabidopsis Brittle1 transport protein and impact of reduced activity on plant metabolism. Plant J. 2008, 56, 51–63. [Google Scholar] [CrossRef]
- Parsons, H.T.; Christiansen, K.; Knierim, B.; Carroll, A.; Ito, J.; Batth, T.S.; Smith-Moritz, A.M.; Morrison, S.; McInerney, P.; Hadi, M.Z.; et al. Isolation and proteomic characterization of the Arabidopsis golgi defines functional and novel components involved in plant cell wall biosynthesis. Plant Physiol. 2012, 159, 12–26. [Google Scholar] [CrossRef]
- Nikolovski, N.; Rubtsov, D.; Segura, M.P.; Miles, G.P.; Stevens, T.J.; Dunkley, T.P.; Munro, S.; Lilley, K.S.; Dupree, P. Putative glycosyltransferases and other plant golgi apparatus proteins are revealed by lopit proteomics. Plant Physiol. 2012, 160, 1037–1051. [Google Scholar] [CrossRef]
- Monne, M.; Daddabbo, L.; Gagneul, D.; Obata, T.; Hielscher, B.; Palmieri, L.; Miniero, D.V.; Fernie, A.R.; Weber, A.P.M.; Palmieri, F. Uncoupling proteins 1 and 2 (UCP1 and UCP2) from Arabidopsis thaliana are mitochondrial transporters of aspartate, glutamate, and dicarboxylates. J. Biol. Chem. 2018, 293, 4213–4227. [Google Scholar] [CrossRef]
- Bernhardt, K.; Wilkinson, S.; Weber, A.P.; Linka, N. A peroxisomal carrier delivers NAD+ and contributes to optimal fatty acid degradation during storage oil mobilization. Plant J. Cell Mol. Biol. 2012, 69, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, L.; Rottensteiner, H.; Girzalsky, W.; Scarcia, P.; Palmieri, F.; Erdmann, R. Identification and functional reconstitution of the yeast peroxisomal adenine nucleotide transporter. Embo J. 2001, 20, 5049–5059. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, C.; Plocharski, B.; Haferkamp, I.; Leroch, M.; Ewald, R.; Bauwe, H.; Riemer, J.; Herrmann, J.M.; Neuhaus, H.E. From endoplasmic reticulum to mitochondria: Absence of the Arabidopsis ATP antiporter endoplasmic reticulum adenylate transporter1 perturbs photorespiration. Plant Cell 2013, 25, 2647–2660. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.C.; Zimmermann, K.; Schorr, S.; Landini, M.; Klemens, P.A.W.; Altensell, J.; Jung, M.; Krause, E.; Nguyen, D.; Helms, V.; et al. Axer is an ATP/ADP exchanger in the membrane of the endoplasmic reticulum. Nat. Commun. 2018, 9, 3489. [Google Scholar] [CrossRef]
- Geigenberger, P.; Riewe, D.; Fernie, A.R. The central regulation of plant physiology by adenylates. Trends Plant Sci. 2010, 15, 98–105. [Google Scholar] [CrossRef]
- Thuswaldner, S.; Lagerstedt, J.O.; Rojas-Stuetz, M.; Bouhidel, K.; Der, C.; Leborgne-Castel, N.; Mishra, A.; Marty, F.; Schoefs, B.; Adamska, I.; et al. Identification, expression, and functional analyses of a thylakoid ATP/ADP carrier from Arabidopsis. J. Biol. Chem. 2007, 282, 8848–8859. [Google Scholar] [CrossRef]
- Comparot-Moss, S.; Denyer, K. The evolution of the starch biosynthetic pathway in cereals and other grasses. J. Exp. Bot. 2009, 60, 2481–2492. [Google Scholar] [CrossRef]
- Beckles, D.M.; Smith, A.M.; ap Rees, T. A cytosolic ADP-glucose pyrophosphorylase is a feature of graminaceous endosperms, but not of other starch-storing organs. Plant Physiol. 2001, 125, 818–827. [Google Scholar] [CrossRef]
- Yin, L.; Lundin, B.; Bertrand, M.; Nurmi, M.; Solymosi, K.; Kangasjarvi, S.; Aro, E.-M.; Schoefs, B.; Spetea, C. Role of thylakoid ATP/ADP carrier in photoinhibition and photoprotection of photosystem II in Arabidopsis. Plant Physiol. 2010, 153, 666–677. [Google Scholar] [CrossRef]
- Tzagoloff, A.; Jang, J.; Glerum, D.M.; Wu, M. Flx1 codes for a carrier protein involved in maintaining a proper balance of flavin nucleotides in yeast mitochondria. J. Biol. Chem. 1996, 271, 7392–7397. [Google Scholar] [CrossRef]
- Klaus, S.M.J.; Kunji, E.R.S.; Bozzo, G.G.; Noiriel, A.; de la Garza, R.D.; Basset, G.J.C.; Ravanel, S.; Rebeille, F.; Gregory, J.F.; Hanson, A.D. Higher plant plastids and cyanobacteria have folate carriers related to those of trypanosomatids. J. Biol. Chem. 2005, 280, 38457–38463. [Google Scholar] [CrossRef] [PubMed]
- Hanson, A.D.; Roje, S. One-carbon metabolism in higher plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001, 52, 119–137. [Google Scholar] [CrossRef] [PubMed]
- Marobbio, C.M.T.; Agrimi, G.; Lasorsa, F.M.; Palmieri, F. Identification and functional reconstitution of yeast mitochondrial carrier for s-adenosylmethionine. Embo J. 2003, 22, 5975–5982. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, D.; Morandini, P.; Ramirez, L.; Soave, C.; Murgia, I. Identification of an Arabidopsis mitoferrinlike carrier protein involved in Fe metabolism. Plant Physiol. Biochem. 2011, 49, 520–529. [Google Scholar] [CrossRef]
- Agrimi, G.; Russo, A.; Pierri, C.L.; Palmieri, F. The peroxisomal NAD+ carrier of Arabidopsis thaliana transports coenzyme A and its derivatives. J. Bioenerg. Biomembr. 2012, 44, 333–340. [Google Scholar] [CrossRef]
- Prohl, C.; Pelzer, W.; Diekert, K.; Kmita, H.; Bedekovics, T.; Kispal, G.; Lill, R. The yeast mitochondrial carrier leu5p and its human homologue graves’ disease protein are required for accumulation of coenzyme a in the matrix. Mol. Cell. Biol. 2001, 21, 1089–1097. [Google Scholar] [CrossRef]
- Fiermonte, G.; Paradies, E.; Todisco, S.; Marobbio, C.M.T.; Palmieri, F. A novel member of solute carrier family 25 (slc25a42) is a transporter of coenzyme a and adenosine 3′,5′-diphosphate in human mitochondria. J. Biol. Chem. 2009, 284, 18152–18159. [Google Scholar] [CrossRef]
- Zallot, R.; Agrimi, G.; Lerma-Ortiz, C.; Teresinski, H.J.; Frelin, O.; Ellens, K.W.; Castegna, A.; Russo, A.; de Crecy-Lagard, V.; Mullen, R.T.; et al. Identification of mitochondrial coenzyme a transporters from maize and Arabidopsis. Plant Physiol. 2013, 162, 581–588. [Google Scholar] [CrossRef]
- Hamel, P.; Saint-Georges, Y.; de Pinto, B.; Lachacinski, N.; Altamura, N.; Dujardin, G. Redundancy in the function of mitochondrial phosphate transport in Saccharomyces cerevisiae and Arabidopsis thaliana. Mol. Microbiol. 2004, 51, 307–317. [Google Scholar] [CrossRef]
- Zhu, W.; Miao, Q.; Sun, D.; Yang, G.; Wu, C.; Huang, J.; Zheng, C. The mitochondrial phosphate transporters modulate plant responses to salt stress via affecting ATP and gibberellin metabolism in Arabidopsis thaliana. PLoS ONE 2012, 7, e43530. [Google Scholar] [CrossRef]
- Jia, F.; Wan, X.; Zhu, W.; Sun, D.; Zheng, C.; Liu, P.; Huang, J. Overexpression of mitochondrial phosphate transporter 3 severely hampers plant development through regulating mitochondrial function in Arabidopsis. PLoS ONE 2015, 10, e0129717. [Google Scholar] [CrossRef] [PubMed]
- Senkler, J.; Senkler, M.; Eubel, H.; Hildebrandt, T.; Lengwenus, C.; Schertl, P.; Schwarzlander, M.; Wagner, S.; Wittig, I.; Braun, H.P. The mitochondrial complexome of Arabidopsis thaliana. Plant J. Cell Mol. Biol. 2017, 89, 1079–1092. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; He, Y.; Zhao, Y.; Xu, Q.; Wu, J.; Ma, H.; Guo, H.; Bai, L.; Zuo, J.; Zhou, J.M.; et al. Regulation of mitochondrial NAD pool via NAD+ transporter 2 is essential for matrix NADH homeostasis and ROS production in Arabidopsis. Sci. China Life Sci. 2019, 62, 991–1002. [Google Scholar] [CrossRef] [PubMed]
- Sweetlove, L.J.; Lytovchenko, A.; Morgan, M.; Nunes-Nesi, A.; Taylor, N.L.; Baxter, C.J.; Eickmeier, I.; Fernie, A.R. Mitochondrial uncoupling protein is required for efficient photosynthesis. Proc. Natl. Acad. Sci. USA 2006, 103, 19587–19592. [Google Scholar] [CrossRef]
- Vercesi, A.E.; Borecky, J.; Maia Ide, G.; Arruda, P.; Cuccovia, I.M.; Chaimovich, H. Plant uncoupling mitochondrial proteins. Annu. Rev. Plant Biol. 2006, 57, 383–404. [Google Scholar] [CrossRef]
- Borecky, J.; Maia, I.G.; Costa, A.D.T.; Jezek, P.; Chaimovich, H.; de Andrade, P.B.M.; Vercesi, A.E.; Arruda, P. Functional reconstitution of Arabidopsis thaliana plant uncoupling mitochondrial protein (AtPUMP1) expressed in Escherichia coli. Febs Lett. 2001, 505, 240–244. [Google Scholar] [CrossRef]
- Palmieri, L.; Picault, N.; Arrigoni, R.; Besin, E.; Palmieri, F.; Hodges, M. Molecular identification of three Arabidopsis thaliana mitochondrial dicarboxylate carrier isoforms: Organ distribution, bacterial expression, reconstitution into liposomes and functional characterization. Biochem. J. 2008, 410, 621–629. [Google Scholar] [CrossRef]
- Barreto, P.; Okura, V.K.; Neshich, I.A.; Maia Ide, G.; Arruda, P. Overexpression of UCP1 in tobacco induces mitochondrial biogenesis and amplifies a broad stress response. BMC Plant Biol. 2014, 14, 144. [Google Scholar] [CrossRef]
- Barreto, P.; Okura, V.; Pena, I.A.; Maia, R.; Maia, I.G.; Arruda, P. Overexpression of mitochondrial uncoupling protein 1 (UCP1) induces a hypoxic response in Nicotiana tabacum leaves. J. Exp. Bot. 2016, 67, 301–313. [Google Scholar] [CrossRef]
- Barreto, P.; Yassitepe, J.; Wilson, Z.A.; Arruda, P. Mitochondrial uncoupling protein 1 overexpression increases yield in Nicotiana tabacum under drought stress by improving source and sink metabolism. Front. Plant Sci. 2017, 8, 1836. [Google Scholar] [CrossRef]
- Sweetlove, L.J.; Beard, K.F.; Nunes-Nesi, A.; Fernie, A.R.; Ratcliffe, R.G. Not just a circle: Flux modes in the plant TCA cycle. Trends Plant Sci. 2010, 15, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, P.; Rugen, N.; Carrie, C.; Elsässer, M.; Finkemeier, I.; Giese, J.; Hildebrandt, T.M.; Kühn, K.; Maurino, V.G.; Ruberti, C.; et al. Single organelle function and organization as estimated from Arabidopsis mitochondrial proteomics. Plant J. Cell Mol. Biol. 2020, 101, 420–441. [Google Scholar] [CrossRef] [PubMed]
- Picault, N.; Palmieri, L.; Pisano, I.; Hodges, M.; Palmieri, F. Identification of a novel transporter for dicarboxylates and tricarboxylates in plant mitochondria–bacterial expression, reconstitution, functional characterization, and tissue distribution. J. Biol. Chem. 2002, 277, 24204–24211. [Google Scholar] [CrossRef] [PubMed]
- Regalado, A.; Pierri, C.L.; Bitetto, M.; Laera, V.L.; Pimentel, C.; Francisco, R.; Passarinho, J.; Chaves, M.M.; Agrimi, G. Characterization of mitochondrial dicarboxylate/tricarboxylate transporters from grape berries. Planta 2013, 237, 693–703. [Google Scholar] [CrossRef]
- Deng, W.; Luo, K.; Li, Z.; Yang, Y. Molecular cloning and characterization of a mitochondrial dicarboxylate/tricarboxylate transporter gene in citrus junos response to aluminum stress. Mitochondrial DNA 2008, 19, 376–384. [Google Scholar]
- Spagnoletta, A.; De Santis, A.; Tampieri, E.; Baraldi, E.; Bachi, A.; Genchi, G. Identification and kinetic characterization of HtDTC, the mitochondrial dicarboxylate-tricarboxylate carrier of jerusalem artichoke tubers. J. Bioenerg. Biomembr. 2006, 38, 57–65. [Google Scholar] [CrossRef]
- Genchi, G.; Spagnoletta, A.; De Santis, A.; Stefanizzi, L.; Palmieri, F. Purification and characterization of the reconstitutively active citrate carrier from maize mitochondria. Plant Physiol. 1999, 120, 841–848. [Google Scholar] [CrossRef]
- Catoni, E.; Schwab, R.; Hilpert, M.; Desimone, M.; Schwacke, R.; Flugge, U.I.; Schumacher, K.; Frommer, W.B. Identification of an Arabidopsis mitochondrial succinate-fumarate translocator. Febs Lett. 2003, 534, 87–92. [Google Scholar] [CrossRef]
- Brito, D.S.; Agrimi, G.; Charton, L.; Brilhaus, D.; Bitetto, M.G.; Lana-Costa, J.; Messina, E.; Nascimento, C.P.; Araujo, E.F.; Viana Pires, M.; et al. Biochemical and functional characterization of a mitochondrial citrate carrier in Arabidopsis thaliana. Biochem. J. 2020, 447, 1759–1777. [Google Scholar] [CrossRef]
- Nunes-Nesi, A.; Fernie, A.R.; Stitt, M. Metabolic and signaling aspects underpinning the regulation of plant carbon nitrogen interactions. Mol. Plant 2010, 3, 973–996. [Google Scholar] [CrossRef]
- Galili, G.; Amir, R.; Fernie, A.R. The regulation of essential amino acid synthesis and accumulation in plants. Annu. Rev. Plant Biol. 2016, 67, 153–178. [Google Scholar] [CrossRef] [PubMed]
- Lawand, S.; Dorne, A.J.; Long, D.; Coupland, G.; Mache, R.; Carol, P. Arabidopsis a bout de souffle, which is homologous with mammalian carnitine acyl carrier, is required for postembryonic growth in the light. Plant Cell 2002, 14, 2161–2173. [Google Scholar] [CrossRef] [PubMed]
- Eisenhut, M.; Planchais, S.; Cabassa, C.; Guivarc’h, A.; Justin, A.M.; Taconnat, L.; Renou, J.P.; Linka, M.; Gagneul, D.; Timm, S.; et al. Arabidopsis a bout de souffle is a putative mitochondrial transporter involved in photorespiratory metabolism and is required for meristem growth at ambient CO2 levels. Plant J. Cell Mol. Biol. 2013, 73, 836–849. [Google Scholar] [CrossRef] [PubMed]
- Porcelli, V.; Vozza, A.; Calcagnile, V.; Gorgoglione, R.; Arrigoni, R.; Fontanesi, F.; Marobbio, C.M.T.; Castegna, A.; Palmieri, F.; Palmieri, L. Molecular identification and functional characterization of a novel glutamate transporter in yeast and plant mitochondria. Biochim. Biophys. Acta Bioenerg. 2018, 1859, 1249–1258. [Google Scholar] [CrossRef]
- Hoyos, M.E.; Palmieri, L.; Wertin, T.; Arrigoni, R.; Polacco, J.C.; Palmieri, F. Identification of a mitochondrial transporter for basic amino acids in Arabidopsis thaliana by functional reconstitution into liposomes and complementation in yeast. Plant J. 2003, 33, 1027–1035. [Google Scholar] [CrossRef]
- Palmieri, L.; Todd, C.D.; Arrigoni, R.; Hoyos, M.E.; Santoro, A.; Polacco, J.C.; Palmieri, F. Arabidopsis mitochondria have two basic amino acid transporters with partially overlapping specificities and differential expression in seedling development. Biochim. Biophys. Acta Bioenerg. 2006, 1757, 1277–1283. [Google Scholar] [CrossRef]
- Catoni, E.; Desimone, M.; Hilpert, M.; Wipf, D.; Kunze, R.; Schneider, A.; Fluegge, U.-I.; Schumacher, K.; Frommer, W.B. Expression pattern of a nuclear encoded mitochondrial arginine-ornithine translocator gene from Arabidopsis. BMC Plant Biol. 2003, 3, 1. [Google Scholar] [CrossRef]
- Taylor, N.L.; Howell, K.A.; Heazlewood, J.L.; Tan, T.Y.; Narsai, R.; Huang, S.; Whelan, J.; Millar, A.H. Analysis of the rice mitochondrial carrier family reveals anaerobic accumulation of a basic amino acid carrier involved in arginine metabolism during seed germination. Plant Physiol. 2010, 154, 691–704. [Google Scholar] [CrossRef]
- Batista-Silva, W.; Heinemann, B.; Rugen, N.; Nunes-Nesi, A.; Araújo, W.L.; Braun, H.P.; Hildebrandt, T.M. The role of amino acid metabolism during abiotic stress release. Plant Cell Environ. 2019, 42, 1630–1644. [Google Scholar] [CrossRef]
- Shaw, G.C.; Cope, J.J.; Li, L.T.; Corson, K.; Hersey, C.; Ackermann, G.E.; Gwynn, B.; Lambert, A.J.; Wingert, R.A.; Traver, D.; et al. Mitoferrin is essential for erythroid iron assimilation. Nature 2006, 440, 96–100. [Google Scholar] [CrossRef]
- Paradkar, P.N.; Zumbrennen, K.B.; Paw, B.H.; Ward, D.M.; Kaplan, J. Regulation of mitochondrial iron import through differential turnover of mitoferrin 1 and mitoferrin 2. Mol. Cell Biol. 2009, 29, 1007–1016. [Google Scholar] [CrossRef] [PubMed]
- Metzendorf, C.; Wu, W.; Lind, M.I. Overexpression of drosophila mitoferrin in l(2)mbn cells results in dysregulation of fer1hch expression. Biochem. J. 2009, 421, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Bashir, K.; Ishimaru, Y.; Nishizawa, N.K. Identification and characterization of the major mitochondrial Fe transporter in rice. Plant Signal. Behav. 2011, 6, 1591–1593. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Dashner, Z.S.; Connolly, E.L. Mitochondrial iron transporters (MIT1 and MIT2) are essential for iron homeostasis and embryogenesis in Arabidopsis thaliana. Front. Plant Sci. 2019, 10, 1449. [Google Scholar] [CrossRef]
- Millar, A.H.; Heazlewood, J.L.; Giglione, C.; Holdsworth, M.J.; Bachmair, A.; Schulze, W.X. The scope, functions, and dynamics of posttranslational protein modifications. Annu. Rev. Plant Biol. 2019, 70, 119–151. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nunes-Nesi, A.; Cavalcanti, J.H.F.; Fernie, A.R. Characterization of In Vivo Function(s) of Members of the Plant Mitochondrial Carrier Family. Biomolecules 2020, 10, 1226. https://doi.org/10.3390/biom10091226
Nunes-Nesi A, Cavalcanti JHF, Fernie AR. Characterization of In Vivo Function(s) of Members of the Plant Mitochondrial Carrier Family. Biomolecules. 2020; 10(9):1226. https://doi.org/10.3390/biom10091226
Chicago/Turabian StyleNunes-Nesi, Adriano, João Henrique F. Cavalcanti, and Alisdair R. Fernie. 2020. "Characterization of In Vivo Function(s) of Members of the Plant Mitochondrial Carrier Family" Biomolecules 10, no. 9: 1226. https://doi.org/10.3390/biom10091226
APA StyleNunes-Nesi, A., Cavalcanti, J. H. F., & Fernie, A. R. (2020). Characterization of In Vivo Function(s) of Members of the Plant Mitochondrial Carrier Family. Biomolecules, 10(9), 1226. https://doi.org/10.3390/biom10091226





