The Influence of Nasturtium officinale R. Br. Agar and Agitated Microshoot Culture Media on Glucosinolate and Phenolic Acid Production, and Antioxidant Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Parent Plant Material
2.2. Initiation of In Vitro Cultures
2.3. Experimental In Vitro Cultures
2.3.1. Agar Microshoot Cultures
2.3.2. Agitated Microshoot Cultures
2.4. Biomass Growth
2.5. Spectrophotometric Analysis of the Total Glucosinolate Pool
2.6. Phenolic acid Analysis
2.7. Total Phenolic Assay
2.8. Antioxidant Capacity
2.8.1. CUPRAC Total Antioxidant Capacity Assay
2.8.2. Ferric Reducing Ability (FRAP) Assay
2.8.3. 1,1-Diphenyl-2-Picrylhydrazyl (DPPH) Radical-Scavenging Activity Assay
2.9. Statistical Model Fitting
3. Results
3.1. The Experimental In Vitro Cultures
3.1.1. Microshoot Appearance and Biomass Growth
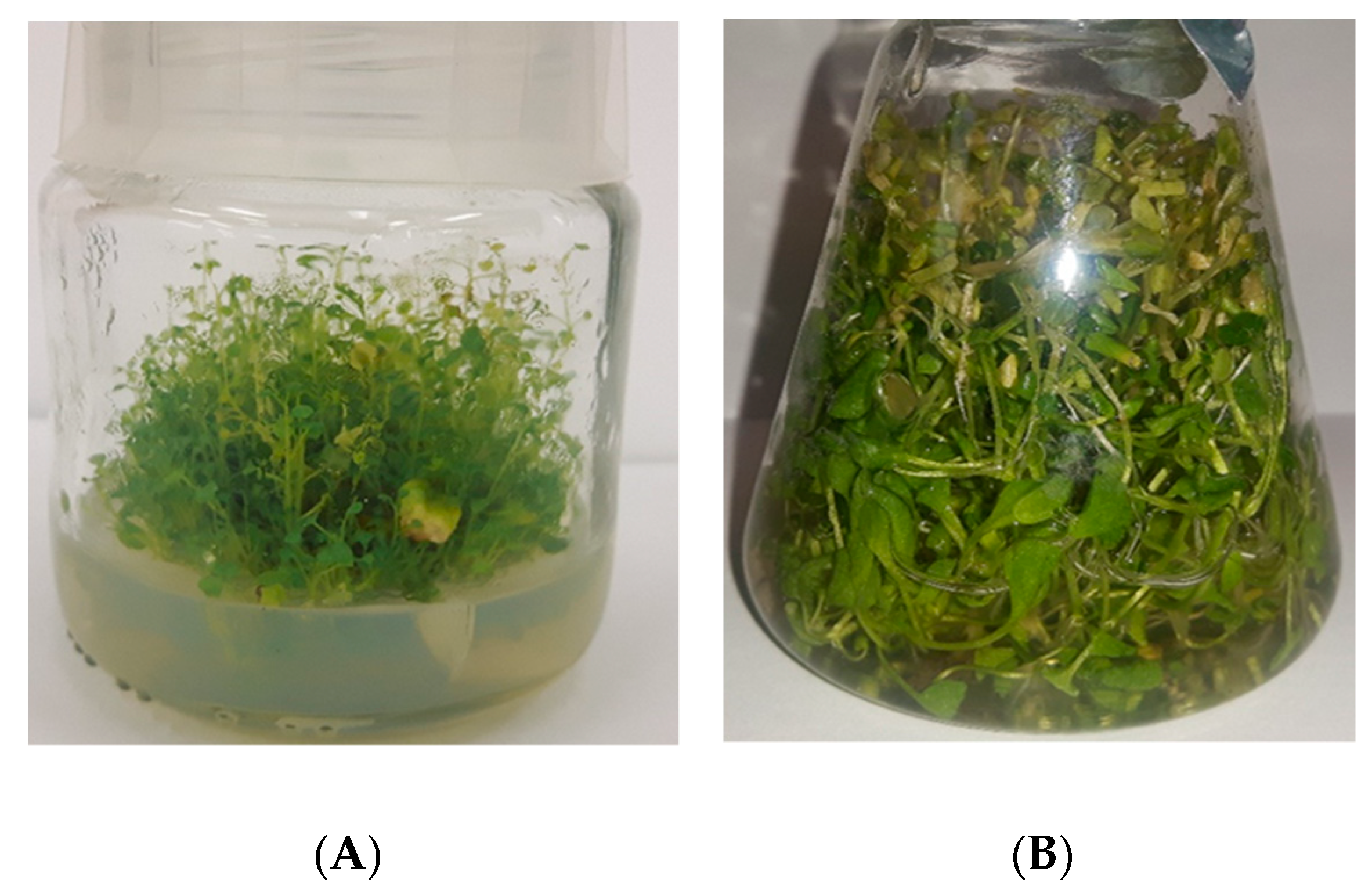
Agar Microshoot Cultures
Agitated Microshoot Cultures
Statistical Analysis
3.1.2. Effect of Supplementation with Different PGRs on Glucosinolate Production
Agar Microshoot Cultures
Agitated Microshoot Cultures
Statistical Analysis
3.1.3. Effect of PGRs on Phenolic Acid Production
Agar Microshoot Cultures
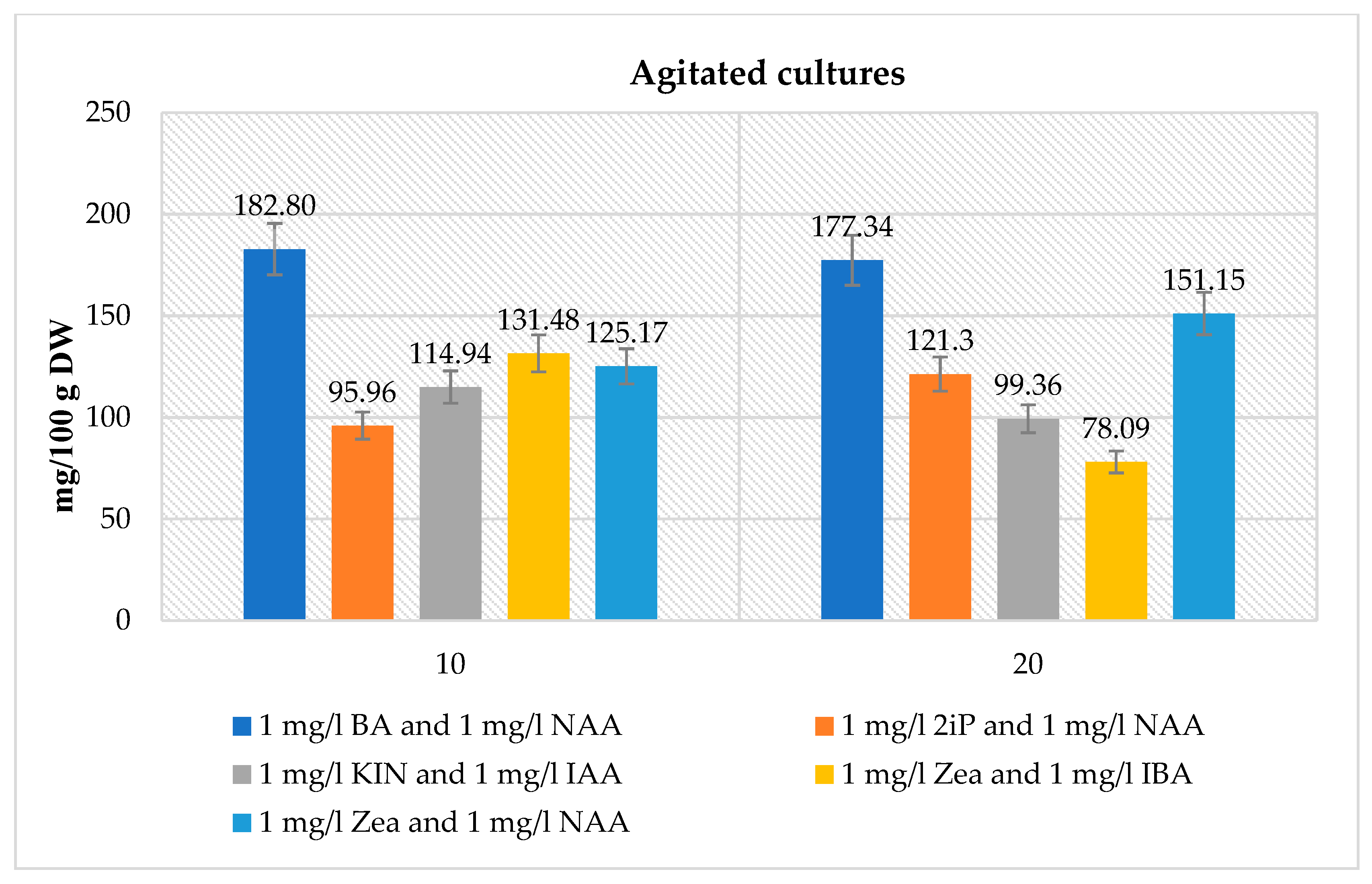
Agitated Microshoot Cultures
Statistical Analysis
- The amounts of gallic, protocatechuic, caffeic, and syringic acids, and the total amount of phenolic acids were significantly higher in the agitated cultures under the same conditions.
- Additions of BA, NAA, Zea, IAA, 2iP, KIN, 2,4-D and IBA caused an increase in many compounds and sometimes a decrease in p-coumaric, o-coumaric, or ferulic acids. This change was correlated with an opposite change of the slope against time (when average content is higher, production in time is lower, and vice versa). For the agitated cultures, the addition of NAA did not much increase the synthesis of gallic, protocatechuic, caffeic, and syringic acids, or the total amount of phenolic acids.
- There were several significant interactions among the added compounds, where the synthesis of the compounds was significantly lower than one could expect from the additivity of positive effects.
3.1.4. The Effect of PGRs on the Antioxidant Potential and Total Phenolic Content of Biomass
Agar Microshoot Cultures
Agitated Microshoot Cultures
Statistical Analysis
3.2. Parent Plant Material
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Klimek-Szczykutowicz, M.; Szopa, A.; Ekiert, H. Chemical composition, traditional and professional use in medicine, application in environmental protection, position in food and cosmetics industries, and biotechnological studies of Nasturtium officinale (watercress)—A review. Fitoterapia 2018, 129, 283–292. [Google Scholar] [CrossRef] [PubMed]
- IUCN The IUCN Red List of Threatened Species. Available online: https://www.iucnredlist.org/ (accessed on 22 January 2020).
- German Comission E Monographie BGA/BfArM, Kommission E. Available online: https://buecher.heilpflanzen-welt.de/BGA-Kommission-E-Monographien/ (accessed on 22 January 2020).
- German Comission D Monographie BGA/BfArM, Kommission D. Available online: https://buecher.heilpflanzen-welt.de/BGA-Kommission-D-Monographien/ (accessed on 22 January 2020).
- EFSA European Food Safety Authority (EFSA). Available online: http://www.efsa.europa.eu/ (accessed on 25 January 2020).
- Teixidor-Toneu, I.; Martin, G.J.; Ouhammou, A.; Puri, R.K.; Hawkins, J.A. An ethnomedicinal survey of a Tashelhit-speaking community in the High Atlas, Morocco. J. Ethnopharmacol. 2016, 188, 96–110. [Google Scholar] [CrossRef]
- Suroowan, S.; Mahomoodally, M.F. A comparative ethnopharmacological analysis of traditional medicine used against respiratory tract diseases in Mauritius. J. Ethnopharmacol. 2016, 177, 61–80. [Google Scholar] [CrossRef] [PubMed]
- Asadi-Samani, M.; Moradi, M.-T.; Mahmoodnia, L.; Alaei, S.; Asadi-Samani, F.; Luther, T. Traditional uses of medicinal plants to prevent and treat diabetes; an updated review of ethnobotanical studies in Iran. J. Nephropathol. 2017, 6, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Bahramikia, S.; Yazdanparast, R. Antioxidant efficacy of Nasturtium officinale extracts using various in vitro assay systems. JAMS J. Acupunct. Meridian Stud. 2010, 3, 283–290. [Google Scholar] [CrossRef]
- Yehuda, H.; Soroka, Y.; Zlotkin-Frušić, M.; Gilhar, A.; Milner, Y.; Tamir, S. Isothiocyanates inhibit psoriasis-related proinflammatory factors in human skin. Inflamm. Res. 2012, 61, 735–742. [Google Scholar] [CrossRef]
- Sadeghi, H.; Mostafazadeh, M.; Sadeghi, H.; Naderian, M.; Barmak, M.J.; Talebianpoor, M.S.; Mehraban, F. In vivo anti-inflammatory properties of aerial parts of Nasturtium officinale. Pharm. Biol. 2014, 52, 169–174. [Google Scholar] [CrossRef]
- Casanova, N.A.; Ariagno, J.I.; López Nigro, M.M.; Mendeluk, G.R.; de los Gette, M.A.; Petenatti, E.; Palaoro, L.A.; Carballo, M.A. In vivo antigenotoxic activity of watercress juice (Nasturtium officinale) against induced DNA damage. J. Appl. Toxicol. 2013, 33, 880–885. [Google Scholar] [CrossRef]
- Jeon, J.; Bong, S.J.; Park, J.S.; Park, Y.K.; Arasu, M.V.; Al-Dhabi, N.A.; Park, S.U. De novo transcriptome analysis and glucosinolate profiling in watercress (Nasturtium officinale R. Br.). BMC Genom. 2017, 18, 1–14. [Google Scholar] [CrossRef]
- Afsharypuor, S.; Salehi, M. Volatile constituents of leaves and stems of Nasturtium officinale R. Br. J. Essent. Oil Res. 2008, 20, 517–518. [Google Scholar] [CrossRef]
- Boligon, A.A.; Janovik, V.; Boligon, A.A.; Pivetta, C.R.; Pereira, R.P.; Rocha, J.B.T.D.; Athayde, M.L. HPLC analysis of polyphenolic compounds and antioxidant activity in Nasturtium officinale. Int. J. Food Prop. 2013, 16, 61–69. [Google Scholar] [CrossRef]
- Anuradha, S.N.; Vilashene, G.; Lalithambigai, J.; Arunkumar, S. “Cosmeceuticals”: An opinion in the direction of pharmaceuticals. Asian J. Pharm. Clin. Res. 2015, 8, 64–69. [Google Scholar]
- Martínez-Sánchez, A.; Gil-Izquierdo, A.; Gil, M.I.; Ferreres, F. A comparative study of flavonoid compounds, vitamin C, and antioxidant properties of baby leaf Brassicaceae species. J. Agric. Food Chem. 2008, 56, 2330–2340. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, P.; Proto, M.C.; Patruno, C.; Sorbo, A.D.; Bifulco, M. The first cosmetic treatise of history. A female point of view. Int. J. Cosmet. Sci. 2008, 30, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Kara, Y. Bioaccumulation of Cu, Zn and Ni from the wastewater by treated Nasturtium officinale. Int. J. Environ. Sci. Technol. 2005, 2, 63–67. [Google Scholar] [CrossRef]
- Duman, F.; Leblebici, Z.; Aksoy, A. Growth and bioaccumulation characteristics of watercress (Nasturtium officinale R. Br.) exposed to cadmium, cobalt and chromium. Chem. Speciat. Bioavailab. 2009, 21, 257–265. [Google Scholar] [CrossRef]
- Li, K.; Lin, L.; Wang, J.; Xia, H.; Liang, D.; Wang, X.; Liao, M.; Wang, L.; Liu, L.; Chen, C.; et al. Hyperaccumulator straw improves the cadmium phytoextraction efficiency of emergent plant Nasturtium officinale. Environ. Monit. Assess. 2017, 189, 374. [Google Scholar] [CrossRef]
- Ozturk, F.; Duman, F.; Leblebici, Z.; Temizgul, R. Arsenic accumulation and biological responses of watercress (Nasturtium officinale R. Br.) exposed to arsenite. Environ. Exp. Bot. 2010, 69, 167–174. [Google Scholar] [CrossRef]
- Cordeiro, C.; Favas, P.J.C.; Pratas, J.; Sarkar, S.K.; Venkatachalam, P. Uranium accumulation in aquatic macrophytes in an uraniferous region: Relevance to natural attenuation. Chemosphere 2016, 156, 76–87. [Google Scholar] [CrossRef]
- Karuppusamy, S. A review on trends in production of secondary metabolites from higher plants by in vitro tissue, organ and cell cultures. J. Med. Plants Res. 2009, 3, 1222–1239. [Google Scholar]
- Wainwright, H.; Marsh, J. The micropropagation of watercress (Rorippa nasturtium-aquaticum L.). J. Hortic. Sci. 1986, 61, 251–256. [Google Scholar] [CrossRef]
- Klimek-Szczykutowicz, M.; Szopa, A.; Blicharska, E.; Dziurka, M.; Komsta, Ł.; Ekiert, H. Bioaccumulation of selected macro- and microelements and their impact on antioxidant properties and accumulation of glucosinolates and phenolic acids in in vitro cultures of Nasturtium officinale (watercress) microshoots. Food Chem. 2019, 300, 125184. [Google Scholar] [CrossRef] [PubMed]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Kokotkiewicz, A.; Bucinski, A.; Luczkiewicz, M. Xanthone, benzophenone and bioflavonoid accumulation in Cyclopia genistoides (L.) Vent. (honeybush) shoot cultures grown on membrane rafts and in a temporary immersion system. Plant. Cell. Tissue Organ. Cult. 2015, 120, 373–378. [Google Scholar] [CrossRef]
- Gallaher, C.M.; Gallaher, D.D.; Peterson, S. Development and validation of a spectrophotometric method for quantification of total glucosinolates in Cruciferous vegetables. J. Agric. Food Chem. 2012, 60, 1358–1362. [Google Scholar] [CrossRef]
- Ellnain-Wojtaszek, M.; Zgórka, G. High-performance liquid chromatography and thin-layer chromatography of phenolic acids from Gingko biloba L. leaves collected within vegetative period. J. Liq. Chromatogr. Relat. Technol. 1999, 22, 1457–1471. [Google Scholar] [CrossRef]
- Sułkowska-Ziaja, K.; Maślanka, A.; Szewczyk, A.; Muszyńska, B. Physiologically active compounds in four species of genus Phellinus. Nat. Prod. Commun. 2017, 12, 363–366. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzym. 1999, 299, 152–178. [Google Scholar]
- Bach, A.; Kapczyńska, A.; Dziurka, K.; Dziurka, M. Phenolic compounds and carbohydrates in relation to bulb formation in Lachenalia “Ronina” and “Rupert” in vitro cultures under different lighting environments. Sci. Hortic. (Amsterdam) 2015, 188, 23–29. [Google Scholar] [CrossRef]
- Özyürek, M.; Güçlü, K.; Bektaşoğlu, B.; Apak, R. Spectrophotometric determination of ascorbic acid by the modified CUPRAC method with extractive separation of flavonoids–La(III) complexes. Anal. Chim. Acta 2007, 588, 88–95. [Google Scholar] [CrossRef]
- Biesaga-Kościelniak, J.; Dziurka, M.; Ostrowska, A.; Mirek, M.; Kościelniak, J.; Janeczko, A. Brassinosteroid improves content of antioxidants in seeds of selected leguminous plants. Aust. J. Crop. Sci. 2014, 8, 378–388. [Google Scholar]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measuer of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Blios, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 26, 1199–1200. [Google Scholar] [CrossRef]
- Szopa, A.; Klimek-Szczykutowicz, M.; Kokotkiewicz, A.; Maślanka, A.; Król, A.; Luczkiewicz, M.; Ekiert, H. Phytochemical and biotechnological studies on Schisandra chinensis cultivar Sadova No. 1—a high utility medicinal plant. Appl. Microbiol. Biotechnol. 2018, 102, 5105–5120. [Google Scholar] [CrossRef] [PubMed]
- Rubin, E.; Aziz, Z.A.; Surugau, N. Glucosinolates content of in vitro grown Nasturtium officinale (watercress). ASM Sci. J. 2018, 11, 132–139. [Google Scholar]
- Giallourou, N.; Oruna-Concha, M.J.; Harbourne, N. Effects of domestic processing methods on the phytochemical content of watercress (Nasturtium officinale). Food Chem. 2016, 212, 411–419. [Google Scholar] [CrossRef]
- Ramawat, K.G.; Mathur, M. Factors afecting the production of secondary metabolites. In Biotechnology: Secondary metabolites, plants and microbes; Ramawat, K.G., Merillon, J.M., Eds.; Science Publisher Inc.: Enfield, NH, USA, 2007; pp. 59–102. [Google Scholar]
- Aires, A.; Carvalho, R.; Rosa, E.A.S.; Saavedra, M.J. Phytochemical characterization and antioxidant properties of baby-leaf watercress produced under organic production system. CYTA-J. Food. 2013, 11, 343–351. [Google Scholar] [CrossRef]
- Zeb, A. Phenolic profile and antioxidant potential of wild watercress (Nasturtium officinale L.). SpringerPlus 2015, 4, 1–7. [Google Scholar] [CrossRef]
- Taveira, M.; Pereira, D.M.; Sousa, C.; Ferreres, F.; Andrade, P.B.; Martins, A.; Pereira, J.A.; Valentão, P. In vitro cultures of Brassica oleracea L. var. costata DC: Potential plant bioreactor for antioxidant phenolic compounds. J. Agric. Food Chem. 2009, 57, 1247–1252. [Google Scholar]
- Szopa, A.; Klimek-Szczykutowicz, M.; Kokotkiewicz, A.; Dziurka, M.; Luczkiewicz, M.; Ekiert, H. Phenolic acid and flavonoid production in agar, agitated and bioreactor-grown microshoot cultures of Schisandra chinensis cv. Sadova No. 1—A valuable medicinal plant. J. Biotechnol. 2019, 305, 61–70. [Google Scholar] [CrossRef]
- Kwiecień, I.; Szydłowska, A.; Kawka, B.; Beerhues, L.; Ekiert, H. Accumulation of biologically active phenolic acids in agitated shoot cultures of three Hypericum perforatum cultivars: ‘Elixir’, ‘Helos’ and ‘Topas.’. Plant. Cell. Tissue Organ. Cult. 2015, 123, 273–281. [Google Scholar]
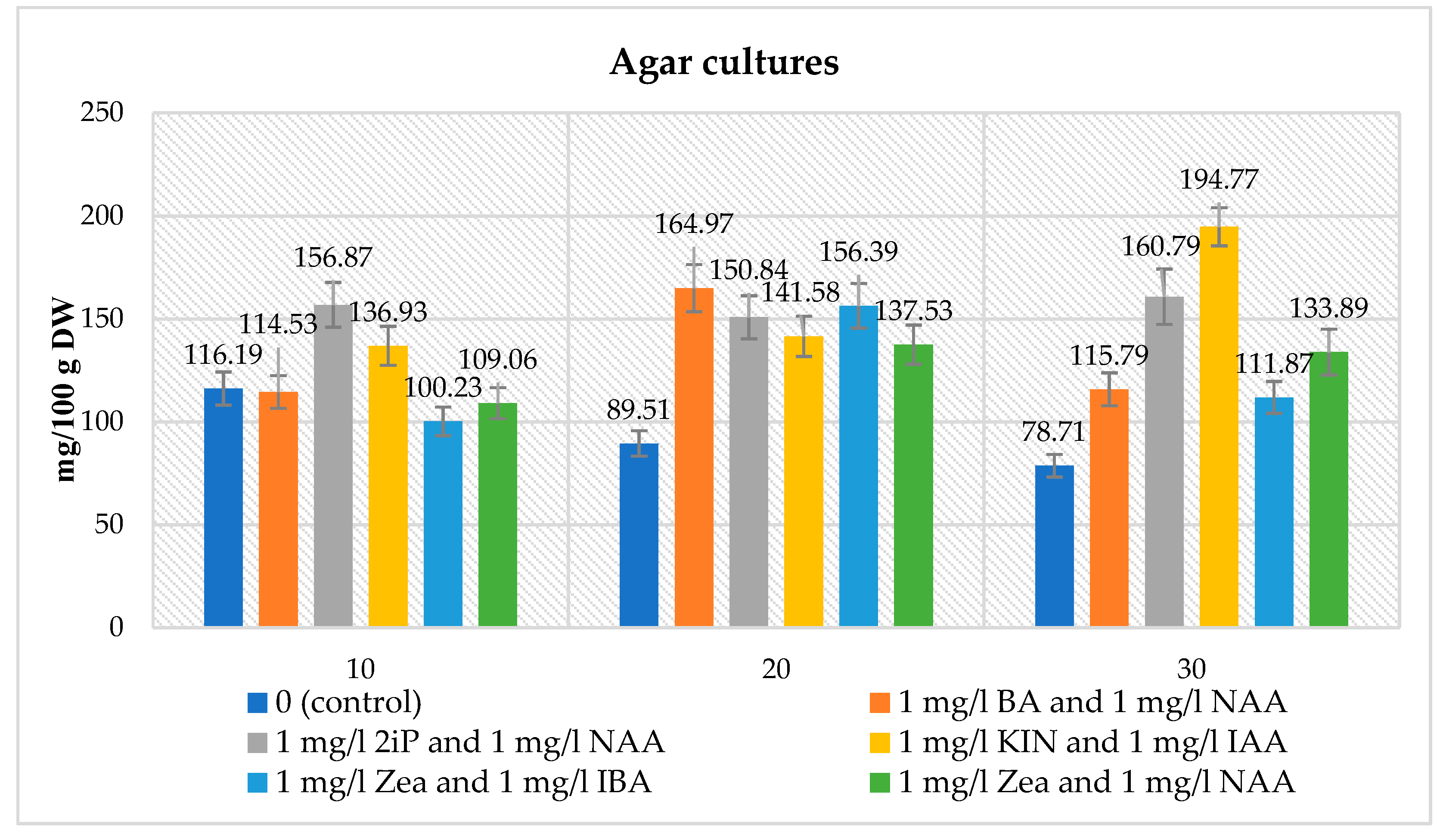
| Type of Microshoot Cultures | Growth Cycles (days) | Min Gi | Max Gi | Increase (fold) | MS Medium Variants |
|---|---|---|---|---|---|
| Agar | 10 | 0.05 ± 0.01 | 1.75 ± 0.03 | 35.00 | 1 mg/L BA and 1 mg/L NAA |
| 20 | 0.37 ± 0.04 | 3.79 ± 0.05 | 10.24 | ||
| 30 | 1.28 ± 0.04 | 5.05 ± 0.14 | 3.95 | ||
| Agitated | 10 | 1.47 ± 0.02 | 5.29 ± 0.07 | 3.60 | 1 mg/L BA and 1 mg/L NAA1 1 mg/L KIN and 1 mg/L IBA |
| 20 | 1.59 ± 0.01 | 10.48 ± 0.08 | 6.59 | 1 mg/L Zea and 1 mg/L NAA |
| Phenolic Acids | Min Content | Max Content | Increase (fold) | Conditions for Max Content | ||
|---|---|---|---|---|---|---|
| Growth Cycle (days) | MS Medium Variant | Gi | ||||
| Caffeic acid | 0.04 ± 0.01 | 16.65 ± 1.59 | 416.25 | 20 | 2 mg/L BA and 1 mg/L NAA | 2.96 ± 0.06 |
| o-Coumaric acid | 0.03 ± 0.01 | 17.34 ± 2.09 | 578.00 | 30 | 1 mg/L 2iP and 1 mg/L IPA | 2.06 ± 0.03 |
| p-Coumaric acid | 2.14 ± 0.18 | 18.95 ± 2.11 | 8.86 | 20 | 1 mg/L KIN and 1 mg/L IPA | 0.42 ± 0.01 |
| Ellagic acid | 2.79 ± 0.25 | 6.56 ± 0.74 | 2.35 | 30 | 1 mg/L 2iP | 1.59 ± 0.05 |
| Ferulic acid | 1.64 ± 0.20 | 18.13 ± 1.13 | 11.05 | 10 | 1 mg/L KIN and 1 mg/L IAA | 0.30 ± 0.02 |
| Gallic acid | 1.40 ± 0.13 | 61.03 ± 5.89 | 43.59 | 30 | 2 mg/l BA and 1 mg/L NAA | 2.95 ± 0.04 |
| Isoferulic acid | 0.02 ± 0.01 | 6.01 ± 0.59 | 300.50 | 10 | 1 mg/L KIN and 1 mg/L IBA | 0.23 ± 0.03 |
| Protocatechuic acid | 0.78 ± 0.08 | 138.40 ± 12.99 | 177.44 | 20 | 2 mg/L BA and 1 mg/L NAA | 2.96 ± 0.06 |
| Rosmarinic acid | 0.07 ± 0.01 | 33.30 ± 3.67 | 475.71 | 20 | 1 mg/L 2iP and 1 mg/L NAA | 1.59 ± 0.01 |
| Syringic acid | 1.65 ± 0.18 | 9.65 ± 0.89 | 5.85 | 20 | 1 mg/L BA and 1 mg/L NAA | 3.79 ± 0.05 |
| Total content | 15.89 ± 1.66 | 237.52 ± 22.56 | 14.95 | 20 | 2 mg/L BA and 1 mg/L NAA | 2.96 ± 0.06 |
| Phenolic Acids | Min Content | Max Content | Increase (fold) | Conditions for Max Content | ||
|---|---|---|---|---|---|---|
| Growth Cycle (days) | MS Medium Variant | Gi | ||||
| Caffeic acid | 0.02 ± 0.01 | 15.30 ± 1.66 | 765.00 | 10 | 1 mg/L Zea and 1 mg/L NAA | 1.75 ± 0.01 |
| o-Coumaric acid | 0.74 ± 0.66 | 14.91 ± 1.35 | 20.15 | 10 | 1 mg/L BA and 1 mg/L NAA | 5.29 ± 0.07 |
| p-Coumaric acid | 4.84 ± 0.45 | 31.66 ± 2.98 | 6.54 | 10 | 1 mg/L BA and 1 mg/L NAA | 5.29 ± 0.07 |
| Ellagic acid | 2.93 ± 0.33 | 8.12 ± 0.79 | 2.69 | 20 | 1 mg/L KIN and 1 mg/L IBA | 7.51 ± 0.03 |
| Ferulic acid | 1.58 ± 1.64 | 38.44 ± 4.12 | 24.33 | 10 | 1 mg/L BA and 1 mg/L NAA | 5.29 ± 0.07 |
| Gallic acid | 29.84 ± 0.28 | 53.34 ± 5.12 | 1.79 | 10 | 1 mg/L Zea and 1 mg/L NAA | 1.75 ± 0.01 |
| Isoferulic acid | 0.02 ± 0.01 | 15.17 ± 1.49 | 758.50 | 10 | 1 mg/L BA and 1 mg/L NAA | 5.29 ± 0.07 |
| Protocatechuic acid | 4.94 ± 0.48 | 132.26 ± 12.02 | 26.77 | 10 | 1 mg/L Zea and 1 mg/L NAA | 1.75 ± 0.01 |
| Rosmarinic acid | 2.95 ± 0.33 | 36.34 ± 3.71 | 12.32 | 20 | 1 mg/L 2iP and 1 mg/L NAA | 1.59 ± 0.01 |
| Syringic acid | 1.65 ± 0.18 | 21.50 ± 2.12 | 13.03 | 10 | 1 mg/L BA and 1 mg/L NAA | 5.29 ± 0.07 |
| Total content | 70.80 ± 7.12 | 236.74 ± 19.30 | 3.34 | 10 | 1 mg/L Zea and 1 mg/L NAA | 1.75 ± 0.01 |
| Culture Type | Method | Min (mmol TE/100 g DW ± SD) | Max (mmol TE/100 g DW ± SD) | Increase (fold) | Conditions for Max Antioxidant Parameters | ||
|---|---|---|---|---|---|---|---|
| Growth Cycle (days) | MS Medium Variant | Gi | |||||
| Agar | CUPRAC | 1.50 ± 0.19 | 4.13 ± 0.80 | 1.69 | 30 | 1 mg/L KIN and 1 mg/L IAA | 2.39 ± 0.05 |
| FRAP | 0.52 ± 0.02 | 1.42 ± 0.02 | 2.73 | 30 | 2 mg/L BA and 1 mg/L NAA | 2.95 ± 0.04 | |
| DPPH | 0.49 ± 0.04 | 30.89 ± 1.82 | 63.04 | 30 | 1 mg/L KIN and 1 mg/L IAA | 2.39 ± 0.05 | |
| F-C | 2.42 ± 0.10 | 8.69 ± 3.44 | 3.59 | 10 | 1 mg/L Zea and 1 mg/L NAA | 3.27 ± 0.05 | |
| Agitated | CUPRAC | 2.52 ± 0.15 | 5.26 ± 0.10 | 2.09 | 20 | 1 mg/L KIN and 1 mg/L IAA | 8.08 ± 0.06 |
| FRAP | 0.51 ± 0.04 | 1.26 ± 0.04 | 2.47 | 20 | 1 mg/L KIN and 1 mg/L IAA | 8.08 ± 0.06 | |
| DPPH | 14.55 ± 0.96 | 25.95 ± 4.07 | 1.78 | 20 | 1 mg/L KIN and 1 mg/L IAA | 8.08 ± 0.06 | |
| F-C | 2.49 ± 0.13 | 5.24 ± 0.20 | 2.10 | 20 | 1 mg/L KIN and 1 mg/L IAA | 8.08 ± 0.06 | |
| Total Glucosinolate Content (mg/100g DW ± SD) | Phenolic Acid Contents (mg/100g DW ± SD) | Antioxidant Activity | ||
|---|---|---|---|---|
| Method | (mmol TE/100 g DW ± SD) | |||
| 499.89 ± 34.59 | Caffeic acid | 7.10 ± 0.65 | CUPRAC | 4.45 ± 0.02 |
| o-Coumaric acid | 2.39 ± 0.19 | |||
| p-Coumaric acid | 0.61 ± 0.05 | FRAP | 0.76 ± 0.08 | |
| Ferulic acid | 14.32 ± 1.43 | |||
| Gallic acid | 13.67 ± 1.12 | DPPH | 26.32 ± 8.23 | |
| Rosmarinic acid | 61.27 ± 5.87 | |||
| Syringic acid | 0.48 ± 0.05 | F-C | 2.70 ± 0.31 | |
| Total content | 99.84 ± 9.65 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klimek-Szczykutowicz, M.; Szopa, A.; Dziurka, M.; Komsta, Ł.; Tomczyk, M.; Ekiert, H. The Influence of Nasturtium officinale R. Br. Agar and Agitated Microshoot Culture Media on Glucosinolate and Phenolic Acid Production, and Antioxidant Activity. Biomolecules 2020, 10, 1216. https://doi.org/10.3390/biom10091216
Klimek-Szczykutowicz M, Szopa A, Dziurka M, Komsta Ł, Tomczyk M, Ekiert H. The Influence of Nasturtium officinale R. Br. Agar and Agitated Microshoot Culture Media on Glucosinolate and Phenolic Acid Production, and Antioxidant Activity. Biomolecules. 2020; 10(9):1216. https://doi.org/10.3390/biom10091216
Chicago/Turabian StyleKlimek-Szczykutowicz, Marta, Agnieszka Szopa, Michał Dziurka, Łukasz Komsta, Michał Tomczyk, and Halina Ekiert. 2020. "The Influence of Nasturtium officinale R. Br. Agar and Agitated Microshoot Culture Media on Glucosinolate and Phenolic Acid Production, and Antioxidant Activity" Biomolecules 10, no. 9: 1216. https://doi.org/10.3390/biom10091216
APA StyleKlimek-Szczykutowicz, M., Szopa, A., Dziurka, M., Komsta, Ł., Tomczyk, M., & Ekiert, H. (2020). The Influence of Nasturtium officinale R. Br. Agar and Agitated Microshoot Culture Media on Glucosinolate and Phenolic Acid Production, and Antioxidant Activity. Biomolecules, 10(9), 1216. https://doi.org/10.3390/biom10091216











