Targeting Endothelial Dysfunction in Eight Extreme-Critically Ill Patients with COVID-19 Using the Anti-Adrenomedullin Antibody Adrecizumab (HAM8101)
Abstract
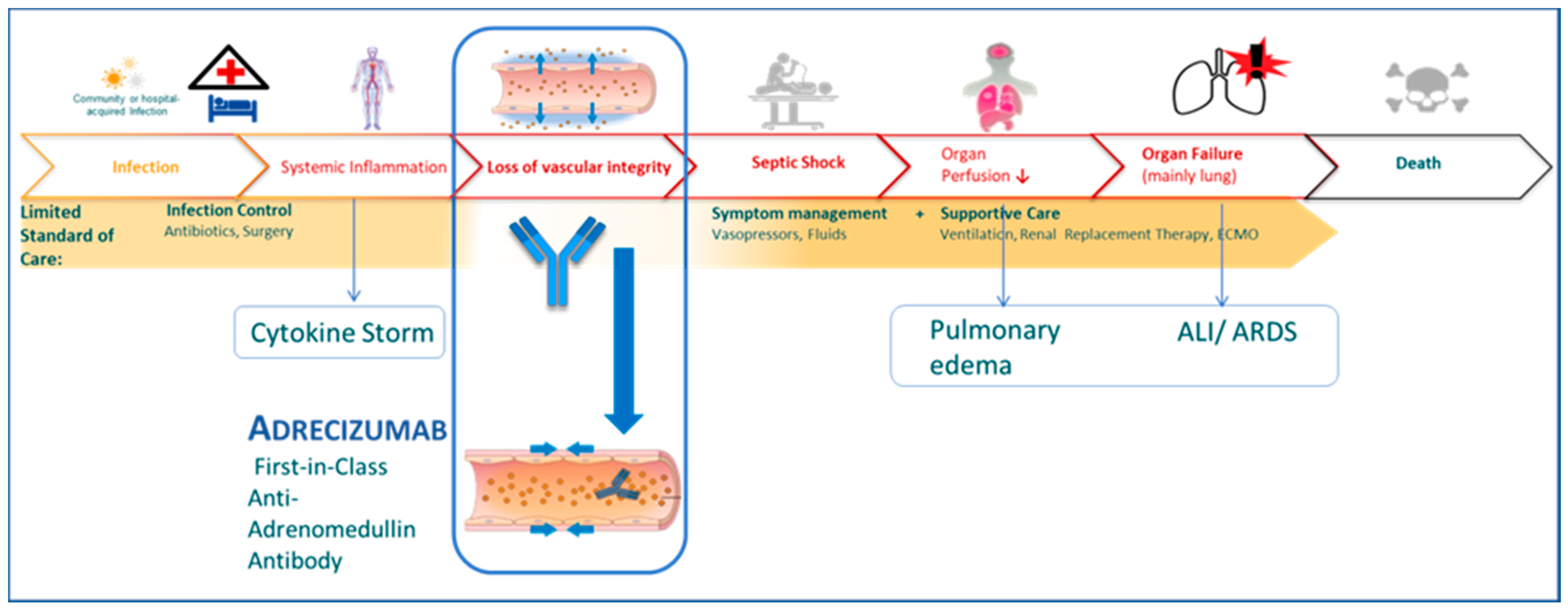
1. Introduction
2. Materials and Methods
2.1. Regulatory
2.2. Patients
2.3. Laboratory Diagnostics
2.4. Administration of Adrecizumab
2.5. Disease Severity Classification
2.6. Clinical Information
3. Results
4. Discussion
4.1. Adrecizumab in COVID-19
4.2. Other Case Series in Critically Ill COVID-19 Patients
4.3. Adrecizumab and Shock
4.4. Study Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shen, C.; Wang, Z.; Zhao, F.; Yang, Y.; Li, J.; Yuan, J.; Wang, F.; Li, D.; Yang, M.; Xing, L.; et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA 2020, 323, 1582–1589. [Google Scholar] [CrossRef]
- WHO. Novel Coronavirus (COVID-19). Available online: https://who.sprinklr.com/ (accessed on 20 April 2020).
- Poston, J.T.; Patel, B.K.; Davis, A.M. Management of critically ill adults with COVID-19. JAMA 2020, 323, 1839–1841. [Google Scholar] [CrossRef]
- Kluge, S.; Janssens, U.; Welte, T.; Weber-Carstens, S.; Marx, G.; Karagiannidis, C. Recommendations for critically ill patients with COVID-19. Med. Klin. Intensivmed. Notfmed. 2020, 115, 175–177. [Google Scholar] [CrossRef] [PubMed]
- Grasselli, G.; Zangrillo, A.; Zanella, A.; Antonelli, M.; Cabrini, L.; Castelli, A.; Cereda, D.; Coluccello, A.; Foti, G.; Fumagalli, R.; et al. COVID-19 Lombardy ICU Network. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA 2020, 323, 1574–1581. [Google Scholar] [CrossRef] [PubMed]
- Voors, A.A.; Kremer, D.; Geven, C.; Ter Maaten, J.M.; Struck, J.; Bergmann, A.; Pickkers, P.; Metra, M.; Mebazaa, A.; Düngen, H.D.; et al. Adrenomedullin in heart failure: Pathophysiology and therapeutic application. Eur. J. Heart Fail. 2019, 21, 163–171. [Google Scholar] [CrossRef]
- Blet, A.; Deniau, B.; Geven, C.; Sadoune, M.; Caillard, A.; Kounde, P.R.; Polidano, E.; Pickkers, P.; Samuel, J.L.; Mebazaa, A. Adrecizumab, a non-neutralizing anti-adrenomedullin antibody, improves haemodynamics and attenuates myocardial oxidative stress in septic rats. Intensive Care Med. Exp. 2019, 7, 25. [Google Scholar] [CrossRef]
- Wu, A.M.; Fossali, T.; Pandolfi, L.; Carsana, L.; Ottolina, D.; Frangipane, V.; Rech, R.; Tosoni, A.; Agarossi, A.; Cogliati, C.; et al. COVID-19: The key role of pulmonary capillary leakage. An observational cohort study. medRxiv 2020. [Google Scholar] [CrossRef]
- Geven, C.; Blet, A.; Kox, M.; Hartmann, O.; Scigalla, P.; Zimmermann, J.; Marx, G.; Laterre, P.F.; Mebazaa, A.; Pickkers, P. A double-blind, placebo-controlled, randomised, multicentre, proof-of-concept and dose-finding phase II clinical trial to investigate the safety, tolerability and efficacy of adrecizumab in patients with septic shock and elevated adrenomedullin concentration (AdrenOSS-2). BMJ Open 2019, 9, e024475. [Google Scholar]
- Adrenomed Announces Positive Top-Line AdrenOSS-2 Phase II Results with Adrecizumab in Septic Shock. Available online: https://adrenomed.com/adrenomed-announces-positive-top-line-adrenoss-2-phase-ii-results-with-adrecizumab-in-septic-shock/ (accessed on 21 April 2020).
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef]
- Deniau, B.; Rehfeld, L.; Santos, K.; Dienelt, A.; Azibani, F.; Sadoune, M.; Kounde, P.R.; Samuel, J.L.; Tolpannen, H.; Lassus, J.; et al. Circulating dipeptidyl peptidase 3 is a myocardial depressant factor: Dipeptidyl peptidase 3 inhibition rapidly and sustainably improves haemodynamics. Eur. J. Heart Fail. 2020, 22, 290–299. [Google Scholar] [CrossRef]
- Takagi, K.; Blet, A.; Levy, B.; Deniau, B.; Azibani, F.; Feliot, E.; Bergmann, A.; Santos, K.; Hartmann, O.; Gayat, E.; et al. Circulating dipeptidyl peptidase 3 and alteration in haemodynamics in cardiogenic shock: Results from the OptimaCC trial. Eur. J. Heart Fail. 2020, 22, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Magliocca, A.; Omland, T.; Latini, R. Dipeptidyl peptidase 3, a biomarker in cardiogenic shock and hopefully much more. Eur. J. Heart Fail. 2020, 22, 300–302. [Google Scholar] [CrossRef] [PubMed]
- Ocaranza, M.P.; Jalil, J.E. On Endogenous Angiotensin II Antagonism in Hypertension: The Role of Dipeptidyl Peptidase III. Hypertension 2016, 68, 552–554. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed]
- Nickel, L.; Seibel, Y.; Frech, M.; Sudhop, T. Changes in the German Medicinal Product Act imposed by the EU regulation on clinical trials. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2017, 60, 804–811. [Google Scholar] [CrossRef]
- Pfefferle, S.; Reucher, S.; Nörz, D.; Lütgehetmann, M. Evaluation of a quantitative RT-PCR assay for the detection of the emerging coronavirus SARS-CoV-2 using a high throughput system. Eur. Surveill. 2020, 25, 2000152. [Google Scholar] [CrossRef]
- Intensive Care National Audit & Research Centre. Available online: https://www.icnarc.org/ (accessed on 21 April 2020).
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Yang, X.; Yu, Y.; Xu, J.; Shu, H.; Xia, J.; Liu, H.; Wu, Y.; Zhang, L.; Yu, Z.; Fang, M.; et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir. Med. 2020, 8, 475–481. [Google Scholar] [CrossRef]
- Bhatraju, P.K.; Ghassemieh, B.J.; Nichols, M.; Kim, R.; Jerome, K.R.; Nalla, A.K.; Greninger, A.L.; Pipavath, S.; Wurfel, M.M.; Evans, L.; et al. Covid-19 in critically ill patients in the Seattle region—Case series. N. Engl. J. Med. 2020, 382, 2012–2022. [Google Scholar] [CrossRef]
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; The Northwell COVID-19 Research Consortium; Barnaby, D.P.; Becker, L.B.; Chelico, J.D.; et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 2020, 323, 2052–2059. [Google Scholar] [CrossRef]
- Geven, C.; van Lier, D.; Blet, A.; Peelen, R.; Elzen, B.T.; Mebazaa, A.; Kox, M.; Pickkers, P. Safety, tolerability and pharmacokinetics/pharmacodynamics of the adrenomedullin antibody adrecizumab in a first-in-human study and during experimental human endotoxaemia in healthy subjects. Br. J. Clin. Pharmacol. 2018, 84, 2129–2141. [Google Scholar] [CrossRef] [PubMed]
- Geven, C.; Bergmann, A.; Kox, M.; Pickkers, P. Vascular effects of adrenomedullin and the anti-adrenomedullin antibody adrecizumab in sepsis. Shock 2018, 50, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Tolppanen, H.; Rivas-Lasarte, M.; Lassus, J.; Sans-Roselló, J.; Hartmann, O.; Lindholm, M.; Arrigo, M.; Tarvasmäki, T.; Köber, L.; Thiele, H.; et al. Adrenomedullin: A marker of impaired hemodynamics, organ dysfunction, and poor prognosis in cardiogenic shock. Ann. Intensive Care 2017, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Trincot, C.E.; Xu, W.; Zhang, H.; Kulikauskas, M.R.; Caranasos, T.G.; Jensen, B.C.; Sabine, A.; Petrova, T.V.; Caron, K.M. Adrenomedullin induces cardiac lymphangiogenesis after myocardial infarction and regulates cardiac edema via connexin 43. Circ. Res. 2019, 124, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Hinrichs, S.; Scherschel, K.; Krüger, S.; Neumann, J.T.; Schwarzl, M.; Yan, I.; Warnke, S.; Ojeda, F.M.; Zeller, T.; Karakas, M.; et al. Precursor proadrenomedullin influences cardiomyocyte survival and local inflammation related to myocardial infarction. Proc. Natl. Acad. Sci. USA 2018, 115, E8727–E8736. [Google Scholar] [CrossRef]
- Müller, H.C.; Witzenrath, M.; Tschernig, T.; Gutbier, B.; Hippenstiel, S.; Santel, A.; Suttorp, N.; Rosseau, S. Adrenomedullin attenuates ventilator-induced lung injury in mice. Thorax 2010, 65, 1077–1084. [Google Scholar] [CrossRef][Green Version]
- Nagaya, N.; Goto, Y.; Satoh, T.; Sumida, H.; Kojima, S.; Miyatake, K.; Kangawa, K. Intravenous adrenomedullin in myocardial function and energy metabolism in patients after myocardial infarction. J. Cardiovasc. Pharmacol. 2002, 39, 754–760. [Google Scholar] [CrossRef]
- Nakamura, R.; Kato, J.; Kitamura, K.; Onitsuka, H.; Imamura, T.; Marutsuka, K.; Asada, Y.; Kangawa, K.; Eto, T. Beneficial effects of adrenomedullin on left ventricular remodeling after myocardial infarction in rats. Cardiovasc. Res. 2002, 56, 373–380. [Google Scholar] [CrossRef]
- Okumura, H.; Nagaya, N.; Itoh, T.; Okano, I.; Hino, J.; Mori, K.; Tsukamoto, Y.; Ishibashi-Ueda, H.; Miwa, S.; Tambara, K.; et al. Adrenomedullin infusion attenuates myocardial ischemia/reperfusion injury through the phosphatidylinositol 3-kinase/Akt-dependent pathway. Circulation 2004, 109, 242–248. [Google Scholar] [CrossRef]
- Nakamura, R.; Kato, J.; Kitamura, K.; Onitsuka, H.; Imamura, T.; Cao, Y.; Marutsuka, K.; Asada, Y.; Kangawa, K.; Eto, T. Adrenomedullin administration immediately after myocardial infarction ameliorates progression of heart failure in rats. Circulation 2004, 110, 426–431. [Google Scholar] [CrossRef]
- Hamid, S.A.; Baxter, G.F. A critical cytoprotective role of endogenous adrenomedullin in acute myocardial infarction. J. Mol. Cell. Cardiol. 2006, 41, 360–363. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 |
|---|---|---|---|---|---|---|---|---|
| Sex | Male | Male | Male | Male | Male | Male | Male | Female |
| Age | 54 | 61 | 73 | 71 | 31 | 76 | 58 | 68 |
| BMI | 29.2 | 31.9 | 26.4 | 32.7 | 41.4 | 30.9 | 27.7 | 39.1 |
| SAPS II score on admission to ICU | 32 | 55 | 37 | 43 | 20 | 64 | 40 | 35 |
| Chronic diseases |
|
|
|
|
|
|
|
|
| Pre-existing RAS-I medication | ACE-inhibitor | ACE-inhibitor | ACE-inhibitor | Angiotensin receptor blocker | None | Angiotensin receptor blocker | ACE-inhibitor | ACE-inhibitor |
| Complication prior to Adrecizumab therapy |
|
|
|
|
|
|
|
|
| Days between symptoms onset and positive testing | 2 | 5 | 14 | 4 | 4 | 2 | 1 | |
| Days between symptom onset and admission | 9 | 5 | 15 | 3 | 5 | 3 | 15 | 3 |
| Days between ICU admission and Adrecizumab therapy | 1 | 2 | 2 | 2 | 1 | 1 | 1 | 3 |
| Most severe disease classification | Critical | Critical | Critical | Critical | Critical | Critical | Critical | Critical |
| Prior CPR | No | No | No | No | No | No | Yes | No |
| Prior treatment with antivirals | Lopinavir/ritonavir | Lopinavir/ritonavir | None | Lopinavir/ritonavir | None | None | None | None |
| Other experimental therapies | None | CytoSorb® | None | CytoSorb® | None | None | None | None |
| Characteristic | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 |
|---|---|---|---|---|---|---|---|---|
| Body weight [kg] | 100 | 100 | 75 | 100 | 125 | 98 | 100 | 100 |
| Dosing group | high-dose [8 mg/kg bw] | high-dose [8 mg/kg bw] | high-dose [8 mg/kg bw] | low-dose [4 mg/kg bw] | high-dose [8 mg/kg bw] | low-dose [4 mg/kg bw] | high-dose [8 mg/kg bw] | high-dose [8 mg/kg bw] |
| Dose Adrecizumab [mg] | 800 | 800 | 600 | 400 | 1000 | 400 | 800 | 800 |
| Bio-ADM [pg/mL] before Adrecizumab | 63.9 | 64.9 | 53.9 | 53.0 | 102.6 | 191.3 | 90.7 | 170.3 |
| day 1 | 264.5 | 377.2 | 403.9 | 239.4 | 226.1 | 678.9 | 388.8 | 515.0 |
| day 2 | 244.6 | n/a | 281.5 | 312.3 | 271.1 | 383.8 | 257.6 | n/a |
| day 3 | 235.6 | 274.7 | 200.2 | 171.8 | 239.8 | 209.9 | 231.1 | 224.8 |
| day 5 | 123.5 | 194.9 | 157.7 | n/a | 246.1 | 130.3 | 167.9 | n/a |
| day 7 | 63.9 | 113.8 | 143.0 | n/a | 155.0 | 118.9 | n/a | n/a |
| day 10–2 | 42.3 | 130.6 | 53.8 | n/a | 134.0 | 170.3 | 92.0 | n/a |
| DPP-3 [ng/mL] before Adrecizumab | 6.92 | 19.3 | 17.9 | 19.0 | 19.0 | 23.5 | 18.3 | 12.5 |
| day 1 | 7.38 | 18.9 | 12.8 | 17.7 | n/a | n/a | n/a | n/a |
| day 3 | 13.22 | 7.84 | n/a | 135.7 | n/a | n/a | n/a | n/a |
| day 5–10 | 9.90 | n/a | n/a | >150 | n/a | 23.5 | n/a | n/a |
| Characteristic | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 |
|---|---|---|---|---|---|---|---|---|
| Days of follow-up | 29 | 27 | 24 | 4 | 21 | 21 | 20 | 13 |
| Current status as of 15 April 2020 | Transferred to normal ward | Alive; still receiving mechanical ventilation | Transferred to normal ward | Deceased–81 h after intervention | Alive; still receiving mechanical ventilation | Alive; still receiving mechanical ventilation | Transferred to normal ward | Alive; still receiving mechanical ventilation |
| Mechanical ventilation | ||||||||
| Onset days before Adrecizumab | 1 | 2 | 1 | 2 | 1 | 1 | 1 | 1 |
| Status | Extubated | De-escalation from BIPAP to intermittent CPAP | Extubated | Deceased | De-escalation from BIPAP to intermittent CPAP | De-escalation from BIPAP to intermittent CPAP | Extubated | De-escalation from BIPAP to intermittent CPAP |
| ECMO | ||||||||
| Onset before/after Adrecizumab | Not received | Same day | Not received | Not received | Same day | Not received | Same day | Not received |
| Removal, days after Adrecizumab | n/a | Still in use | n/a | n/a | 19 | n/a | 6 | n/a |
| PaO2/FiO2 | ||||||||
| before Adrecizumab | 126 | 90 | 181 | 108 | 107 | 213 | 75 | 160 |
| best value within 12 h | 244 | 127 | 224 | 122 | 133 | 215 | 113 | 241 |
| mean value day 1 | 204 | 105 | 184 | 142 | 81 | 171 | 132 | 168 |
| mean value day 2 | 186 | 96 | 161 | 116 | 87 | 162 | 163 | 166 |
| mean value day 3 | 218 | 106 | 177 | 93 | 78 | 165 | 155 | 185 |
| mean value day 5 | 216 | 122 | 190 | n/a | 171 | 181 | 192 | 196 |
| mean value day 7 | 225 | 98 | 209 | n/a | 162 | 97 | 316 | 220 |
| mean value day 10 | 259 | 78 | 226 | n/a | 168 | 134 | 348 | 236 |
| mean value day 12 | 249 | 84 | 240 | n/a | 176 | 157 | 295 | 230 |
| Noradrenaline [µg/kg/min] | ||||||||
| before Adrecizumab | 0.02 | 0.20 | 0.14 | 0.14 | 0.11 | 0.16 | 0.52 | 0.06 |
| highest dose day 1 | 0.10 | 0.96 | 0.17 | 0.40 | 0.11 | 0.30 | 0.33 | 0.07 |
| highest dose day 2 | 0.14 | 0.48 | 0.16 | 1.60 | 0.11 | 0.11 | 0.28 | 0.04 |
| highest dose day 3 | 0.12 | 0.48 | 0.15 | 4.00 | 0.13 | 0.07 | 0.09 | 0.06 |
| highest dose day 5 | 0 | 0.44 | 0.26 | n/a | 0.10 | 0.09 | 0.10 | 0.08 |
| highest dose day 7 | 0 | 0.20 | 0.16 | n/a | 0.09 | 0.20 | 0.11 | 0.02 |
| highest dose day 10 | 0.25 | 0.44 | 0 | n/a | 0.23 | 0.31 | 0 | 0 |
| highest dose day 12 | 0 | 0.40 | 0 | n/a | 0.25 | 0.37 | 0 | 0 |
| SOFA score | ||||||||
| before Adrecizumab | 12 | 14 | 12 | 12 | 16 | 12 | 13 | 14 |
| day 1 | 11 | 16 | 12 | 16 | 16 | 12 | 12 | 14 |
| day 2 | 10 | 17 | 11 | 18 | 16 | 12 | 14 | 13 |
| day 3 | 10 | 17 | 11 | n/a | 15 | 11 | 14 | 13 |
| day 5 | 10 | 18 | 10 | n/a | 14 | 13 | 11 | 12 |
| day 7 | 6 | 17 | 10 | n/a | 14 | 12 | 13 | 13 |
| day 10 | 7 | 19 | 6 | n/a | 12 | 13 | 10 | 4 |
| day 12 | 9 | 18 | 6 | n/a | 14 | 13 | 5 | 7 |
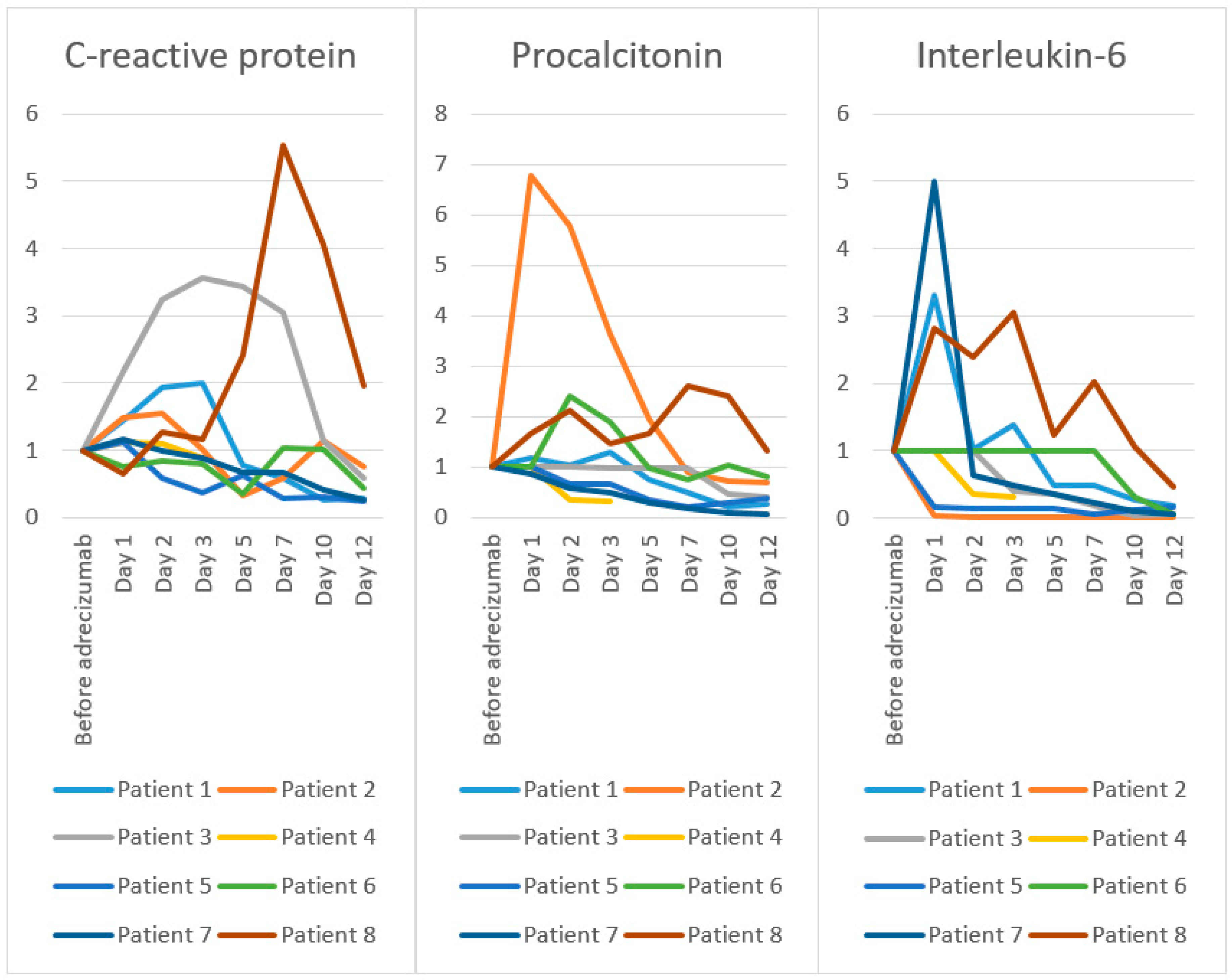
| C-reactive protein [mg/L]; Reference value < 5 mg/L | ||||||||
| before Adrecizumab | 105 | 186 | 86 | 304 | 72 | 284 | 137 | 37 |
| day 1 | 151 | 277 | 186 | 339 | 80 | 213 | 160 | 24 |
| day 2 | 203 | 286 | 279 | 334 | 42 | 239 | 136 | 47 |
| day 3 | 210 | 189 | 307 | 266 | 27 | 224 | 122 | 43 |
| day 5 | 82 | 61 | 296 | n/a | 45 | 100 | 91 | 89 |
| day 7 | 61 | 108 | 261 | n/a | 20 | 292 | 91 | 205 |
| day 10 | 27 | 213 | 97 | n/a | 22 | 286 | 56 | 150 |
| day 12 | 29 | 141 | 51 | n/a | 14 | 122 | 35 | 72 |
| Procalcitonin [µg/L]; Reference value < 0.5µg/L | ||||||||
| before Adrecizumab | 0.47 | 2.56 | n/a | 1297 | 2.29 | n/a | 2.76 | 0.15 |
| day 1 | 0.55 | 17.33 | 0.49 | n/a | n/a | 4.08 | 2.37 | 0.25 |
| day 2 | 0.49 | 14.8 | n/a | 456.8 | 1.51 | 9.86 | 1.64 | 0.32 |
| day 3 | 0.6 | 9.33 | 0.48 | 413.1 | n/a | 7.74 | 1.38 | 0.22 |
| day 5 | 0.35 | 4.96 | n/a | n/a | 0.79 | 4.02 | 0.77 | 0.25 |
| day 7 | 0.23 | 2.27 | n/a | n/a | 0.51 | 3.08 | 0.51 | 0.39 |
| day 10 | 0.1 | 1.87 | 0.23 | n/a | 0.67 | 4.21 | 0.26 | 0.36 |
| day 12 | 0.12 | 1.75 | 0.20 | n/a | 0.86 | 3.35 | 0.20 | 0.20 |
| Interleukin-6 [ng/L]; Reference value < 7 ng/L | ||||||||
| before Adrecizumab | 129 | 18,825 | n/a | 1297 | 304 | n/a | 364 | 33 |
| day 1 | 426 | 781 | 1052 | n/a | 50 | n/a | 427 | 93 |
| day 2 | 132 | 106 | n/a | 457 | 46 | n/a | 232 | 79 |
| day 3 | 179 | 37 | 421 | 413 | 41 | n/a | 173 | 101 |
| day 5 | 62 | 104 | 382 | n/a | 43 | n/a | 131 | 41 |
| day 7 | 62 | 132 | 192 | n/a | 15 | 1078 | 84 | 67 |
| day 10 | 33 | 129 | 34 | n/a | 36 | 335 | 32 | 35 |
| day 12 | 24 | 94 | 63 | n/a | 49 | 58 | 21 | 15 |
| Lactate [mmol/L]; Reference value 0.5–2.2 mmol/L | ||||||||
| before Adrecizumab | 1.1 | 3.4 | 0.9 | 1.3 | 2.3 | 3.0 | 2.2 | 0.7 |
| day 1 | 1.4 | 3.6 | 1.0 | 2.5 | 2.4 | 1.9 | 1.5 | 0.7 |
| day 2 | 1.2 | 3.0 | 1.4 | 7.0 | 1.8 | 2.1 | 1.0 | 0.6 |
| day 3 | 1.3 | 2.2 | 1.5 | 12.0 | 2.2 | 1.3 | 0.9 | 1.0 |
| day 5 | 1.4 | 1.7 | 1.3 | n/a | 1.5 | 2.0 | 1.0 | 0.8 |
| day 7 | 0.9 | 1.5 | 1.3 | n/a | 1.2 | 1.3 | 1.0 | 0.8 |
| day 10 | n/a | 1.8 | 1.2 | n/a | 1.6 | 1.6 | 1.0 | 0.4 |
| day 12 | n/a | 1.8 | 1.1 | n/a | 1.4 | 1.1 | 1.2 | 0.5 |
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | |
|---|---|---|---|---|---|---|---|---|
| Vital and hospitalization status | ||||||||
| Before Adrecizumab | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| day 1 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| day 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| day 7 | 3 | 3 | 3 | 5 | 3 | 3 | 3 | 3 |
| day 12 | 3 | 3 | 3 | n/a | 3 | 3 | 3 | 3 |
| last day of follow-up | 1 | 3 | 1 | n/a | 3 | 3 | 1 | 3 |
| Circulation status | ||||||||
| Before Adrecizumab | 1 | 2 | 2 | 2 | 2 | 2 | 3 | 1 |
| day 1 | 1 | 3 | 2 | 2 | 2 | 2 | 2 | 1 |
| day 3 | 2 | 3 | 2 | 3 | 2 | 1 | 1 | 1 |
| day 7 | 0 | 2 | 2 | 3 | 1 | 2 | 2 | 1 |
| day 12 | 0 | 2 | 0 | n/a | 2 | 2 | 0 | 0 |
| last day of follow-up | 0 | 3 | 0 | n/a | 0 | 3 | 0 | 0 |
| Ventilation status | ||||||||
| Before Adrecizumab | 3 | 3 | 3 | 3 | 4 | 3 | 4 | 2 |
| day 1 | 3 | 4 | 2 | 3 | 4 | 3 | 4 | 2 |
| day 3 | 3 | 4 | 3 | 3 | 4 | 3 | 4 | 2 |
| day 7 | 3 | 4 | 3 | 3 | 4 | 3 | 2 | 2 |
| day 12 | 1 | 4 | 0 | n/a | 4 | 3 | 0 | 2 |
| last day of follow-up | 0 | 4 | 0 | n/a | 2 | 2 | 0 | 2 |
| Mean PaO2/FiO2 | ||||||||
| Before Adrecizumab | 2 | 3 | 2 | 2 | 2 | 1 | 3 | 2 |
| day 1 | 1 | 2 | 2 | 2 | 3 | 2 | 2 | 2 |
| day 3 | 1 | 2 | 2 | 3 | 3 | 2 | 2 | 2 |
| day 7 | 1 | 3 | 1 | 3 | 2 | 3 | 0 | 1 |
| day 12 | 1 | 3 | 1 | n/a | 2 | 2 | 1 | 1 |
| last day of follow-up | 0 | 3 | 0 | n/a | 1 | 2 | 0 | 0 |
| Total score | ||||||||
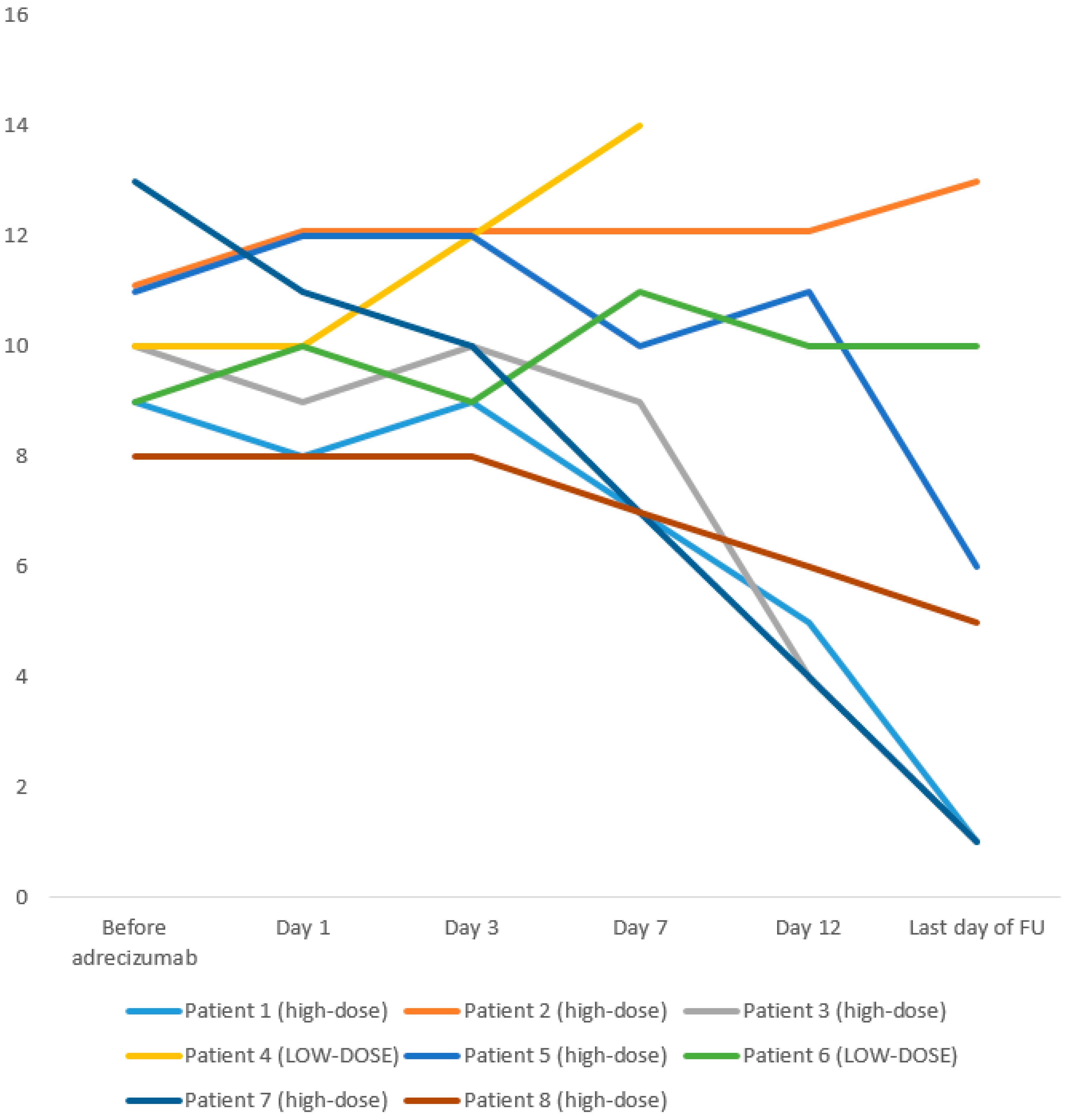
| Before Adrecizumab | 9 | 11 | 10 | 10 | 11 | 9 | 13 | 8 |
| day 1 | 8 | 12 | 9 | 10 | 12 | 10 | 11 | 8 |
| day 3 | 9 | 12 | 10 | 12 | 12 | 9 | 10 | 8 |
| day 7 | 7 | 12 | 9 | 14 | 10 | 11 | 7 | 7 |
| day 12 | 5 | 12 | 4 | n/a | 11 | 10 | 4 | 6 |
| last day of follow-up | 1 | 13 | 1 | n/a | 6 | 10 | 1 | 5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karakas, M.; Jarczak, D.; Becker, M.; Roedl, K.; Addo, M.M.; Hein, F.; Bergmann, A.; Zimmermann, J.; Simon, T.-P.; Marx, G.; et al. Targeting Endothelial Dysfunction in Eight Extreme-Critically Ill Patients with COVID-19 Using the Anti-Adrenomedullin Antibody Adrecizumab (HAM8101). Biomolecules 2020, 10, 1171. https://doi.org/10.3390/biom10081171
Karakas M, Jarczak D, Becker M, Roedl K, Addo MM, Hein F, Bergmann A, Zimmermann J, Simon T-P, Marx G, et al. Targeting Endothelial Dysfunction in Eight Extreme-Critically Ill Patients with COVID-19 Using the Anti-Adrenomedullin Antibody Adrecizumab (HAM8101). Biomolecules. 2020; 10(8):1171. https://doi.org/10.3390/biom10081171
Chicago/Turabian StyleKarakas, Mahir, Dominik Jarczak, Martin Becker, Kevin Roedl, Marylyn M. Addo, Frauke Hein, Andreas Bergmann, Jens Zimmermann, Tim-Philipp Simon, Gernot Marx, and et al. 2020. "Targeting Endothelial Dysfunction in Eight Extreme-Critically Ill Patients with COVID-19 Using the Anti-Adrenomedullin Antibody Adrecizumab (HAM8101)" Biomolecules 10, no. 8: 1171. https://doi.org/10.3390/biom10081171
APA StyleKarakas, M., Jarczak, D., Becker, M., Roedl, K., Addo, M. M., Hein, F., Bergmann, A., Zimmermann, J., Simon, T.-P., Marx, G., Lütgehetmann, M., Nierhaus, A., & Kluge, S. (2020). Targeting Endothelial Dysfunction in Eight Extreme-Critically Ill Patients with COVID-19 Using the Anti-Adrenomedullin Antibody Adrecizumab (HAM8101). Biomolecules, 10(8), 1171. https://doi.org/10.3390/biom10081171






