Functionalizable Antifouling Coatings as Tunable Platforms for the Stress-Driven Manipulation of Living Cell Machinery
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Preparation of RGD-Functionalized Coatings
2.2.1. Preparation of pCB-Based Polymer Brushes
2.2.2. Preparation of the Carboxy-Functional OEG-Based Self-Assembled Monolayer (OEG-SAM)
2.3. Surface Characterization by SPR
2.4. Cell Culture
2.5. Assessment of Cell Death
2.6. Localization and Quantification of Endogenous YAP
2.7. Spinning Disk Confocal Microscopy
2.8. Statistical Analysis
3. Results
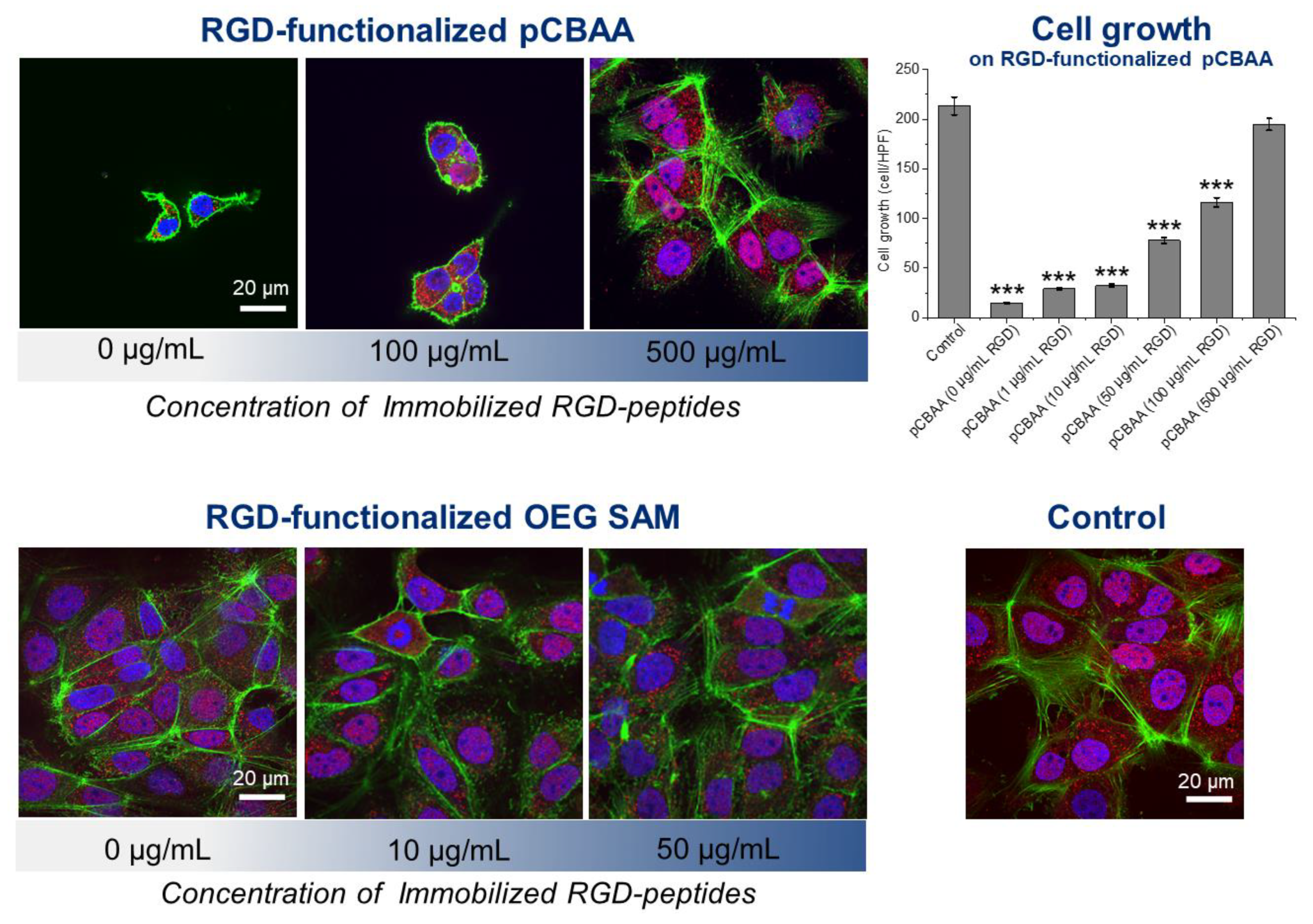
3.1. Antifouling Properties of RGD-Functionalized Coatings
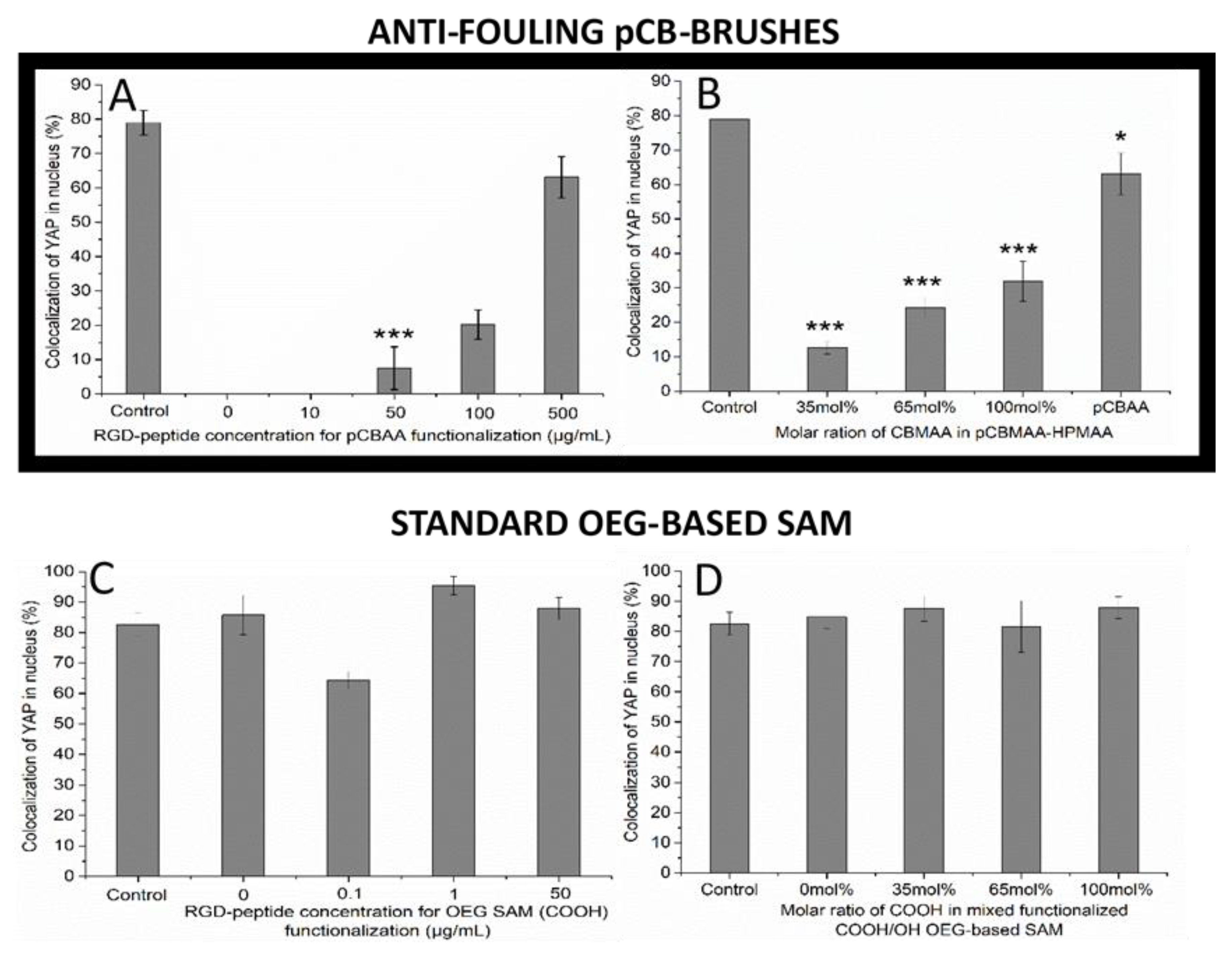
3.2. Modulation of Mechanotransduction Signaling
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wolfenson, H.; Yang, B.; Sheetz, M.P. Steps in Mechanotransduction Pathways that Control Cell Morphology. Annu. Rev. Physiol. 2019, 81, 585–605. [Google Scholar] [CrossRef] [PubMed]
- Lansky, Z.; Mutsafi, Y.; Houben, L.; Ilani, T.; Armony, G.; Wolf, S.G.; Fass, D. 3D mapping of native extracellular matrix reveals cellular responses to the microenvironment. JSBX 2019, 1, 100002. [Google Scholar] [CrossRef] [PubMed]
- Cobbaut, M.; Karagil, S.; Bruno, L.; Diaz de la Loza, M.D.C.; Mackenzie, F.E.; Stolinski, M.; Elbediwy, A. Dysfunctional Mechanotransduction through the YAP/TAZ/Hippo Pathway as a Feature of Chronic Disease. Cells 2020, 9, 151. [Google Scholar] [CrossRef] [PubMed]
- Gautrot, J.E.; Malmström, J.; Sundh, M.; Margadant, C.; Sonnenberg, A.; Sutherland, D.S. The Nanoscale Geometrical Maturation of Focal Adhesions Controls Stem Cell Differentiation and Mechanotransduction. Nano Lett. 2014, 14, 3945–3952. [Google Scholar] [CrossRef] [PubMed]
- Martino, F.; Perestrelo, A.R.; Vinarský, V.; Pagliari, S.; Forte, G. Cellular Mechanotransduction: From Tension to Function. Front. Physiol. 2018, 9, 824. [Google Scholar] [CrossRef] [PubMed]
- Elbediwy, A.; Vincent-Mistiaen, Z.I.; Spencer-Dene, B.; Stone, R.K.; Boeing, S.; Wculek, S.K.; Cordero, J.; Tan, E.H.; Ridgway, R.; Brunton, V.G.; et al. Integrin signalling regulates YAP and TAZ to control skin homeostasis. Development 2016, 143, 1674–1687. [Google Scholar] [CrossRef]
- Mori, M.; Triboulet, R.; Mohseni, M.; Schlegelmilch, K.; Shrestha, K.; Camargo, F.D.; Gregory, R.I. Hippo signaling regulates microprocessor and links cell-density-dependent miRNA biogenesis to cancer. Cell 2014, 156, 893–906. [Google Scholar] [CrossRef]
- Nardone, G.; Oliver-De La Cruz, J.; Vrbsky, J.; Martini, C.; Pribyl, J.; Skládal, P.; Pešl, M.; Caluori, G.; Pagliari, S.; Martino, F.; et al. YAP regulates cell mechanics by controlling focal adhesion assembly. Nat. Commun. 2017, 8, 15321. [Google Scholar] [CrossRef]
- Piccolo, S.; Dupont, S.; Cordenonsi, M. The biology of YAP/TAZ: Hippo signaling and beyond. Physiol. Rev. 2014, 94, 1287–1312. [Google Scholar] [CrossRef]
- Shreberk-Shaked, M.; Oren, M. New insights into YAP/TAZ nucleo-cytoplasmic shuttling: New cancer therapeutic opportunities? Mol. Oncol. 2019, 13, 1335–1341. [Google Scholar] [CrossRef]
- Halder, G.; Dupont, S.; Piccolo, S. Transduction of mechanical and cytoskeletal cues by YAP and TAZ. Nat. Rev. Mol. cell Biol. 2012, 13, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, M.C.; Halder, G. Regulation of the Hippo pathway by cell architecture and mechanical signals. Semin. Cell Dev. Biol. 2012, 23, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Thomasy, S.M.; Morgan, J.T.; Wood, J.A.; Murphy, C.J.; Russell, P. Substratum stiffness and latrunculin B modulate the gene expression of the mechanotransducers YAP and TAZ in human trabecular meshwork cells. Exp. Eye Res. 2013, 113, 66–73. [Google Scholar] [CrossRef]
- Aragona, M.; Panciera, T.; Manfrin, A.; Giulitti, S.; Michielin, F.; Elvassore, N.; Dupont, S.; Piccolo, S. A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell 2013, 154, 1047–1059. [Google Scholar] [CrossRef] [PubMed]
- Wada, K.; Itoga, K.; Okano, T.; Yonemura, S.; Sasaki, H. Hippo pathway regulation by cell morphology and stress fibers. Development 2011, 138, 3907–3914. [Google Scholar] [CrossRef] [PubMed]
- Gibson, W.T.; Gibson, M.C. Cell topology, geometry, and morphogenesis in proliferating epithelia. Curr. Top. Dev. Biol. 2009, 89, 87–114. [Google Scholar] [CrossRef]
- Zhao, B.; Wei, X.; Li, W.; Udan, R.S.; Yang, Q.; Kim, J.; Xie, J.; Ikenoue, T.; Yu, J.; Li, L.; et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Gene. Dev. 2007, 21, 2747–2761. [Google Scholar] [CrossRef] [PubMed]
- Varelas, X.; Samavarchi-Tehrani, P.; Narimatsu, M.; Weiss, A.; Cockburn, K.; Larsen, B.G.; Rossant, J.; Wrana, J.L. The Crumbs complex couples cell density sensing to Hippo-dependent control of the TGF-beta-SMAD pathway. Dev. Cell 2010, 19, 831–844. [Google Scholar] [CrossRef]
- Monteiro, A.I.; Kollmetz, T.; Malmstrom, J. Engineered systems to study the synergistic signaling between integrin-mediated mechanotransduction and growth factors (Review). Biointerphases 2018, 13, 06d302. [Google Scholar] [CrossRef]
- Poręba, R.; de los Santos Pereira, A.; Pola, R.; Jiang, S.; Pop-Georgievski, O.; Sedláková, Z.; Schönherr, H. “Clickable” and Antifouling Block Copolymer Brushes as a Versatile Platform for Peptide-Specific Cell Attachment. Macromol. Biosci. 2020, 20, 1900354. [Google Scholar] [CrossRef]
- Goor, O.J.G.M.; Brouns, J.E.P.; Dankers, P.Y.W. Introduction of anti-fouling coatings at the surface of supramolecular elastomeric materials via post-modification of reactive supramolecular additives. Polym. Chem. 2017, 8, 5228–5238. [Google Scholar] [CrossRef]
- Pape, A.C.H.; Ippel, B.D.; Dankers, P.Y.W. Cell and Protein Fouling Properties of Polymeric Mixtures Containing Supramolecular Poly(ethylene glycol) Additives. Langmuir 2017, 33, 4076–4082. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Liu, Y.; Zhang, Y.; Ren, B.; Xu, J.; Zheng, J. Synthesis and Characterization of Ultralow Fouling Poly(N-acryloyl-glycinamide) Brushes. Langmuir 2017, 33, 13964–13972. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wang, H.; Wang, Y.; Gui, T.; Wang, K.; Gao, C. Creation of antifouling microarrays by photopolymerization of zwitterionic compounds for protein assay and cell patterning. Biosens. Bioelectro. 2018, 102, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Leigh, B.L.; Cheng, E.; Xu, L.; Andresen, C.; Hansen, M.R.; Guymon, C.A. Photopolymerizable Zwitterionic Polymer Patterns Control Cell Adhesion and Guide Neural Growth. Biomacromolecules 2017, 18, 2389–2401. [Google Scholar] [CrossRef]
- Hersel, U.; Dahmen, C.; Kessler, H. RGD modified polymers: Biomaterials for stimulated cell adhesion and beyond. Biomaterials 2003, 24, 4385–4415. [Google Scholar] [CrossRef]
- Chien, H.W.; Fu, S.W.; Shih, A.Y.; Tsai, W.B. Modulation of the stemness and osteogenic differentiation of human mesenchymal stem cells by controlling RGD concentrations of poly(carboxybetaine) hydrogel. Biotechnol. J. 2014, 9, 1613–1623. [Google Scholar] [CrossRef]
- Rodda, A.E.; Ercole, F.; Glattauer, V.; Gardiner, J.; Nisbet, D.R.; Healy, K.E.; Forsythe, J.S.; Meagher, L. Low Fouling Electrospun Scaffolds with Clicked Bioactive Peptides for Specific Cell Attachment. Biomacromolecules 2015, 16, 2109–2118. [Google Scholar] [CrossRef]
- Vaisocherová-Lísalová, H.; Víšová, I.; Ermini, M.L.; Špringer, T.; Song, X.C.; Mrázek, J.; Lamačová, J.; Scott Lynn, N.; Šedivák, P.; Homola, J. Low-fouling surface plasmon resonance biosensor for multi-step detection of foodborne bacterial pathogens in complex food samples. Biosens. Bioelectron. 2016, 80, 84–90. [Google Scholar] [CrossRef]
- Vaisocherova, H.; Sipova, H.; Visova, I.; Bockova, M.; Springer, T.; Laura Ermini, M.; Song, X.; Krejcik, Z.; Chrastinova, L.; Pastva, O.; et al. Rapid and sensitive detection of multiple microRNAs in cell lysate by low-fouling surface plasmon resonance biosensor. Biosens. Bioelectron. 2015, 70, 226–231. [Google Scholar] [CrossRef]
- Víšová, I.; Smolková, B.; Uzhytchak, M.; Vrabcová, M.; Zhigunova, Y.; Houska, M.; Surman, F.; de los Santos Pereira, A.; Lunov, O.; Dejneka, A.; et al. Modulation of Living Cell Behavior with Ultra-Low Fouling Polymer Brush Interfaces. Macromol. Biosci. 2020, 20, 1900351. [Google Scholar] [CrossRef] [PubMed]
- Lokanathan, A.R.; Zhang, S.; Regina, V.R.; Cole, M.A.; Ogaki, R.; Dong, M.; Besenbacher, F.; Meyer, R.L.; Kingshott, P. Mixed poly (ethylene glycol) and oligo (ethylene glycol) layers on gold as nonfouling surfaces created by backfilling. Biointerphases 2011, 6, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Vaisocherova, H.; Brynda, E.; Homola, J. Functionalizable low-fouling coatings for label-free biosensing in complex biological media: Advances and applications. Anal. Bioanal. Chem. 2015, 407, 3927–3953. [Google Scholar] [CrossRef] [PubMed]
- Lísalová, H.; Brynda, E.; Houska, M.; Víšová, I.; Mrkvová, K.; Song, X.C.; Gedeonová, E.; Surman, F.; Riedel, T.; Pop-Georgievski, O.; et al. Ultralow-Fouling Behavior of Biorecognition Coatings Based on Carboxy-Functional Brushes of Zwitterionic Homo- and Copolymers in Blood Plasma: Functionalization Matters. Anal. Chem. 2017, 89, 3524–3531. [Google Scholar] [CrossRef]
- Smolkova, B.; Lunova, M.; Lynnyk, A.; Uzhytchak, M.; Churpita, O.; Jirsa, M.; Kubinova, S.; Lunov, O.; Dejneka, A. Non-Thermal Plasma, as a New Physicochemical Source, to Induce Redox Imbalance and Subsequent Cell Death in Liver Cancer Cell Lines. Cell. Physiol. Biochem. 2019, 52, 119–140. [Google Scholar] [CrossRef]
- March, S.; Ramanan, V.; Trehan, K.; Ng, S.; Galstian, A.; Gural, N.; Scull, M.A.; Shlomai, A.; Mota, M.M.; Fleming, H.E.; et al. Micropatterned coculture of primary human hepatocytes and supportive cells for the study of hepatotropic pathogens. Nat. Protoc. 2015, 10, 2027–2053. [Google Scholar] [CrossRef]
- Jelinek, M.; Kocourek, T.; Jurek, K.; Jelinek, M.; Smolková, B.; Uzhytchak, M.; Lunov, O. Preliminary Study of Ge-DLC Nanocomposite Biomaterials Prepared by Laser Codeposition. Nanomaterials 2019, 9, 451. [Google Scholar] [CrossRef]
- Heggestad, J.T.; Fontes, C.M.; Joh, D.Y.; Hucknall, A.M.; Chilkoti, A. In Pursuit of Zero 2.0: Recent Developments in Nonfouling Polymer Brushes for Immunoassays. Adv. Mater. 2020, 32, 1903285. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, G.; Hein, R.; Liu, N.; Luo, X.; Davis, J.J. Antifouling Strategies for Selective In Vitro and In Vivo Sensing. Chem. Rev. 2020, 120, 3852–3889. [Google Scholar] [CrossRef]
- Tocce, E.J.; Broderick, A.H.; Murphy, K.C.; Liliensiek, S.J.; Murphy, C.J.; Lynn, D.M.; Nealey, P.F. Functionalization of reactive polymer multilayers with RGD and an antifouling motif: RGD density provides control over human corneal epithelial cell-substrate interactions. J. Biomed. Mater. Res. Part A 2012, 100, 84–93. [Google Scholar] [CrossRef]
- Yu, S.; Zuo, X.; Shen, T.; Duan, Y.; Mao, Z.; Gao, C. A density gradient of VAPG peptides on a cell-resisting surface achieves selective adhesion and directional migration of smooth muscle cells over fibroblasts. Acta Biomater. 2018, 72, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Vaisocherová-Lísalová, H.; Surman, F.; Víšová, I.; Vala, M.; Špringer, T.; Ermini, M.L.; Šípová, H.; Šedivák, P.; Houska, M.; Riedel, T.; et al. Copolymer Brush-Based Ultralow-Fouling Biorecognition Surface Platform for Food Safety. Anal. Chem. 2016, 88, 10533–10539. [Google Scholar] [CrossRef]
- Vaisocherova, H.; Sevcu, V.; Adam, P.; Spackova, B.; Hegnerova, K.; Pereira, A.D.; Rodriguez-Emmenegger, C.; Riedel, T.; Houska, M.; Brynda, E.; et al. Functionalized ultra-low fouling carboxy- and hydroxy-functional surface platforms: Functionalization capacity, biorecognition capability and resistance to fouling from undiluted biological media. Biosens. Bioelectron. 2014, 51, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Brault, N.D.; White, A.D.; Taylor, A.D.; Yu, Q.; Jiang, S. Directly Functionalizable Surface Platform for Protein Arrays in Undiluted Human Blood Plasma. Anal. Chem. 2013, 85, 1447–1453. [Google Scholar] [CrossRef]
- Low, B.C.; Pan, C.Q.; Shivashankar, G.V.; Bershadsky, A.; Sudol, M.; Sheetz, M. YAP/TAZ as mechanosensors and mechanotransducers in regulating organ size and tumor growth. FEBS Lett. 2014, 588, 2663–2670. [Google Scholar] [CrossRef] [PubMed]
- Dupont, S.; Morsut, L.; Aragona, M.; Enzo, E.; Giulitti, S.; Cordenonsi, M.; Zanconato, F.; Le Digabel, J.; Forcato, M.; Bicciato, S.; et al. Role of YAP/TAZ in mechanotransduction. Nature 2011, 474, 179–183. [Google Scholar] [CrossRef]
- Lin, X.; Jain, P.; Wu, K.; Hong, D.; Hung, H.-C.; O’Kelly, M.B.; Li, B.; Zhang, P.; Yuan, Z.; Jiang, S. Ultralow Fouling and Functionalizable Surface Chemistry Based on Zwitterionic Carboxybetaine Random Copolymers. Langmuir 2019, 35, 1544–1551. [Google Scholar] [CrossRef]
- Abercrombie, M. Contact inhibition and malignancy. Nature 1979, 281, 259–262. [Google Scholar] [CrossRef]
- Flammang, P.; Santos, R.; Aldred, N.; Gorb, S. (Eds.) Biological and Biomimetic Adhesives: Challenges and Opportunities; Royal Society of Chemistry: London, UK, 2013. [Google Scholar]
- Kolahi, K.S.; Donjacour, A.; Liu, X.; Lin, W.; Simbulan, R.K.; Bloise, E.; Maltepe, E.; Rinaudo, E. Effect of Substrate Stiffness on Early Mouse Embryo Development. PLoS ONE 2012, 7, e41717. [Google Scholar] [CrossRef]
- Durán-Pastén, M.L.; Cortes, D.; Valencia-Amaya, A.E.; King, S.; González-Gómez, G.H.; Hautefeuille, M. Cell Culture Platforms with Controllable Stiffness for Chick Embryonic Cardiomyocytes. Biomimetics 2019, 4, 33. [Google Scholar] [CrossRef]
- Moroishi, T.; Hansen, C.G.; Guan, K.L. The emerging roles of YAP and TAZ in cancer. Nat. Rev. Cancer 2015, 15, 73–79. [Google Scholar] [CrossRef]
- Víšová, I.; Vrabcová, M.; Forinová, M.; Zhignuova, Y.; Mironov, V.; Houska, M.; Bittrich, E.; Eichhorn, K.-J.; Hashim, H.; Schovanek, P.; et al. Surface Preconditioning Influences the Antifouling Capabilities of Zwitterionic and Nonionic Polymer Brushes. Langmuir 2020, in press. [Google Scholar] [CrossRef]
- Vaisocherová, H.; Zhang, Z.; Yang, W.; Cao, Z.; Cheng, G.; Taylor, A.D.; Piliarik, M.; Homola, J.; Jiang, S. Functionalizable surface platform with reduced nonspecific protein adsorption from full blood plasma-Material selection and protein immobilization optimization. Biosens. Bioelectron. 2008, 24, 1924–1930. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Cao, Z. Ultralow-Fouling, Functionalizable, and Hydrolyzable Zwitterionic Materials and Their Derivatives for Biological Applications. Adv. Mater. 2010, 22, 920–932. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Yuan, Z.; He, Y.; Hung, H.-C.; Jiang, S. Zwitterionic Nanoconjugate Enables Safe and Efficient Lymphatic Drug Delivery. Nano Lett. 2020, 20, 4693–4699. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Yuan, Z.; Jain, P.; Hung, H.-C.; He, Y.; Lin, X.; McMullen, P.; Jiang, S. De Novo Design of Functional Zwitterionic Biomimetic Material for Immunomodulation. Sci. Adv. 2020, 6, eaba0754. [Google Scholar] [CrossRef]
- Lin, X.; Boit, M.O.; Wu, K.; Jain, P.; Liu, E.J.; Hsieh, Y.-F.; Zhou, O.; Li, B.; Hung, H.-C.; Jiang, S. Zwitterionic carboxybetaine polymers extend the shelf-life of human platelets. Acta Biomater. 2020, 109, 51–60. [Google Scholar] [CrossRef]

| RGD Peptide Concentrations Used for Immobilization (µg/mL) | Fouling on pCBAA (ng/cm2) | RGD Peptide Concentrations Used for Immobilization (µg/mL) | Fouling on OEG-Based SAM (ng/cm2) |
|---|---|---|---|
| 0 | Undetectable 1 | 0 | 20.1 |
| 1 | Undetectable 1 | 0.1 | 32.7 |
| 50 | Undetectable 1 | 1 | 25.0 |
| 500 | Undetectable 1 | 50 | 11.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Víšová, I.; Smolková, B.; Uzhytchak, M.; Vrabcová, M.; Chafai, D.E.; Houska, M.; Pastucha, M.; Skládal, P.; Farka, Z.; Dejneka, A.; et al. Functionalizable Antifouling Coatings as Tunable Platforms for the Stress-Driven Manipulation of Living Cell Machinery. Biomolecules 2020, 10, 1146. https://doi.org/10.3390/biom10081146
Víšová I, Smolková B, Uzhytchak M, Vrabcová M, Chafai DE, Houska M, Pastucha M, Skládal P, Farka Z, Dejneka A, et al. Functionalizable Antifouling Coatings as Tunable Platforms for the Stress-Driven Manipulation of Living Cell Machinery. Biomolecules. 2020; 10(8):1146. https://doi.org/10.3390/biom10081146
Chicago/Turabian StyleVíšová, Ivana, Barbora Smolková, Mariia Uzhytchak, Markéta Vrabcová, Djamel Eddine Chafai, Milan Houska, Matěj Pastucha, Petr Skládal, Zdeněk Farka, Alexandr Dejneka, and et al. 2020. "Functionalizable Antifouling Coatings as Tunable Platforms for the Stress-Driven Manipulation of Living Cell Machinery" Biomolecules 10, no. 8: 1146. https://doi.org/10.3390/biom10081146
APA StyleVíšová, I., Smolková, B., Uzhytchak, M., Vrabcová, M., Chafai, D. E., Houska, M., Pastucha, M., Skládal, P., Farka, Z., Dejneka, A., & Vaisocherová-Lísalová, H. (2020). Functionalizable Antifouling Coatings as Tunable Platforms for the Stress-Driven Manipulation of Living Cell Machinery. Biomolecules, 10(8), 1146. https://doi.org/10.3390/biom10081146