Cellular and Molecular Mechanism of Cardiac Regeneration: A Comparison of Newts, Zebrafish, and Mammals
Abstract
1. Introduction
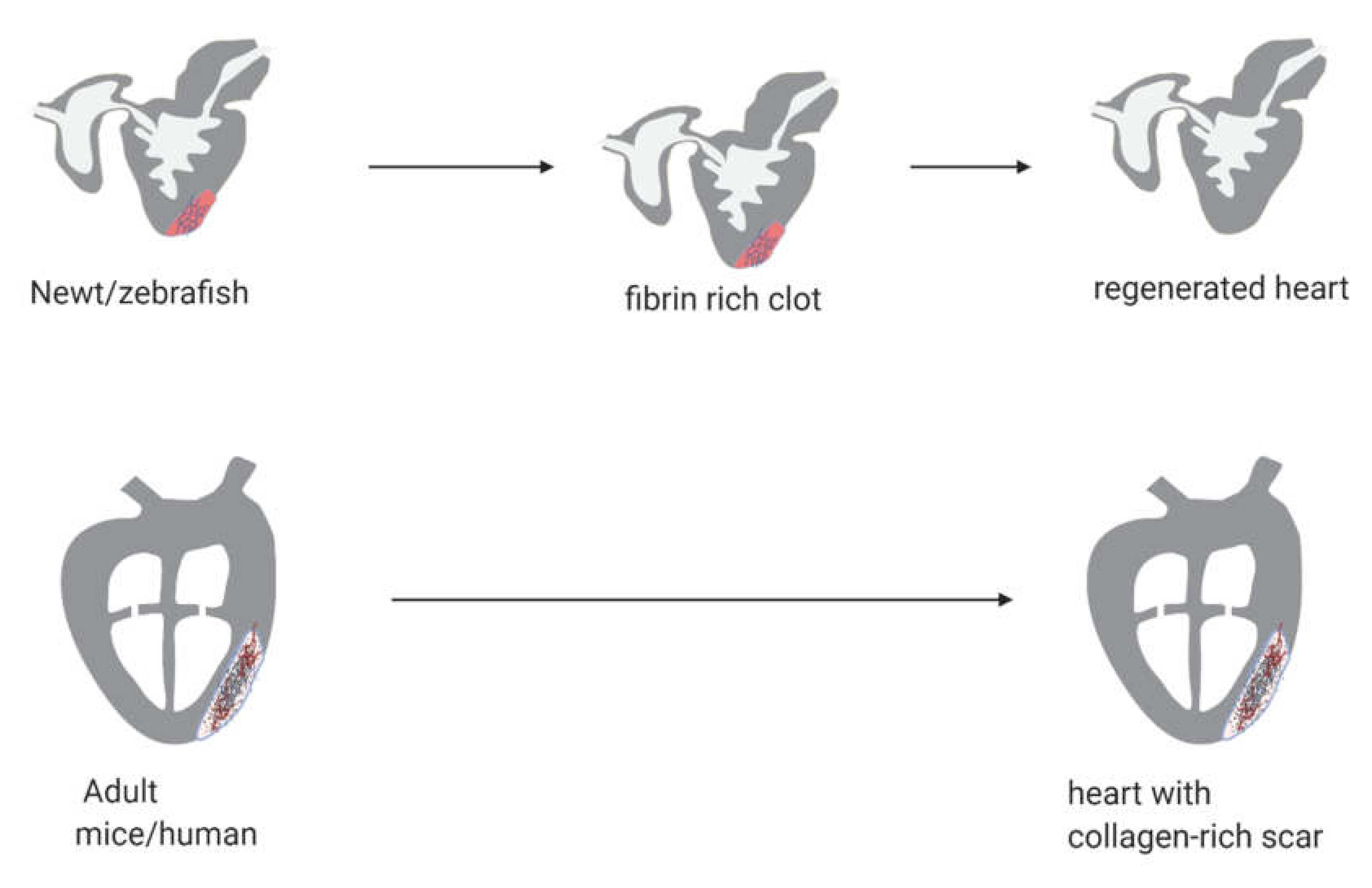
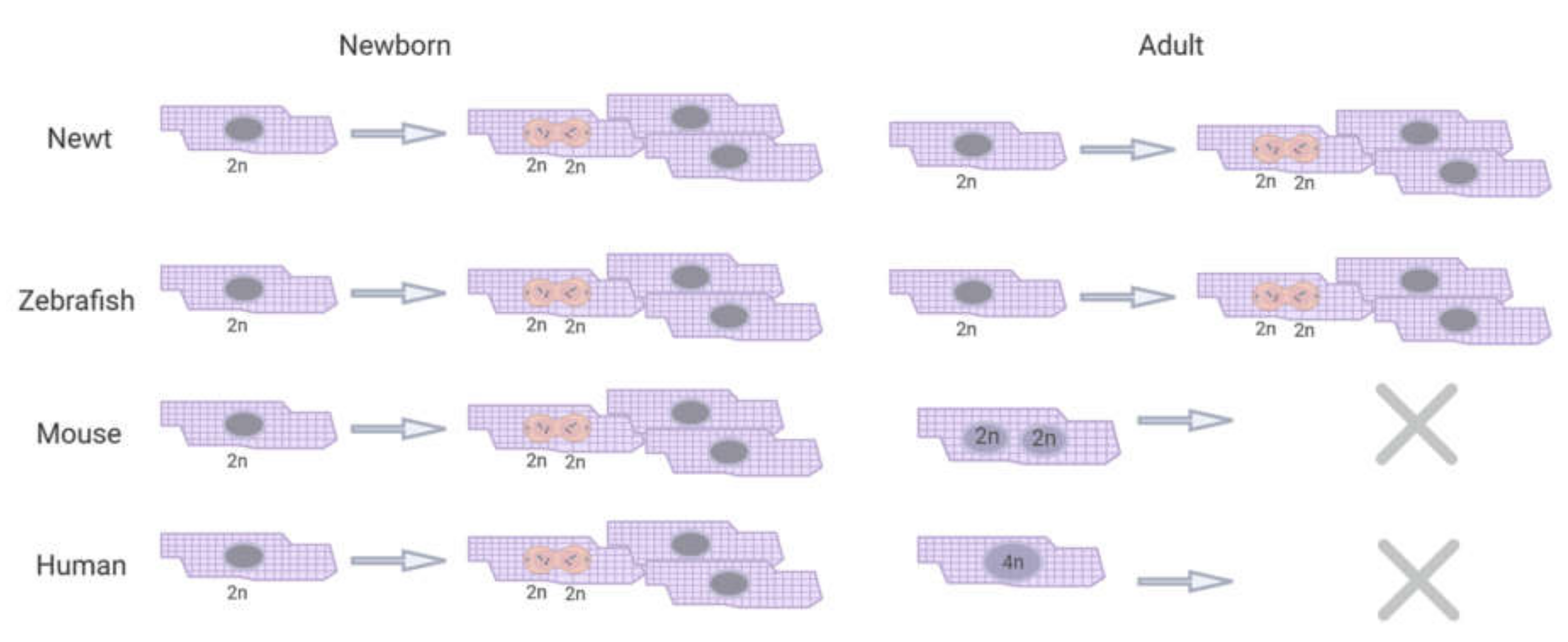
2. Cardiac Regeneration in Experimental Animal Models
2.1. Newts
2.2. Zebrafish
2.3. Mice
2.4. Humans
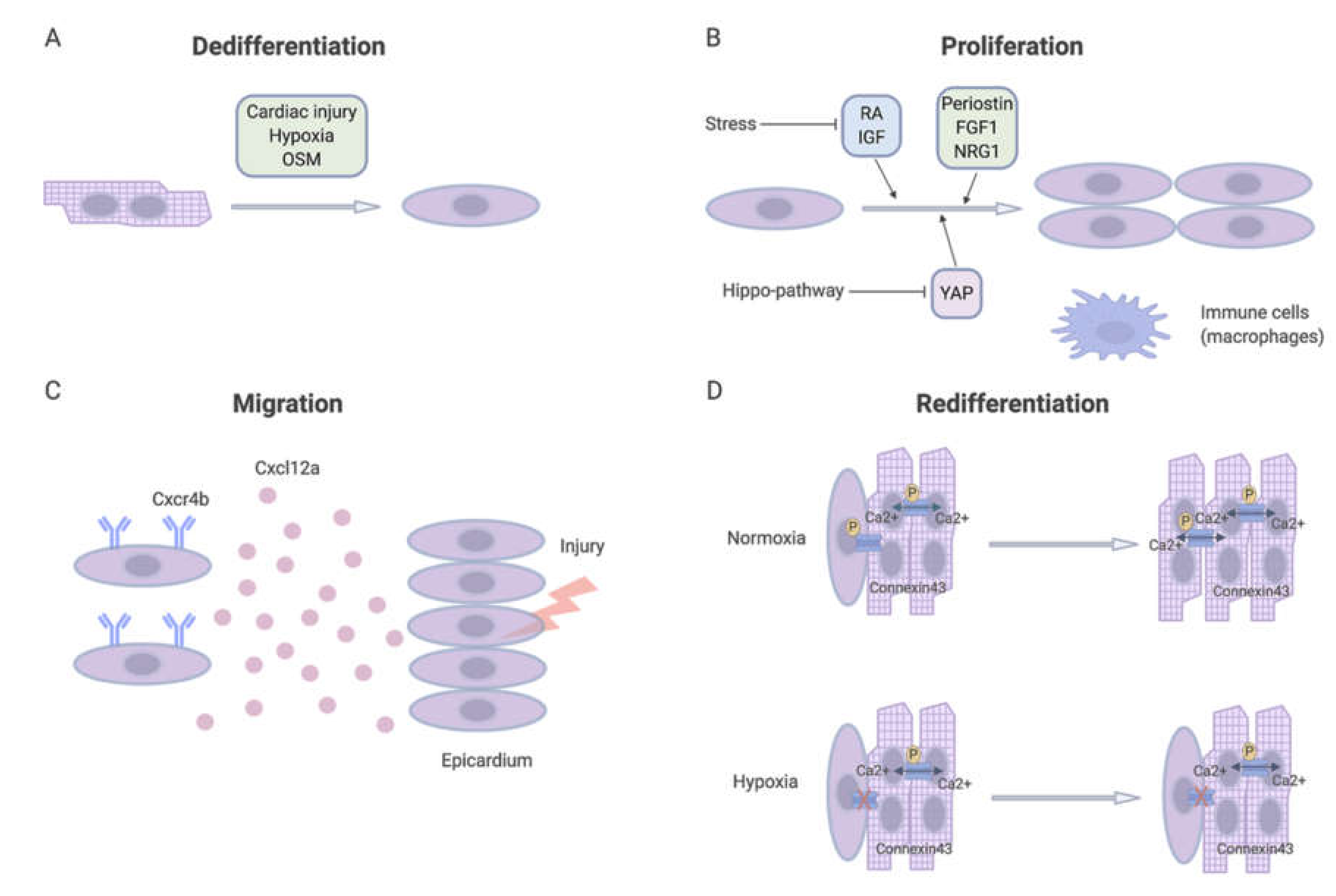
3. Four Fundamental Processes During Cardiac Regeneration
3.1. Dedifferentiation Factors
3.1.1. Hypoxia
3.1.2. Oncostatin M
3.2. Proliferation Factors
3.2.1. Cyclins
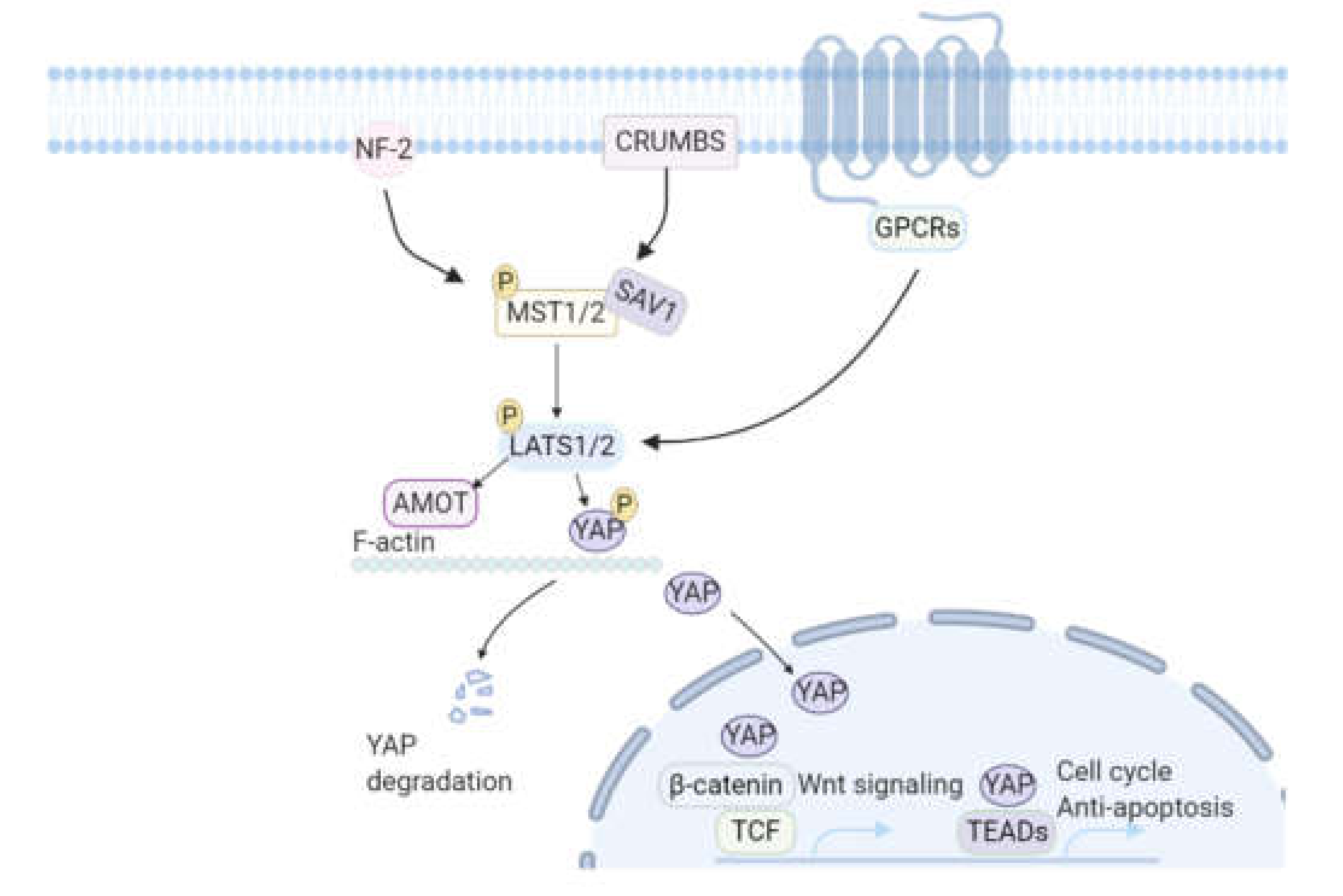
3.2.2. The Hippo-YAP Pathway
3.2.3. P38 MAP Kinase
3.2.4. Meis1
3.2.5. Extracellular Matrix Composition
3.2.6. Neuregulin 1
3.2.7. Neural Factors
3.2.8. Retinoic Acid
3.2.9. IGF
3.3. Migration Factors
Inflammation
3.4. Re-Differentiation Factors
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mozaffarian, D.; Benjamin, E.; Go, A.; Arnett, D.; Blaha, M.; Cushman, M.; Das, S.; Ferranti, S.; Després, J.; Fullerton, H.; et al. Heart disease and stroke statistics-2016 update: A report from the american heart association. Circulation 2016, 135, e146–e603. [Google Scholar] [CrossRef] [PubMed]
- Gemberling, M.; Karra, R.; Dickson, A.; Poss, K. Nrg1 is an injury-induced cardiomyocyte mitogen for the endogenous heart regeneration program in zebrafish. ELife 2015, 4. [Google Scholar] [CrossRef] [PubMed]
- Niederberger, P.; Farine, E.; Raillard, M.; Dornbierer, M.; Freed, D.H.; Large, S.R.; Chew, H.C.; MacDonald, P.S.; Messer, S.J.; White, C.W.; et al. Heart Transplantation With Donation After Circulatory Death. Circ. Heart Fail. 2019, 12, e005517. [Google Scholar] [CrossRef] [PubMed]
- Degterev, A.; Huang, Z.; Boyce, M.; Li, Y.; Jagtap, P.; Mizushima, N.; Cuny, G.D.; Mitchison, T.J.; Moskowitz, M.A.; Yuan, J. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat. Chem. Biol. 2005, 1, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Choo, E.H.; Lee, J.H.; Park, E.H.; Park, H.E.; Jung, N.C.; Kim, T.H.; Koh, Y.S.; Kim, E.; Seung, K.B.; Park, C.; et al. Infarcted Myocardium-Primed Dendritic Cells Improve Remodeling and Cardiac Function After Myocardial Infarction by Modulating the Regulatory T Cell and Macrophage Polarization. Circulation 2017, 135, 1444–1457. [Google Scholar] [CrossRef]
- Hausenloy, D.J.; Garcia-Dorado, D.; Botker, H.E.; Davidson, S.M.; Downey, J.; Engel, F.B.; Jennings, R.; Lecour, S.; Leor, J.; Madonna, R.; et al. Novel targets and future strategies for acute cardioprotection: Position Paper of the European Society of Cardiology Working Group on Cellular Biology of the Heart. Cardiovasc. Res. 2017, 113, 564–585. [Google Scholar] [CrossRef]
- Madonna, R.; Van Laake, L.W.; Botker, H.E.; Davidson, S.M.; De Caterina, R.; Engel, F.B.; Eschenhagen, T.; Fernandez-Aviles, F.; Hausenloy, D.J.; Hulot, J.S.; et al. ESC Working Group on Cellular Biology of the Heart: Position paper for Cardiovascular Research: Tissue engineering strategies combined with cell therapies for cardiac repair in ischaemic heart disease and heart failure. Cardiovasc. Res. 2019, 115, 488–500. [Google Scholar] [CrossRef]
- Sluijter, J.P.G.; Davidson, S.M.; Boulanger, C.M.; Buzas, E.I.; de Kleijn, D.P.V.; Engel, F.B.; Giricz, Z.; Hausenloy, D.J.; Kishore, R.; Lecour, S.; et al. Extracellular vesicles in diagnostics and therapy of the ischaemic heart: Position Paper from the Working Group on Cellular Biology of the Heart of the European Society of Cardiology. Cardiovasc. Res. 2018, 114, 19–34. [Google Scholar] [CrossRef]
- Madonna, R.; Van Laake, L.W.; Davidson, S.M.; Engel, F.B.; Hausenloy, D.J.; Lecour, S.; Leor, J.; Perrino, C.; Schulz, R.; Ytrehus, K.; et al. Position Paper of the European Society of Cardiology Working Group Cellular Biology of the Heart: Cell-based therapies for myocardial repair and regeneration in ischemic heart disease and heart failure. Eur. Heart J. 2016, 37, 1789–1798. [Google Scholar] [CrossRef]
- Bergmann, O.; Bhardwaj, R.D.; Bernard, S.; Zdunek, S.; Barnabe-Heider, F.; Walsh, S.; Zupicich, J.; Alkass, K.; Buchholz, B.A.; Druid, H.; et al. Evidence for cardiomyocyte renewal in humans. Science 2009, 324, 98–102. [Google Scholar] [CrossRef]
- Jopling, C.; Sleep, E.; Raya, M.; Marti, M.; Raya, A.; Izpisua Belmonte, J.C. Zebrafish heart regeneration occurs by cardiomyocyte dedifferentiation and proliferation. Nature 2010, 464, 606–609. [Google Scholar] [CrossRef] [PubMed]
- Simon, H.G.; Odelberg, S. Assessing cardiomyocyte proliferative capacity in the newt heart and primary culture. Methods Mol. Biol. 2015, 1290, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Witman, N.; Murtuza, B.; Davis, B.; Arner, A.; Morrison, J. Recapitulation of developmental cardiogenesis governs the morphological and functional regeneration of adult newt hearts following injury. Dev. Biol. 2011, 354, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Mercer, S.; Odelberg, S.; Simon, H. A dynamic spatiotemporal extracellular matrix facilitates epicardial-mediated vertebrate heart regeneration. Dev. Biol. 2013, 382, 457–469. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, K.; Holdway, J.; Werdich, A.; Anderson, R.; Fang, Y.; Egnaczyk, G.; Evans, T.; Macrae, C.; Stainier, D.; Poss, K. Primary contribution to zebrafish heart regeneration by gata4(+) cardiomyocytes. Nature 2010, 464, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Haubner, B.J.; Schneider, J.; Schweigmann, U.; Schuetz, T.; Dichtl, W.; Velik-Salchner, C.; Stein, J.I.; Penninger, J.M. Functional Recovery of a Human Neonatal Heart After Severe Myocardial Infarction. Circ. Res. 2016, 118, 216–221. [Google Scholar] [CrossRef]
- Porrello, E.; Mahmoud, A.; Simpson, E.; Johnson, B.; Grinsfelder, D.; Canseco, D.; Mammen, P.; Rothermal, B.; Olson, E.; Sadek, H. Regulation of neonatal and adult mammalian heart regeneration by the miR-15 family. Proc. Natl. Acad. Sci. USA 2012, 110, 187–192. [Google Scholar] [CrossRef]
- Porrello, E.; Mahmoud, A.; Simpson, E.; Hill, J.; Richardson, J.; Olson, E.; Sadek, H. Transient regenerative potential of the neonatal mouse heart. Science 2011, 331, 1078–1080. [Google Scholar] [CrossRef]
- Ingason, A.; Goldstone, A.; Paulsen, M.; Thakore, A.; Truong, V.; Edwards, B.; Eskandari, A.; Bollig, T.; Steele, A.; Woo, Y. Angiogenesis precedes cardiomyocyte migration in regenerating mammalian hearts. J. Thorac. Cardiovasc. Surg. 2018, 155, 1128–1129. [Google Scholar] [CrossRef]
- Chen, Z.; Xie, J.; Hao, H.; Lin, H.; Wang, L.; Zhang, Y.; Chen, L.; Cao, S.; Huang, X.; Liao, W.; et al. Ablation of periostin inhibits post-infarction myocardial regeneration in neonatal mice mediated by the phosphatidylinositol 3 kinase/glyocogen synthase kinase 3β/cyclin D1 signalling pathway. Cardiovasc. Res. 2017, 113, 620–632. [Google Scholar] [CrossRef]
- Lam, N.T.; Sadek, H.A. Neonatal heart regeneration: Comprehensive literature review. Circulation 2018, 138, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Stainier, D.Y.; Lee, R.K.; Fishman, M.C. Cardiovascular development in the zebrafish. I. Myocardial fate map and heart tube formation. Development 1993, 119, 31–40. [Google Scholar] [PubMed]
- Bednarek, D.; González-Rosa, J.; Guzmán-Martínez, G.; Gutiérrez-Gutiérrez, Ó.; Aguado, T.; Sánchez-Ferrer, C.; Marques, I.; Galardi Castilla, M.; de Diego, I.; Gómez, M.; et al. Telomerase is essential for zebrafish heart regeneration. Cell Rep. 2015, 12, 1691–1703. [Google Scholar] [CrossRef] [PubMed]
- Jazwinska, A.; Sallin, P. Regeneration versus scarring in vertebrate appendages and heart. J. Pathol. 2016, 238, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Wills, A.A.; Holdway, J.E.; Major, R.J.; Poss, K.D. Regulated addition of new myocardial and epicardial cells fosters homeostatic cardiac growth and maintenance in adult zebrafish. Development 2008, 135, 183–192. [Google Scholar] [CrossRef]
- Bergmann, O.; Zdunek, S.; Felker, A.; Salehpour, M.; Alkass, K.; Bernard, S.; Sjostrom, S.; Szewczykowska, M.; Jackowska, T.; Dos Remedios, C.; et al. Dynamics of cell generation and turnover in the human heart. Cell 2015, 161, 1566–1575. [Google Scholar] [CrossRef]
- Walsh, S.; Ponten, A.; Fleischmann, B.K.; Jovinge, S. Cardiomyocyte cell cycle control and growth estimation in vivo--an analysis based on cardiomyocyte nuclei. Cardiovasc. Res. 2010, 86, 365–373. [Google Scholar] [CrossRef]
- Soonpaa, M.H.; Kim, K.K.; Pajak, L.; Franklin, M.; Field, L.J. Cardiomyocyte DNA synthesis and binucleation during murine development. Am. J. Physiol. 1996, 271, H2183–H2189. [Google Scholar] [CrossRef]
- Hesse, M.; Doengi, M.; Becker, A.; Kimura, K.; Voeltz, N.; Stein, V.; Fleischmann, B.K. Midbody Positioning and Distance Between Daughter Nuclei Enable Unequivocal Identification of Cardiomyocyte Cell Division in Mice. Circ. Res. 2018, 123, 1039–1052. [Google Scholar] [CrossRef]
- Leone, M.; Engel, F.B. Pseudo-bipolar spindle formation and cell division in postnatal binucleated cardiomyocytes. J. Mol. Cell Cardiol. 2019, 134, 69–73. [Google Scholar] [CrossRef]
- Hirose, K.; Payumo, A.Y.; Cutie, S.; Hoang, A.; Zhang, H.; Guyot, R.; Lunn, D.; Bigley, R.B.; Yu, H.; Wang, J.; et al. Evidence for hormonal control of heart regenerative capacity during endothermy acquisition. Science 2019, 364, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Trinh, L.A.; Stainier, D.Y. Fibronectin regulates epithelial organization during myocardial migration in zebrafish. Dev. Cell 2004, 6, 371–382. [Google Scholar] [CrossRef]
- Piatkowski, T.; Muhlfeld, C.; Borchardt, T.; Braun, T. Reconstitution of the myocardium in regenerating newt hearts is preceded by transient deposition of extracellular matrix components. Stem Cells Dev. 2013, 22, 1921–1931. [Google Scholar] [CrossRef] [PubMed]
- Poss, K.D.; Wilson, L.G.; Keating, M.T. Heart regeneration in zebrafish. Science 2002, 298, 2188–2190. [Google Scholar] [CrossRef] [PubMed]
- Flink, I.L. Cell cycle reentry of ventricular and atrial cardiomyocytes and cells within the epicardium following amputation of the ventricular apex in the axolotl, Amblystoma mexicanum: Confocal microscopic immunofluorescent image analysis of bromodeoxyuridine-labeled nuclei. Anat. Embryol. 2002, 205, 235–244. [Google Scholar] [CrossRef]
- Suetsugu-Maki, R.; Maki, N.; Nakamura, K.; Sumanas, S.; Zhu, J.; Del Rio-Tsonis, K.; Tsonis, P.A. Lens regeneration in axolotl: New evidence of developmental plasticity. BMC Biol. 2012, 10, 103. [Google Scholar] [CrossRef]
- Laube, F.; Heister, M.; Scholz, C.; Borchardt, T.; Braun, T. Re-programming of newt cardiomyocytes is induced by tissue regeneration. J. Cell Sci. 2006, 119, 4719–4729. [Google Scholar] [CrossRef]
- Gonzalez-Rosa, J.M.; Mercader, N. Cryoinjury as a myocardial infarction model for the study of cardiac regeneration in the zebrafish. Nat. Protoc. 2012, 7, 782–788. [Google Scholar] [CrossRef]
- Wang, J.; Panakova, D.; Kikuchi, K.; Holdway, J.E.; Gemberling, M.; Burris, J.S.; Singh, S.P.; Dickson, A.L.; Lin, Y.F.; Sabeh, M.K.; et al. The regenerative capacity of zebrafish reverses cardiac failure caused by genetic cardiomyocyte depletion. Development 2011, 138, 3421–3430. [Google Scholar] [CrossRef]
- Parente, V.; Balasso, S.; Pompilio, G.; Verduci, L.; Colombo, G.I.; Milano, G.; Guerrini, U.; Squadroni, L.; Cotelli, F.; Pozzoli, O.; et al. Hypoxia/reoxygenation cardiac injury and regeneration in zebrafish adult heart. PLoS ONE 2013, 8, e53748. [Google Scholar] [CrossRef]
- Oberpriller, J.O.; Oberpriller, J.C. Response of the adult newt ventricle to injury. J. Exp. Zool. 1974, 187, 249–253. [Google Scholar] [CrossRef]
- Kikuchi, K.; Holdway, J.; Major, R.; Blum, N.; Dahn, R.; Begemann, G.; Poss, K. Retinoic acid production by endocardium and epicardium is an injury response essential for zebrafish heart regeneration. Dev. Cell 2011, 20, 397–404. [Google Scholar] [CrossRef]
- Smart, N.; Risebro, C.A.; Melville, A.A.; Moses, K.; Schwartz, R.J.; Chien, K.R.; Riley, P.R. Thymosin beta4 induces adult epicardial progenitor mobilization and neovascularization. Nature 2007, 445, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Lepilina, A.; Coon, A.N.; Kikuchi, K.; Holdway, J.E.; Roberts, R.W.; Burns, C.G.; Poss, K.D. A dynamic epicardial injury response supports progenitor cell activity during zebrafish heart regeneration. Cell 2006, 127, 607–619. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.B.; Tucci, V.; Uchiyama, J.; Fabian, N.J.; Lin, M.C.; Bayliss, P.E.; Neuberg, D.S.; Zhdanova, I.V.; Kishi, S. Differential effects of genotoxic stress on both concurrent body growth and gradual senescence in the adult zebrafish. Aging Cell 2007, 6, 209–224. [Google Scholar] [CrossRef] [PubMed]
- Olivetti, G.; Cigola, E.; Maestri, R.; Corradi, D.; Lagrasta, C.; Gambert, S.R.; Anversa, P. Aging, cardiac hypertrophy and ischemic cardiomyopathy do not affect the proportion of mononucleated and multinucleated myocytes in the human heart. J. Mol. Cell Cardiol. 1996, 28, 1463–1477. [Google Scholar] [CrossRef]
- Sallin, P.; de Preux Charles, A.S.; Duruz, V.; Pfefferli, C.; Jazwinska, A. A dual epimorphic and compensatory mode of heart regeneration in zebrafish. Dev. Biol. 2015, 399, 27–40. [Google Scholar] [CrossRef]
- Choi, W.Y.; Gemberling, M.; Wang, J.; Holdway, J.E.; Shen, M.C.; Karlstrom, R.O.; Poss, K.D. In vivo monitoring of cardiomyocyte proliferation to identify chemical modifiers of heart regeneration. Development 2013, 140, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Jopling, C.; Suñé, G.; Faucherre, A.; Fabregat, C.; Izpisua Belmonte, J.C. Hypoxia induces myocardial regeneration in zebrafish. Circulation 2012, 126, 3017–3027. [Google Scholar] [CrossRef] [PubMed]
- Nakada, Y.; Canseco, D.C.; Thet, S.; Abdisalaam, S.; Asaithamby, A.; Santos, C.X.; Shah, A.M.; Zhang, H.; Faber, J.E.; Kinter, M.T.; et al. Hypoxia induces heart regeneration in adult mice. Nature 2017, 541, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Kubin, T.; Pöling, J.; Kostin, S.; Gajawada, P.; Hein, S.; Rees, W.; Wietelmann, A.; Tanaka, M.; Lörchner, H.; Schimanski, S.; et al. Oncostatin M is a major mediator of cardiomyocyte dedifferentiation and remodeling. Cell Stem Cell 2011, 9, 420–432. [Google Scholar] [CrossRef] [PubMed]
- Poling, J.; Gajawada, P.; Lorchner, H.; Polyakova, V.; Szibor, M.; Bottger, T.; Warnecke, H.; Kubin, T.; Braun, T. The Janus face of OSM-mediated cardiomyocyte dedifferentiation during cardiac repair and disease. Cell Cycle 2012, 11, 439–445. [Google Scholar] [CrossRef] [PubMed]
- van den Heuvel, S.; Harlow, E. Distinct roles for cyclin-dependent kinases in cell cycle control. Science 1993, 262, 2050–2054. [Google Scholar] [CrossRef] [PubMed]
- Pagano, M.; Pepperkok, R.; Verde, F.; Ansorge, W.; Draetta, G. Cyclin A is required at two points in the human cell cycle. EMBO J. 1992, 11, 961–971. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, M.; Shirato, H.; Nakajima, K.; Kojima, M.; Takahashi, M.; Kubota, M.; Suzuki-Migishima, R.; Motegi, Y.; Yokoyama, M.; Takeuchi, T. Jumonji downregulates cardiac cell proliferation by repressing cyclin D1 expression. Dev. Cell 2003, 5, 85–97. [Google Scholar] [CrossRef]
- Pasumarthi, K.B.; Nakajima, H.; Nakajima, H.O.; Soonpaa, M.H.; Field, L.J. Targeted expression of cyclin D2 results in cardiomyocyte DNA synthesis and infarct regression in transgenic mice. Circ. Res. 2005, 96, 110–118. [Google Scholar] [CrossRef]
- Flink, I.L.; Oana, S.; Maitra, N.; Bahl, J.J.; Morkin, E. Changes in E2F complexes containing retinoblastoma protein family members and increased cyclin-dependent kinase inhibitor activities during terminal differentiation of cardiomyocytes. J. Mol. Cell Cardiol. 1998, 30, 563–578. [Google Scholar] [CrossRef]
- Yoshizumi, M.; Lee, W.S.; Hsieh, C.M.; Tsai, J.C.; Li, J.; Perrella, M.A.; Patterson, C.; Endege, W.O.; Schlegel, R.; Lee, M.E. Disappearance of cyclin A correlates with permanent withdrawal of cardiomyocytes from the cell cycle in human and rat hearts. J. Clin. Investig. 1995, 95, 2275–2280. [Google Scholar] [CrossRef]
- Mohamed, T.M.A.; Ang, Y.S.; Radzinsky, E.; Zhou, P.; Huang, Y.; Elfenbein, A.; Foley, A.; Magnitsky, S.; Srivastava, D. Regulation of Cell Cycle to Stimulate Adult Cardiomyocyte Proliferation and Cardiac Regeneration. Cell 2018, 173, 104–116. [Google Scholar] [CrossRef]
- Chaudhry, H.; Dashoush, N.; Tang, H.; Zhang, L.; Wang, X.; Wu, E.; Wolgemuth, D. Cyclin A2 mediates cardiomyocyte mitosis in the postmitotic myocardium. J. Biol. Chem. 2004, 279, 35858–35866. [Google Scholar] [CrossRef]
- Brooks, G.; Poolman, R.A.; McGill, C.J.; Li, J.M. Expression and activities of cyclins and cyclin-dependent kinases in developing rat ventricular myocytes. J. Mol. Cell Cardiol. 1997, 29, 2261–2271. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.J.; Koh, G.Y. Differential and dramatic changes of cyclin-dependent kinase activities in cardiomyocytes during the neonatal period. J. Mol. Cell Cardiol. 1997, 29, 1767–1777. [Google Scholar] [CrossRef] [PubMed]
- Woo, Y.; Panlilio, C.; Cheng, R.; Liao, G.; Atluri, P.; Hsu, V.; Cohen, J.; Chaundhry, H. Therapeutic delivery of cyclin A2 induces myocardial regeneration and enhances cardiac function in ischemic heart failure. Circulation 2006, 114, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Soonpaa, M.; Koh, G.; Pajak, L.; Jing, S.; Wang, H.; Franklin, M.; Kim, K.; Field, L. Cyclin D1 overexpression promotes cardiomyocyte DNA synthesis and multinucleation in transgenic mice. J. Clin. Investig. 1997, 99, 2644–2654. [Google Scholar] [CrossRef]
- Hassink, R.J.; Pasumarthi, K.B.; Nakajima, H.; Rubart, M.; Soonpaa, M.H.; de la Riviere, A.B.; Doevendans, P.A.; Field, L.J. Cardiomyocyte cell cycle activation improves cardiac function after myocardial infarction. Cardiovasc. Res. 2008, 78, 18–25. [Google Scholar] [CrossRef]
- Zhang, N.; Bai, H.; David, K.K.; Dong, J.; Zheng, Y.; Cai, J.; Giovannini, M.; Liu, P.; Anders, R.A.; Pan, D. The Merlin/NF2 tumor suppressor functions through the YAP oncoprotein to regulate tissue homeostasis in mammals. Dev. Cell 2010, 19, 27–38. [Google Scholar] [CrossRef]
- Hao, Y.; Chun, A.; Cheung, K.; Rashidi, B.; Yang, X. Tumor suppressor LATS1 is a negative regulator of oncogene YAP. J. Biol. Chem. 2008, 283, 5496–5509. [Google Scholar] [CrossRef]
- Heallen, T.; Morikawa, Y.; Leach, J.; Tao, G.; Willerson, J.T.; Johnson, R.L.; Martin, J.F. Hippo signaling impedes adult heart regeneration. Development 2013, 140, 4683–4690. [Google Scholar] [CrossRef]
- Xin, M.; Kim, Y.; Sutherland, L.B.; Murakami, M.; Qi, X.; McAnally, J.; Porrello, E.R.; Mahmoud, A.I.; Tan, W.; Shelton, J.M.; et al. Hippo pathway effector Yap promotes cardiac regeneration. Proc. Natl. Acad. Sci. USA 2013, 110, 13839–13844. [Google Scholar] [CrossRef]
- Zhao, B.; Wei, X.; Li, W.; Udan, R.S.; Yang, Q.; Kim, J.; Xie, J.; Ikenoue, T.; Yu, J.; Li, L.; et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007, 21, 2747–2761. [Google Scholar] [CrossRef]
- von Gise, A.; Lin, Z.; Schlegelmilch, K.; Honor, L.B.; Pan, G.M.; Buck, J.N.; Ma, Q.; Ishiwata, T.; Zhou, B.; Camargo, F.D.; et al. YAP1, the nuclear target of Hippo signaling, stimulates heart growth through cardiomyocyte proliferation but not hypertrophy. Proc. Natl. Acad. Sci. USA 2012, 109, 2394–2399. [Google Scholar] [CrossRef]
- Zhao, B.; Ye, X.; Yu, J.; Li, L.; Li, W.; Li, S.; Yu, J.; Lin, J.D.; Wang, C.Y.; Chinnaiyan, A.M.; et al. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 2008, 22, 1962–1971. [Google Scholar] [CrossRef] [PubMed]
- Heallen, T.; Zhang, M.; Wang, J.; Bonilla-Claudio, M.; Klysik, E.; Johnson, R.L.; Martin, J.F. Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science 2011, 332, 458–461. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Yang, G.; Zablocki, D.; Liu, J.; Hong, C.; Kim, S.J.; Soler, S.; Odashima, M.; Thaisz, J.; Yehia, G.; et al. Activation of Mst1 causes dilated cardiomyopathy by stimulating apoptosis without compensatory ventricular myocyte hypertrophy. J. Clin. Investig. 2003, 111, 1463–1474. [Google Scholar] [CrossRef] [PubMed]
- Monroe, T.O.; Hill, M.C.; Morikawa, Y.; Leach, J.P.; Heallen, T.; Cao, S.; Krijger, P.H.; de Laat, W.; Wehrens, X.H.; Rodney, G.G. YAP partially reprograms chromatin accessibility to directly induce adult cardiogenesis in vivo. Dev. Cell 2019, 48, 765–779. [Google Scholar] [CrossRef]
- Engel, F.; Schebesta, M.; Duong, M.; Lu, G.; Ren, S.; Madwed, J.; Jiang, H.; Wang, Y.; Keating, M. p38 MAP kinase inhibition enables proliferation of adult mammalian cardiomyocytes. Genes Dev. 2005, 19, 1175–1187. [Google Scholar] [CrossRef]
- Engel, F.; Hsieh, P.; Lee, R.; Keating, M. FGF1/p38 MAP kinase inhibitor therapy induces cardiomyocyte mitosis, reduces scarring, and rescues function after myocardial infarction. Proc. Natl. Acad. Sci. USA 2006, 103, 15546–15551. [Google Scholar] [CrossRef]
- Mahmoud, A.; Kocabas, F.; Muralidhar, S.; Kimura, W.; Koura, A.; Thet, S.; Porrello, E.; Sadek, H. Meis1 regulates postnatal cardiomyocyte cell cycle arrest. Nature 2013, 497, 249–253. [Google Scholar] [CrossRef]
- Wamstad, J.; Alexander, J.; Truty, R.; Shrikumar, A.; Li, F.; Eilertson, K.; Ding, H.; Wylie, J.; Pico, A.; Capra, J.; et al. Dynamic and coordinated epigenetic regulation of developmental transitions in the cardiac lineage. Cell 2012, 151, 206–220. [Google Scholar] [CrossRef]
- González-Lázaro, M.; Roselló-Díez, A.; Delgado, I.; Carramolino, L.; Sanguino, M.; Giovinazzo, G.; Torres, M. Two new targeted alleles for the comprehensive analysis of Meis1 functions in the mouse. Genesis 2014, 52, 967–975. [Google Scholar] [CrossRef]
- Yahalom-Ronen, Y.; Rajchman, D.; Sarig, R.; Geiger, B.; Tzahor, E. Reduced matrix rigidity promotes neonatal cardiomycoyte dedifferentiation, proliferation and clonal expansion. ELife 2015, 4, e07455. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wang, Z.; Missinato, M.; Park, D.; Long, D.; Liu, H.; Zeng, X.; Yates, N.; Kim, K.; Wang, Y. Decellularized zebrafish cardiac extracellular matrix induces mammalian heart regeneration. Sci. Adv. 2016, 2, e1600844. [Google Scholar] [CrossRef]
- Bassat, E.; Mutlak, Y.; Genzelinakh, A.; Shadrin, I.; Baruch-Umansky, K.; Yifa, O.; Kain, D.; Rajchman, D.; Leach, J.; Bassat, D.; et al. The extracellular matrix protein Agrin promotes heart regeneration in mice. Nature 2017. [Google Scholar] [CrossRef] [PubMed]
- Vinarsky, V.; Atkinson, D.; Stevenson, T.; Keating, M.; Odelberg, S. Normal newt limb regeneration requires matrix metalloproteinase function. Dev. Biol. 2005, 279, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Chablais, F.; Jazwinska, A. The regenerative capacity of the zebrafish heart is dependent on TGFbeta signaling. Development 2012, 139, 1921–1930. [Google Scholar] [CrossRef]
- Elosegui-Artola, A.; Andreu, I.; Beedle, A.E.M.; Lezamiz, A.; Uroz, M.; Kosmalska, A.J.; Oria, R.; Kechagia, J.Z.; Rico-Lastres, P.; Le Roux, A.L.; et al. Force Triggers YAP Nuclear Entry by Regulating Transport across Nuclear Pores. Cell 2017, 171, 1397–1410. [Google Scholar] [CrossRef]
- Conway, S.J.; Molkentin, J.D. Periostin as a heterofunctional regulator of cardiac development and disease. Curr. Genomics. 2008, 9, 548–555. [Google Scholar] [CrossRef]
- Matsui, Y.; Morimoto, J.; Uede, T. Role of matricellular proteins in cardiac tissue remodeling after myocardial infarction. World J. Biol. Chem. 2010, 1, 69–80. [Google Scholar] [CrossRef]
- Kuhn, B.; del Monte, F.; Hajjar, R.J.; Chang, Y.S.; Lebeche, D.; Arab, S.; Keating, M.T. Periostin induces proliferation of differentiated cardiomyocytes and promotes cardiac repair. Nat. Med. 2007, 13, 962–969. [Google Scholar] [CrossRef]
- Grego-Bessa, J.; Luna-Zurita, L.; del Monte, G.; Bolos, V.; Melgar, P.; Arandilla, A.; Garratt, A.N.; Zang, H.; Mukouyama, Y.S.; Chen, H.; et al. Notch signaling is essential for ventricular chamber development. Dev. Cell 2007, 12, 415–429. [Google Scholar] [CrossRef]
- Bersell, K.; Arab, S.; Haring, B.; Kühn, B. Neuregulin1/ErbB4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell 2009, 138, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Wadugu, B.; Kühn, B. The role of neuregulin/ErbB2/ErbB4 signaling in the heart with special focus on effects on cardiomyocyte proliferation. Am. J. Physiol. 2012, 302, H2139–H2147. [Google Scholar] [CrossRef] [PubMed]
- D′Uva, G.; Aharonov, A.; Lauriola, M.; Kain, D.; Yahalom-Ronen, Y.; Carvalho, S.; Weisinger, K.; Bassat, E.; Rajchman, D.; Yifa, O.; et al. ERBB2 triggers mammalian heart regeneration by promoting cardiomyocyte dedifferentiation and proliferation. Nat. Cell Biol. 2015, 17, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.I.; O’Meara, C.C.; Gemberling, M.; Zhao, L.; Bryant, D.M.; Zheng, R.; Gannon, J.B.; Cai, L.; Choi, W.Y.; Egnaczyk, G.F.; et al. Nerves Regulate Cardiomyocyte Proliferation and Heart Regeneration. Dev. Cell 2015, 34, 387–399. [Google Scholar] [CrossRef] [PubMed]
- Kaye, D.; Vaddadi, G.; Gruskin, S.; Du, X.; Esler, M. Reduced myocardial nerve growth factor expression in human and experimental heart failure. Circ. Res. 2000, 86, 80–84. [Google Scholar] [CrossRef]
- Lam, N.; Currie, P.; Lieschke, G.; Rosenthal, N.; Kaye, D. Nerve growth factor stimulates cardiac regeneration via cardiomyocyte proliferation in experimental heart failure. PLoS ONE 2012, 7, e53210. [Google Scholar] [CrossRef]
- Shefer, G.; Oron, U.; Irintchev, A.; Werning, A.; Halevy, O. Skeletal muscle cell activation by low-energy laser irradiation: A role for the MAPK/ERK pathway. J. Cell. Physiol. 2001, 187, 7380. [Google Scholar] [CrossRef]
- Oberpriller, J.O.; Oberpriller, J.C.; Matz, D.; Soonpaa, M. Stimulation of proliferative events in the adult amphibian cardiac myocyte. Ann. N. Y. Acad. Sci. 1995, 752, 30–46. [Google Scholar] [CrossRef]
- Lavine, K.J.; Yu, K.; White, A.C.; Zhang, X.; Smith, C.; Partanen, J.; Ornitz, D.M. Endocardial and epicardial derived FGF signals regulate myocardial proliferation and differentiation in vivo. Dev. Cell 2005, 8, 85–95. [Google Scholar] [CrossRef]
- Li, P.; Cavallero, S.; Gu, Y.; Chen, T.H.; Hughes, J.; Hassan, A.B.; Bruning, J.C.; Pashmforoush, M.; Sucov, H.M. IGF signaling directs ventricular cardiomyocyte proliferation during embryonic heart development. Development 2011, 138, 1795–1805. [Google Scholar] [CrossRef]
- Huang, Y.; Harrison, M.; Osorio, A.; Kim, J.; Baugh, A.; Duan, C.; Sucov, H.; Lien, C. Igf signaling is required for cardiomyocyte proliferation during zebrafish heart development and regeneration. PLoS ONE 2013, 8, e67266. [Google Scholar] [CrossRef] [PubMed]
- Sallin, P.; Jazwinska, A. Acute stress is detrimental to heart regeneration in zebrafish. Open Biol. 2016, 6. [Google Scholar] [CrossRef]
- Itou, J.; Oishi, I.; Kawakami, H.; Glass, T.J.; Richter, J.; Johnson, A.; Lund, T.C.; Kawakami, Y. Migration of cardiomyocytes is essential for heart regeneration in zebrafish. Development 2012, 139, 4133–4142. [Google Scholar] [CrossRef] [PubMed]
- Dona, E.; Barry, J.D.; Valentin, G.; Quirin, C.; Khmelinskii, A.; Kunze, A.; Durdu, S.; Newton, L.R.; Fernandez-Minan, A.; Huber, W.; et al. Directional tissue migration through a self-generated chemokine gradient. Nature 2013, 503, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Venkiteswaran, G.; Lewellis, S.W.; Wang, J.; Reynolds, E.; Nicholson, C.; Knaut, H. Generation and dynamics of an endogenous, self-generated signaling gradient across a migrating tissue. Cell 2013, 155, 674–687. [Google Scholar] [CrossRef] [PubMed]
- Segers, V.F.; Tokunou, T.; Higgins, L.J.; MacGillivray, C.; Gannon, J.; Lee, R.T. Local delivery of protease-resistant stromal cell derived factor-1 for stem cell recruitment after myocardial infarction. Circulation 2007, 116, 1683–1692. [Google Scholar] [CrossRef]
- Saxena, A.; Fish, J.E.; White, M.D.; Yu, S.; Smyth, J.W.; Shaw, R.M.; DiMaio, J.M.; Srivastava, D. Stromal cell-derived factor-1alpha is cardioprotective after myocardial infarction. Circulation 2008, 117, 2224–2231. [Google Scholar] [CrossRef] [PubMed]
- de Preux Charles, A.; Bise, T.; Baier, F.; Marro, J.; Jazwinska, A. Distinct effects of inflammation on preconditioning and regeneration of the adult zebrafish heart. Open Biol. 2016, 6. [Google Scholar] [CrossRef]
- Aurora, A.B.; Porrello, E.R.; Tan, W.; Mahmoud, A.I.; Hill, J.A.; Bassel-Duby, R.; Sadek, H.A.; Olson, E.N. Macrophages are required for neonatal heart regeneration. J. Clin. Investig. 2014, 124, 1382–1392. [Google Scholar] [CrossRef]
- Lavine, K.J.; Epelman, S.; Uchida, K.; Weber, K.J.; Nichols, C.G.; Schilling, J.D.; Ornitz, D.M.; Randolph, G.J.; Mann, D.L. Distinct macrophage lineages contribute to disparate patterns of cardiac recovery and remodeling in the neonatal and adult heart. Proc. Natl. Acad. Sci. USA 2014, 111, 16029–16034. [Google Scholar] [CrossRef]
- Lien, C.L.; Schebesta, M.; Makino, S.; Weber, G.J.; Keating, M.T. Gene expression analysis of zebrafish heart regeneration. PLoS Biol. 2006, 4, e260. [Google Scholar] [CrossRef] [PubMed]
- Ley, K. M1 Means Kill; M2 Means Heal. J. Immunol. 2017, 199, 2191–2193. [Google Scholar] [CrossRef]
- Vagnozzi, R.J.; Maillet, M.; Sargent, M.A.; Khalil, H.; Johansen, A.K.Z.; Schwanekamp, J.A.; York, A.J.; Huang, V.; Nahrendorf, M.; Sadayappan, S.; et al. An acute immune response underlies the benefit of cardiac stem cell therapy. Nature 2020, 577, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.R.; Hippenmeyer, S.; Saadat, L.V.; Luo, L.; Weissman, I.L.; Ardehali, R. Existing cardiomyocytes generate cardiomyocytes at a low rate after birth in mice. Proc. Natl. Acad. Sci. USA 2014, 111, 8850–8855. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.E.; Li, L.; Xia, X.; Fu, W.; Liao, Q.; Lan, C.; Yang, D.; Chen, H.; Yue, R.; Zeng, C.; et al. Dedifferentiation, Proliferation, and Redifferentiation of Adult Mammalian Cardiomyocytes After Ischemic Injury. Circulation 2017, 136, 834–848. [Google Scholar] [CrossRef] [PubMed]
- Puente, B.N.; Kimura, W.; Muralidhar, S.A.; Moon, J.; Amatruda, J.F.; Phelps, K.L.; Grinsfelder, D.; Rothermel, B.A.; Chen, R.; Garcia, J.A.; et al. The oxygen-rich postnatal environment induces cardiomyocyte cell-cycle arrest through DNA damage response. Cell 2014, 157, 565–579. [Google Scholar] [CrossRef]
- Bettencourt-Dias, M.; Mittnacht, S.; Brockes, J. Heterogeneous proliferative potential in regenerative adult newt cardiomyocytes. J. Cell Sci. 2003, 116, 4001–4009. [Google Scholar] [CrossRef]
- González-Rosa, J.; Sharpe, M.; Field, D.; Soonpaa, M.; Field, L.; Burns, C.E.; Burns, C.G. Myocardial polyploidization creates a barrier to heart regeneration in zebrafish. Dev. Cell 2018, 44, 433–446. [Google Scholar] [CrossRef]
- Marshall, L.N.; Vivien, C.J.; Girardot, F.; Pericard, L.; Scerbo, P.; Palmier, K.; Demeneix, B.A.; Coen, L. Stage-dependent cardiac regeneration in Xenopus is regulated by thyroid hormone availability. Proc. Natl. Acad. Sci. USA 2019, 116, 3614–3623. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Wit, L.; Fang, J.; Neef, K.; Xiao, J.; A. Doevendans, P.; Schiffelers, R.M.; Lei, Z.; Sluijter, J.P.G. Cellular and Molecular Mechanism of Cardiac Regeneration: A Comparison of Newts, Zebrafish, and Mammals. Biomolecules 2020, 10, 1204. https://doi.org/10.3390/biom10091204
de Wit L, Fang J, Neef K, Xiao J, A. Doevendans P, Schiffelers RM, Lei Z, Sluijter JPG. Cellular and Molecular Mechanism of Cardiac Regeneration: A Comparison of Newts, Zebrafish, and Mammals. Biomolecules. 2020; 10(9):1204. https://doi.org/10.3390/biom10091204
Chicago/Turabian Stylede Wit, Lousanne, Juntao Fang, Klaus Neef, Junjie Xiao, Pieter A. Doevendans, Raymond M. Schiffelers, Zhiyong Lei, and Joost P.G. Sluijter. 2020. "Cellular and Molecular Mechanism of Cardiac Regeneration: A Comparison of Newts, Zebrafish, and Mammals" Biomolecules 10, no. 9: 1204. https://doi.org/10.3390/biom10091204
APA Stylede Wit, L., Fang, J., Neef, K., Xiao, J., A. Doevendans, P., Schiffelers, R. M., Lei, Z., & Sluijter, J. P. G. (2020). Cellular and Molecular Mechanism of Cardiac Regeneration: A Comparison of Newts, Zebrafish, and Mammals. Biomolecules, 10(9), 1204. https://doi.org/10.3390/biom10091204




