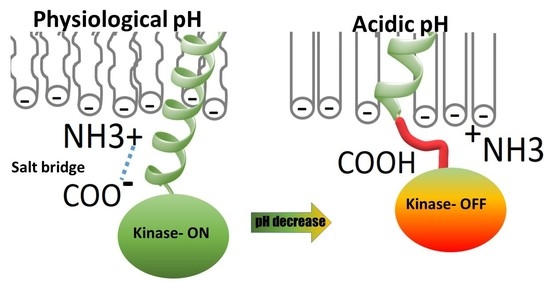
A Transmembrane Histidine Kinase Functions as a pH Sensor
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Activity Measurements
2.2. Statistics
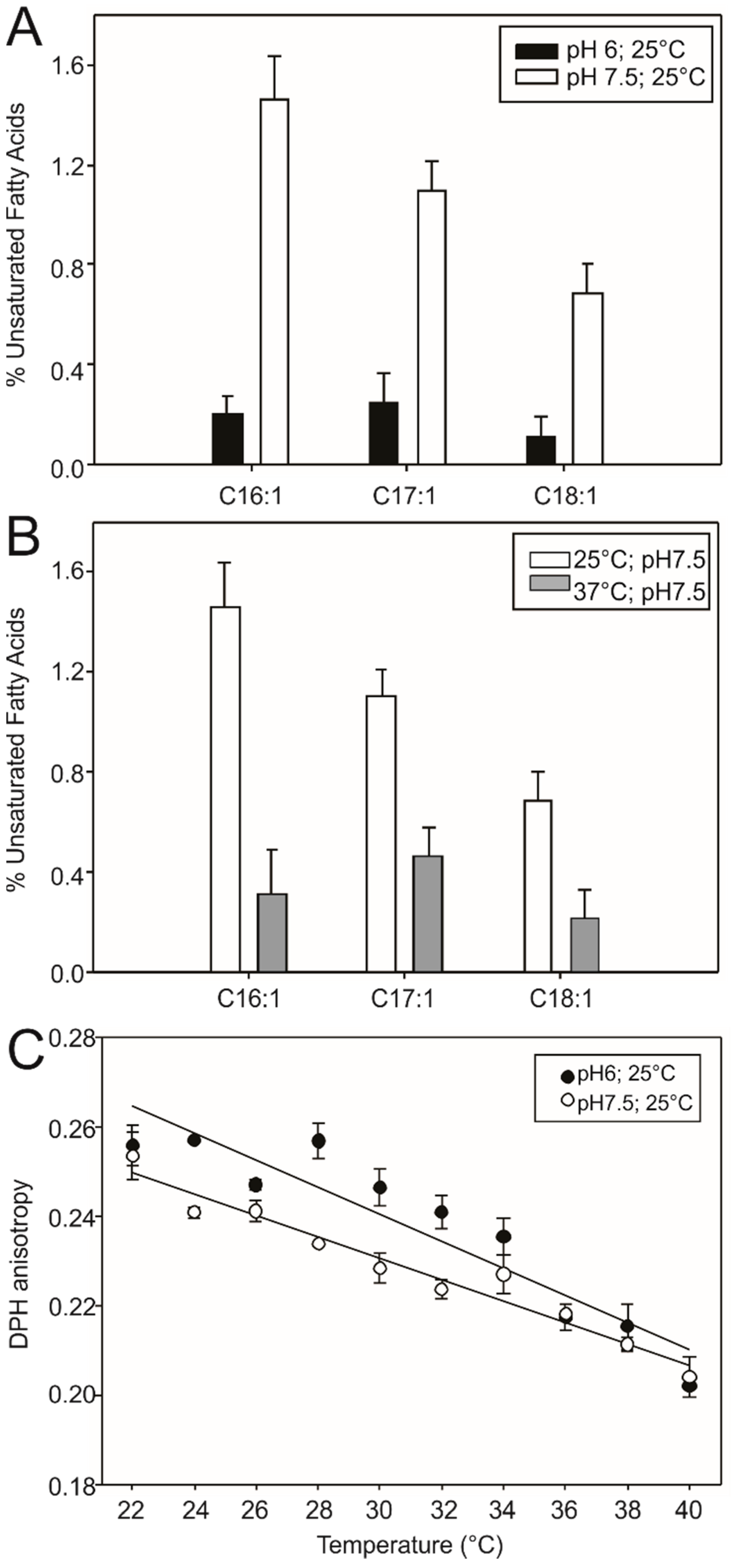
2.3. Fluorescence Anisotropy
2.4. Synthetic Peptides
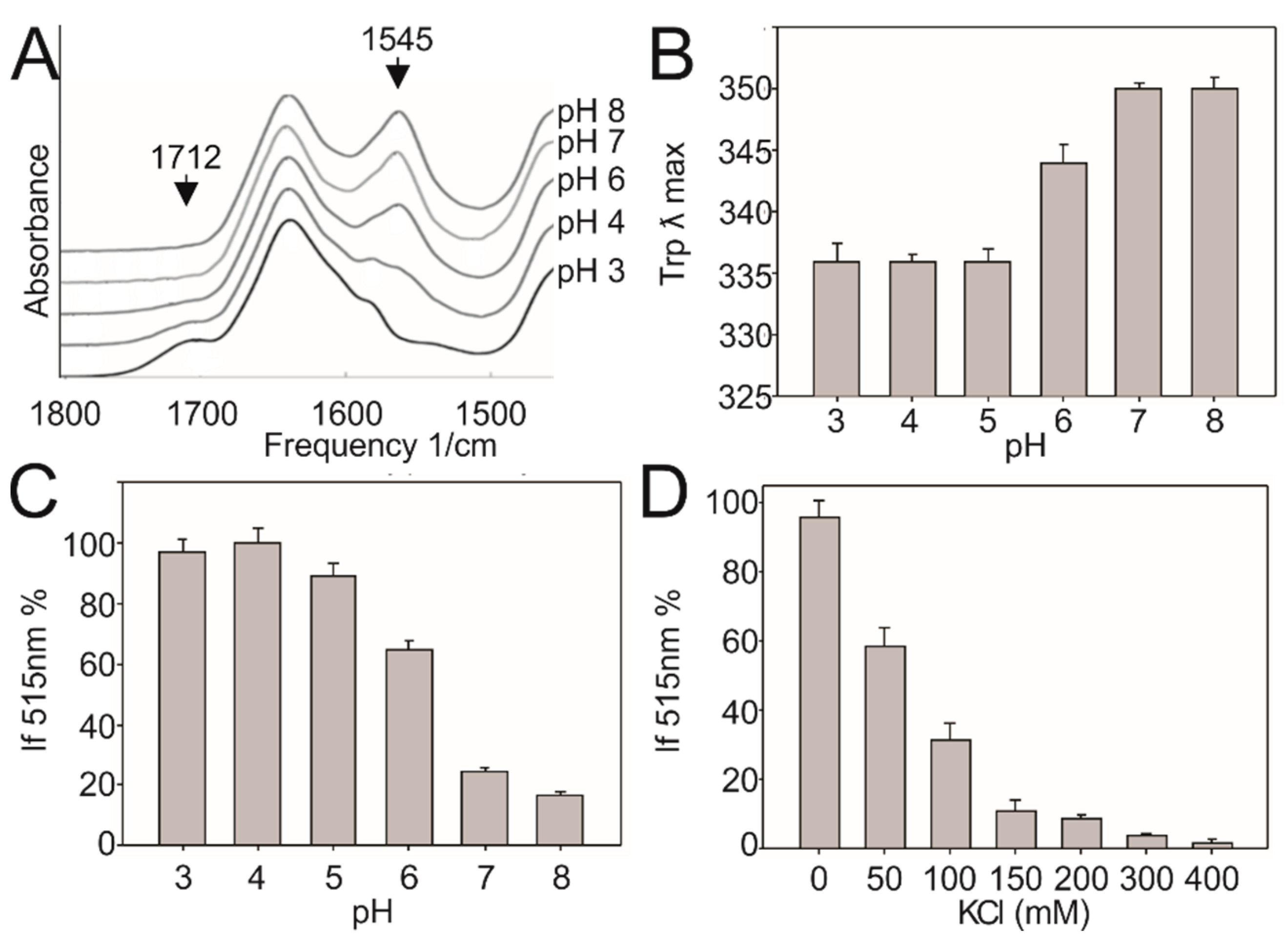
2.5. ATR-FTIR Measurement
2.6. Förster Resonance Energy Transfer (FRET) Measurement
3. Results
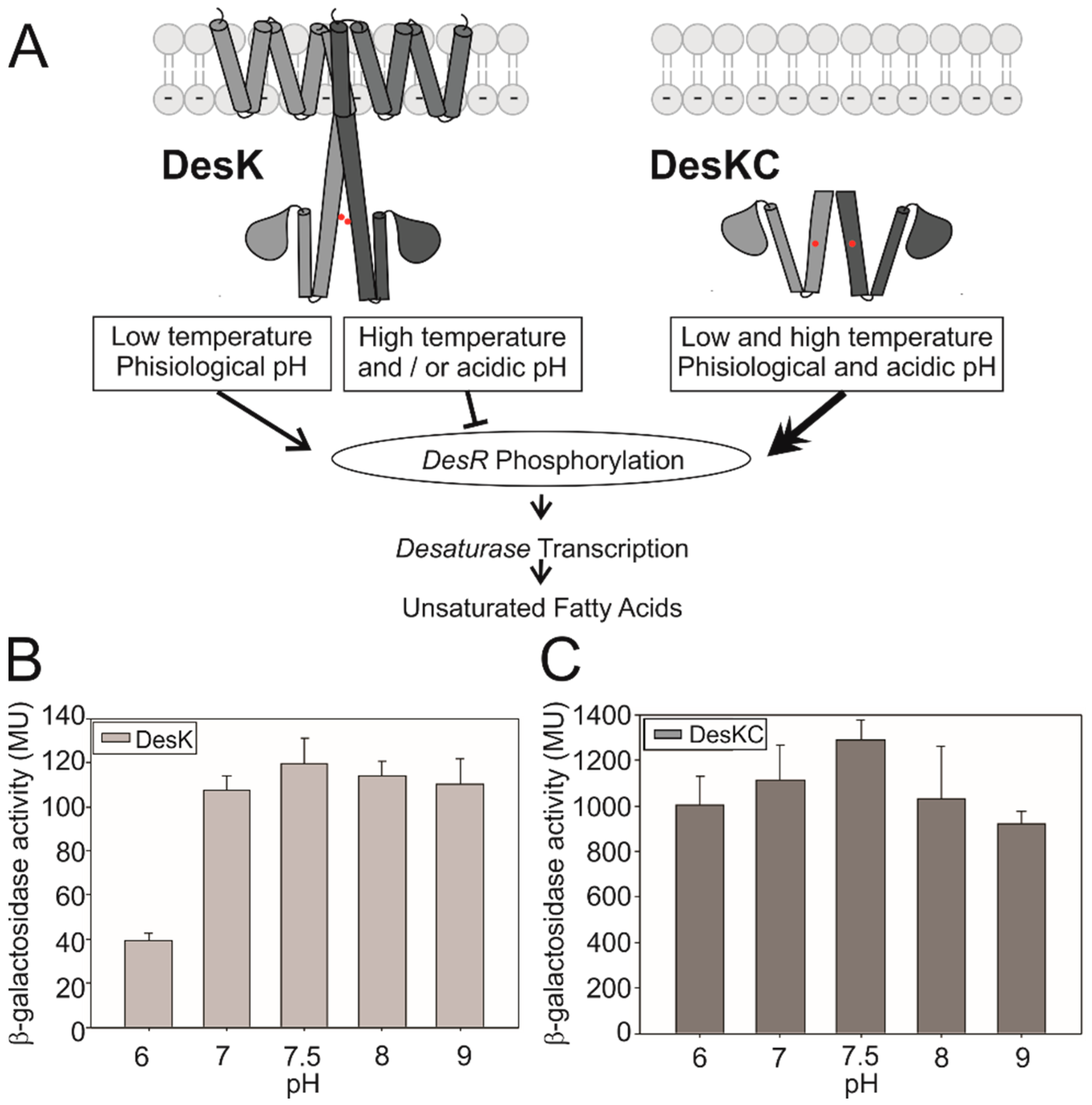
3.1. DesK Senses pH
3.2. Searching for the pH Sensing Motif
3.3. Low pH Triggers Linker Structural Changes That Turn off Kinase Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Inda, M.E.; Almada, J.C.; Vazquez, D.B.; Bortolotti, A.; Fernández, A.; Ruysschaert, J.M.; Cybulski, L.E. Driving the catalytic activity of a transmembrane thermosensor kinase. Cell. Mol. Life Sci. 2019, 1–8. [Google Scholar] [CrossRef]
- Albanesi, D.; Martín, M.; Trajtenberg, F.; Mansilla, M.C.; Haouz, A.; Alzari, P.M.; De Mendoza, D.; Buschiazzo, A. Structural plasticity and catalysis regulation of a thermosensor histidine kinase. Proc. Natl. Acad. Sci. 2009, 106, 16185–16190. [Google Scholar] [CrossRef] [PubMed]
- Cybulski, L.E.; Del Solar, G.; Craig, P.O.; Espinosa, M.; De Mendoza, D.; Torregrossa, P.; Buhl, L.; Bancila, M.; Durbec, P.; Schäfer, C.; et al. Bacillus subtilisDesR Functions as a Phosphorylation-activated Switch to Control Membrane Lipid Fluidity. J. Boil. Chem. 2004, 279, 39340–39347. [Google Scholar] [CrossRef] [PubMed]
- Albanesi, D.; Mansilla, M.C.; de Mendoza, D. The Membrane Fluidity Sensor DesK of. J. Bacteriol. 2004, 186, 2655–2663. [Google Scholar] [CrossRef] [PubMed]
- Altabe, S.G.; Aguilar, P.S.; Caballero, G.M.; De Mendoza, D. The Bacillus subtilis Acyl Lipid Desaturase Is a Δ5 Desaturase. J. Bacteriol. 2003, 185, 3228–3231. [Google Scholar] [CrossRef]
- Aguilar, P.S.; Hernandez-Arriaga, A.M.; Cybulski, L.E.; Erazo, A.C.; De Mendoza, D. Molecular basis of thermosensing: A two-component signal transduction thermometer in Bacillus subtilis. EMBO J. 2001, 20, 1681–1691. [Google Scholar] [CrossRef]
- Rousk, J.; Brookes, P.C.; Bååth, E. Contrasting Soil pH Effects on Fungal and Bacterial Growth Suggest Functional Redundancy in Carbon Mineralization. Appl. Environ. Microbiol. 2009, 75, 1589–1596. [Google Scholar] [CrossRef]
- Slessarev, E.W.; Lin, Y.; Bingham, N.L.; Johnson, J.; Dai, Y.; Schimel, J.P.; Chadwick, O.A. Water balance creates a threshold in soil pH at the global scale. Nature 2016, 540, 567–569. [Google Scholar] [CrossRef]
- Spizizen, J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc. Natl. Acad. Sci. USA 1958, 44, 1072–1078. [Google Scholar] [CrossRef]
- Harwood, C.; Cutting, S.M. Molecular Biological Methods for Bacillus; John Wiley & Sons: Chichester, UK, 1990; ISBN 0471923931 9780471923930. [Google Scholar]
- Martin, M.; Albanesi, D.; Alzari, P.M.; De Mendoza, D. Functional in vitro assembly of the integral membrane bacterial thermosensor DesK. Protein Expr. Purif. 2009, 66, 39–45. [Google Scholar] [CrossRef]
- Dawaliby, R.; Trubbia, C.; Delporte, C.; Noyon, C.; Ruysschaert, J.-M.; Van Antwerpen, P.; Govaerts, C. Phosphatidylethanolamine Is a Key Regulator of Membrane Fluidity in Eukaryotic Cells. J. Boil. Chem. 2015, 291, 3658–3667. [Google Scholar] [CrossRef] [PubMed]
- Cybulski, L.E.; Martin, M.; Mansilla, M.C.; Fernández, A.; De Mendoza, D. Membrane Thickness Cue for Cold Sensing in a Bacterium. Curr. Boil. 2010, 20, 1539–1544. [Google Scholar] [CrossRef] [PubMed]
- Harms, M.J.; Castañeda, C.A.; Schlessman, J.L.; Sue, G.R.; Isom, D.G.; Cannon, B.R.; Bertrand García-Moreno, E. The pKa Values of Acidic and Basic Residues Buried at the Same Internal Location in a Protein Are Governed by Different Factors. J. Mol. Boil. 2009, 389, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Marqusee, S.; Baldwin, R.L. Helix stabilization by Glu-. Lys+ salt bridges in short peptides of de novo design. Proc. Natl. Acad. Sci. USA 1987, 84, 8898–8902. [Google Scholar] [CrossRef] [PubMed]
- Inda, M.E.; Vandenbranden, M.; Fernández, A.; De Mendoza, D.; Ruysschaert, J.-M.; Cybulski, L.E. A lipid-mediated conformational switch modulates the thermosensing activity of DesK. Proc. Natl. Acad. Sci. USA 2014, 111, 3579–3584. [Google Scholar] [CrossRef]
- Goormaghtigh, E.; Vigneron, L.; Knibiehler, M.; Lazdunski, C.; Ruysschaert, J. Secondary structure of the membrane-bound form of the pore-forming domain of colicin A. An attenuated total-reflection polarized Fourier-transform infrared spectroscopy study. Eur. J. Biochem. 1991, 202, 1299–1305. [Google Scholar] [CrossRef]
- Minnikin, D.E.; Abdolrahimzadeh, H. Effect of pH on the Proportions of Polar Lipids, in Chemostat Cultures of Bacillus subtilis. J. Bacteriol. 1974, 120, 999–1003. [Google Scholar] [CrossRef]
- Uttlová, P.; Pinkas, D.; Bechyňková, O.; Fišer, R.; Svobodová, J.; Seydlová, G. Bacillus subtilis alters the proportion of major membrane phospholipids in response to surfactin exposure. Biochim. Biophys. Acta (BBA)-Biomembr. 2016, 1858, 2965–2971. [Google Scholar] [CrossRef]
- Inda, M.E.; Oliveira, R.G.; De Mendoza, D.; Cybulski, L.E. The Single Transmembrane Segment of Minimal Sensor DesK Senses Temperature via a Membrane-Thickness Caliper. J. Bacteriol. 2016, 198, 2945–2954. [Google Scholar] [CrossRef]
- Cotter, P.D.; Hill, C. Surviving the Acid Test: Responses of Gram-Positive Bacteria to Low pH. Microbiol. Mol. Boil. Rev. 2003, 67, 429–453. [Google Scholar] [CrossRef]
- Kundu, A.; Yamaguchi, S.; Tahara, T. Evaluation of pH at Charged Lipid/Water Interfaces by Heterodyne-Detected Electronic Sum Frequency Generation. J. Phys. Chem. Lett. 2014, 5, 762–766. [Google Scholar] [CrossRef] [PubMed]
- Inda, M.E.; Mimee, M.; Lu, T.K. Cell-based biosensors for immunology, inflammation, and allergy. J. Allergy Clin. Immunol. 2019, 144, 645–647. [Google Scholar] [CrossRef] [PubMed]

| Linker variant | i 32 | j 34 | i + 4 36 | j + 4 38 | 41 | 42 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| WT | K | S | R | K | E | R | E | R | L | E | E |
| A3 | K | S | R | A | E | A | E | A | L | E | E |
| Q3 | K | S | R | K | Q | R | Q | R | L | Q | E |
| Linker bridge+ | E | S | R | K | K | R | E | R | L | E | E |
| Linker bridge− | K | S | E | K | K | E | E | R | L | E | E |
| Residue | pKa in Bulk Solution | pKa Close to the Membrane |
|---|---|---|
| Glu 36 | 3.64 | 5.43 |
| Glu 38 | 3.89 | 5.83 |
| Glu 41 | 3.57 | 5.59 |
| Glu 42 | 4.18 | 5.84 |
| Protein | i | j | i + 3 | i + 4 | j + 4 | Organism | Uniprot ID Code | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| DesK | K | S | R | K | E | R | E | R | L | Bacillus subtilis | O34757 |
| BvgS | K | R | A | E | R | A | L | N | D | Bacillus pertussis | P16575 |
| Kcnk1 | E | E | Q | K | Q | S | E | P | F | Mouse | O08581 |
| MscL | R | K | K | E | E | P | A | A | A | Escherichia coli | P0A742 |
| TRPA1.2 | R | F | K | K | E | Q | M | E | Q | Chimpanzee | H2R465 |
| Osm9 | E | L | W | R | A | Q | V | V | A | Rabbit | Q9XSM3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bortolotti, A.; Vazquez, D.B.; Almada, J.C.; Inda, M.E.; Drusin, S.I.; Villalba, J.M.; Moreno, D.M.; Ruysschaert, J.M.; Cybulski, L.E. A Transmembrane Histidine Kinase Functions as a pH Sensor. Biomolecules 2020, 10, 1183. https://doi.org/10.3390/biom10081183
Bortolotti A, Vazquez DB, Almada JC, Inda ME, Drusin SI, Villalba JM, Moreno DM, Ruysschaert JM, Cybulski LE. A Transmembrane Histidine Kinase Functions as a pH Sensor. Biomolecules. 2020; 10(8):1183. https://doi.org/10.3390/biom10081183
Chicago/Turabian StyleBortolotti, Ana, Daniela Belén Vazquez, Juan Cruz Almada, Maria Eugenia Inda, Salvador Iván Drusin, Juan Manuel Villalba, Diego M. Moreno, Jean Marie Ruysschaert, and Larisa Estefania Cybulski. 2020. "A Transmembrane Histidine Kinase Functions as a pH Sensor" Biomolecules 10, no. 8: 1183. https://doi.org/10.3390/biom10081183
APA StyleBortolotti, A., Vazquez, D. B., Almada, J. C., Inda, M. E., Drusin, S. I., Villalba, J. M., Moreno, D. M., Ruysschaert, J. M., & Cybulski, L. E. (2020). A Transmembrane Histidine Kinase Functions as a pH Sensor. Biomolecules, 10(8), 1183. https://doi.org/10.3390/biom10081183