Expression and Purification of Recombinant GHK Tripeptides Are Able to Protect against Acute Cardiotoxicity from Exposure to Waterborne-Copper in Zebrafish
Abstract
1. Introduction
1.1. GHK Tripeptide and Its Applications
1.2. The Toxicity of Copper
1.3. GHK and Copper Ion Complex Formation
1.4. Zebrafish as an Animal Model to Study the GHK Tripeptide Effect on Chelating Copper Toxicity
2. Materials and Methods
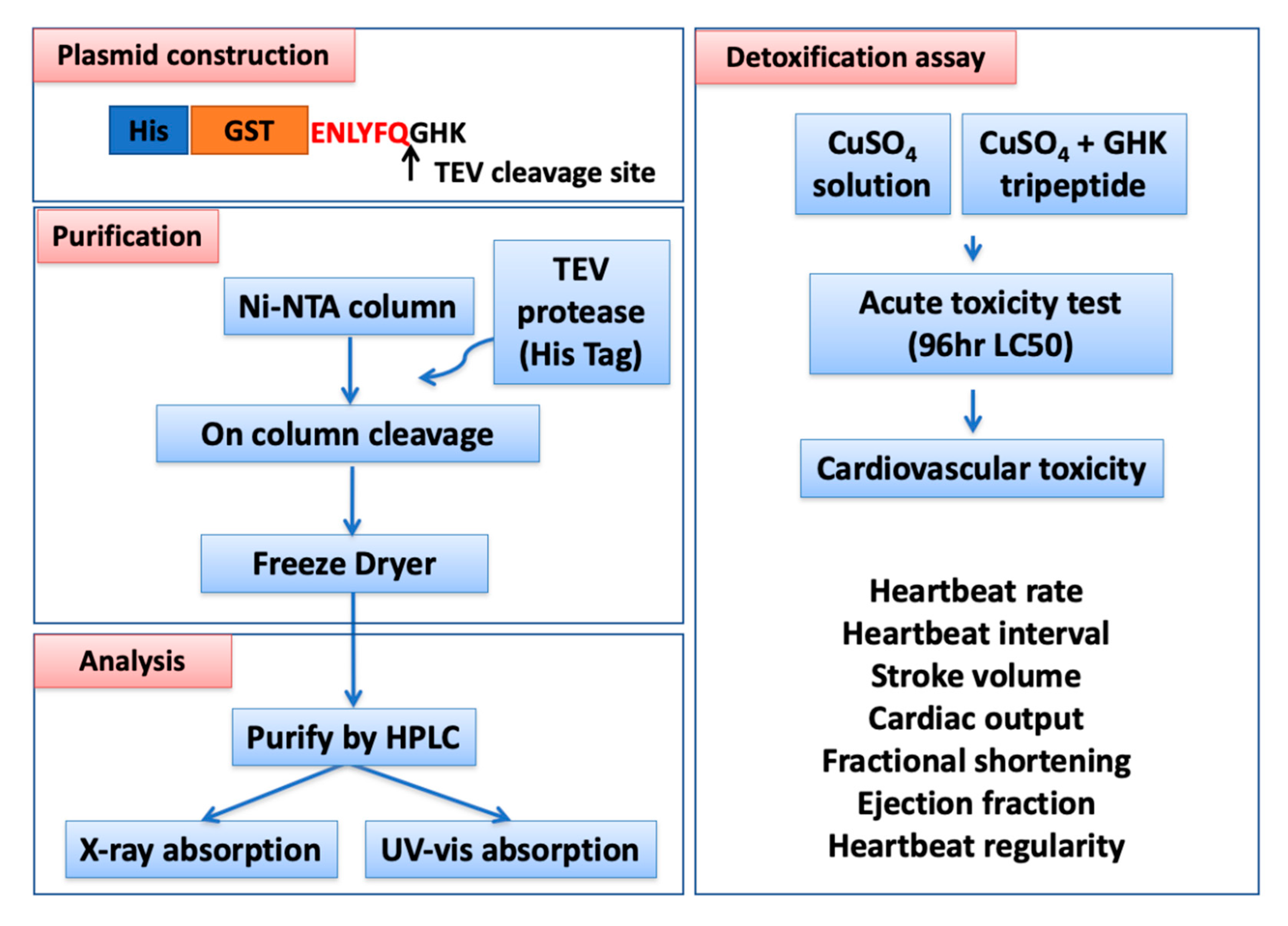
2.1. Plasmid Construction
2.2. Protein Expression in E. coli
2.3. Protein Purification by HisTrip Column and 6XHis Tag TEV Cleavage
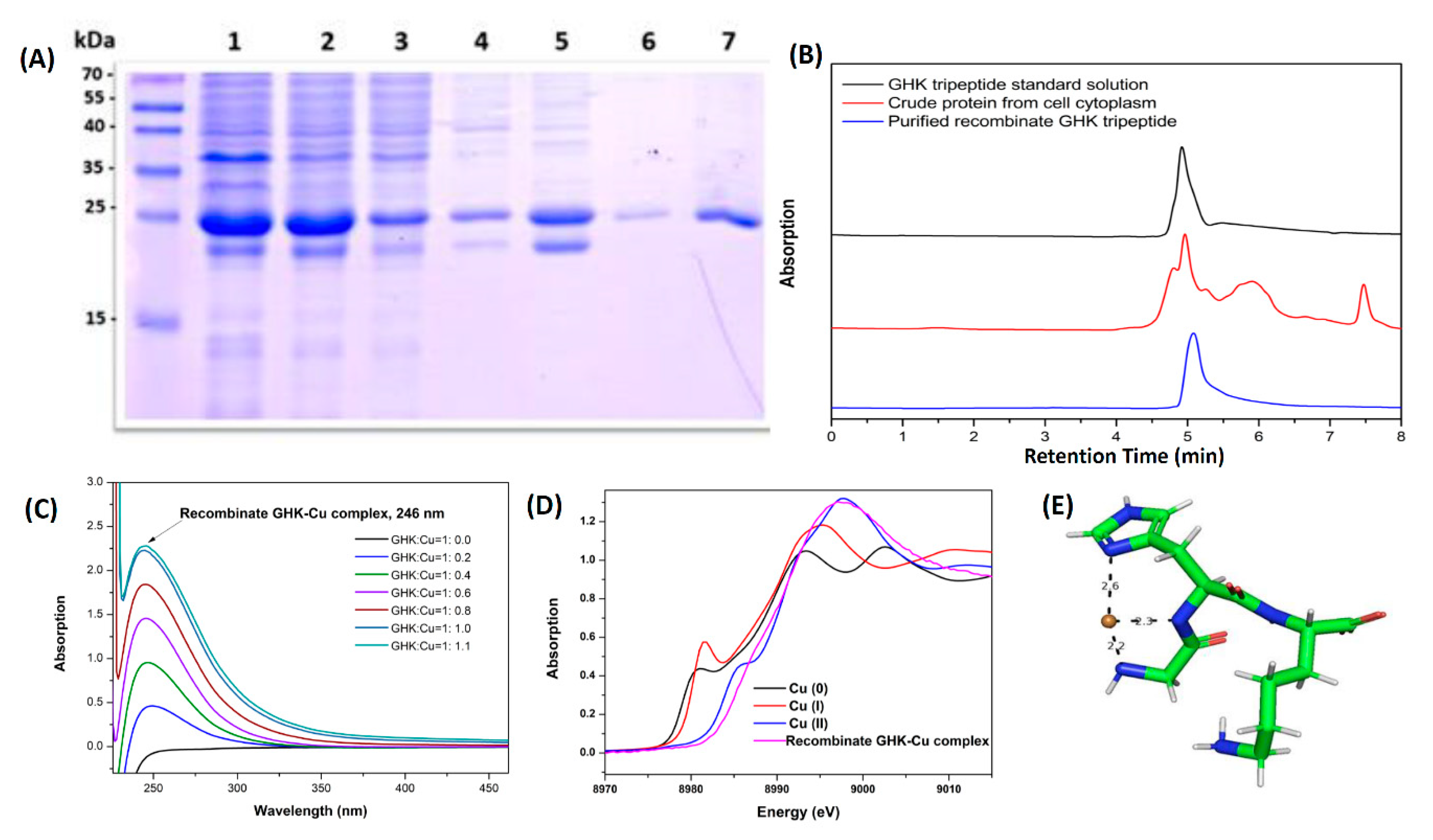
2.4. SDS-PAGE
2.5. UV-vis Spectra
2.6. X-Ray Absorption Spectroscopy
2.7. Determine the Concentrations and Molecular Weight of Recombinant GHK Peptide by HPLC
2.8. Zebrafish Maintenance and Ethics
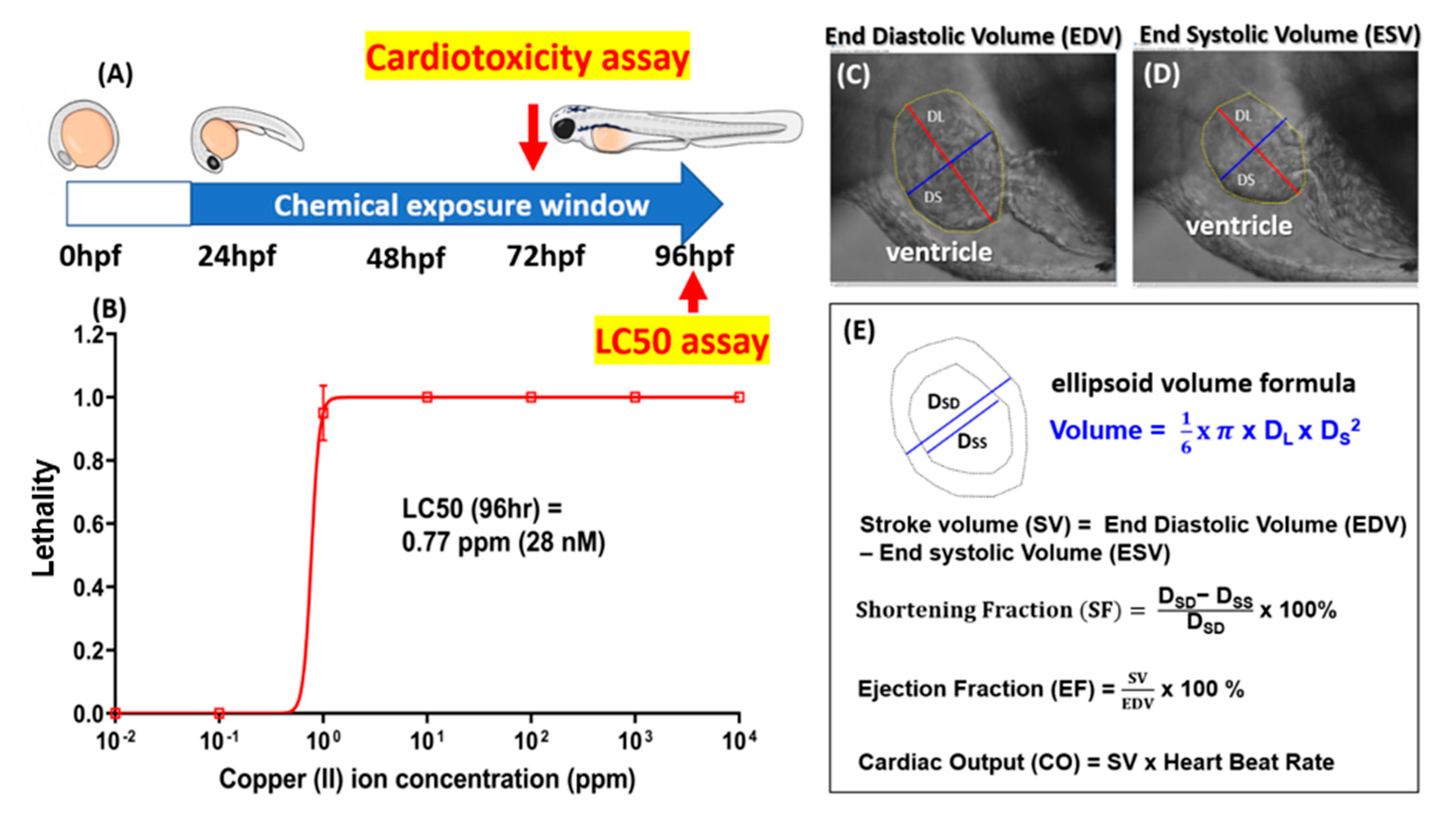
2.9. Zebrafish Acute Toxicity Test
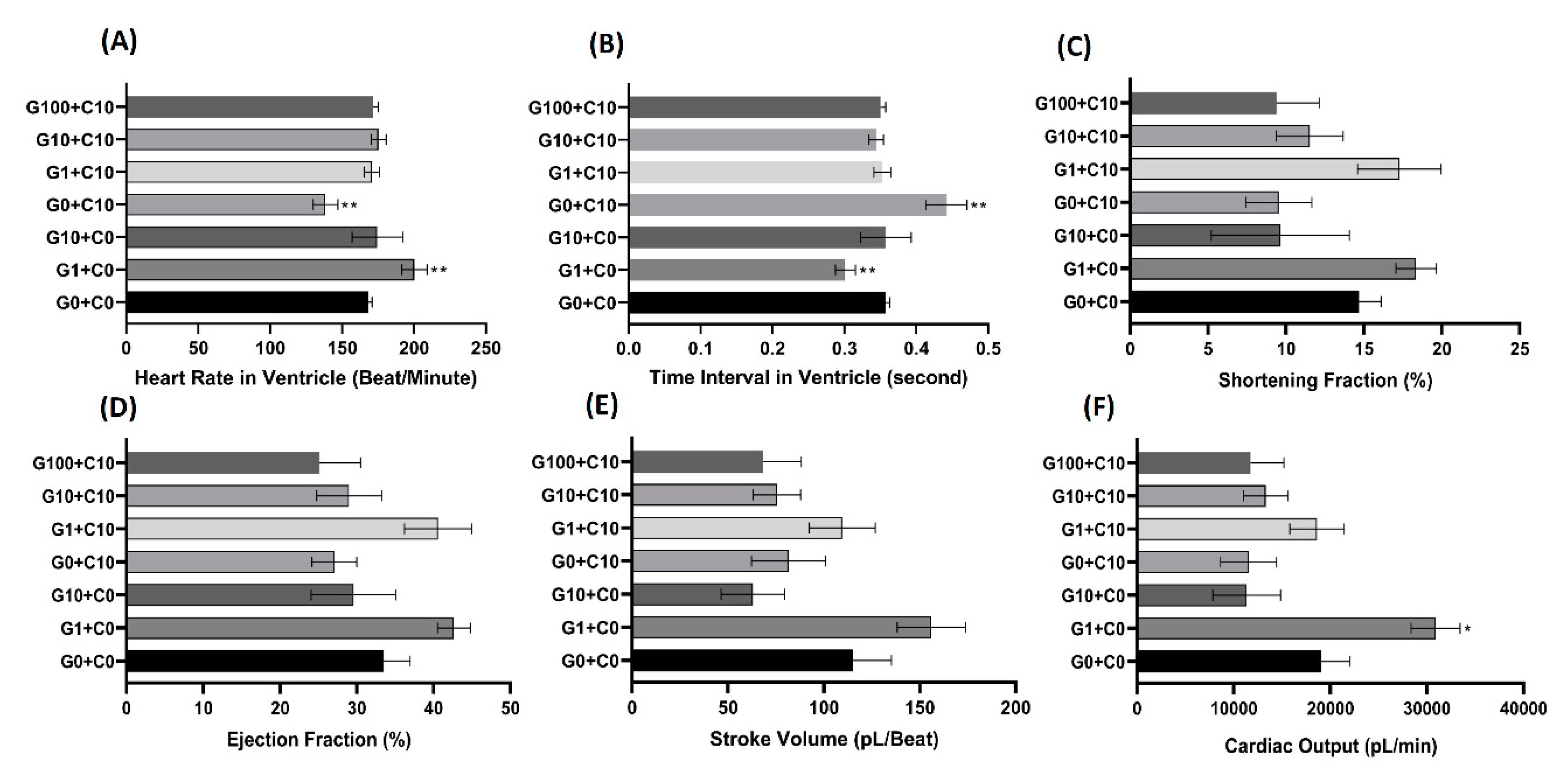
2.10. Zebrafish Cardiac Performance Assay
2.11. Zebrafish Cardiac Performance Endpoint Calculation
2.12. Statistics
3. Results
3.1. Overview of Experimental Design and Workflow
3.2. Expression, Purification, and Characterization of GHK Tripeptide
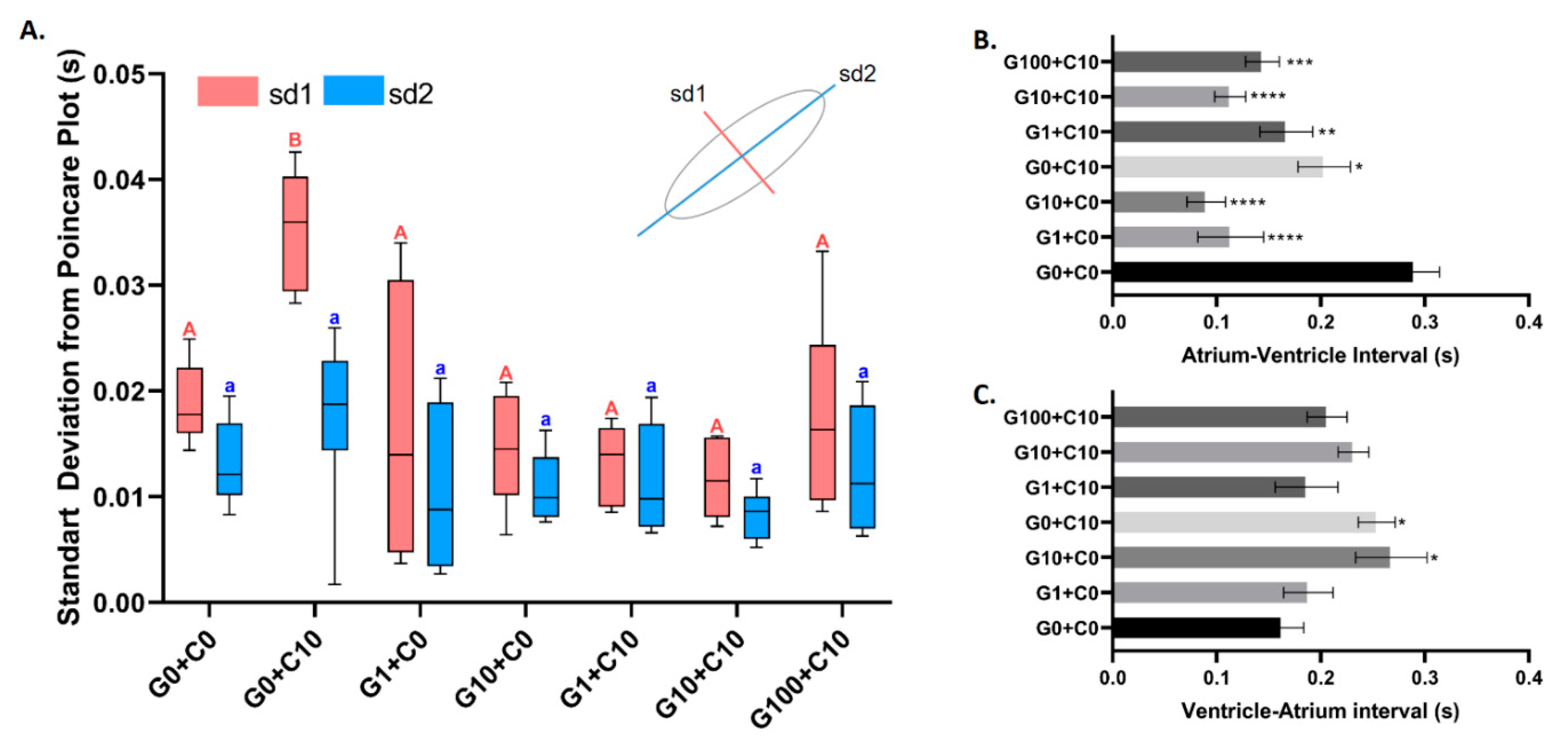
3.3. Bioassay of GHK Tripeptide on Protecting Copper Toxicity in Zebrafish
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| GHK Tripeptide Production Methods | ||
|---|---|---|
| Method | Recombinant expression (this study) | Solid-phase peptide synthesis (SPPS) |
| Productivity | Good reproducibility—recombinant GHK is overexpressed in E. coli successfully. High efficiency—large-scale and high-density E. coli cultures are achieved by a fermentation process. Simplifies purification by using one-step affinity column chromatography. | The SPPS method is mature—the porous solid resin matrix is stable and makes the reactants easily proceed in the voids. The polymerization of six amino acids can be completed in one day. |
| Sustainable | Eliminating the use of toxic chemical reagents and organic solvents. | Organic solvents like reprotoxic dimethylformamide are required. |
| Limitation | Cloning and molecular microbiology technology are required. Unable to prepare nonproteinogenic amino acids (e.g., D-form amino acids). | Length limited to 70 amino acid monomers or less. The price of peptide raw materials. Chemical synthesis yield. |
References
- Pickart, L.; Vasquez-Soltero, J.M.; Margolina, A. GHK peptide as a natural modulator of multiple cellular pathways in skin regeneration. Biomed. Res. Int. 2015, 2015, 648108. [Google Scholar] [CrossRef]
- Park, J.-R.; Lee, H.; Kim, S.-I.; Yang, S.-R. The tri-peptide GHK-Cu complex ameliorates lipopolysaccharide-induced acute lung injury in mice. Oncotarget 2016, 7, 58405. [Google Scholar] [CrossRef]
- Pickart, L.; MM, T. Tripeptide in human serum which prolongs survival of normal liver cells and stimulates growth in neoplastic liver. Nat. New Biol. 1973, 243, 85–87. [Google Scholar] [PubMed]
- Pickart, L.; Thaler, M.M. Growth-modulating tripeptide (glycylhistidyllysine): Associaton with copper and iron in plasma, and stimulation of adhesiveness and growth of hepatoma cells in culture by tripeptide-metal ion complexes. J. Cell. Physiol. 1980, 102, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Barra, R. Effects of glycyl-histidyl-lysine on Morris hepatoma 7777 cells. Cytobios 1987, 52, 99–107. [Google Scholar] [PubMed]
- Pickart, L. The use of glycylhistidyllysine in culture systems. In Vitro 1981, 17, 459–466. [Google Scholar] [CrossRef]
- Lassila, O.; Lambris, J.D.; Gisler, R.H. A role for Lys-His-Gly-NH2 in avian and murine B cell development. Cell. Immunol. 1989, 122, 319–328. [Google Scholar] [CrossRef]
- Pešáková, V.; Novotná, J.; Milan, A. Effect of the tripeptide glycyl-L-histidyl-L-lysine on the proliferation and synthetic activity of chick embryo chondrocytes. Biomaterials 1995, 16, 911–915. [Google Scholar] [CrossRef]
- Errick, J.E.; Ing, K.W.; Eggo, M.C.; Burrow, G.N. Growth and differentiation in cultured human thyroid cells: Effects of epidermal growth factor and thyrotropin. In Vitro Cell. Dev. Biol. 1986, 22, 28–36. [Google Scholar] [CrossRef]
- Godet, D.; Marie, P. Effects of the tripeptide glycyl-L-histidyl-L-lysine copper complex on osteoblastic cell spreading, attachment and phenotype. Cell. Mol. Biol. 1995, 41, 1081. [Google Scholar]
- Pickart, L.; Lovejoy, S. Biological activity of human plasma copper-binding growth factor glycyl-l-histidyl-l-lysine. In Methods in Enzymology; Barnes, D., Sirbasku, D.A., Eds.; Elsevier: Amsterdam, The Netherlands, 1987; Volume 147, pp. 314–328. [Google Scholar]
- Conato, C.; Gavioli, R.; Guerrini, R.; Kozłowski, H.; Młynarz, P.; Pasti, C.; Pulidori, F.; Remelli, M. Copper complexes of glycyl-histidyl-lysine and two of its synthetic analogues: Chemical behaviour and biological activity. Biochim. Et Biophys. Acta 2001, 1526, 199–210. [Google Scholar] [CrossRef]
- Gullino, P.M. Microenvironment and angiogenic response. In Angiogenesis; Steiner, R., Weisz, P.B., Langer, R., Eds.; Birkhäuser: Basel, Switzerland, 1992; pp. 125–128. [Google Scholar]
- Sage, E.H.; Vernon, R.B. Regulation of angiogenesis by extracellular matrix: The growth and the glue. J. Hypertens. Suppl. Off. J. Int. Soc. Hypertens. 1994, 12, S145. [Google Scholar]
- Miller, D.; DeSilva, D.; Pickart, L.; Aust, S. Effects of glycyl-histidyl-lysyl chelated Cu (II) on ferritin dependent lipid peroxidation. In Antioxidants in Therapy and Preventive Medicine; Emerit, I., Packer, L., Auclair, C., Eds.; Springer: Boston, MA, USA, 1990; pp. 79–84. [Google Scholar]
- Buffoni, F.; Pino, R.; Dal Pozzo, A. Effect of tripeptide-copper complexes on the process of skin wound healing and on cultured fibroblasts. Arch. Int. De Pharmacodyn. Et De Ther. 1995, 330, 345–360. [Google Scholar]
- Pohunkova, H.; Stehí, J.; Vachal, J.; Adam, M. Morphological features of bone healing under the effect of collagen-graft—glycosaminoglycan copolymer supplemented with the tripeptide Gly-His-Lys. Biomaterials 1996, 17, 1567–1574. [Google Scholar] [CrossRef]
- Maquart, F.-X.; Pickart, L.; Laurent, M.; Gillery, P.; Monboisse, J.-C.; Borel, J.-P. Stimulation of collagen synthesis in fibroblast cultures by the tripeptide-copper complex glycyl-L-histidyl-L-lysine-Cu2+. FEBS Lett. 1988, 238, 343–346. [Google Scholar] [CrossRef]
- Finkley, M.; Appa, Y.; Bhandarkar, S. Copper Peptide and Skin Cosmeceuticals and Active Cosmetics: Drugs vs. Cosmetics; Elsner, P., Maibach, H., Eds.; Marcel Dekker: New York, NY, USA, 2005; pp. 550–561. [Google Scholar]
- Pickart, L.; Vasquez-Soltero, J.M.; Margolina, A. GHK-Cu may prevent oxidative stress in skin by regulating copper and modifying expression of numerous antioxidant genes. Cosmetics 2015, 2, 236–247. [Google Scholar] [CrossRef]
- Pickart, L.; Vasquez-Soltero, J.M.; Margolina, A. The human tripeptide GHK-Cu in prevention of oxidative stress and degenerative conditions of aging: Implications for cognitive health. Oxidative Med. Cell. Longev. 2012, 2012, 324832. [Google Scholar] [CrossRef]
- Pickart, L. The human tri-peptide GHK and tissue remodeling. J. Biomater. Sci. Polym. Ed. 2008, 19, 969–988. [Google Scholar] [CrossRef]
- Gómez-Mendikute, A.; Cajaraville, M. Comparative effects of cadmium, copper, paraquat and benzo [a] pyrene on the actin cytoskeleton and production of reactive oxygen species (ROS) in mussel haemocytes. Toxicol. Vitro 2003, 17, 539–546. [Google Scholar] [CrossRef]
- Davis, A.; Smith, G. Fallout over Disneyland. Earth Isl. J. 2002, 17, 28–29. [Google Scholar]
- Zietz, B. Prevalence of elevated copper concentrations in tap water in two areas of Germany used for infant feeding and possible health implications. Eur. J. Med. Res. 1999, 4, 298. [Google Scholar] [PubMed]
- Philips, N.; Hwang, H.; Chauhan, S.; Leonardi, D.; Gonzalez, S. Stimulation of cell proliferation and expression of matrixmetalloproteinase-1 and interluekin-8 genes in dermal fibroblasts by copper. Connect. Tissue Res. 2010, 51, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Sabullah, M.; Shukor, M.Y.; Sulaiman, M.R.; Shamaan, N.; Syed, M.; Khalid, A.; Ahmad, S. The effect of copper on the ultrastructure of Puntius javanicus hepatocyte. Aust. J. Basic Appl. Sci. 2014, 8, 245–251. [Google Scholar]
- Pelgrom, S.; Lock, R.; Balm, P.; Bonga, S.W. Integrated physiological response of tilapia, Oreochromis mossambicus, to sublethal copper exposure. Aquat. Toxicol. 1995, 32, 303–320. [Google Scholar] [CrossRef]
- Monteiro, S.M.; Oliveira, E.; Fontaínhas-Fernandes, A.; Sousa, M. Effects of sublethal and lethal copper concentrations on the gill epithelium ultrastructure of Nile tilapia, Oreochromis niloticus. Zool. Stud. 2012, 51, 977–987. [Google Scholar]
- Heath, A.G. Effects of waterborne copper or zinc on the osmoregulatory response of bluegill to a hypertonic NaCl challenge. Comp. Biochem. Physiol. Part. C Comp. Pharmacol. 1987, 88, 307–311. [Google Scholar] [CrossRef]
- De Romaña, D.L.; Olivares, M.; Uauy, R.; Araya, M. Risks and benefits of copper in light of new insights of copper homeostasis. J. Trace Elem. Med. Biol. 2011, 25, 3–13. [Google Scholar] [CrossRef]
- Lefer, D.J.; Granger, D.N. Oxidative stress and cardiac disease. Am. J. Med. 2000, 109, 315–323. [Google Scholar] [CrossRef]
- Serruys, P.W.; Farooq, V.; Kalesan, B.; de Vries, T.; Buszman, P.; Linke, A.; Ischinger, T.; Klauss, V.; Eberli, F.; Wijns, W. Improved safety and reduction in stent thrombosis associated with biodegradable polymer-based biolimus-eluting stents versus durable polymer-based sirolimus-eluting stents in patients with coronary artery disease: Final 5-year report of the LEADERS (Limus Eluted From A Durable Versus ERodable Stent Coating) randomized, noninferiority trial. Jacc: Cardiovasc. Interv. 2013, 6, 777–789. [Google Scholar]
- Hernandez, P.P.; Undurraga, C.; Gallardo, V.E.; Mackenzie, N.; Allende, M.L.; Reyes, A.E. Sublethal concentrations of waterborne copper induce cellular stress and cell death in zebrafish embryos and larvae. Biol. Res. 2011, 44, 7–15. [Google Scholar] [CrossRef][Green Version]
- Johnson, A.; Carew, E.; Sloman, K. The effects of copper on the morphological and functional development of zebrafish embryos. Aquat. Toxicol. 2007, 84, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Stanley, A.S.R.S.A. Studies on the toxicological effects of bimetals on the cladoceran, Daphnia magna and examination of histopathological effects through Transmission Electron Microscopy (TEM). J. Chem. Pharm. Res. 2015, 7, 506–511. [Google Scholar]
- Curtis, T.; Williamson, R.; Depledge, M. The initial mode of action of copper on the cardiac physiology of the blue mussel, Mytilus edulis. Aquat. Toxicol. 2001, 52, 29–38. [Google Scholar] [CrossRef]
- Darwish, H.M.; Cheney, J.C.; Schmitt, R.C.; Ettinger, M.J. Mobilization of copper (II) from plasma components and mechanisms of hepatic copper transport. Am. J. Physiol. Gastrointest. Liver Physiol. 1984, 246, G72–G79. [Google Scholar] [CrossRef] [PubMed]
- Pickart, L.; Freedman, J.H.; Loker, W.J.; Peisach, J.; Perkins, C.M.; Stenkamp, R.E.; Weinstein, B. Growth-modulating plasma tripeptide may function by facilitating copper uptake into cells. Nature 1980, 288, 715–717. [Google Scholar] [CrossRef] [PubMed]
- Hartter, D.; Barnea, A. Brain tissue accumulates 67copper by two ligand-dependent saturable processes. A high affinity, low capacity and a low affinity, high capacity process. J. Biol. Chem. 1988, 263, 799–805. [Google Scholar]
- Antholine, W.E.; Petering, D.H.; Pickart, L. ESR studies of the interaction of copper (II) GHK, histidine, and Ehrlich cells. J. Inorg. Biochem. 1989, 35, 215–224. [Google Scholar] [CrossRef]
- Perkins, C.M.; Rose, N.J.; Weinstein, B.; Stenkamp, R.E.; Jensen, L.H.; Pickart, L. The structure of a copper complex of the growth factor glycyl-L-histidyl-L-lysine at 1.1 Å resolution. Inorg. Chim. Acta 1984, 82, 93–99. [Google Scholar] [CrossRef]
- Kremennaya, M.; Soldatov, M.; Podkovyrina, Y.S.; Dadasheva, I.; Soldatov, A. X-ray spectroscopy study of the Cu (II) GHK peptide complex. J. Struct. Chem. 2017, 58, 1213–1219. [Google Scholar] [CrossRef]
- Lau, S.-J.; Sarkar, B. The interaction of copper (II) and glycyl-L-histidyl-L-lysine, a growth-modulating tripeptide from plasma. Biochem. J. 1981, 199, 649–656. [Google Scholar] [CrossRef]
- Pickart, L. Iamin: A human growth factor with multiple wound-healing properties. In Biology of Copper Complexes; Sorenson, J., Ed.; Humana Press: Totowa, NJ, USA, 1987; pp. 273–285. [Google Scholar]
- Pickart, L.R. Use of GHL-Cu as a wound-healing and anti-inflammatory agent. US4760051A, 26 July 1988. [Google Scholar]
- Pickart, L.; Vasquez-Soltero, J.M.; Margolina, A. The effect of the human peptide GHK on gene expression relevant to nervous system function and cognitive decline. Brain Sci. 2017, 7, 20. [Google Scholar] [CrossRef]
- Badenhorst, T.; Svirskis, D.; Merrilees, M.; Bolke, L.; Wu, Z. Effects of GHK-Cu on MMP and TIMP expression, collagen and elastin production, and facial wrinkle parameters. J. Aging Sci. 2016, 4, 166. [Google Scholar] [CrossRef]
- Wegrowski, Y.; Maquart, F.; Borel, J. Stimulation of sulfated glycosaminoglycan synthesis by the tripeptide-copper complex glycyl-L-histidyl-L-lysine-Cu2+. Life Sci. 1992, 51, 1049–1056. [Google Scholar] [CrossRef]
- Siméon, A.; Emonard, H.; Hornebeck, W.; Maquart, F.-X. The tripeptide-copper complex glycyl-L-histidyl-L-lysine-Cu2+ stimulates matrix metalloproteinase-2 expression by fibroblast cultures. Life Sci. 2000, 67, 2257–2265. [Google Scholar] [CrossRef]
- Ehrlich, H. Stimulation of skin healing in immunosuppressed rats. In Proceedings of the Symposium on Collagen and Skin Repair, Reims, France, September 1991. [Google Scholar]
- Maquart, F.-X.; Bellon, G.; Chaqour, B.; Wegrowski, J.; Patt, L.M.; Trachy, R.E.; Monboisse, J.-C.; Chastang, F.; Birembaut, P.; Gillery, P. In vivo stimulation of connective tissue accumulation by the tripeptide-copper complex glycyl-L-histidyl-L-lysine-Cu2+ in rat experimental wounds. J. Clin. Investig. 1993, 92, 2368–2376. [Google Scholar] [CrossRef]
- Pickart, L.; Margolina, A. Regenerative and protective actions of the GHK-Cu peptide in the light of the new gene data. Int. J. Mol. Sci. 2018, 19, 1987. [Google Scholar] [CrossRef]
- Zheng, M.; Lu, J.; Zhao, D. Effects of starch-coating of magnetite nanoparticles on cellular uptake, toxicity and gene expression profiles in adult zebrafish. Sci. Total Environ. 2018, 622, 930–941. [Google Scholar] [CrossRef]
- Westerfield, M. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Danio Rerio). Available online: http://zfin.org/zf_info/zfbook/zfbk.html (accessed on 18 August 2020).
- de Oliveira, G.M.T.; de Oliveira, E.M.N.; Pereira, T.C.B.; Papaléo, R.M.; Bogo, M.R. Implications of exposure to dextran-coated and uncoated iron oxide nanoparticles to developmental toxicity in zebrafish. J. Nanopart. Res. 2017, 19, 389. [Google Scholar] [CrossRef]
- Vargas, R.A.; Sarmiento, K.; Vásquez, I.C. Zebrafish (danio rerio): A potential model for toxinological studies. Zebrafish 2015, 12, 320–326. [Google Scholar] [CrossRef]
- Chakraborty, C.; Sharma, A.R.; Sharma, G.; Lee, S.-S. Zebrafish: A complete animal model to enumerate the nanoparticle toxicity. J. Nanobiotechnol. 2016, 14, 1–13. [Google Scholar] [CrossRef]
- Perrone, L.; Mothes, E.; Vignes, M.; Mockel, A.; Figueroa, C.; Miquel, M.-C.; Maddelein, M.-L.; Faller, P. Copper Transfer from Cu-Ab to Human Serum Albumin Inhibits Aggregation, Radical Production and Reduces Ab Toxicity. ChemBioChem 2010, 11, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Dave, G.; Xiu, R. Toxicity of mercury, copper, nickel, lead, and cobalt to embryos and larvae of zebrafish, Brachydanio rerio. Arch. Environ. Contam. Toxicol. 1991, 21, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Hernández, P.P.; Moreno, V.; Olivari, F.A.; Allende, M.L. Sub-lethal concentrations of waterborne copper are toxic to lateral line neuromasts in zebrafish (Danio rerio). Hear. Res. 2006, 213, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Griffitt, R.J.; Weil, R.; Hyndman, K.A.; Denslow, N.D.; Powers, K.; Taylor, D.; Barber, D.S. Exposure to copper nanoparticles causes gill injury and acute lethality in zebrafish (Danio rerio). Environ. Sci. Technol. 2007, 41, 8178–8186. [Google Scholar] [CrossRef]
- Sampurna, B.P.; Audira, G.; Juniardi, S.; Lai, Y.-H.; Hsiao, C.-D. A simple imagej-based method to measure cardiac rhythm in zebrafish embryos. Inventions 2018, 3, 21. [Google Scholar] [CrossRef]
- Van Dalen, E.C.; van den Brug, M.; Caron, H.N.; Kremer, L.C. Anthracycline-induced cardiotoxicity: Comparison of recommendations for monitoring cardiac function during therapy in paediatric oncology trials. Eur. J. Cancer 2006, 42, 3199–3205. [Google Scholar] [CrossRef]
- Cikes, M.; Solomon, S.D. Beyond ejection fraction: An integrative approach for assessment of cardiac structure and function in heart failure. Eur. Heart J. 2016, 37, 1642–1650. [Google Scholar] [CrossRef]
- La Fontaine, S.; Ackland, M.L.; Mercer, J.F. Mammalian copper-transporting P-type ATPases, ATP7A and ATP7B: Emerging roles. Int. J. Biochem. Cell Biol. 2010, 42, 206–209. [Google Scholar] [CrossRef]
- Pereira, T.C.B.; Campos, M.M.; Bogo, M.R. Copper toxicology, oxidative stress and inflammation using zebrafish as experimental model. J. Appl. Toxicol. 2016, 36, 876–885. [Google Scholar] [CrossRef]
- Gaasch, W.H.; Carroll, J.D.; Blaustein, A.S.; Bing, O. Myocardial relaxation: Effects of preload on the time course of isovolumetric relaxation. Circulation 1986, 73, 1037–1041. [Google Scholar] [CrossRef]
- Guilhermino, L.; Diamantino, T.C.; Ribeiro, R.; Gonçalves, F.; Soares, A.M. Suitability of Test Media Containing EDTA for the Evaluation of Acute Metal Toxicity toDaphnia magnaStraus. Ecotoxicol. Environ. Saf. 1997, 38, 292–295. [Google Scholar] [CrossRef] [PubMed]
- Tubbing, D.M.; Admiraal, W.; Cleven, R.F.; Iqbal, M.; van de Meent, D.; Verweij, W. The contribution of complexed copper to the metabolic inhibition of algae and bacteria in synthetic media and river water. Water Res. 1994, 28, 37–44. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsiao, C.-D.; Wu, H.-H.; Malhotra, N.; Liu, Y.-C.; Wu, Y.-H.; Lin, Y.-N.; Saputra, F.; Santoso, F.; Chen, K.H.-C. Expression and Purification of Recombinant GHK Tripeptides Are Able to Protect against Acute Cardiotoxicity from Exposure to Waterborne-Copper in Zebrafish. Biomolecules 2020, 10, 1202. https://doi.org/10.3390/biom10091202
Hsiao C-D, Wu H-H, Malhotra N, Liu Y-C, Wu Y-H, Lin Y-N, Saputra F, Santoso F, Chen KH-C. Expression and Purification of Recombinant GHK Tripeptides Are Able to Protect against Acute Cardiotoxicity from Exposure to Waterborne-Copper in Zebrafish. Biomolecules. 2020; 10(9):1202. https://doi.org/10.3390/biom10091202
Chicago/Turabian StyleHsiao, Chung-Der, Hsin-Hui Wu, Nemi Malhotra, Yen-Ching Liu, Ying-Hsuan Wu, Yu-Nung Lin, Ferry Saputra, Fiorency Santoso, and Kelvin H.-C. Chen. 2020. "Expression and Purification of Recombinant GHK Tripeptides Are Able to Protect against Acute Cardiotoxicity from Exposure to Waterborne-Copper in Zebrafish" Biomolecules 10, no. 9: 1202. https://doi.org/10.3390/biom10091202
APA StyleHsiao, C.-D., Wu, H.-H., Malhotra, N., Liu, Y.-C., Wu, Y.-H., Lin, Y.-N., Saputra, F., Santoso, F., & Chen, K. H.-C. (2020). Expression and Purification of Recombinant GHK Tripeptides Are Able to Protect against Acute Cardiotoxicity from Exposure to Waterborne-Copper in Zebrafish. Biomolecules, 10(9), 1202. https://doi.org/10.3390/biom10091202








