Recent Advances in the Biosynthesis of Carbazoles Produced by Actinomycetes
Abstract
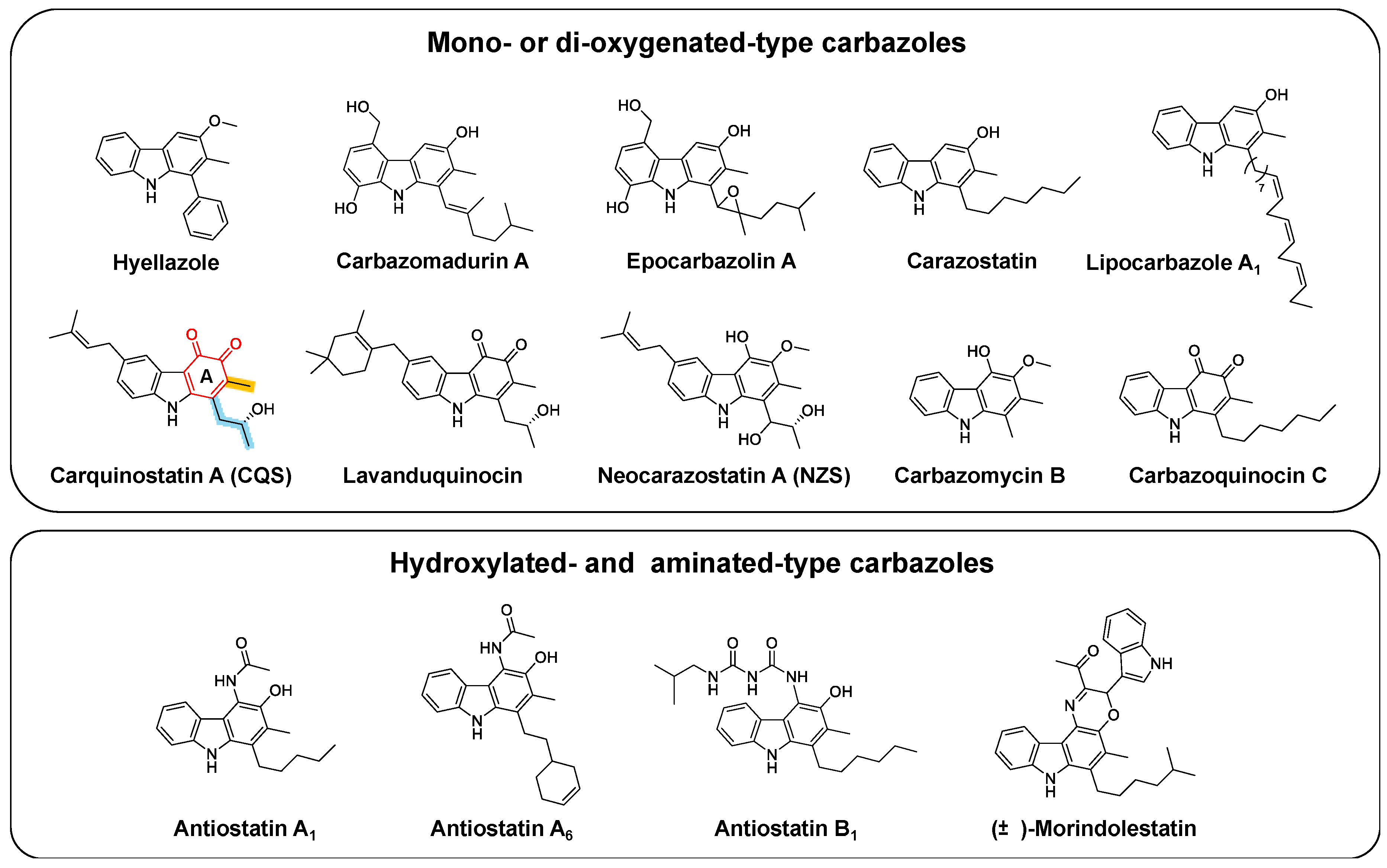
1. Introduction
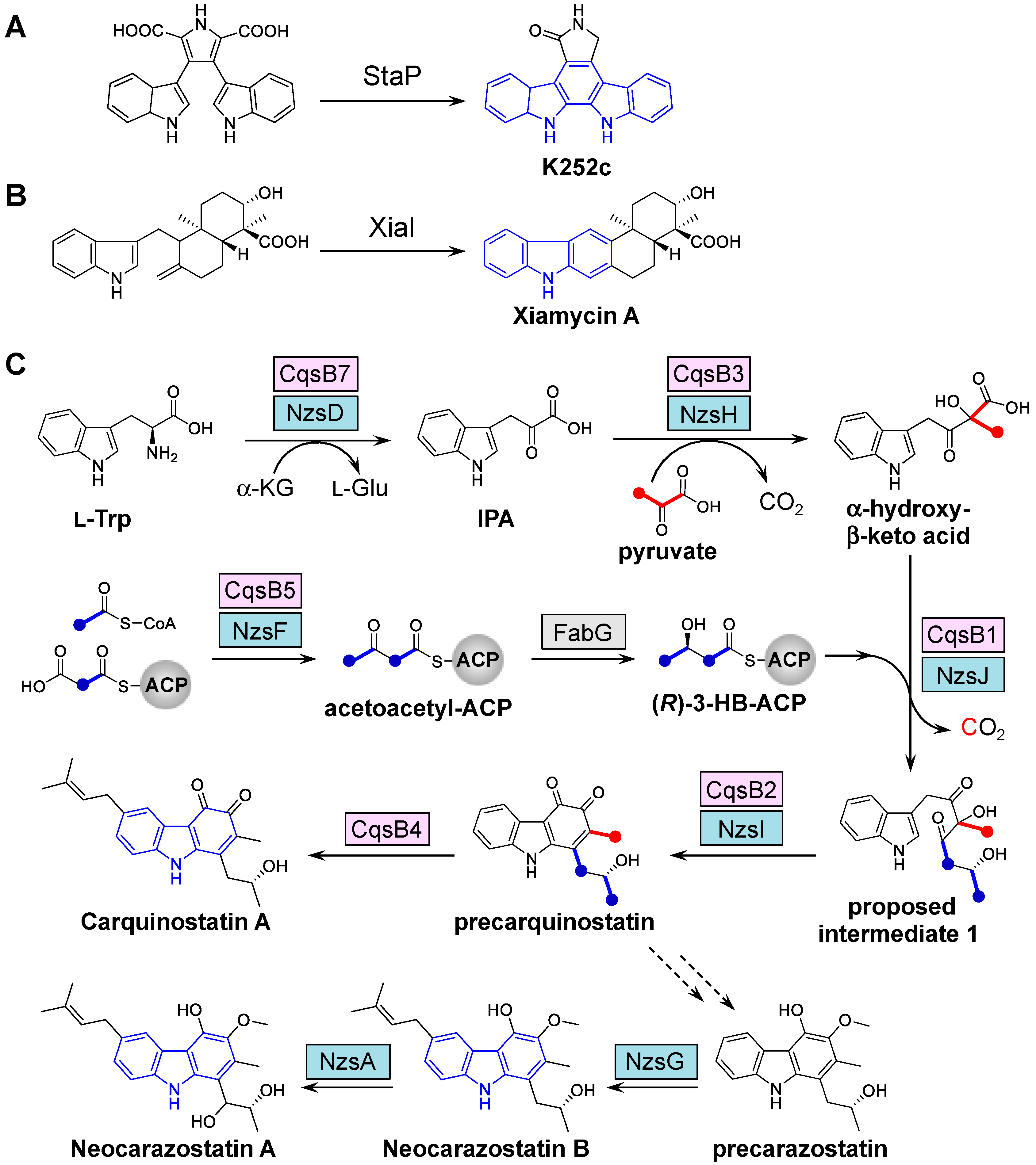
2. Elucidation of the Carbazole Biosynthetic Pathway
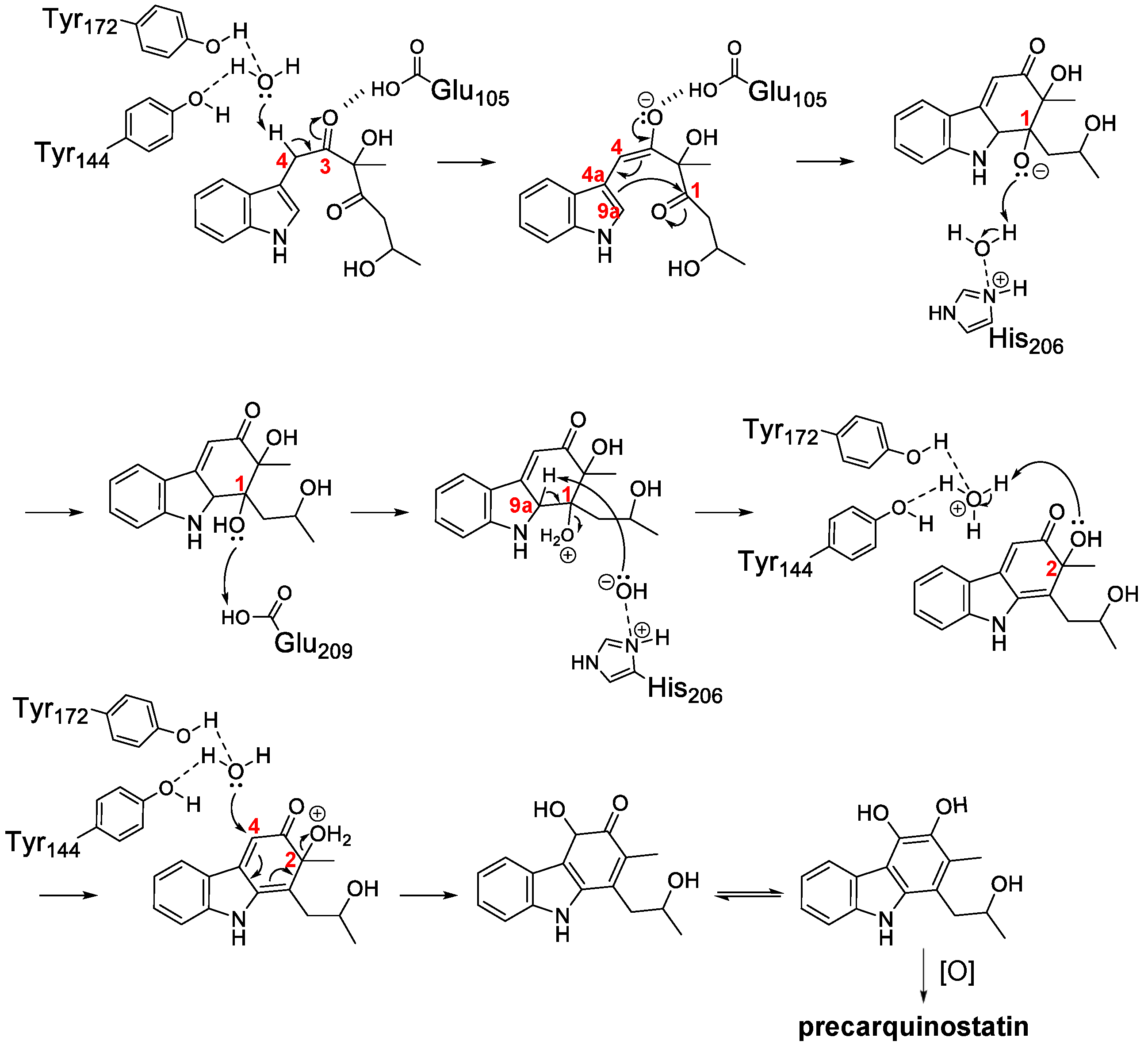
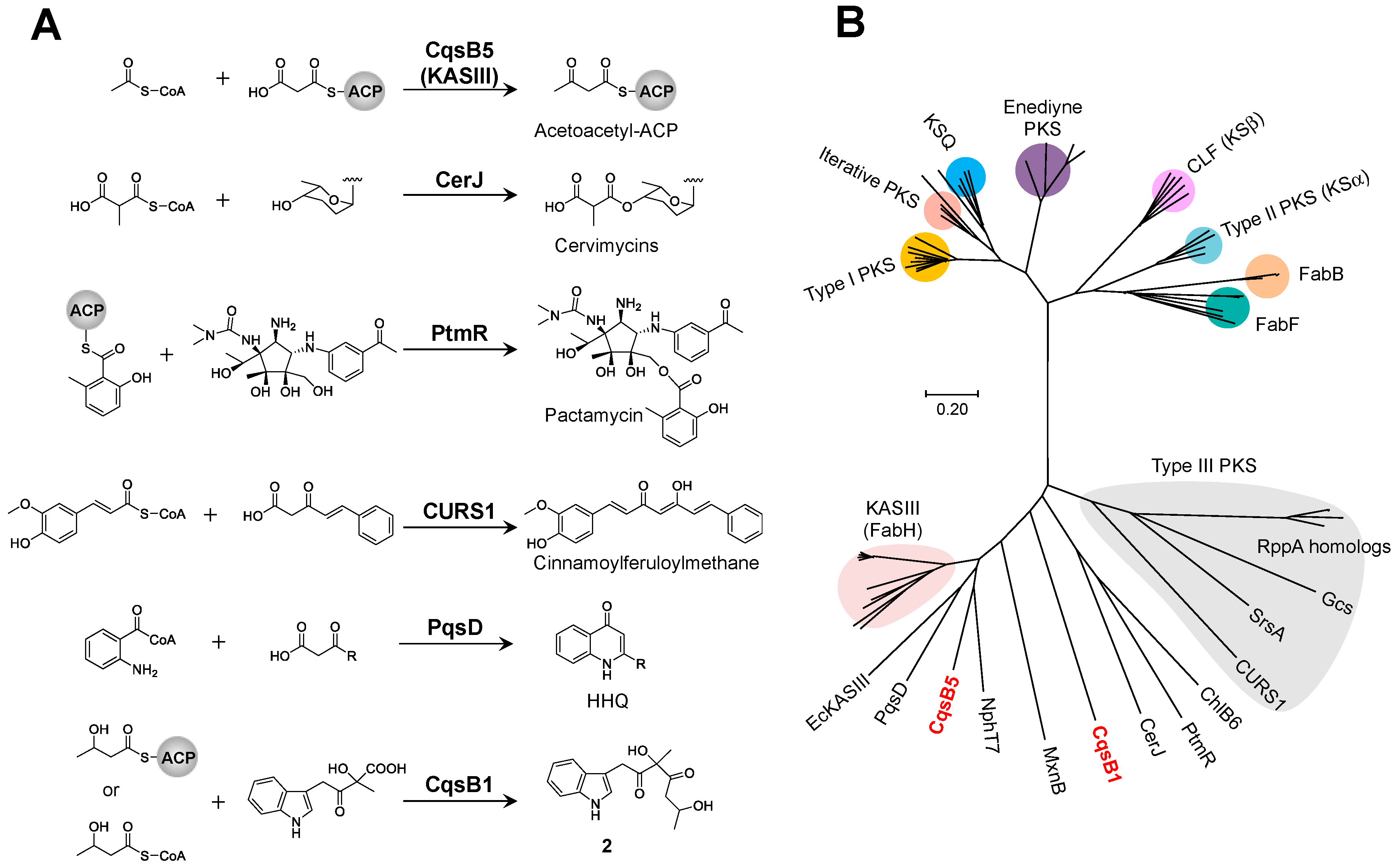
2.1. Discovery of the Carbazole Synthase CqsB2
2.2. Reconstitution of Carbazole Backbone
3. Enzymatic Modification of the Carbazole Skeleton
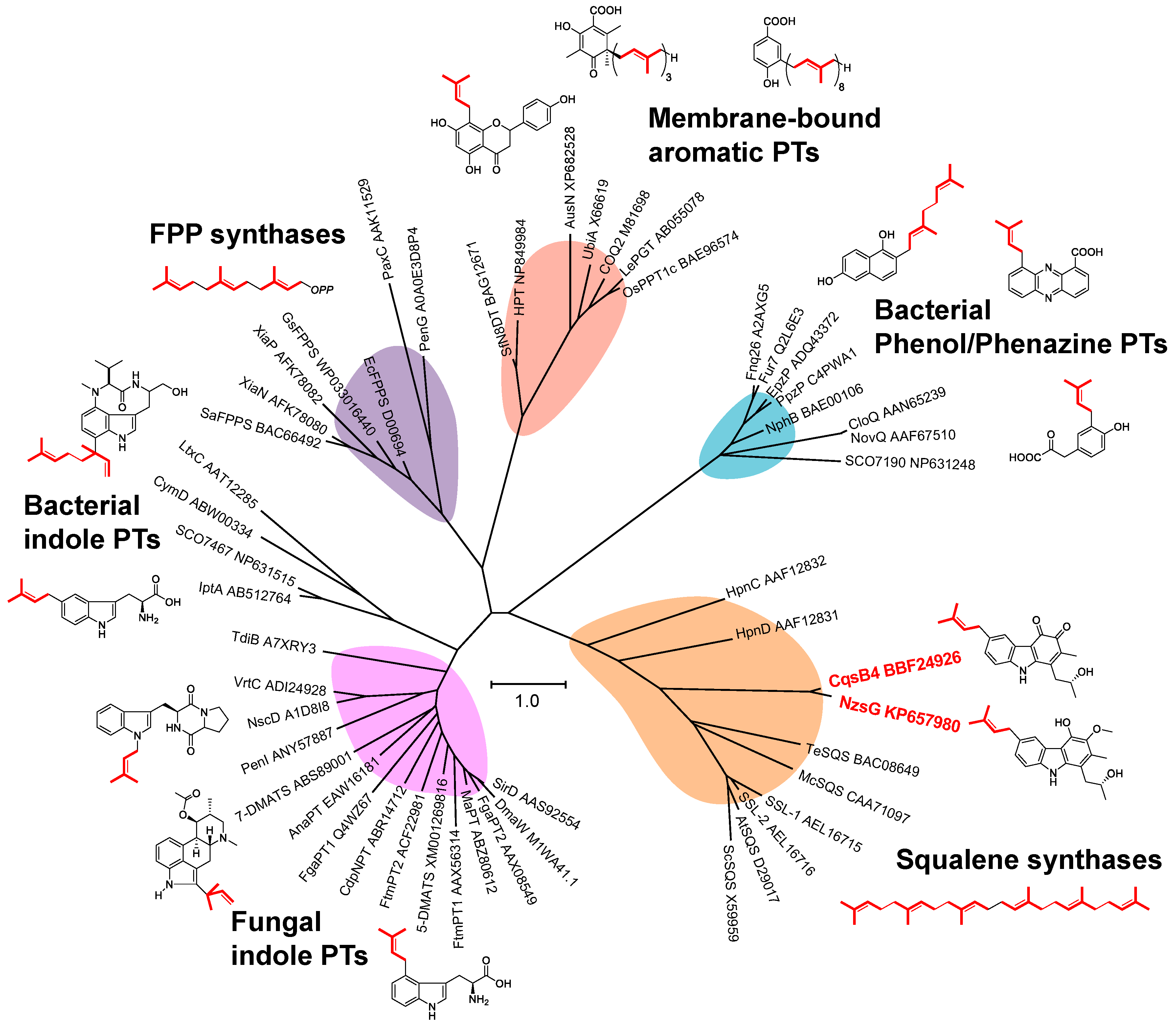
3.1. New-Type Carbazole Prenyltransferase
3.2. Isopentenyl Diphosphate Isomerase
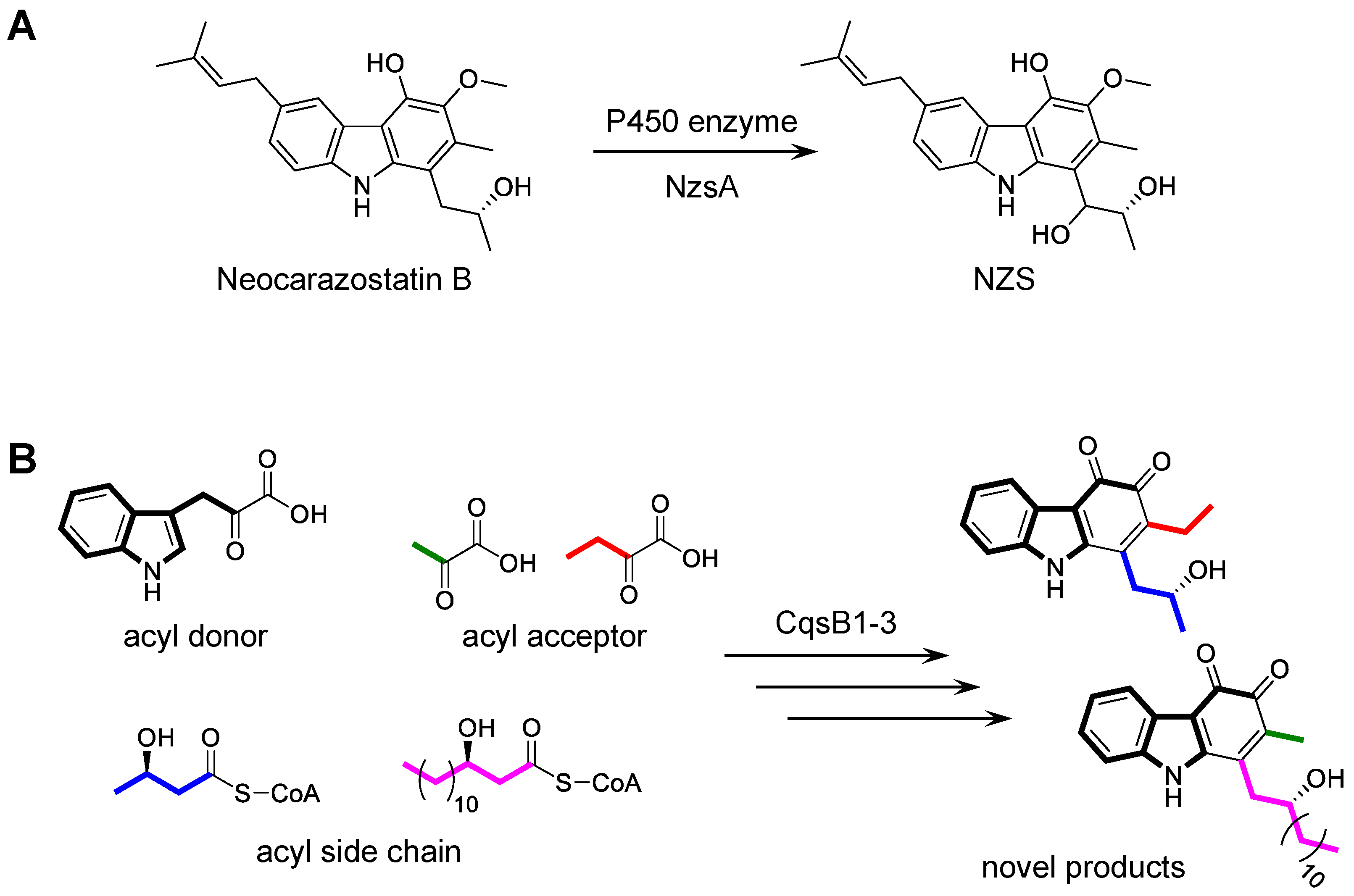
3.3. Biotransformation of Carbazole Derivatives
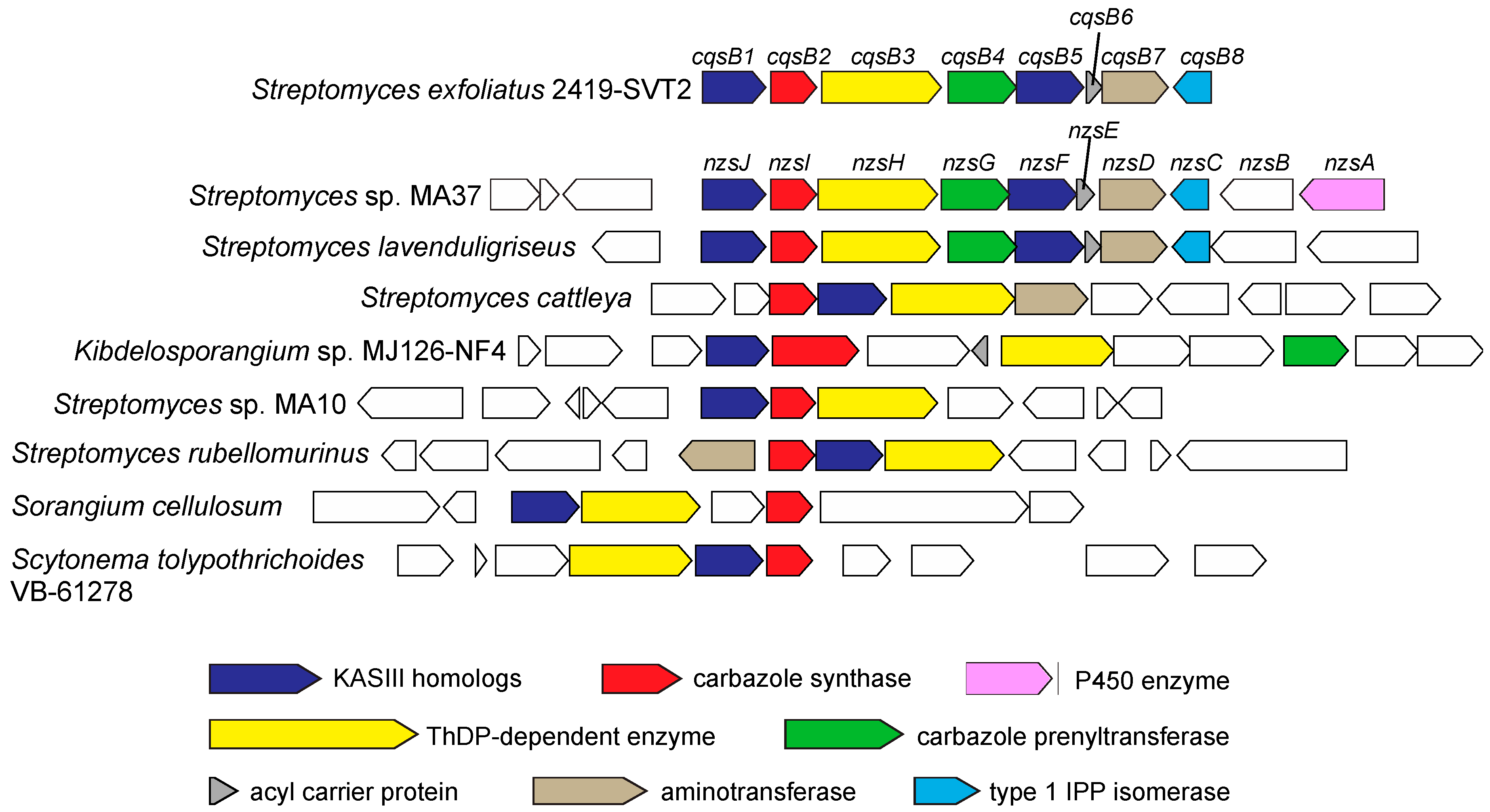
4. Conserved Gene Clusters Distributed in Bacteria
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schmidt, A.W.; Reddy, K.R.; Knölker, H.J. Occurrence, Biogenesis, and Synthesis of Biologically Active Carbazole Alkaloids. Chem. Rev. 2012, 112, 3193–3328. [Google Scholar] [CrossRef] [PubMed]
- Alkhalaf, L.M.; Ryan, K.S. Biosynthetic Manipulation of Tryptophan in Bacteria: Pathways and Mechanisms. Chem. Boil. 2015, 22, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Cardellina, J.H.; Kirkup, M.P.; Moore, R.E.; Mynderse, J.S.; Seff, K.; Simmons, C.J. Hyellazone and chlorohyellazole, two novel carbazoles from the blue-green alga Hyella caespitosa born. et flah. Tetrahedron Lett. 1979, 20, 4915–4916. [Google Scholar] [CrossRef]
- Kotoda, N.; Shin-ya, K.; Furihata, K.; Hayakawa, Y.; Seto, H. Isolation and structure elucidation of novel neuronal cell protecting substances, carbazomadurins A and B produced by Actinomadura madurae. J. Antibiot. 1997, 50, 770–772. [Google Scholar] [CrossRef][Green Version]
- Nihei, Y.; Yamamoto, H.; Hasegawa, M.; Hanada, M.; Fukagawa, Y.; Oki, T. Epocarbazolins a and b, novel 5-lipoxygenase inhibitors. J. Antibiot. 1993, 46, 25–33. [Google Scholar] [CrossRef]
- Kato, S.; Kawai, H.; Kawasaki, T.; Toda, Y.; Urata, T.; Hayakawa, Y. Studies on free radical scavenging substances from microorganisms. I. Carazostatin, a new free radical scavenger produced by Streptomyces chromofuscus DC 118. J. Antibiot. 1989, 42, 1879–1881. [Google Scholar] [CrossRef]
- Kathrin, S.; Nachtigall, J.; Hänchen, A.; Nicholson, G.; Goodfellow, M.; Süssmuth, R.D.; Fiedler, H.P. Lipocarbazoles, Secondary Metabolites from Tsukamurella pseudospumae Acta 1857 with Antioxidative Activity. J. Nat. Prod. 2009, 72, 1768–1772. [Google Scholar] [CrossRef]
- Mo, C.J.; Shin-ya, K.; Furihata, K.; Shimazu, A.; Hayakawa, Y.; Seto, H.; Furihata, K.; Furihata, K. Isolation and structural elucidation of antioxidative agents, antiostatins A1 to A4 and B2 to B5. J. Antibiot. 1990, 43, 1337–1340. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Zhan, Z.J.; Zhang, H.; Qi, H.; Zhang, L.Q.; Chen, S.X.; Gan, L.S.; Wang, J.D.; Ma, L.F. Morindolestatin, Naturally Occurring Dehydromorpholinocarbazole Alkaloid from Soil-Derived Bacterium of the Genus Streptomyces. Org. Lett. 2020, 22, 1113–1116. [Google Scholar] [CrossRef]
- Shin-ya, K.; Tanaka, M.; Furihata, K.; Hayakawa, Y.; Seto, H. Structure of carquinostatin a, a new neuronal cell protecting substance produced by Streptomyces exfoliatus. Tetrahedron Lett. 1993, 34, 4943–4944. [Google Scholar] [CrossRef]
- Shin-ya, K.; Kunigami, T.; Kim, J.S.; Furihata, K.; Hayakawa, Y.; Seto, H. Carquinostatin B, a New Neuronal Cell-protecting Substance Produced by Streptomyces exfoliatus. Biosci. Biotechnol. Biochem. 1997, 61, 1768–1769. [Google Scholar] [CrossRef] [PubMed]
- Grammel, H.; Wolf, H.; Gilles, E.D.; Huth, F.; Laatsch, H. Carbazole antibiotics synthesis in a Streptomyces tendae bald mutant, created by acriflavine treatment. Z. Nat. C 1998, 53, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Shin-ya, K.; Shimizu, S.; Kunigami, T.; Furihata, K.; Furihata, K.; Seto, H. A New Neuronal Cell Protecting Substance, Lavanduquinocin, Produced by Streptomyces viridochromogenes. J. Antibiot. 1995, 48, 574–578. [Google Scholar] [CrossRef]
- Kato, S.; Shindo, K.; Kataoka, Y.; Yamagishi, Y.; Mochizuki, J. Studies on free radical scavenging substances from microorganisms. II. Neocarazostatins A, B and C, novel free radical scavengers. J. Antibiot. 1991, 44, 903–907. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Shin-ya, K.; Furihata, K.; Seto, H. Isolation and Structural Elucidation of Antioxidative Substances, Carbazoquinocins A to F. J. Antibiot. 1995, 48, 326–328. [Google Scholar] [CrossRef]
- Sakano, K.; Nakamura, S. New antibiotics, carbazomycins A and B. II. Structural elucidation. J. Antibiot. 1980, 33, 961–966. [Google Scholar] [CrossRef]
- Naid, T.; Kitahara, T.; Kaneda, M.; Nakamura, S. Carbazomycins C, D, E and F, minor components of the carbazomycin complex. J. Antibiot. 1987, 40, 157–164. [Google Scholar] [CrossRef]
- Kaneda, M.; Naid, T.; Kitahara, T.; Nakamura, S.; Hirata, T.; Suga, T. Carbazomycins G and H, novel carbazomycin congeners containing a quinol moiety. J. Antibiot. 1988, 41, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Intaraudom, C.; Rachtawee, P.; Suvannakad, R.; Pittayakhajonwut, P. Antimalarial and antituberculosis substances from Streptomyces sp. BCC26924. Tetrahedron 2011, 67, 7593–7597. [Google Scholar] [CrossRef]
- Hammond, B.; Kontos, H.A.; Hess, M.L. Oxygen radicals in the adult respiratory distress syndrome, in myocardial ischemia and reperfusion injury, and in cerebral vascular damage. Can. J. Physiol. Pharmacol. 1985, 63, 173–187. [Google Scholar] [CrossRef]
- Cerutti, P. Prooxidant states and tumor promotion. Science 1985, 227, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Writing Group; Edaravone (MCI-186) ALS 19 Study Group. Safety and efficacy of edaravone in well defined patients with amyotrophic lateral sclerosis: A randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2017, 16, 505–512. [Google Scholar] [CrossRef]
- Makino, M.; Sugimoto, H.; Shiro, Y.; Asamizu, S.; Onaka, H.; Nagano, S. Crystal structures and catalytic mechanism of cytochrome P450 StaP that produces the indolocarbazole skeleton. Proc. Natl. Acad. Sci. USA 2007, 104, 11591–11596. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, Q.; Li, S.; Zhu, Y.; Zhang, G.; Zhang, H.; Tian, X.; Zhang, S.; Ju, J.; Zhang, C. Identification and Characterization of Xiamycin A and Oxiamycin Gene Cluster Reveals an Oxidative Cyclization Strategy Tailoring Indolosesquiterpene Biosynthesis. J. Am. Chem. Soc. 2012, 134, 8996–9005. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Baunach, M.; Ding, L.; Hertweck, C. Bacterial Synthesis of Diverse Indole Terpene Alkaloids by an Unparalleled Cyclization Sequence†. Angew. Chem. Int. Ed. 2012, 51, 10293–10297. [Google Scholar] [CrossRef]
- Yamasaki, K.; Kaneda, M.; Watanabe, K.; Ueki, Y.; Ishimaru, K.; Nakamura, S.; Nomi, R.; Yoshida, N.; Nakajima, T. New antibiotics, carbazomycins A and B. III. Taxonomy and biosynthesis. J. Antibiot. 1983, 36, 552–558. [Google Scholar] [CrossRef]
- Kaneda, M.; Kitahara, T.; Yamasaki, K.; Nakamura, S. Biosynthesis of carbazomycin B. II. Origin of the whole carbon skeleton. J. Antibiot. 1990, 43, 1623–1626. [Google Scholar] [CrossRef]
- Orihara, N.; Furihata, K.; Seto, H. Studies on the biosynthesis of terpenoidal compounds produced by actinomycetes. 2. Biosynthesis of carquinostatin B via the non-mevalonate pathway in Streptomyces exfoliatus. J. Antibiot. 1997, 50, 979–981. [Google Scholar] [CrossRef][Green Version]
- Huang, S.; Elsayed, S.S.; Lv, M.; Tabudravu, J.; Rateb, M.E.; Gyampoh, R.; Kyeremeh, K.; Ebel, R.; Jaspars, M.; Deng, Z.; et al. Biosynthesis of Neocarazostatin A Reveals the Sequential Carbazole Prenylation and Hydroxylation in the Tailoring Steps. Chem. Biol. 2015, 22, 1633–1642. [Google Scholar] [CrossRef]
- Su, L.; Lv, M.; Kyeremeh, K.; Deng, Z.; Deng, H.; Yu, Y. A ThDP-dependent enzymatic carboligation reaction involved in Neocarazostatin A tricyclic carbazole formation. Org. Biomol. Chem. 2016, 14, 8679–8684. [Google Scholar] [CrossRef]
- Su, L.; Zhang, R.; Kyeremeh, K.; Deng, Z.; Deng, H.; Yu, Y. Dissection of the neocarazostatin: A C 4 alkyl side chain biosynthesis by in vitro reconstitution. Org. Biomol. Chem. 2017, 15, 3843–3848. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Tomita, T.; Shin-ya, K.; Nishiyama, M.; Kuzuyama, T. An Unprecedented Cyclization Mechanism in the Biosynthesis of Carbazole Alkaloids in Streptomyces. Angew. Chem. Int. Ed. 2019, 58, 13349–13353. [Google Scholar] [CrossRef] [PubMed]
- Ames, B.D.; Korman, T.P.; Zhang, W.; Smith, P.; Vu, T.; Tang, Y.; Tsai, S.C. Crystal structure and functional analysis of tetracenomycin ARO/CYC: Implications for cyclization specificity of aromatic polyketides. Proc. Natl. Acad. Sci. USA 2008, 105, 5349–5354. [Google Scholar] [CrossRef]
- Lee, M.Y.; Ames, B.D.; Tsai, S.C. Insight into the Molecular Basis of Aromatic Polyketide Cyclization: Crystal Structure and in Vitro Characterization of WhiE-ORFVI. Biochemistry 2012, 51, 3079–3091. [Google Scholar] [CrossRef] [PubMed]
- Radauer, C.; Lackner, P.; Breiteneder, H. The Bet v 1 fold: An ancient, versatile scaffold for binding of large, hydrophobic ligands. BMC Evol. Boil. 2008, 8, 286. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Su, L.; Fang, Q.; Tabudravu, J.N.; Yang, X.; Rickaby, K.; Trembleau, L.; Kyeremeh, K.; Deng, Z.; Deng, H.; et al. Enzymatic Reconstitution and Biosynthetic Investigation of the Bacterial Carbazole Neocarazostatin A. J. Org. Chem. 2019, 84, 16323–16328. [Google Scholar] [CrossRef]
- Proteau, P.J.; Li, Y.; Chen, J.; Williamson, R.T.; Gould, S.J.; Laufer, R.S.; Dmitrienko, G.I. Isoprekinamycin Is a Diazobenzo[a]fluorene Rather than a Diazobenzo[b]fluorene. J. Am. Chem. Soc. 2000, 122, 8325–8326. [Google Scholar] [CrossRef]
- Hertweck, C.; Luzhetskyy, A.; Rebets, Y.; Bechthold, A. Type II polyketide synthases: Gaining a deeper insight into enzymatic teamwork. Nat. Prod. Rep. 2007, 24, 162–190. [Google Scholar] [CrossRef]
- Shen, Y.; Yoon, P.; Yu, T.W.; Floss, H.G.; Hopwood, D.; Moore, B.S. Ectopic expression of the minimal whiE polyketide synthase generates a library of aromatic polyketides of diverse sizes and shapes. Proc. Natl. Acad. Sci. USA 1999, 96, 3622–3627. [Google Scholar] [CrossRef]
- Sun, Y.; Hahn, F.; Demydchuk, Y.; Chettle, J.; Tosin, M.; Osada, H.; Leadlay, P.F. In vitro reconstruction of tetronate RK-682 biosynthesis. Nat. Methods 2009, 6, 99–101. [Google Scholar] [CrossRef]
- Pistorius, D.; Ullrich, A.; Lucas, S.; Hartmann, R.W.; Kazmaier, U.; Müller, R. Biosynthesis of 2-Alkyl-4(1H)-Quinolones in Pseudomonas aeruginosa: Potential for Therapeutic Interference with Pathogenicity. ChemBioChem 2011, 12, 850–853. [Google Scholar] [CrossRef] [PubMed]
- Katsuyama, Y.; Miyazono, K.I.; Tanokura, M.; Ohnishi, Y.; Horinouchi, S. Structural and Biochemical Elucidation of Mechanism for Decarboxylative Condensation of β-Keto Acid by Curcumin Synthase. J. Boil. Chem. 2010, 286, 6659–6668. [Google Scholar] [CrossRef] [PubMed]
- Bretschneider, T.; Zocher, G.; Unger, M.; Scherlach, K.; Stehle, T.; Hertweck, C. A ketosynthase homolog uses malonyl units to form esters in cervimycin biosynthesis. Nat. Methods 2011, 8, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Abugrain, M.E.; Brumsted, C.J.; Osborn, A.R.; Philmus, B.; Mahmud, T. A Highly Promiscuous ß-Ketoacyl-ACP Synthase (KAS) III-like Protein Is Involved in Pactamycin Biosynthesis. ACS Chem. Boil. 2017, 12, 362–366. [Google Scholar] [CrossRef]
- Rock, C.O.; Cronan, J.E. Escherichia coli as a model for the regulation of dissociable (type II) fatty acid biosynthesis. Biochim. Biophys. Acta (BBA) Lipids Lipid Metab. 1996, 1302, 1–16. [Google Scholar] [CrossRef]
- Willadsen, P.; Eggerer, H. Substrate Stereochemistry of the Enoyl-CoA Hydratase Reaction. JBIC J. Boil. Inorg. Chem. 1975, 54, 247–252. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Tello, M.; Kuzuyama, T.; Heide, L.E.; Noel, J.P.; Richard, S.B. The ABBA family of aromatic prenyltransferases: Broadening natural product diversity. Cell. Mol. Life Sci. 2008, 65, 1459–1463. [Google Scholar] [CrossRef]
- Kuzuyama, T.; Noel, J.P.; Richard, S.B. Structural basis for the promiscuous biosynthetic prenylation of aromatic natural products. Nature 2005, 435, 983–987. [Google Scholar] [CrossRef]
- Metzger, U.; Schall, C.; Zocher, G.; Unsöld, I.; Stec, E.; Li, S.M.; Heide, L.E.; Stehle, T. The structure of dimethylallyl tryptophan synthase reveals a common architecture of aromatic prenyltransferases in fungi and bacteria. Proc. Natl. Acad. Sci. USA 2009, 106, 14309–14314. [Google Scholar] [CrossRef]
- Roose, B.W.; Christianson, D.W. Structural Basis of Tryptophan Reverse N-Prenylation Catalyzed by CymD. Biochemistry 2019, 58, 3232–3242. [Google Scholar] [CrossRef] [PubMed]
- Yazaki, K.; Kunihisa, M.; Fujisaki, T.; Sato, F. Geranyl Diphosphate:4-Hydroxybenzoate Geranyltransferase from Lithospermum erythrorhizon. J. Boil. Chem. 2001, 277, 6240–6246. [Google Scholar] [CrossRef] [PubMed]
- Melzer, M.; Heide, L.E. Characterization of Polyprenyldiphosphate: 4-Hydroxybenzoate Polyprenyltransferase from Escherichia coli. Biochim. Biophys. Acta (BBA) Lipids Lipid Metab. 1994, 1212, 93–102. [Google Scholar] [CrossRef]
- Heide, L.E. Prenyl transfer to aromatic substrates: Genetics and enzymology. Curr. Opin. Chem. Boil. 2009, 13, 171–179. [Google Scholar] [CrossRef]
- Botta, B.; Vitali, A.; Menendez, P.; Misiti, D.; Monache, G.D. Prenylated Flavonoids: Pharmacology and Biotechnology. Curr. Med. Chem. 2005, 12, 713–739. [Google Scholar] [CrossRef]
- Ramos-Valdivia, A.C.; Van Der Heijden, R.; Verpoorte, R. Isopentenyl diphosphate isomerase: A core enzyme in isoprenoid biosynthesis. A review of its biochemistry and function. Nat. Prod. Rep. 1997, 14, 591–603. [Google Scholar] [CrossRef]
- Kaneda, K.; Kuzuyama, T.; Takagi, M.; Hayakawa, Y.; Seto, H. An unusual isopentenyl diphosphate isomerase found in the mevalonate pathway gene cluster from Streptomyces sp. strain CL190. Proc. Natl. Acad. Sci. USA 2000, 98, 932–937. [Google Scholar] [CrossRef]
- Takagi, M.; Kuzuyama, T.; Takahashi, S.; Seto, H. A Gene Cluster for the Mevalonate Pathway from Streptomyces sp. Strain CL190. J. Bacteriol. 2000, 182, 4153–4157. [Google Scholar] [CrossRef]
- Kobayashi, M. Biosynthetic Studies on the Prenylated Indole Compounds Produced by Streptomyces. Ph.D. Thesis, The University of Tokyo, Tokyo, Japan, 2018. UTokyo Repository. [Google Scholar] [CrossRef]
- Choshi, T.; Uchida, Y.; Kubota, Y.; Nobuhiro, J.; Takeshita, M.; Hatano, T.; Hibino, S. Lipase-catalyzed asymmetric synthesis of desprenyl-carquinostatin A and descycloavandulyl-lavanduquinocin. Chem. Pharm. Bull. 2007, 55, 1060–1064. [Google Scholar] [CrossRef]
- Bailey, T.L.; Johnson, J.; Grant, C.E.; Noble, W.S. The MEME Suite. Nucleic Acids Res. 2015, 43, W39–W49. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobayashi, M.; Kuzuyama, T. Recent Advances in the Biosynthesis of Carbazoles Produced by Actinomycetes. Biomolecules 2020, 10, 1147. https://doi.org/10.3390/biom10081147
Kobayashi M, Kuzuyama T. Recent Advances in the Biosynthesis of Carbazoles Produced by Actinomycetes. Biomolecules. 2020; 10(8):1147. https://doi.org/10.3390/biom10081147
Chicago/Turabian StyleKobayashi, Masaya, and Tomohisa Kuzuyama. 2020. "Recent Advances in the Biosynthesis of Carbazoles Produced by Actinomycetes" Biomolecules 10, no. 8: 1147. https://doi.org/10.3390/biom10081147
APA StyleKobayashi, M., & Kuzuyama, T. (2020). Recent Advances in the Biosynthesis of Carbazoles Produced by Actinomycetes. Biomolecules, 10(8), 1147. https://doi.org/10.3390/biom10081147




