Advanced Hydrogels as Wound Dressings
Abstract
1. Introduction
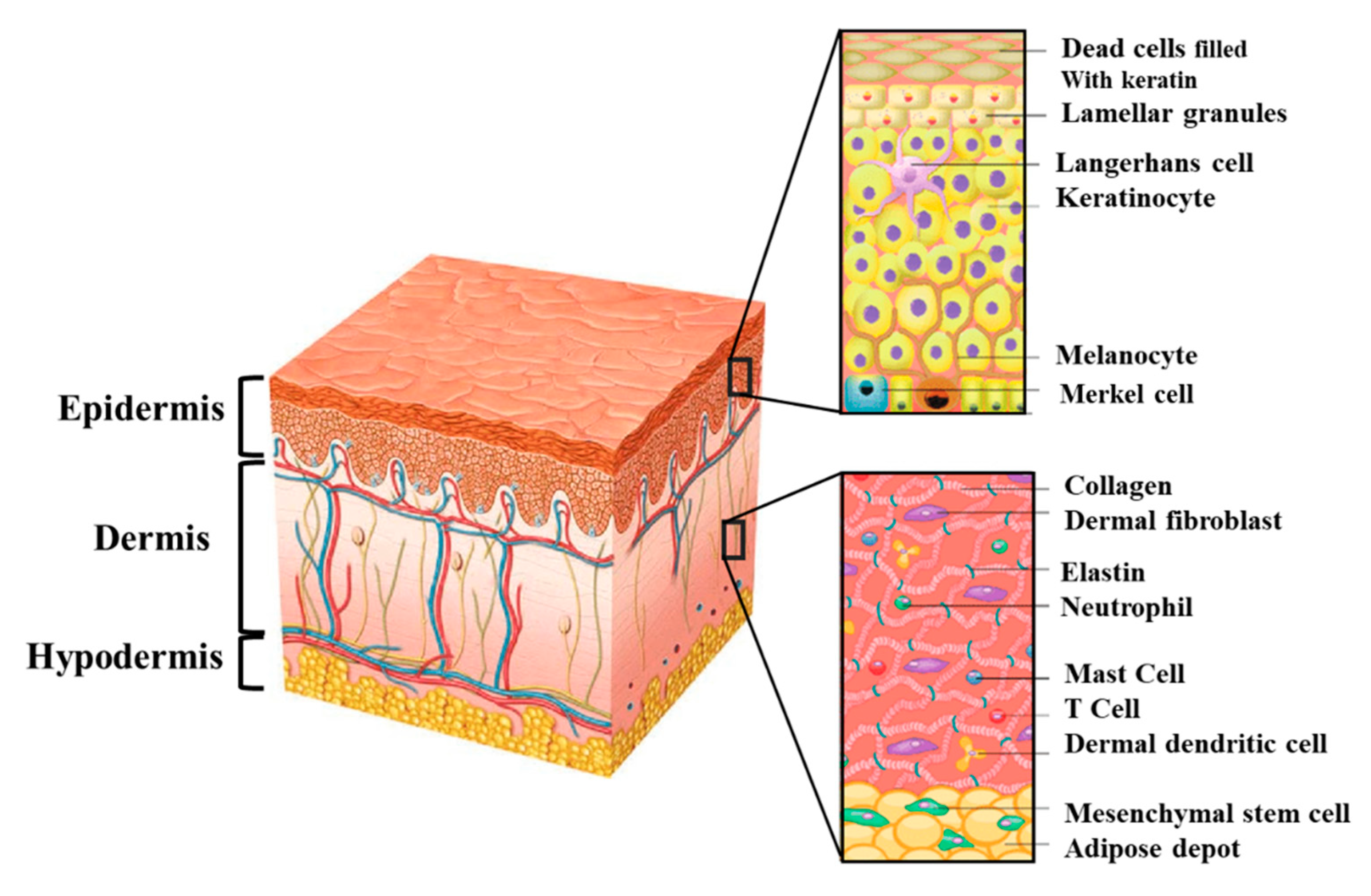
2. Skin Structure
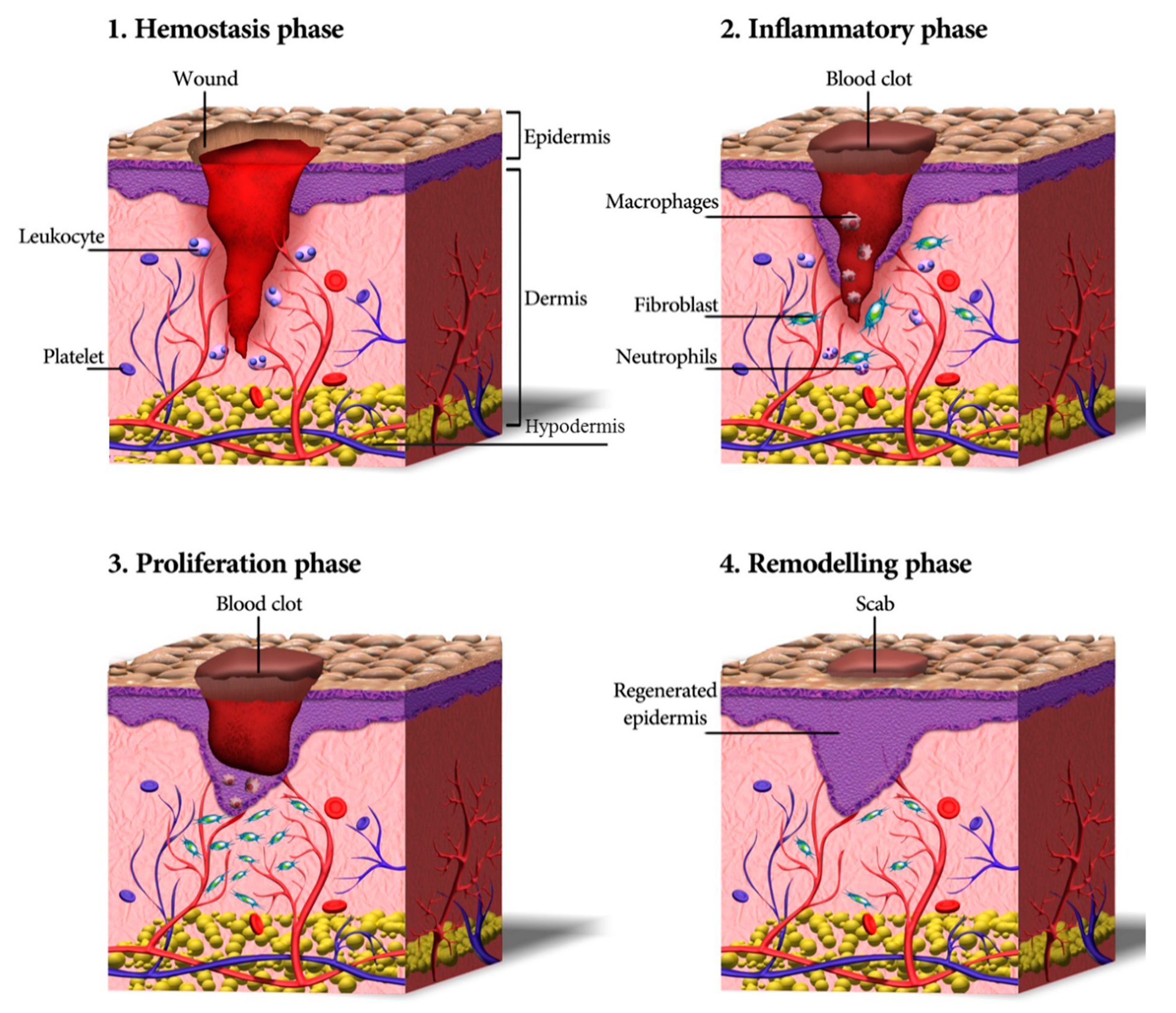
3. Skin Wound Healing
4. Types of Wounds
5. Applications of Hydrogels for Wound Healing
5.1. Sprayable “In Situ” Forming Hydrogels for Wound Applications
5.1.1. Methacrylated Gelatin
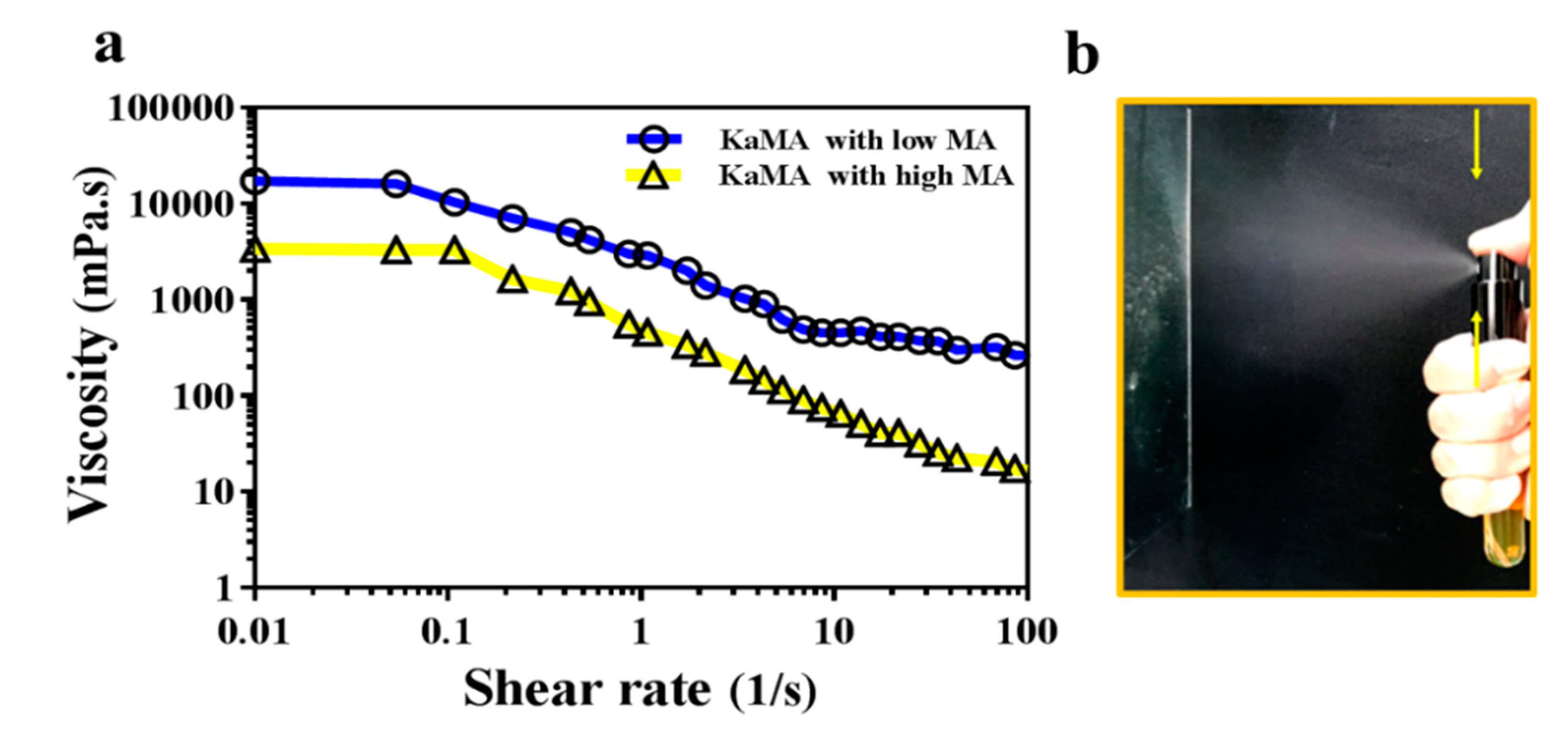
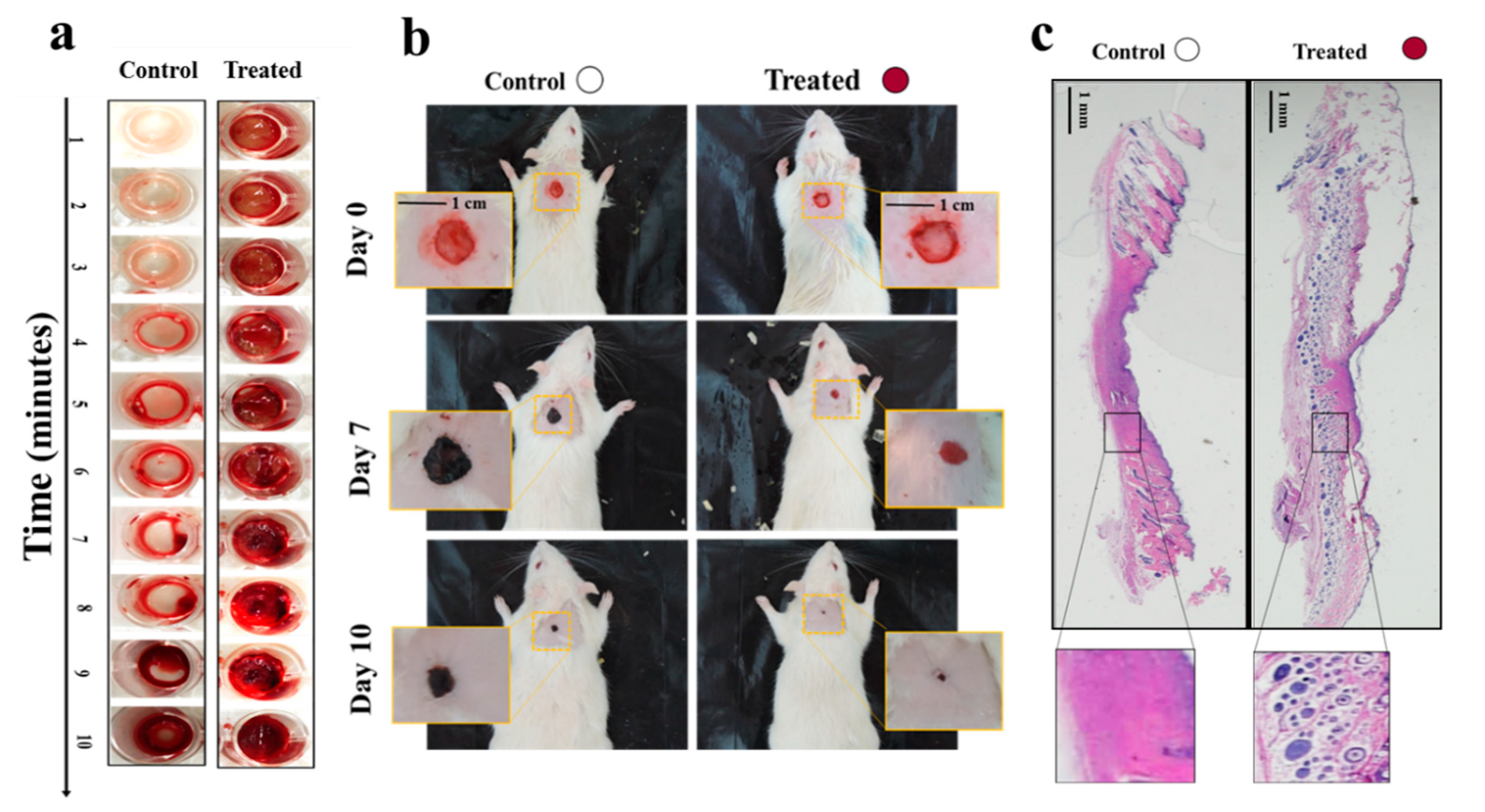
5.1.2. Methacrylated Kappa-Carrageenan
5.1.3. Chitosan
5.2. Acellular Hydrogel-Based Wound Dressings
5.2.1. Commercial Dermal Substitutes Based on Natural Hydrogels
5.2.2. Commercial Dermal Substitutes Based on Synthetic Hydrogels
5.3. Hydrogel Dressings with Integrated Sensors
6. Conclusions and Future Direction
Author Contributions
Funding
Conflicts of Interest
References
- Vig, K.; Chaudhari, A.; Tripathi, S.; Dixit, S.; Sahu, R.; Pillai, S.; Dennis, V.A.; Singh, S.R. Advances in skin regeneration using tissue engineering. Int. J. Mol. Sci. 2017, 18, 789. [Google Scholar] [CrossRef] [PubMed]
- Herndon, D.N.; Barrow, R.E.; Rutan, R.L.; Rutan, T.C.; Desai, M.H.; Abston, S. A comparison of conservative versus early excision. Therapies in severely burned patients. Ann. Surg. 1989, 209, 547. [Google Scholar] [CrossRef] [PubMed]
- Schiestl, C.; Stiefel, D.; Meuli, M. Giant naevus, giant excision, eleg (i) ant closure? Reconstructive surgery with Integra Artificial Skin® to treat giant congenital melanocytic naevi in children. J. Plast. Reconst. Aesthet. Surg. 2010, 63, 610–615. [Google Scholar] [CrossRef] [PubMed]
- Schiestl, C.; Neuhaus, K.; Biedermann, T.; Böttcher-Haberzeth, S.; Reichmann, E.; Meuli, M. Novel treatment for massive lower extremity avulsion injuries in children: Slow, but effective with good cosmesis. Eur. J. Pediatr. Surg. 2011, 21, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Böttcher-Haberzeth, S.; Kapoor, S.; Meuli, M.; Neuhaus, K.; Biedermann, T.; Reichmann, E.; Schiestl, C. Osmotic expanders in children: No filling–no control–no problem? Eur. J. Pediatr. Surg. 2011, 21, 163–167. [Google Scholar] [CrossRef]
- Barbour, J.R.; Schweppe, M.; Seung-Jun, O. Lower-extremity burn reconstruction in the child. J. Carnio. Surg. 2008, 19, 976–988. [Google Scholar] [CrossRef]
- Berman, B.; Viera, M.H.; Amini, S.; Huo, R.; Jones, I.S. Prevention and management of hypertrophic scars and keloids after burns in children. J. Carnio. Surg. 2008, 19, 989–1006. [Google Scholar] [CrossRef]
- Klar, A.S.; Böttcher-Haberzeth, S.; Biedermann, T.; Schiestl, C.; Reichmann, E.; Meuli, M. Analysis of blood and lymph vascularization patterns in tissue-engineered human dermo-epidermal skin analogs of different pigmentation. Pediatr. Surg. Int. 2014, 30, 223–231. [Google Scholar] [CrossRef]
- Klar, A.S.; Güven, S.; Biedermann, T.; Luginbühl, J.; Böttcher-Haberzeth, S.; Meuli-Simmen, C.; Meuli, M.; Martin, I.; Scherberich, A.; Reichmann, E. Tissue-engineered dermo-epidermal skin grafts prevascularized with adipose-derived cells. Biomaterials 2014, 35, 5065–5078. [Google Scholar] [CrossRef]
- Klar, A.S.; Michalak-Mićka, K.; Biedermann, T.; Simmen-Meuli, C.; Reichmann, E.; Meuli, M. Characterization of M1 and M2 polarization of macrophages in vascularized human dermo-epidermal skin substitutes in vivo. Pediatr. Surg. Int. 2018, 34, 129–135. [Google Scholar] [CrossRef]
- Augustine, R.; Kalarikkal, N.; Thomas, S. Advancement of wound care from grafts to bioengineered smart skin substitutes. Prog. Biomater. 2014, 3, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Halim, A.S.; Khoo, T.L.; Yussof, S.J.M. Biologic and synthetic skin substitutes: An overview. Indian J. Plast. Surg. 2010, 43, S23–S28. [Google Scholar] [CrossRef]
- Balakrishnan, B.; Mohanty, M.; Umashankar, P.; Jayakrishnan, A. Evaluation of an in situ forming hydrogel wound dressing based on oxidized alginate and gelatin. Biomaterials 2005, 26, 6335–6342. [Google Scholar] [CrossRef] [PubMed]
- Holland, T.; Chaouk, H.; Asfaw, B.; Goodrich, S.; Hunter, A.; Francis, V. Spray hydrogel wound dressings. Google Patents No. 09/960,449, 2002. [Google Scholar]
- Gao, W.; Zhang, Y.; Zhang, Q.; Zhang, L. Nanoparticle-hydrogel: A hybrid biomaterial system for localized drug delivery. Ann. Biomed. Eng. 2016, 44, 2049–2061. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, A.D.; Ferguson, M.W. Tissue engineering of replacement skin: The crossroads of biomaterials, wound healing, embryonic development, stem cells and regeneration. J. R. Soc. Interface 2007, 4, 413–437. [Google Scholar] [CrossRef]
- Kolarsick, P.A.; Kolarsick, M.A.; Goodwin, C. Anatomy and physiology of the skin. J. Dermatol. Nurs. Assoc. 2011, 3, 203–213. [Google Scholar] [CrossRef]
- Chu, D. Overview of Biology, Development, and Structure of Skin; Wolff, K., Goldsmith, L.A., Katz, S.I., Gilchrest, B.A., Paller, A.S., Leffell, D.J., Eds.; McGraw-Hill: New York, NY, USA, 2008. [Google Scholar]
- Standring, S. Gray’s Anatomy E-book: The Anatomical Basis of Clinical Practice; Elsevier Health Sciences: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Aumailley, M.; Rousselle, P. Laminins of the dermo–epidermal junction. Matrix Biol. 1999, 18, 19–28. [Google Scholar] [CrossRef]
- Timpl, R.; Brown, J.C. Supramolecular assembly of basement membranes. Bioessays 1996, 18, 123–132. [Google Scholar] [CrossRef]
- Fleischmajer, R.; Kühn, K.; Sato, Y.; MacDonald, E.D., II; Perlish, J.S.; Pan, T.-C.; Chu, M.-L.; Kishiro, Y.; Oohashi, T.; Bernier, S.M. There is temporal and spatial expression of α1 (IV), α2 (IV), α5 (IV), α6 (IV) collagen chains and β1 integrins during the development of the basal lamina in an “in vitro” skin model. J. Investig. Dermatol. 1997, 109, 527–533. [Google Scholar] [CrossRef]
- Smola, H.; Stark, H.-J.; Thiekötter, G.; Mirancea, N.; Krieg, T.; Fusenig, N.E. Dynamics of basement membrane formation by keratinocyte–fibroblast interactions in organotypic skin culture. Exp. Cell. Res. 1998, 239, 399–410. [Google Scholar] [CrossRef]
- Yamane, Y.; Yaoita, H.; Couchman, J.R. Basement membrane proteoglycans are of epithelial origin in rodent skin. J. Invest. Dermatol. 1996, 106, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Rittié, L. Cellular mechanisms of skin repair in humans and other mammals. J. Cell Commun. Signal. 2016, 10, 103–120. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.F.; Sousa, A.; Barrias, C.C.; Bayat, A.; Granja, P.L.; Bártolo, P.J. Advances in bioprinted cell-laden hydrogels for skin tissue engineering. Biomanufacturing Rev. 2017, 2, 1. [Google Scholar] [CrossRef]
- Wallig, M.A.; Bolon, B.; Haschek, W.M.; Rousseaux, C.G. Fundamentals of Toxicologic Pathology; Academic Press: Cambridge, MA, USA, 2017. [Google Scholar]
- Clayton, K.; Vallejo, A.F.; Davies, J.; Sirvent, S.; Polak, M.E. Langerhans cells—Programmed by the epidermis. Front. Immunol. 2017, 8, 1676. [Google Scholar] [CrossRef]
- Massella, D.; Argenziano, M.; Ferri, A.; Guan, J.; Giraud, S.; Cavalli, R.; Barresi, A.A.; Salaün, F. Bio-functional textiles: Combining pharmaceutical nanocarriers with fibrous materials for innovative dermatological therapies. Pharmaceutics 2019, 11, 403. [Google Scholar] [CrossRef]
- Wallace, H.A.; Basehore, B.M.; Zito, P.M. Wound healing phases. StatPearls 2019. Available online: https://europepmc.org/article/NBK/NBK470443 (accessed on 4 May 2020).
- Gonzalez, A.C.d.O.; Costa, T.F.; Andrade, Z.D.A.; Medrado, A.R.A.P. Wound healing—A literature review. An. Bras. Dermatol. 2016, 91, 614–620. [Google Scholar] [CrossRef]
- Yun, S.-H.; Sim, E.-H.; Goh, R.-Y.; Park, J.-I.; Han, J.-Y. Platelet activation: The mechanisms and potential biomarkers. Biomed Res. Int. 2016, 2016. [Google Scholar] [CrossRef]
- Rasche, H. Haemostasis and thrombosis: An overview. Eur. Heart J. Suppl. 2001, 3, Q3–Q7. [Google Scholar] [CrossRef]
- Versteeg, H.H.; Heemskerk, J.W.; Levi, M.; Reitsma, P.H. New fundamentals in hemostasis. Physiol. Rev. 2013, 93, 327–358. [Google Scholar] [CrossRef]
- Rossaint, J.; Margraf, A.; Zarbock, A. Role of platelets in leukocyte recruitment and resolution of inflammation. Front. Immunol. 2018, 9, 2712. [Google Scholar] [CrossRef] [PubMed]
- Schultz, G.S.; Chin, G.A.; Moldawer, L.; Diegelmann, R.F. Principles of wound healing. In Mechanisms of Vascular Disease: A Reference Book for Vascular Specialists [Internet]; University of Adelaide Press: Adelaide, Australia, 2011. [Google Scholar]
- Midwood, K.S.; Williams, L.V.; Schwarzbauer, J.E. Tissue repair and the dynamics of the extracellular matrix. Int. J. Biochem. Cell Biol. 2004, 36, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- desJardins-Park, H.E.; Foster, D.S.; Longaker, M.T. Fibroblasts and wound healing: An update. Future Med. 2018. [Google Scholar] [CrossRef] [PubMed]
- Bowden, L.; Byrne, H.; Maini, P.; Moulton, D. A morphoelastic model for dermal wound closure. Biomech. Mod. Mechan. 2016, 15, 663–681. [Google Scholar] [CrossRef]
- Lazarus, G.S.; Cooper, D.M.; Knighton, D.R.; Margolis, D.J.; Percoraro, R.E.; Rodeheaver, G.; Robson, M.C. Definitions and guidelines for assessment of wounds and evaluation of healing. Wound Repair Regener. 1994, 2, 165–170. [Google Scholar] [CrossRef]
- Percival, N.J. Classification of wounds and their management. Surgery (Oxf.) 2002, 20, 114–117. [Google Scholar] [CrossRef]
- Golinko, M.S.; Clark, S.; Rennert, R.; Flattau, A.; Boulton, A.J.; Brem, H. Wound emergencies: The importance of assessment, documentation, and early treatment using a wound electronic medical record. Ostomy/Wound Manag. 2009, 55, 54. [Google Scholar]
- Moore, K.; McCallion, R.; Searle, R.J.; Stacey, M.C.; Harding, K.G. Prediction and monitoring the therapeutic response of chronic dermal wounds. Int. Wound J. 2006, 3, 89–98. [Google Scholar] [CrossRef]
- Uccioli, L.; Izzo, V.; Meloni, M.; Vainieri, E.; Ruotolo, V.; Giurato, L. Non-healing foot ulcers in diabetic patients: General and local interfering conditions and management options with advanced wound dressings. J. Wound Care 2015, 24, 35–42. [Google Scholar] [CrossRef]
- Nicodemus, G.D.; Bryant, S.J. Cell encapsulation in biodegradable hydrogels for tissue engineering applications. Tissue Eng. Part B 2008, 14, 149–165. [Google Scholar] [CrossRef]
- Boateng, J.S.; Matthews, K.H.; Stevens, H.N.; Eccleston, G.M. Wound healing dressings and drug delivery systems: A review. J. Pharm. Sci. 2008, 97, 2892–2923. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Jaiswal, M. Design and in vitro investigation of nanocomposite hydrogel based in situ spray dressing for chronic wounds and synthesis of silver nanoparticles using green chemistry. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- van Vlierberghe, S.; Dubruel, P.; Schacht, E. Biopolymer-based hydrogels as scaffolds for tissue engineering applications: A review. Biomacromolecules 2011, 12, 1387–1408. [Google Scholar] [CrossRef] [PubMed]
- Bilici, C.; Can, V.; Noöchel, U.; Behl, M.; Lendlein, A.; Okay, O. Melt-processable shape-memory hydrogels with self-healing ability of high mechanical strength. Macromol. 2016, 49, 7442–7449. [Google Scholar] [CrossRef]
- Koehler, J.; Brandl, F.P.; Goepferich, A.M. Hydrogel wound dressings for bioactive treatment of acute and chronic wounds. Eur. Polym. J. 2018, 100, 1–11. [Google Scholar] [CrossRef]
- Gupta, P.; Vermani, K.; Garg, S. Hydrogels: From controlled release to pH-responsive drug delivery. Drug Discov. Today 2002, 7, 569–579. [Google Scholar] [CrossRef]
- Tavakoli, S.; Mokhtari, H.; Kharaziha, M.; Kermanpour, A.; Talebi, A.; Moshtaghian, J. A multifunctional nanocomposite spray dressing of Kappa-carrageenan-polydopamine modified ZnO/L-glutamic acid for diabetic wounds. Mater. Sci. Eng. C 2020, 110837. [Google Scholar] [CrossRef]
- Jones, A.; Vaughan, D. Hydrogel dressings in the management of a variety of wound types: A review. J. Orthop. Nurs. 2005, 9, S1–S11. [Google Scholar] [CrossRef]
- Dhivya, S.; Padma, V.V.; Santhini, E. Wound dressings–A review. BioMedicine 2015, 5. [Google Scholar] [CrossRef]
- White, R. Wound dressings and other topical treatment modalities in bioburden control. J. Wound Care 2011, 20, 431–439. [Google Scholar] [CrossRef]
- Pereira, R.F.; Barrias, C.C.; Bártolo, P.J.; Granja, P.L. Cell-instructive pectin hydrogels crosslinked via thiol-norbornene photo-click chemistry for skin tissue engineering. Acta Biomater. 2018, 66, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Mihaila, S.M.; Gaharwar, A.K.; Reis, R.L.; Marques, A.P.; Gomes, M.E.; Khademhosseini, A. Photocrosslinkable kappa-carrageenan hydrogels for tissue engineering applications. Adv. Healthc. Mater. 2013, 2, 895–907. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.F.; Sousa, A.; Barrias, C.C.; Bártolo, P.J.; Granja, P.L. A single-component hydrogel bioink for bioprinting of bioengineered 3D constructs for dermal tissue engineering. Mater. Horiz. 2018, 5, 1100–1111. [Google Scholar] [CrossRef]
- Chouhan, D.; Lohe, T.u.; Samudrala, P.K.; Mandal, B.B. In situ forming injectable silk fibroin hydrogel promotes skin regeneration in full thickness burn wounds. Adv. Healthc. Mater. 2018, 7, 1801092. [Google Scholar] [CrossRef]
- Tavakoli, S.; Kharaziha, M.; Kermanpur, A.; Mokhtari, H. Sprayable and injectable visible-light Kappa-carrageenan hydrogel for in-situ soft tissue engineering. Int. J. Biol. Macromol. 2019, 138, 590–601. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Sun, X.; Wang, Z.; Guo, S.; Yu, G.; Yang, H. Synthesis and properties of gelatin methacryloyl (GelMA) hydrogels and their recent applications in load-bearing tissue. Polymers 2018, 10, 1290. [Google Scholar] [CrossRef]
- Tondera, C.; Hauser, S.; Krüger-Genge, A.; Jung, F.; Neffe, A.T.; Lendlein, A.; Klopfleisch, R.; Steinbach, J.; Neuber, C.; Pietzsch, J. Gelatin-based hydrogel degradation and tissue interaction in vivo: Insights from multimodal preclinical imaging in immunocompetent nude mice. Theranostics 2016, 6, 2114. [Google Scholar] [CrossRef]
- Neumann, P.; Zur, B.; Ehrenreich, Y. Gelatin-based sprayable foam as a skin substitute. J. Biomed. Mater. Res. 1981, 15, 9–18. [Google Scholar] [CrossRef]
- Zhao, X.; Lang, Q.; Yildirimer, L.; Lin, Z.Y.; Cui, W.; Annabi, N.; Ng, K.W.; Dokmeci, M.R.; Ghaemmaghami, A.M.; Khademhosseini, A. Photocrosslinkable gelatin hydrogel for epidermal tissue engineering. Adv. Healthc. Mater. 2016, 5, 108–118. [Google Scholar] [CrossRef]
- Annabi, N.; Rana, D.; Sani, E.S.; Portillo-Lara, R.; Gifford, J.L.; Fares, M.M.; Mithieux, S.M.; Weiss, A.S. Engineering a sprayable and elastic hydrogel adhesive with antimicrobial properties for wound healing. Biomaterials 2017, 139, 229–243. [Google Scholar] [CrossRef]
- Yegappan, R.; Selvaprithiviraj, V.; Amirthalingam, S.; Jayakumar, R. Carrageenan based hydrogels for drug delivery, tissue engineering and wound healing. Carbohydr. Polym. 2018, 198, 385–400. [Google Scholar] [CrossRef] [PubMed]
- Mokhtari, H.; Kharaziha, M.; Karimzadeh, F.; Tavakoli, S. An injectable mechanically robust hydrogel of Kappa-carrageenan-dopamine functionalized graphene oxide for promoting cell growth. Carbohydr. Polym. 2019, 214, 234–249. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, F.; Oveisi, Z.; Samani, S.M.; Amoozgar, Z. Chitosan based hydrogels: Characteristics and pharmaceutical applications. Res. Pharm. Sci. 2015, 10, 1. [Google Scholar] [PubMed]
- Peniche, C.; Argüelles-Monal, W.; Peniche, H.; Acosta, N. Chitosan: An attractive biocompatible polymer for microencapsulation. Macromol. Biosci. 2003, 3, 511–520. [Google Scholar] [CrossRef]
- Ong, S.-Y.; Wu, J.; Moochhala, S.M.; Tan, M.-H.; Lu, J. Development of a chitosan-based wound dressing with improved hemostatic and antimicrobial properties. Biomaterials 2008, 29, 4323–4332. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Saito, Y.; Yura, H.; Ishikawa, K.; Kurita, A.; Akaike, T.; Ishihara, M. Photocrosslinkable chitosan as a biological adhesive. J. Biomed. Mater. Res. 2000, 49, 289–295. [Google Scholar] [CrossRef]
- Gholizadeh, H.; Messerotti, E.; Pozzoli, M.; Cheng, S.; Traini, D.; Young, P.; Kourmatzis, A.; Caramella, C.; Ong, H.X. Application of a thermosensitive in situ gel of chitosan-based nasal spray loaded with tranexamic acid for localised treatment of nasal wounds. AAPS PharmSciTech 2019, 20, 299. [Google Scholar] [CrossRef]
- Mattioli-Belmonte, M.; Zizzi, A.; Lucarini, G.; Giantomassi, F.; Biagini, G.; Tucci, G.; Orlando, F.; Provinciali, M.; Carezzi, F.; Morganti, P. Chitin nanofibrils linked to chitosan glycolate as spray, gel, and gauze preparations for wound repair. J. Bioact. Comp. Polym. 2007, 22, 525–538. [Google Scholar] [CrossRef]
- Hampton, S. A new spray-on chitosan FH02™ dressing for venous leg ulcers: An evaluation. J. Clin. Nurs. 2018, 32, 25–28. [Google Scholar]
- Sharpe, A.; Tickle, J.; Hampton, S.; Gray, D. A multicentre evaluation of a new chitosan FH02™ spray-on dressing in patients with chronic wounds in the UK. J. Commun. Nurs. 2018, 32. Available online: https://lqdspray.com/wp-content/uploads/2018/06/Multicentre-Evaluation.pdf (accessed on 4 May 2020).
- Dai, T.; Tanaka, M.; Huang, Y.-Y.; Hamblin, M.R. Chitosan preparations for wounds and burns: Antimicrobial and wound-healing effects. Expert Rev. Anti-Infect. Ther. 2011, 9, 857–879. [Google Scholar] [CrossRef] [PubMed]
- Mogoşanu, G.D.; Grumezescu, A.M. Natural and synthetic polymers for wounds and burn dressing. Int. J. Pharm. 2014, 463, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Cascone, S.; Lamberti, G. Hydrogel-based commercial products for biomedical applications: A review. Int. J. Pharm. 2020, 573, 118803. [Google Scholar] [CrossRef] [PubMed]
- Varshney, L. Role of natural polysaccharides in radiation formation of PVA–hydrogel wound dressing. Nucl. Instrum. Methods Phys. Res. Sect. B 2007, 255, 343–349. [Google Scholar] [CrossRef]
- Schiavon, M.; Francescon, M.; Drigo, D.; Salloum, G.; Baraziol, R.; Tesei, J.; Fraccalanza, E.; Barbone, F. The use of Integra dermal regeneration template versus flaps for reconstruction of full-thickness scalp defects involving the calvaria: A cost–benefit analysis. Aesthet. Plast. Surg. 2016, 40, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Myers, S.; Navsaria, H.; Ojeh, N. Skin engineering and keratinocyte stem cell therapy. In Tissue Engineering; Elsevier: Amsterdam, The Netherlands, 2014; pp. 497–528. [Google Scholar]
- Shakespeare, P.G. The role of skin substitutes in the treatment of burn injuries. Clin. Dermatol. 2005, 23, 413–418. [Google Scholar] [CrossRef]
- Stiefel, D.; Schiestl, C.; Meuli, M. Integra Artificial Skin® for burn scar revision in adolescents and children. Burns 2010, 36, 114–120. [Google Scholar] [CrossRef]
- Broussard, K.C.; Powers, J.G. Wound dressings: Selecting the most appropriate type. Am. J. Clin. Dermatol. 2013, 14, 449–459. [Google Scholar] [CrossRef]
- Yao, M.; Attalla, K.; Ren, Y.; French, M.A.; Driver, V.R. Ease of use, safety, and efficacy of integra bilayer wound matrix in the treatment of diabetic foot ulcers in an outpatient clinical setting: A prospective pilot study. J. Am. Pod. Med. Assoc. 2013, 103, 274–280. [Google Scholar] [CrossRef]
- Bottcher-Haberzeth, S.; Biedermann, T.; Schiestl, C.; Hartmann-Fritsch, F.; Schneider, J.; Reichmann, E.; Meuli, M. Matriderm (R) 1 mm versus Integra (R) Single Layer 1.3 mm for one-step closure of full thickness skin defects: A comparative experimental study in rats. Pediatr. Surg. Int. 2012, 28, 171–177. [Google Scholar] [CrossRef]
- Kolokythas, P.; Aust, M.; Vogt, P.; Paulsen, F. Dermal subsitute with the collagen-elastin matrix Matriderm in burn injuries: A comprehensive review. Handchir. Mikrochir. Plast. Chir. Organ Dtsch. Arb. Handchir. Organ Dtsch. Arb. Mikrochir. Peripher. Nerven GefasseOrgan V 2008, 40, 367–371. [Google Scholar]
- Min, J.H.; Yun, I.S.; Lew, D.H.; Roh, T.S.; Lee, W.J. The use of matriderm and autologous skin graft in the treatment of full thickness skin defects. Arch. Plast. Surg. 2014, 41, 330. [Google Scholar] [CrossRef] [PubMed]
- Haslik, W.; Kamolz, L.-P.; Nathschläger, G.; Andel, H.; Meissl, G.; Frey, M. First experiences with the collagen-elastin matrix Matriderm® as a dermal substitute in severe burn injuries of the hand. Burns 2007, 33, 364–368. [Google Scholar] [CrossRef] [PubMed]
- Cervelli, V.; Brinci, L.; Spallone, D.; Tati, E.; Palla, L.; Lucarini, L.; de Angelis, B. The use of MatriDerm® and skin grafting in post-traumatic wounds. Int. Wound J. 2011, 8, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Ryssel, H.; Germann, G.; Kloeters, O.; Gazyakan, E.; Radu, C. Dermal substitution with Matriderm® in burns on the dorsum of the hand. Burns 2010, 36, 1248–1253. [Google Scholar] [CrossRef]
- de Vries, H.; Zeegelaar, J.; Middelkoop, E.; Gijsbers, G.; van Marle, J.; Wildevuur, C.; Westerhof, W. Reduced wound contraction and scar formation in punch biopsy wounds. Native collagen dermal substitutes. A clinical study. Br. J. Dermatol. 1995, 132, 690–697. [Google Scholar] [CrossRef]
- Schneider, J.; Biedermann, T.; Widmer, D.; Montano, I.; Meuli, M.; Reichmann, E.; Schiestl, C. Matriderm® versus Integra®: A comparative experimental study. Burns 2009, 35, 51–57. [Google Scholar] [CrossRef]
- Catoira, M.C.; Fusaro, L.; di Francesco, D.; Ramella, M.; Boccafoschi, F. Overview of natural hydrogels for regenerative medicine applications. J. Mater. Sci. Mater. Med. 2019, 30, 115. [Google Scholar] [CrossRef]
- Hartmann-Fritsch, F.; Biedermann, T.; Braziulis, E.; Luginbühl, J.; Pontiggia, L.; Böttcher-Haberzeth, S.; van Kuppevelt, T.H.; Faraj, K.A.; Schiestl, C.; Meuli, M. Collagen hydrogels strengthened by biodegradable meshes are a basis for dermo-epidermal skin grafts intended to reconstitute human skin in a one-step surgical intervention. J. Tissue Eng. Regener. Med. 2016, 10, 81–91. [Google Scholar] [CrossRef]
- Seal, B.; Otero, T.; Panitch, A. Polymeric biomaterials for tissue and organ regeneration. Mater. Sci. Eng. R Rep. 2001, 34, 147–230. [Google Scholar] [CrossRef]
- Shahrokhi, S.; Arno, A.; Jeschke, M.G. The use of dermal substitutes in burn surgery: Acute phase. Wound Repair Regener. 2014, 22, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Li, J.K.; Wang, N.; Wu, X.S. Poly (vinyl alcohol) nanoparticles prepared by freezing–thawing process for protein/peptide drug delivery. J. Control. Release 1998, 56, 117–126. [Google Scholar] [CrossRef]
- Kamoun, E.A.; Kenawy, E.-R.S.; Chen, X. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J. Adv. Res. 2017, 8, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Zhai, M.; Yoshii, F.; Kume, T.; Hashim, K. Syntheses of PVA/starch grafted hydrogels by irradiation. Carbohydr. Polym. 2002, 50, 295–303. [Google Scholar] [CrossRef]
- Khil, M.S.; Cha, D.I.; Kim, H.Y.; Kim, I.S.; Bhattarai, N. Electrospun nanofibrous polyurethane membrane as wound dressing. J. Biomed. Mater. Res. Part B 2003, 67, 675–679. [Google Scholar] [CrossRef] [PubMed]
- Cheshire, P.A.; Herson, M.R.; Cleland, H.; Akbarzadeh, S. Artificial dermal templates: A comparative study of NovoSorb™ Biodegradable Temporising Matrix (BTM) and Integra® Dermal Regeneration Template (DRT). Burns 2016, 42, 1088–1096. [Google Scholar] [CrossRef]
- Abed, S.; Dantzer, E.; Souraud, J.; Brissy, S.; Fournier, B.; Boyé, T.; Guennoc, B.; Morand, J. The place of skin substitutes in surgical treatment of necrotising cellulitis: Seven cases. Ann. Dermatol. Venereol. 2013, 141, 49–52. [Google Scholar]
- Danielsson, P.; Fredriksson, C.; Huss, F. A novel concept for treating large necrotizing fasciitis wounds with bilayer dermal matrix, split-thickness skin grafts, and negative pressure wound therapy. Wounds 2009, 21, 215–220. [Google Scholar]
- Wagstaff, M.J.; Salna, I.M.; Caplash, Y.; Greenwood, J.E. Biodegradable Temporising Matrix (BTM) for the reconstruction of defects following serial debridement for necrotising fasciitis: A case series. Burns Open 2019, 3, 12–30. [Google Scholar] [CrossRef]
- Damkat-Thomas, L.; Greenwood, J.E.; Wagstaff, M.J. A synthetic biodegradable temporising matrix in degloving lower extremity trauma reconstruction: A case report. Plast. Reconst. Surg. Glob. Open 2019, 7. [Google Scholar] [CrossRef]
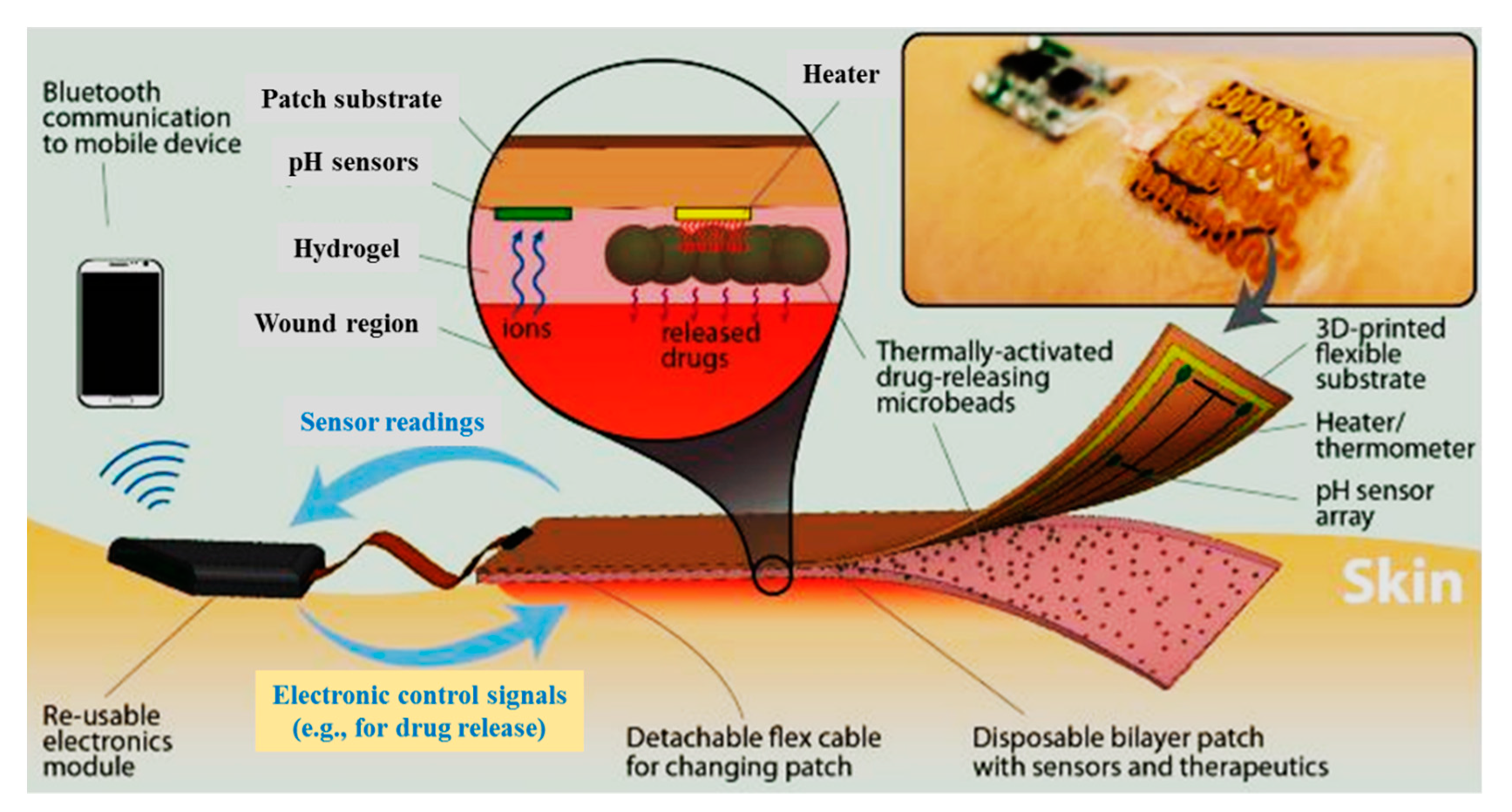
- Derakhshandeh, H.; Kashaf, S.S.; Aghabaglou, F.; Ghanavati, I.O.; Tamayol, A. Smart bandages: The future of wound care. Trends Biotechnol. 2018, 36, 1259–1274. [Google Scholar] [CrossRef]
- Mir, M.; Ali, M.N.; Barakullah, A.; Gulzar, A.; Arshad, M.; Fatima, S.; Asad, M. Synthetic polymeric biomaterials for wound healing: A review. Prog. Biomater. 2018, 7, 1–21. [Google Scholar] [CrossRef]
- Mehmood, N.; Hariz, A.; Fitridge, R.; Voelcker, N.H. Applications of modern sensors and wireless technology in effective wound management. J. Biomed. Mater. Res. Part B 2014, 102, 885–895. [Google Scholar] [CrossRef] [PubMed]
- Najafabadi, A.H.; Tamayol, A.; Annabi, N.; Ochoa, M.; Mostafalu, P.; Akbari, M.; Nikkhah, M.; Rahimi, R.; Dokmeci, M.R.; Sonkusale, S. Biodegradable nanofibrous polymeric substrates for generating elastic and flexible electronics. Adv. Mater. 2014, 26, 5823–5830. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, M.; Rahimi, R.; Ziaie, B. Flexible sensors for chronic wound management. IEEE Rev. Biomed. Eng. 2013, 7, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Abu-Raya, Y.S.; Haick, H. Advanced materials for health monitoring with skin-based wearable devices. Adv. Healthc. Mater. 2017, 6, 1700024. [Google Scholar] [CrossRef]
- Feiner, R.; Wertheim, L.; Gazit, D.; Kalish, O.; Mishal, G.; Shapira, A.; Dvir, T. A stretchable and flexible cardiac tissue–electronics hybrid enabling multiple drug release, sensing, and stimulation. Small 2019, 15, 1805526. [Google Scholar] [CrossRef]
- Pang, Q.; Lou, D.; Li, S.; Wang, G.; Qiao, B.; Dong, S.; Ma, L.; Gao, C.; Wu, Z. Smart flexible electronics-integrated wound dressing for real-time monitoring and on-demand treatment of infected wounds. Adv. Sci. 2020, 7, 1902673. [Google Scholar] [CrossRef]
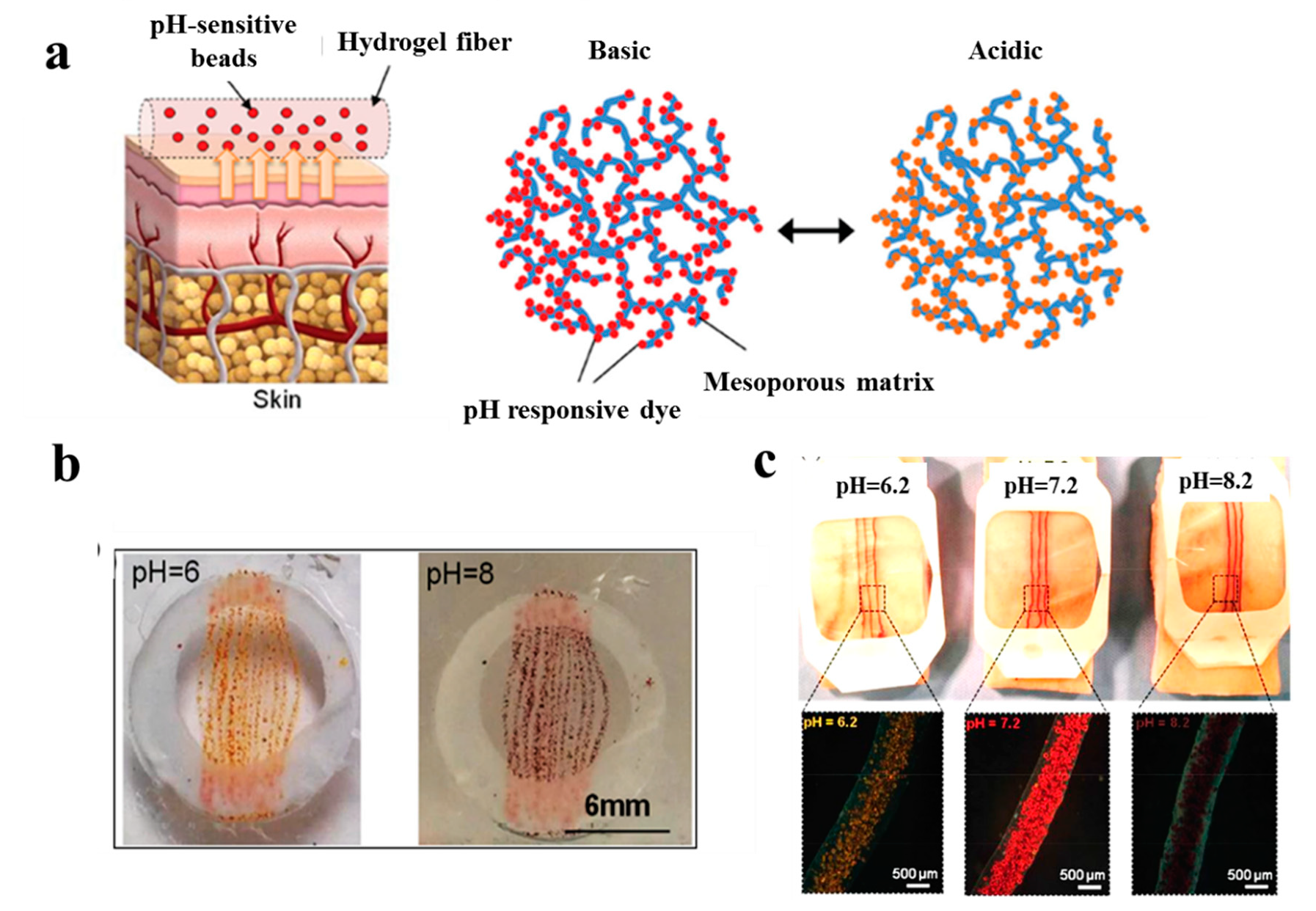
- Tamayol, A.; Akbari, M.; Zilberman, Y.; Comotto, M.; Lesha, E.; Serex, L.; Bagherifard, S.; Chen, Y.; Fu, G.; Ameri, S.K. Flexible pH-sensing hydrogel fibers for epidermal applications. Adv. Healthc. Mater. 2016, 5, 711–719. [Google Scholar] [CrossRef]
- Percival, S.L.; McCarty, S.; Hunt, J.A.; Woods, E.J. The effects of pH on wound healing, biofilms, and antimicrobial efficacy. Wound Repair Regener. 2014, 22, 174–186. [Google Scholar] [CrossRef]
- Jones, E.M.; Cochrane, C.A.; Percival, S.L. The effect of pH on the extracellular matrix and biofilms. Adv. Wound Care 2015, 4, 431–439. [Google Scholar] [CrossRef]
- Sadat Ebrahimi, M.M.; Schoönherr, H. Enzyme-sensing chitosan hydrogels. Langmuir 2014, 30, 7842–7850. [Google Scholar] [CrossRef] [PubMed]
- Occhiuzzi, C.; Ajovalasit, A.; Sabatino, M.A.; Dispenza, C.; Marrocco, G. RFID epidermal sensor including hydrogel membranes for wound monitoring and healing. Proceedings of IEEE International Conference on RFID (RFID), San Diego, CA, USA, 15 April 2015; pp. 182–188. [Google Scholar]
- Mostafalu, P.; Tamayol, A.; Rahimi, R.; Ochoa, M.; Khalilpour, A.; Kiaee, G.; Yazdi, I.K.; Bagherifard, S.; Dokmeci, M.R.; Ziaie, B. Smart bandage for monitoring and treatment of chronic wounds. Small 2018, 14, 1703509. [Google Scholar] [CrossRef] [PubMed]


| Feature | Description |
|---|---|
| No toxic component | Free from toxic materials that can damage and lead to dire consequences |
| Prevention of bacterial infection | Preventing bacterial infections, which could impair wound healing and prolong its duration |
| Adhesiveness | Providing an optimum amount of adhesive material to the wound site (excessive adhesive sustains an injury) |
| Moisture | Maintaining the optimum moisture level to promote cell migration and proliferation |
| Thermal insulation | Maintaining the optimal temperature of wound site to reduce pain |
| Absorption of excess exudate | Regulating the amount of exudates present in the wound |
| Oxygen permeability | Allowing the diffusion of oxygen to the wound bed to accelerate cell activity |
| Mechanical and physical properties | Resembling the structure of native skin |
| Minimal tissue trauma (pain) | Minimizing patient pain during application and removal |
| Cost effectiveness | Providing an affordable wound dressing |
| Free availability | Accessible for all patients or healthcare centers |
| Product | Company | Main Component | Application |
|---|---|---|---|
| Algisite | Smith and Nephew | Alginate | Lacerations, abrasions, skin tears and minor burn wounds |
| Medihoney | Derma Sciences | Alginate | Partial to full-thickness wounds and burns |
| Kaltostat | Convatec | Alginate | Moderate to heavily exuding wounds chronic and acute wounds |
| NU-GEL | Systagenix | Alginate | Management of chronic wounds throughout all stages of healing |
| Condress | Smith and Nephew | Collagen | Chronic and acute wounds |
| Helix3-cm | Amerx Health Care | Collagen | Chronic and acute wounds |
| DermaFilm | DermaRite | Hydrocolloids | Abrasions, closed surgical wounds, superficial ulcers, and skin grafts |
| Comfeel | Coloplast | Hydrocolloids | Designed for difficult-to-dress areas |
| CovaWound | Covalon | Hydrocolloids | Pressure, leg and foot ulcers, superficial partial-thickness burns |
| Inadine | Systagenix | Polyethylene glycol | Open wounds that may become infected |
| Sofargen | Sofar | Colloidal silica | For abrasions, grazes, first- and second-degree burns and injuries |
| Cutimed | Bsn Medical | Dialkylcarbamoyl-chloride | Treatment of necrotic and sloughy tissues in chronic wounds |
| Kendall | Cardinal Health | Glycerin formulation | First- and second-degree burns and partial- and full-thickness wounds |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tavakoli, S.; Klar, A.S. Advanced Hydrogels as Wound Dressings. Biomolecules 2020, 10, 1169. https://doi.org/10.3390/biom10081169
Tavakoli S, Klar AS. Advanced Hydrogels as Wound Dressings. Biomolecules. 2020; 10(8):1169. https://doi.org/10.3390/biom10081169
Chicago/Turabian StyleTavakoli, Shima, and Agnes S. Klar. 2020. "Advanced Hydrogels as Wound Dressings" Biomolecules 10, no. 8: 1169. https://doi.org/10.3390/biom10081169
APA StyleTavakoli, S., & Klar, A. S. (2020). Advanced Hydrogels as Wound Dressings. Biomolecules, 10(8), 1169. https://doi.org/10.3390/biom10081169






