Development of Recombinant Immunotoxins for Hairy Cell Leukemia
Abstract
1. Introduction to Hairy Cell Leukemia
2. Diagnosis of HCL
3. Differentiation of HCL from Variants
4. When Treatment Is Indicated in HCL
5. Criteria for Response in HCL
6. Minimal Residual Disease (MRD)
7. Introduction to Recombinant Immunotoxins
8. Plant vs. Bacterial Toxins
9. LMB-2 Targeting CD25
10. Development of anti-CD22 Recombinant Immunotoxins for HCL
11. Construction of Recombinant Immunotoxin Moxetumomab Pasudotox
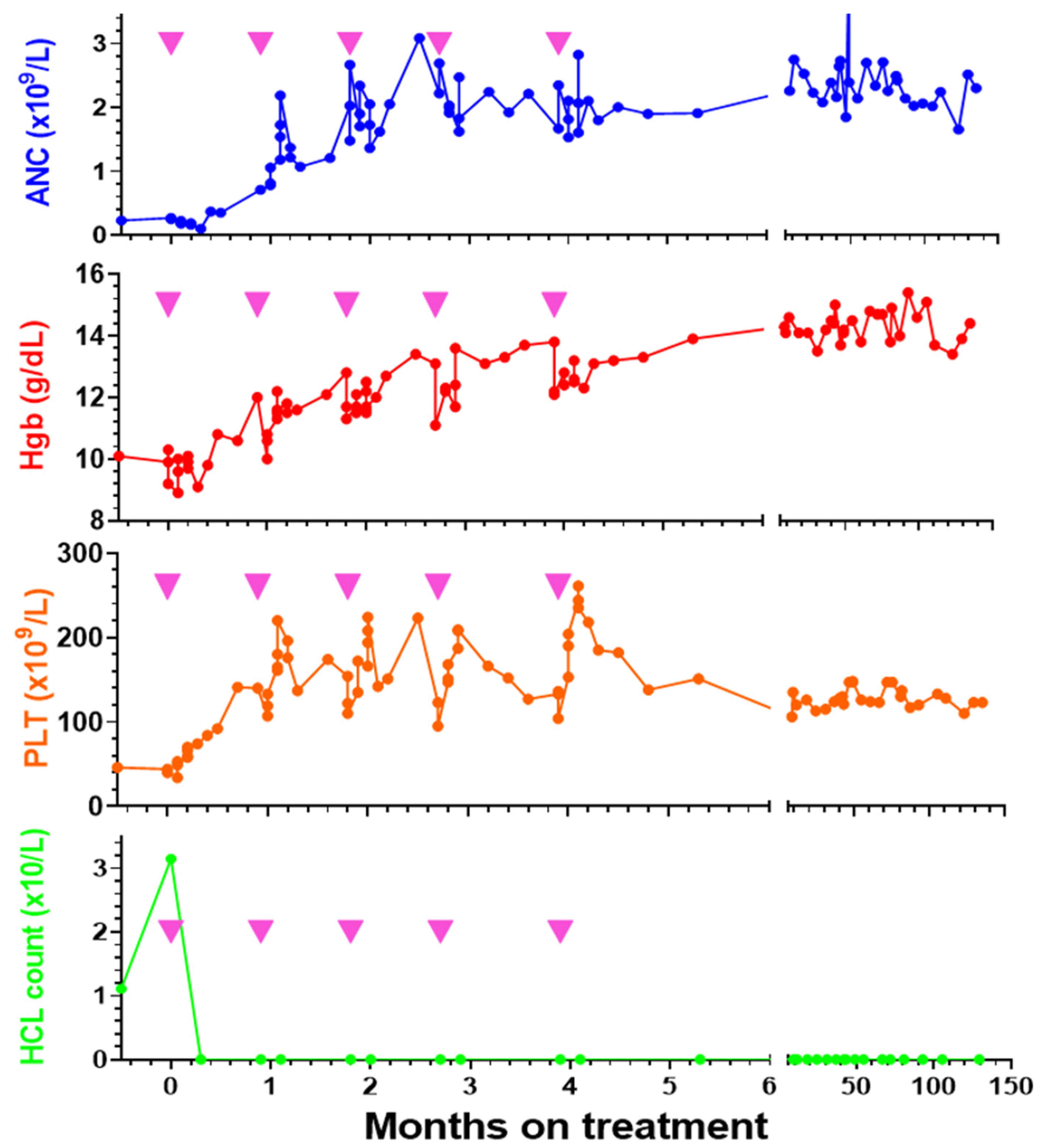
12. Moxe Phase 1 Clinical Results
13. Expansion of the Phase 1 Trial of Moxe
14. Pharmacokinetics of Moxe by Bioassay
15. Antidrug Antibodies and Moxe Pharmacokinetics
16. Pivotal Phase 3 Testing of Moxe, Trial Design
17. Moxe Phase 3 Pivotal Trial Results
18. Mechanism and Prevention of HUS and CLS with Moxe
19. ADA and Pharmacokinetics of Moxe during Phase 3 Testing
20. Further Development of Moxe for HCL
Funding
Acknowledgments
Conflicts of Interest
References
- Bouroncle, B.A.; Wiseman, B.K.; Doan, C.A. Leukemic reticuloendotheliosis. Blood 1958, 13, 609–630. [Google Scholar] [CrossRef] [PubMed]
- Bouroncle, B.A. Leukemic reticuloendotheliosis (hairy cell leukemia). Blood 1979, 53, 412–436. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Tiacci, E.; Trifonov, V.; Schiavoni, G.; Holmes, A.; Kern, W.; Martelli, M.P.; Pucciarini, A.; Bigerna, B.; Pacini, R.; Wells, V.A.; et al. BRAF mutations in hairy-cell leukemia. N. Engl. J. Med. 2011, 364, 2305–2315. [Google Scholar] [CrossRef]
- Tiacci, E.; Schiavoni, G.; Forconi, F.; Santi, A.; Trentin, L.; Ambrosetti, A.; Cecchini, D.; Sozzi, E.; Francia di Celle, P.; Di Bello, C.; et al. Simple genetic diagnosis of hairy cell leukemia by sensitive detection of the BRAF-V600E mutation. Blood 2012, 119, 192–195. [Google Scholar] [CrossRef]
- Tiacci, E.; Pettirossi, V.; Schiavoni, G.; Falini, B. Genomics of Hairy Cell Leukemia. J. Clin. Oncol. 2017, 35, 1002–1010. [Google Scholar] [CrossRef]
- Xi, L.; Arons, E.; Navarro, W.; Calvo, K.R.; Stetler-Stevenson, M.; Raffeld, M.; Kreitman, R.J. Both variant and IGHV4-34-expressing hairy cell leukemia lack the BRAF V600E mutation. Blood 2012, 119, 3330–3332. [Google Scholar] [CrossRef]
- Durham, B.H.; Getta, B.; Dietrich, S.; Taylor, J.; Won, H.; Bogenberger, J.M.; Scott, S.; Kim, E.; Chung, Y.R.; Chung, S.S.; et al. Genomic analysis of hairy cell leukemia identifies novel recurrent genetic alterations. Blood 2017, 130, 1644–1648. [Google Scholar] [CrossRef]
- Maitre, E.; Bertrand, P.; Maingonnat, C.; Viailly, P.J.; Wiber, M.; Naguib, D.; Salaun, V.; Cornet, E.; Damaj, G.; Sola, B.; et al. New generation sequencing of targeted genes in the classical and the variant form of hairy cell leukemia highlights mutations in epigenetic regulation genes. Oncotarget 2018, 9, 28866–28876. [Google Scholar] [CrossRef]
- Weston-Bell, N.J.; Tapper, W.; Gibson, J.; Bryant, D.; Moreno, Y.; John, M.; Ennis, S.; Kluin-Nelemans, H.C.; Collins, A.R.; Sahota, S.S. Exome Sequencing in Classic Hairy Cell Leukaemia Reveals Widespread Variation in Acquired Somatic Mutations between Individual Tumours Apart from the Signature BRAF V(600)E Lesion. PLoS ONE 2016, 11, e0149162. [Google Scholar] [CrossRef]
- Green-Lott, A.M.; Singaraju, R.; Liu, M.L.; Ascensao, J. Hairy Cell Leukemia and Ground Water Contamination with Industrial Solvents: A Case Report. Mil. Med. 2020. [Google Scholar] [CrossRef]
- Aristeguieta, C.; de Perio, M.A. Three cases of hairy cell leukemia in coal miners. Leuk. Lymphoma 2011, 52, 2391–2392. [Google Scholar] [CrossRef]
- Hardell, L.; Eriksson, M.; Nordstrom, M. Exposure to pesticides as risk factor for non-Hodgkin’s lymphoma and hairy cell leukemia: Pooled analysis of two Swedish case-control studies. Leuk. Lymphoma 2002, 43, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- Paydas, S. Hairy cell leukemia and bladder cancer in a patient: Relation with dye exposure and review of the literaure. Adv. Hematol. 2009, 2009, 812960. [Google Scholar] [CrossRef] [PubMed]
- Tadmor, T.; Polliack, A. Epidemiology and environmental risk in hairy cell leukemia. Best Pract. Res. Clin. Haematol. 2015, 28, 175–179. [Google Scholar] [CrossRef]
- Golomb, H.M.; Catovsky, D.; Golde, D.W. Hairy cell leukemia: A clinical review based on 71 cases. Ann. Intern. Med. 1978, 89, 677–683. [Google Scholar] [CrossRef]
- Spiers, A.S.; Moore, D.; Cassileth, P.A.; Harrington, D.P.; Cummings, F.J.; Neiman, R.S.; Bennett, J.M.; MJ, O.C. Remissions in hairy-cell leukemia with pentostatin (2′-deoxycoformycin). N. Engl. J. Med. 1987, 316, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Johnston, J.B.; Glazer, R.I.; Pugh, L.; Israels, L.G. The treatment of hairy-cell leukaemia with 2′-deoxycoformycin. Br. J. Haematol. 1986, 63, 525–534. [Google Scholar] [CrossRef]
- Grever, M.; Kopecky, K.; Foucar, M.K.; Head, D.; Bennett, J.M.; Hutchison, R.E.; Corbett, W.E.; Cassileth, P.A.; Habermann, T.; Golomb, H.; et al. Randomized comparison of pentostatin versus interferon alfa-2a in previously untreated patients with hairy cell leukemia: An intergroup study. J. Clin. Oncol. 1995, 13, 974–982. [Google Scholar] [CrossRef]
- Piro, L.D.; Carrera, C.J.; Carson, D.A.; Beutler, E. Lasting remissions in hairy-cell leukemia induced by a single infusion of 2-chlorodeoxyadenosine. N. Engl. J. Med. 1990, 322, 1117–1121. [Google Scholar] [CrossRef]
- Saven, A.; Burian, C.; Koziol, J.A.; Piro, L.D. Long-term follow-up of patients with hairy cell leukemia after cladribine treatment. Blood 1998, 92, 1918–1926. [Google Scholar] [CrossRef] [PubMed]
- Robak, T.; Blasinska-Morawiec, M.; Blonski, J.; Hellmann, A.; Halaburda, K.; Konopka, L.; Kotlarek-Haus, S.; Potoczek, S.; Hansz, J.; Dmoszynska, A.; et al. 2-chlorodeoxyadenosine (cladribine) in the treatment of hairy cell leukemia and hairy cell leukemia variant: 7-year experience in Poland. Eur. J. Haematol. 1999, 62, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Else, M.; Dearden, C.E.; Matutes, E.; Garcia-Talavera, J.; Rohatiner, A.Z.; Johnson, S.A.; O’Connor, N.T.; Haynes, A.; Osuji, N.; Forconi, F.; et al. Long-term follow-up of 233 patients with hairy cell leukaemia, treated initially with pentostatin or cladribine, at a median of 16 years from diagnosis. Br. J. Haematol. 2009, 145, 733–740. [Google Scholar] [CrossRef]
- Goodman, G.R.; Beutler, E.; Saven, A. Cladribine in the treatment of hairy-cell leukaemia. Best Pract. Res. Clin. Haematol. 2003, 16, 101–116. [Google Scholar] [CrossRef]
- Flinn, I.W.; Kopecky, K.J.; Foucar, M.K.; Head, D.; Bennett, J.M.; Hutchison, R.E.; Corbett, W.E.N.; Cassileth, P.A.; Habermann, T.; Golomb, H.; et al. Long-term results in hairy cell leukemia treated with pentostatin. Proc. Am. Soc. Clin. Oncol. 1997, 90 (Suppl. 1), 578a. [Google Scholar]
- Wierda, W.G.; Byrd, J.C.; Abramson, J.S.; Bhat, S.; Bociek, G.; Brander, D.; Brown, J.; Chanan-Khan, A.; Coutre, S.E.; Davis, R.S.; et al. Hairy Cell Leukemia, Version 2.2018, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2017, 15, 1414–1427. [Google Scholar] [CrossRef] [PubMed]
- Grever, M.R.; Abdel-Wahab, O.; Andritsos, L.A.; Banerji, V.; Barrientos, J.; Blachly, J.S.; Call, T.G.; Catovsky, D.; Dearden, C.; Demeter, J.; et al. Consensus Guidelines for the Diagnosis and Management of Patients with Classic Hairy Cell Leukemia. Blood 2017, 129, 553–560. [Google Scholar] [CrossRef]
- Seymour, J.F.; Kurzrock, R.; Freireich, E.J.; Estey, E.H. 2-chlorodeoxyadenosine induces durable remissions and prolonged suppression of CD4+ lymphocyte counts in patients with hairy cell leukemia. Blood 1994, 83, 2906–2911. [Google Scholar] [CrossRef]
- Seymour, J.F.; Talpaz, M.; Kurzrock, R. Response duration and recovery of CD4+ lymphocytes following deoxycoformycin in interferon-alpha-resistant hairy cell leukemia: 7- year follow-up. Leukemia 1997, 11, 42–47. [Google Scholar] [CrossRef][Green Version]
- Tadmor, T. Purine analog toxicity in patients with hairy cell leukemia. Leuk. Lymphoma 2011, 52 (Suppl. 2), 38–42. [Google Scholar] [CrossRef]
- Cheson, B.D.; Vena, D.A.; Foss, F.M.; Sorensen, J.M. Neurotoxicity of purine analogs: A review. J. Clin. Oncol. 1994, 12, 2216–2228. [Google Scholar] [CrossRef] [PubMed]
- Getta, B.M.; Woo, K.M.; Devlin, S.; Park, J.H.; Abdel-Wahab, O.; Saven, A.; Rai, K.; Tallman, M.S. Treatment outcomes and secondary cancer incidence in young patients with hairy cell leukaemia. Br. J. Haematol. 2016, 175, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Arons, E.; Suntum, T.; Stetler-Stevenson, M.; Kreitman, R.J. VH4-34+ hairy cell leukemia, a new variant with poor prognosis despite standard therapy. Blood 2009, 114, 4687–4695. [Google Scholar] [CrossRef] [PubMed]
- Kreitman, R.J.; Wilson, W.; Calvo, K.R.; Arons, E.; Roth, L.; Sapolsky, J.; Zhou, H.; Raffeld, M.; Stetler-Stevenson, M. Cladribine with immediate rituximab for the treatment of patients with variant hairy cell leukemia. Clin. Cancer Res. 2013, 19, 6873–6881. [Google Scholar] [CrossRef]
- Matutes, E.; Martinez-Trillos, A.; Campo, E. Hairy cell leukaemia-variant: Disease features and treatment. Best Pract. Res. Clin. Haematol. 2015, 28, 253–263. [Google Scholar] [CrossRef]
- Quest, G.R.; Johnston, J.B. Clinical features and diagnosis of hairy cell leukemia. Best Pract. Res. Clin Haematol. 2015, 28, 180–192. [Google Scholar] [CrossRef]
- Bigorra, L.; Larriba, I.; Gutierrez-Gallego, R. The hairy cell leukaemia oxymoron: Monocytotic monocytopenia. Clin. Chem. Lab. Med. 2020. [Google Scholar] [CrossRef]
- Shao, H.; Calvo, K.R.; Grönborg, M.; Tembhare, P.R.; Kreitman, R.J.; Stetler-Stevenson, M.; Yuan, C.M. Distinguishing Hairy Cell Leukemia Variant from Hairy Cell Leukemia: Development and Validation of Diagnostic Criteria. Leuk. Res. 2013, 37, 401–409. [Google Scholar] [CrossRef]
- Wotherspoon, A.; Attygalle, A.; Mendes, L.S. Bone marrow and splenic histology in hairy cell leukaemia. Best Pract. Res. Clin. Haematol. 2015, 28, 200–207. [Google Scholar] [CrossRef]
- Gupta, G.K.; Sun, X.; Yuan, C.M.; Stetler-Stevenson, M.; Kreitman, R.J.; Maric, I. The Usefulness of Novel Dual Color Immunohistochemistry in Detection of Minimal Hairy Cell Leukemia in Bone Marrow. Am. J. Clin. Pathol. 2019, 153, 322–327. [Google Scholar] [CrossRef]
- Guerrini, F.; Paolicchi, M.; Ghio, F.; Ciabatti, E.; Grassi, S.; Salehzadeh, S.; Ercolano, G.; Metelli, M.R.; Del Re, M.; Iovino, L.; et al. The Droplet Digital PCR: A New Valid Molecular Approach for the Assessment of B-RAF V600E Mutation in Hairy Cell Leukemia. Front. Pharmacol. 2016, 7, 363. [Google Scholar] [CrossRef] [PubMed]
- Arons, E.; Adams, S.; Pastan, I.; Kreitman, R.J. Class II human leukocyte antigen DRB1*11 in hairy cell leukaemia patients with and without haemolytic uremic syndrome. Br. J. Haematol. 2014, 166, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Cawley, J.C.; Burns, G.F.; Hayhoe, F.G. A chronic lymphoproliferative disorder with distinctive features: A distinct variant of hairy-cell leukaemia. Leuk. Res. 1980, 4, 547–559. [Google Scholar] [CrossRef]
- Matutes, E. Immunophenotyping and differential diagnosis of hairy cell leukemia. Hematol. Oncol. Clin. N. Am. 2006, 20, 1051–1063. [Google Scholar] [CrossRef] [PubMed]
- Ravandi, F.; O’Brien, S.; Jorgensen, J.; Pierce, S.; Faderl, S.; Ferrajoli, A.; Koller, C.; Challagundla, P.; York, S.; Brandt, M.; et al. Phase 2 study of cladribine followed by rituximab in patients with hairy cell leukemia. Blood 2011, 118, 3818–3823. [Google Scholar] [CrossRef] [PubMed]
- Matutes, E.; Oscier, D.; Montalban, C.; Berger, F.; CalletBauchu, E.; Dogan, A.; Felman, P.; Franco, V.; Iannitto, E.; Mollejo, M.; et al. Splenic marginal zone lymphoma proposals for a revision of diagnostic, staging and therapeutic criteria. Leukemia 2008, 22, 487–495. [Google Scholar] [CrossRef]
- Jain, P.; Ok, C.Y.; Konoplev, S.; Patel, K.P.; Jorgensen, J.; Estrov, Z.; Luthra, R.; Kantarjian, H.; Ravandi, F. Relapsed Refractory BRAF-Negative, IGHV4-34-Positive Variant of Hairy Cell Leukemia: A Distinct Entity? J. Clin. Oncol. 2016, 34, e57–e60. [Google Scholar] [CrossRef]
- Arons, E.; Kreitman, R.J. Molecular variant of hairy cell leukemia with poor prognosis. Leuk. Lymphoma 2011, 52 (Suppl. 2), 99–102. [Google Scholar] [CrossRef]
- Poret, N.; Fu, Q.; Guihard, S.; Cheok, M.; Miller, K.; Zeng, G.; Quesnel, B.; Troussard, X.; Galiegue-Zouitina, S.; Shelley, C.S. CD38 in Hairy Cell Leukemia Is a Marker of Poor Prognosis and a New Target for Therapy. Cancer Res. 2015, 75, 3902–3911. [Google Scholar] [CrossRef]
- Jain, D.; Dorwal, P.; Gajendra, S.; Pande, A.; Mehra, S.; Sachdev, R. CD5 positive hairy cell leukemia: A rare case report with brief review of literature. Cytom. B Clin. Cytom. 2016, 90, 467–472. [Google Scholar] [CrossRef]
- Shackelford, R.E.; Heldmann, M.; Eskandari, F.; Joshi, N.; Browning, J.; Maxwell, N.; Coteligam, J. Marked retroperitoneal lymphadenopathy in hairy cell leukemia: A case report. Case Rep. Oncol. 2013, 6, 493–496. [Google Scholar] [CrossRef]
- Cortazar, J.M.; DeAngelo, D.J.; Pinkus, G.S.; Morgan, E.A. Morphological and immunophenotypical features of hairy cell leukaemia involving lymph nodes and extranodal tissues. Histopathology 2017, 71, 112–124. [Google Scholar] [CrossRef]
- Wang, L.; Tadros, A.S.; Hoh, C.K.; Wang, H.Y. CD10-Positive Hairy Cell Leukemia Involving Multiple Deep Lymph Nodes. Clin. Lymphoma Myeloma Leuk. 2016, 16, e51–e53. [Google Scholar] [CrossRef] [PubMed]
- Tallman, M.S.; Hakimian, D.; Rademaker, A.W.; Zanzig, C.; Wollins, E.; Rose, E.; Peterson, L.C. Relapse of hairy cell leukemia after 2-chlorodeoxyadenosine: Long-term follow-up of the Northwestern University experience. Blood 1996, 88, 1954–1959. [Google Scholar] [CrossRef] [PubMed]
- Kreitman, R.J.; Wilson, W.H.; Robbins, D.; Margulies, I.; Stetler-Stevenson, M.; Waldmann, T.A.; Pastan, I. Responses in refractory hairy cell leukemia to a recombinant immunotoxin. Blood 1999, 94, 3340–3348. [Google Scholar] [CrossRef] [PubMed]
- Kreitman, R.J.; Wilson, W.H.; White, J.D.; Stetler-Stevenson, M.; Jaffe, E.S.; Waldmann, T.A.; Pastan, I. Phase I trial of recombinant immunotoxin Anti-Tac(Fv)-PE38 (LMB-2) in patients with hematologic malignancies. J. Clin. Oncol. 2000, 18, 1614–1636. [Google Scholar] [CrossRef]
- Kreitman, R.J.; Wilson, W.H.; Bergeron, K.; Raggio, M.; Stetler-Stevenson, M.; FitzGerald, D.J.; Pastan, I. Efficacy of the Anti-CD22 Recombinant Immunotoxin BL22 in Chemotherapy-Resistant Hairy-Cell Leukemia. New Engl. J. Med. 2001, 345, 241–247. [Google Scholar] [CrossRef]
- Kreitman, R.J.; Squires, D.R.; Stetler-Stevenson, M.; Noel, P.; Fitzgerald, D.J.; Wilson, W.H.; Pastan, I. Phase I trial of recombinant immunotoxin RFB4(dsFv)-PE38 (BL22) in patients with B-cell malignancies. J. Clin. Oncol. 2005, 23, 6719–6729. [Google Scholar] [CrossRef]
- Kreitman, R.J.; Stetler-Stevenson, M.; Margulies, I.; Noel, P.; FitzGerald, D.J.P.; Wilson, W.H.; Pastan, I. Phase II trial of recombinant immunotoxin RFB4(dsFv)-PE38 (BL22) in patients with hairy cell leukemia. J. Clin. Oncol. 2009, 27, 2983–2990. [Google Scholar] [CrossRef]
- Kreitman, R.J.; Tallman, M.S.; Robak, T.; Coutre, S.; Wilson, W.H.; Stetler-Stevenson, M.; FitzGerald, D.J.; Lechleider, R.; Pastan, I. Phase I trial of anti-CD22 recombinant immunotoxin moxetumomab pasudotox (CAT-8015 or HA22) in patients with hairy cell leukemia. J. Clin. Oncol. 2012, 30, 1822–1828. [Google Scholar] [CrossRef]
- Resolution Consensus. Consensus resolution: Proposed criteria for evaluation of response to treatment in hairy cell leukemia. Leukemia 1987, 1, 405. [Google Scholar]
- Cheson, B.D.; Sorensen, J.M.; Vena, D.A.; Montello, M.J.; Barrett, J.A.; Damasio, E.; Tallman, M.; Annino, L.; Connors, J.; Coiffier, B.; et al. Treatment of hairy cell leukemia with 2-chlorodeoxyadenosine via the Group C protocol mechanism of the National Cancer Institute: A report of 979 patients. J. Clin. Oncol. 1998, 16, 3007–3015. [Google Scholar] [CrossRef] [PubMed]
- Chadha, P.; Rademaker, A.W.; Mendiratta, P.; Kim, B.; Evanchuk, D.M.; Hakimian, D.; Peterson, L.C.; Tallman, M.S. Treatment of hairy cell leukemia with 2-chlorodeoxyadenosine (2-CdA): Long-term follow-up of the Northwestern University experience. Blood 2005, 106, 241–246. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kreitman, R.J.; Tallman, M.S.; Robak, T.; Coutre, S.; Wilson, W.H.; Stetler-Stevenson, M.; FitzGerald, D.J.; Santiago, L.; Gao, G.; Lanasa, M.C.; et al. Minimal residual hairy cell leukemia eradication with moxetumomab pasudotox: Phase 1 results and long-term follow-up. Blood 2018, 131, 2331–2334. [Google Scholar] [CrossRef] [PubMed]
- Kreitman, R.J.; Dearden, C.; Zinzani, P.L.; Delgado, J.; Karlin, L.; Robak, T.; Gladstone, D.E.; le Coutre, P.; Dietrich, S.; Gotic, M.; et al. Moxetumomab Pasudotox in Relapsed/Refractory Hairy Cell Leukemia. Leukemia 2018, 32, 1768–1777. [Google Scholar] [CrossRef]
- Burotto, M.; Stetler-Stevenson, M.; Arons, E.; Zhou, H.; Wilson, W.; Kreitman, R.J. Bendamustine and Rituximab in Relapsed and Refractory Hairy Cell Leukemia. Clin. Cancer Res. 2013, 19, 6313–6321. [Google Scholar] [CrossRef]
- Chihara, D.; Arons, E.; Stetler-Stevenson, M.; Yuan, C.; Wang, H.; Zhou, H.; Raffeld, M.; Xi, L.; Steinberg, S.M.; Feurtado, J.; et al. Randomized phase II study of 1st-line cladribine with concurrent or delayed rituximab in patients with hairy cell leukemia. J. Clin. Oncol. 2020, in press. [Google Scholar] [CrossRef]
- Tiacci, E.; Park, J.H.; De Carolis, L.; Chung, S.S.; Broccoli, A.; Scott, S.; Zaja, F.; Devlin, S.; Pulsoni, A.; Chung, Y.R.; et al. Targeting Mutant BRAF in Relapsed or Refractory Hairy-Cell Leukemia. New Engl. J. Med. 2015, 373, 1733–1747. [Google Scholar] [CrossRef]
- Sarid, N.; Ahmad, H.N.; Wotherspoon, A.; Dearden, C.E.; Else, M.; Catovsky, D. An unusual indication for splenectomy in hairy cell leukaemia: A report of three cases with persistent splenomegaly after chemoimmunotherapy. Br. J. Haematol. 2015, 171, 784–787. [Google Scholar] [CrossRef]
- Matsushita, K.; Margulies, I.; Onda, M.; Nagata, S.; Stetler-Stevenson, M.; Kreitman, R.J. Soluble CD22 as a Tumor Marker for Hairy Cell Leukemia. Blood 2008, 112, 2272–2277. [Google Scholar] [CrossRef]
- Kreitman, R.J. Immunoconjugates and new molecular targets in hairy cell leukemia. Hematol. Am. Soc. Hematol. Educ. Program. 2012, 2012, 660–666. [Google Scholar] [CrossRef]
- Tallman, M.S.; Hakimian, D.; Kopecky, K.J.; Wheaton, S.; Wollins, E.; Foucar, K.; Cassileth, P.A.; Habermann, T.; Grever, M.; Rowe, J.M.; et al. Minimal residual disease in patients with hairy cell leukemia in complete remission treated with 2-chlorodeoxyadenosine or 2- deoxycoformycin and prediction of early relapse. Clin. Cancer Res. 1999, 5, 1665–1670. [Google Scholar] [PubMed]
- Chihara, D.; Kreitman, R.J. Treatment of Hairy cell leukemia. Exp. Rev. Hematol. 2020. submitted. [Google Scholar]
- Kreitman, R.J.; Pastan, I. Antibody fusion proteins: Anti-CD22 recombinant immunotoxin moxetumomab pasudotox. Clin. Cancer Res. 2011, 17, 6398–6405. [Google Scholar] [CrossRef] [PubMed]
- Pastan, I.; Hassan, R.; FitzGerald, D.J.P.; Kreitman, R.J. Immunotoxin therapy of cancer. Nat. Rev. Cancer 2006, 6, 559–565. [Google Scholar] [CrossRef]
- Kreitman, R.J.; Pastan, I. Immunotoxins in the treatment of refractory hairy cell leukemia. Hematol. Oncol. Clin. N. Am. 2006, 20, 1137–1151. [Google Scholar] [CrossRef]
- Endo, Y.; Mitsui, K.; Motizuki, M.; Tsurugi, K. The mechanism of action of ricin and related toxic lectins on eukaryotic ribosomes. J. Biol. Chem. 1987, 262, 5908–5912. [Google Scholar]
- Yamaizumi, M.; Mekada, E.; Uchida, T.; Okada, Y. One molecule of diphtheria toxin fragment A introduced into a cell can kill the cell. Cell 1978, 15, 245–250. [Google Scholar] [CrossRef]
- Du, X.; Youle, R.J.; FitzGerald, D.J.; Pastan, I. Pseudomonas exotoxin A-mediated apoptosis is Bak dependent and preceded by the degradation of Mcl-1. Mol. Cell Biol. 2010, 30, 3444–3452. [Google Scholar] [CrossRef]
- Messmann, R.A.; Vitetta, E.S.; Headlee, D.; Senderowicz, A.M.; Figg, W.D.; Schindler, J.; Michiel, D.F.; Creekmore, S.; Steinberg, S.M.; Kohler, D.; et al. A phase I study of combination therapy with immunotoxins IgG-HD37- deglycosylated ricin A chain (dgA) and IgG-RFB4-dgA (Combotox) in patients with refractory CD19(+), CD22(+) B cell lymphoma. Clin. Cancer Res. 2000, 6, 1302–1313. [Google Scholar]
- Amlot, P.L.; Stone, M.J.; Cunningham, D.; Fay, J.; Newman, J.; Collins, R.; May, R.; McCarthy, M.; Richardson, J.; Ghetie, V.; et al. A phase I study of an anti-CD22-deglycosylated ricin A chain immunotoxin in the treatment of B-cell lymphomas resistant to conventional therapy. Blood 1993, 82, 2624–2633. [Google Scholar] [CrossRef] [PubMed]
- Kreitman, R.J. Getting plant toxins to fuse. Leuk. Res. 1997, 21, 997–999. [Google Scholar] [CrossRef]
- Bacha, P.; Williams, D.P.; Waters, C.; Williams, J.M.; Murphy, J.R.; Strom, T.B. Interleukin 2 receptor-targeted cytotoxicity: Interleukin 2 receptor-mediated action of a diphtheria toxin-related interleukin 2 fusion protein. J. Exp. Med. 1988, 167, 612–622. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Beers, R.; FitzGerald, D.J.; Pastan, I. Differential cellular internalization of anti-CD19 and-CD22 immunotoxins results in different cytotoxic activity. Cancer Res. 2008, 68, 6300–6305. [Google Scholar] [CrossRef] [PubMed]
- Ogata, M.; Fryling, C.M.; Pastan, I.; FitzGerald, D.J. Cell-mediated cleavage of Pseudomonas exotoxin between Arg279 and Gly280 generates the enzymatically active fragment which translocates to the cytosol. J. Biol. Chem. 1992, 267, 25396–25401. [Google Scholar]
- Fryling, C.; Ogata, M.; FitzGerald, D. Characterization of a cellular protease that cleaves Pseudomonas exotoxin. Infect. Immun. 1992, 60, 497–502. [Google Scholar] [CrossRef]
- Chiron, M.F.; Fryling, C.M.; FitzGerald, D.J. Cleavage of Pseudomonas exotoxin and diphtheria toxin by a furin-like enzyme prepared from beef liver. J. Biol. Chem. 1994, 269, 18167–18176. [Google Scholar]
- McKee, M.L.; FitzGerald, D.J. Reduction of furin-nicked Pseudomonas exotoxin A: An unfolding story. Biochemistry 1999, 38, 16507–16513. [Google Scholar] [CrossRef]
- Kreitman, R.J.; Pastan, I. Importance of the glutamate residue of KDEL in increasing the cytotoxicity of Pseudomonas exotoxin derivatives and for increased binding to the KDEL receptor. Biochem. J. 1995, 307, 29–37. [Google Scholar] [CrossRef]
- Hessler, J.L.; Kreitman, R.J. An early step in Pseudomonas exotoxin action is removal of the terminal lysine residue, which allows binding to the KDEL receptor. Biochemistry 1997, 36, 14577–14582. [Google Scholar] [CrossRef]
- Theuer, C.; Kasturi, S.; Pastan, I. Domain II of Pseudomonas exotoxin A arrests the transfer of translocating nascent chains into mammalian microsomes. Biochemistry 1994, 33, 5894–5900. [Google Scholar] [CrossRef] [PubMed]
- Theuer, C.P.; Buchner, J.; FitzGerald, D.; Pastan, I. The N-terminal region of the 37-kDa translocated fragment of Pseudomonas exotoxin A aborts translocation by promoting its own export after microsomal membrane insertion. Proc. Natl. Acad. Sci. USA 1993, 90, 7774–7778. [Google Scholar] [CrossRef] [PubMed]
- Webb, T.R.; Cross, S.H.; McKie, L.; Edgar, R.; Vizor, L.; Harrison, J.; Peters, J.; Jackson, I.J. Diphthamide modification of eEF2 requires a J-domain protein and is essential for normal development. J. Cell Sci. 2008, 121, 3140–3145. [Google Scholar] [CrossRef] [PubMed]
- Carroll, S.F.; Collier, R.J. Active site of Pseudomonas aeruginosa exotoxin A. Glutamic acid 553 is photolabeled by NAD and shows functional homology with glutamic acid 148 of diphtheria toxin. J. Biol. Chem. 1987, 262, 8707–8711. [Google Scholar] [PubMed]
- Brinkmann, U.; Brinkmann, E.; Gallo, M.; Pastan, I. Cloning and characterization of a cellular apoptosis susceptibility gene, the human homologue to the yeast chromosome segregation gene CSE1. Proc. Natl. Acad. Sci. USA 1995, 92, 10427–10431. [Google Scholar] [CrossRef]
- Keppler-Hafkemeyer, A.; Kreitman, R.J.; Pastan, I. Apoptosis induced by immunotoxins used in the treatment of hematologic malignancies. Int. J. Cancer 2000, 87, 86–94. [Google Scholar] [CrossRef]
- Decker, T.; Oelsner, M.; Kreitman, R.J.; Salvatore, G.; Wang, Q.C.; Pastan, I.; Peschel, C.; Licht, T. Induction of Caspase-Dependent Programmed Cell Death in B-Cell Chronic Lymphocytic Leukemia Cells by Anti-CD22 Immunotoxins. Blood 2004, 103, 2718–2726. [Google Scholar] [CrossRef]
- Antignani, A.; Segal, D.; Simon, N.; Kreitman, R.J.; Huang, D.; FitzGerald, D.J. Essential Role for Bim in mediating the apoptotic and anti-tumor activities of immunotoxins. Oncogene 2017, 36, 4953–4962. [Google Scholar] [CrossRef]
- Chaudhary, V.K.; Queen, C.; Junghans, R.P.; Waldmann, T.A.; FitzGerald, D.J.; Pastan, I. A recombinant immunotoxin consisting of two antibody variable domains fused to Pseudomonas exotoxin. Nature 1989, 339, 394–397. [Google Scholar] [CrossRef]
- Kreitman, R.J.; Batra, J.K.; Seetharam, S.; Chaudhary, V.K.; FitzGerald, D.J.; Pastan, I. Single-chain immunotoxin fusions between anti-Tac and Pseudomonas exotoxin: Relative importance of the two toxin disulfide bonds. Bioconj. Chem. 1993, 4, 112–120. [Google Scholar] [CrossRef]
- Kreitman, R.J.; Chaudhary, V.K.; Waldmann, T.; Willingham, M.C.; FitzGerald, D.J.; Pastan, I. The recombinant immunotoxin anti-Tac(Fv)-Pseuodomonas exotoxin 40 is cytotoxic toward peripheral blood malignant cells from patients with adult T-cell leukemia. Proc. Natl. Acad. Sci. USA 1990, 87, 8291–8295. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.; Li, J.; Ghetie, M.; Ghetie, V.; May, R.D.; Till, M.; Brown, A.N.; Relf, M.; Knowles, P.; Uhr, J.W.; et al. Evaluation of four CD22 antibodies as ricin A chain-containing immunotoxins for the in vivo therapy of human B-cell leukemias and lymphomas. Int. J. Cancer 1988, 42, 792–797. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, E.; Amlot, P.; Pastan, I.; FitzGerald, D.J. Recombinant RFB4 immunotoxins exhibit potent cytotoxic activity for CD22-bearing cells and tumors. Blood 1997, 90, 2020–2026. [Google Scholar] [CrossRef] [PubMed]
- Kreitman, R.J.; Bailon, P.; Chaudhary, V.K.; FitzGerald, D.J.P.; Pastan, I. Recombinant immunotoxins containing anti-Tac(Fv) and derivatives of Pseudomonas exotoxin produce complete regression in mice of an interleukin-2 receptor-expressing human carcinoma. Blood 1994, 83, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Kreitman, R.J.; Chaudhary, V.K.; Waldmann, T.A.; Hanchard, B.; Cranston, B.; FitzGerald, D.J.P.; Pastan, I. Cytotoxic activities of recombinant immunotoxins composed of Pseudomonas toxin or diphtheria toxin toward lymphocytes from patients with adult T-cell leukemia. Leukemia 1993, 7, 553–562. [Google Scholar]
- Robbins, D.H.; Margulies, I.; Stetler-Stevenson, M.; Kreitman, R.J. Hairy cell leukemia, a B-cell neoplasm which is particularly sensitive to the cytotoxic effect of anti-Tac(Fv)-PE38 (LMB-2). Clin. Cancer Res. 2000, 6, 693–700. [Google Scholar]
- Clark, E.A.; Giltiay, N.V. CD22: A Regulator of Innate and Adaptive B Cell Responses and Autoimmunity. Front. Immunol. 2018, 9, 2235. [Google Scholar] [CrossRef]
- Enterina, J.R.; Jung, J.; Macauley, M.S. Coordinated roles for glycans in regulating the inhibitory function of CD22 on B cells. Biomed. J. 2019, 42, 218–232. [Google Scholar] [CrossRef]
- Kreitman, R.J.; Margulies, I.; Stetler-Stevenson, M.; Wang, Q.C.; FitzGerald, D.J.P.; Pastan, I. Cytotoxic activity of disulfide-stabilized recombinant immunotoxin RFB4(dsFv)-PE38 (BL22) towards fresh malignant cells from patients with B-cell leukemias. Clin. Cancer Res. 2000, 6, 1476–1487. [Google Scholar]
- Alderson, R.F.; Kreitman, R.J.; Chen, T.; Yeung, P.; Herbst, R.; Fox, J.A.; Pastan, I. CAT-8015: A second-generation pseudomonas exotoxin A-based immunotherapy targeting CD22-expressing hematologic malignancies. Clin. Cancer Res. 2009, 15, 832–839. [Google Scholar] [CrossRef]
- Kreitman, R.J.; Wang, Q.C.; FitzGerald, D.J.P.; Pastan, I. Complete regression of human B-cell lymphoma xenografts in mice treated with recombinant anti-CD22 immunotoxin RFB4(dsFv)-PE38 at doses tolerated by Cynomolgus monkeys. Int. J. Cancer 1999, 81, 148–155. [Google Scholar] [CrossRef]
- Kreitman, R.J.; Hansen, H.J.; Jones, A.L.; FitzGerald, D.J.P.; Goldenberg, D.M.; Pastan, I. Pseudomonas exotoxin-based immunotoxins containing the antibody LL2 or LL2-Fab’ induce regression of subcutaneous human B-cell lymphoma in mice. Cancer Res. 1993, 53, 819–825. [Google Scholar] [PubMed]
- Theuer, C.P.; Kreitman, R.J.; FitzGerald, D.J.; Pastan, I. Immunotoxins made with a recombinant form of Pseudomonas exotoxin A that do not require proteolysis for activity. Cancer Res. 1993, 53, 340–347. [Google Scholar] [PubMed]
- Mansfield, E.; Chiron, M.F.; Amlot, P.; Pastan, I.; FitzGerald, D.J. Recombinant RFB4 single-chain immunotoxin that is cytotoxic towards CD22-positive cells. Biochem. Soc. Trans. 1997, 25, 709–714. [Google Scholar] [CrossRef]
- Moake, J.L. Thrombotic thrombocytopenic purpura and the hemolytic uremic syndrome. Arch Pathol. Lab. Med. 2002, 126, 1430–1433. [Google Scholar]
- Salvatore, G.; Beers, R.; Margulies, I.; Kreitman, R.J.; Pastan, I. Improved Cytotoxic activity towards cell lines and fresh leukemia cells of a mutant anti-CD22 immunotoxin obtained by antibody phage display. Clin. Cancer Res. 2002, 8, 995–1002. [Google Scholar]
- Kuan, C.; Pai, L.H.; Pastan, I. Immunotoxins containing Pseudomonas exotoxin targeting LeY damage human endothelial cells in an antibody-specific mode: Relevance to vascular leak syndrome. Clin. Cancer Res. 1995, 1, 1589–1594. [Google Scholar]
- Lindstrom, A.L.; Erlandsen, S.L.; Kersey, J.H.; Pennell, C.A. An in vitro model for toxin-mediated vascular leak syndrome: Ricin toxin A chain increases the permeability of human endothelial cell monolayers. Blood 1997, 90, 2323–2334. [Google Scholar] [CrossRef]
- Vitetta, E.S. Immunotoxins and vascular leak syndrome. Cancer J. 2000, 6, S218–S224. [Google Scholar]
- Hassan, R.; Bullock, S.; Premkumar, A.; Kreitman, R.J.; Kindler, H.; Willingham, M.; Pastan, I. Phase I study of SS1P, a recombinant anti-mesothelin immunotoxin given as a bolus I.V. infusion to patients with mesothelin-expressing mesothelioma, ovarian, and pancreatic cancers. Clin. Cancer Res. 2007, 13, 5144–5149. [Google Scholar] [CrossRef]
- Kreitman, R.J.; Hassan, R.; FitzGerald, D.J.; Pastan, I. Phase I Trial of Continuous Infusion Anti-Mesothelin Recombinant Immunotoxin SS1P. Clin. Cancer Res. 2009, 15, 5274–5279. [Google Scholar] [CrossRef] [PubMed]
- Pai, L.H.; Wittes, R.; Setser, A.; Willingham, M.C.; Pastan, I. Treatment of advanced solid tumors with immunotoxin LMB-1: An antibody linked to Pseudomonas exotoxin. Nat. Med. 1996, 2, 350–353. [Google Scholar] [CrossRef] [PubMed]
- Kreitman, R.J.; Stetler-Stevenson, M.; Jaffe, E.S.; Conlon, K.C.; Steinberg, S.M.; Wilson, W.; Waldmann, T.A.; Pastan, I. Complete Remissions of Adult T-cell Leukemia with Anti-CD25 Recombinant Immunotoxin LMB-2 and Chemotherapy to Block Immunogenicity. Clin. Cancer Res. 2016, 22, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Sausville, E.A.; Headlee, D.; Stetler-Stevenson, M.; Jaffe, E.S.; Solomon, D.; Figg, W.D.; Herdt, J.; Kopp, W.C.; Rager, H.; Steinberg, S.M.; et al. Continuous infusion of the anti-CD22 immunotoxin IgG-RFB4-SMPT-dgA in patients with B-cell lymphoma: A phase I study. Blood 1995, 85, 3457–3465. [Google Scholar] [CrossRef] [PubMed]
- Wayne, A.; Bhojwani, D.; Richards, K.; Stetler-Stevenson, M.; Silverman, L.B.; Jehac, S.; Pui, C.; McDevitt, J.; FitzGerald, D.J.; Kreitman, R.J.; et al. Complete Remissions In 3 of 12 Patients with Pediatric Acute Lymphoblastic Leukemia (ALL) During Phase I Testing of the Anti-CD22 Immunotoxin Moxetumomab Pasudotox. Am. Soc. Hematol. 2010. [Google Scholar] [CrossRef]
- Wayne, A.S.; Shah, N.N.; Bhojwani, D.; Silverman, L.B.; Whitlock, J.A.; Stetler-Stevenson, M.; Sun, W.; Liang, M.; Yang, J.; Chang, L.; et al. Phase 1 study of the anti-CD22 immunotoxin moxetumomab pasudotox for childhood acute lymphoblastic leukemia. Blood 2017, 130, 1620–1627. [Google Scholar] [CrossRef]
- Feurtado, J.; Kreitman, R.J. Moxetumomab Pasudotox: Clinical Experience in Relapsed/Refractory Hairy Cell Leukemia. Clin. J. Oncol. Nurs. 2019, 23, E52–E59. [Google Scholar] [CrossRef]
- Vainshtein, I.; Sun, B.; Roskos, L.K.; Liang, M. A novel approach to assess domain specificity of anti-drug antibodies to moxetumomab pasudotox, an immunotoxin with two functional domains. J. Immunol. Methods 2020, 477, 112688. [Google Scholar] [CrossRef]
- Kuruvilla, D.; Chia, Y.L.; Balic, K.; Yao, N.S.; Kreitman, R.J.; Pastan, I.; Li, X.; Standifer, N.; Liang, M.; Tseng, C.; et al. Population Pharmacokinetics, Efficacy, and Safety of Moxetumomab Pasudotox in Patients With Relapsed or Refractory Hairy Cell Leukemia. Br. J. Clin. Pharmacol. 2020, 86, 1367–1376. [Google Scholar] [CrossRef]
- Hassan, R.; Williams-Gould, J.; Watson, T.; Pai-Scherf, L.; Pastan, I. Pretreatment with Rituximab Does Not Inhibit the Human Immune Response against the Immunogenic Protein LMB-1. Clin. Cancer Res. 2004, 10, 16–18. [Google Scholar] [CrossRef]
- Nieva, J.; Bethel, K.; Saven, A. Phase 2 study of rituximab in the treatment of cladribine-failed patients with hairy cell leukemia. Blood 2003, 102, 810–813. [Google Scholar] [CrossRef] [PubMed]
- Tiacci, E.; Carolis, L.D.; Simonetti, E.; Zaja, F.; Capponi, M.; Ambrosetti, A.; Lucia, E.; Antolino, A.; Pulsoni, A.; Ferrari, S.; et al. The BRAF inhibitor vemurafenib plus rituximab produces a high rate of deep and durable responses in relapsed/refractory hairy cell leukemia: Updated results of a phase-2 trial. Hematol. Oncol. 2019, 37, 110–111. [Google Scholar] [CrossRef]

| Consensus Guidelines [26,27] | Variations | |
|---|---|---|
| Complete remission (CR) | ||
| CBC | ANC >1.5, Hgb >11, and Plt 100 | Hgb ≥12 for males [62] |
| CBC duration | One resolved CBC is sufficient | Resolved CBCs ≥4 weeks [19,59,60,62,64,65,66] |
| HCL cells | Absent in blood and marrow by morphology | |
| Spleen | Absent on exam | Resolved by CT/MRI [65] |
| Lymph nodes | Not specified | Resolved to ≤2cm in short axis by imaging/exam [66] |
| CR, MRD-free | ||
| Bone marrow | IHC Negative by CD20, DBA.44, or VE1 | IHC and flow cytometry negative |
| Partial response (PR) | ||
| CBC | Same as CR | ≥50% improvement in ANC, Hgb, and Plt [21,55,56,57,58,59,60,62,63] |
| CBC duration | No minimum duration | Resolved CBC ≥4 weeks [19,59,60,62,64,65,66] |
| Blood HCL | Not specified | ≥50% reduction [19,21,68] |
| Marrow HCL | ≥50% reduction in infiltration | Avoid due to heterogeneity [64,65] |
| Organomegaly | ≥50% reduction | Require CT/MRI imaging due to bias [65] |
| Progressive disease (PD) | ||
| CBC | ≥25% decline in ANC, Hgb, or Plt, due to HCL | |
| Symptoms | Increase in symptoms related to disease | |
| Organomegaly | ≥25% increase in organomegaly | |
| Lymph nodes | Not specified | New lymph nodes or ≥25% increase in existing nodes [65,71] |
| Blood HCL | Not specified | ≥50% increase in HCL cells or absolute lymphocytes [65,71] |
| Stable disease | Neither CR, PR, nor PD |
| Phase 1 | Phase 3 | |
|---|---|---|
| Patients treated | 49 | 80 |
| Eligibility, clinical | Cytopenias or Symptomatic splenomegaly | Cytopenias or Symptomatic splenomegaly |
| Eligibility, ADA | Negative test for ADA | Testing not needed |
| Doses tested (μg/Kg QOD ×3) | 5, 10, 20, and 30 (n = 3 each) | 40 (n = 80) |
| 40 (n = 4), 50 (n = 33) | ||
| Patient ages | 40–77 (median 57) | 34–84 (median 60) |
| Male-Female ratio | 41:8 | 68:12 |
| HCLv | 2 (4%) | 3 (3.8%) |
| Prior purine analog courses | ||
| 1 | 4 (8%) | 10 (13%) |
| 2 | 24 (49%) | 30 (38%) |
| ≥3 | 21 (43%) | 40 (50%) |
| Prior rituximab | 30 (61%) | 60 (75%) |
| Prior splenectomy | 8 (16%) | 5 (6%) |
| ORR | 42 (86%) | 60 (75%) |
| CR rate | 28 (57%) | 33 (41%) |
| Toxicity, dose level assessed | 50 μg/Kg × 3 (n = 33) | 40 μg/Kg × 3 (n = 80) |
| Grades 1–4, 3–4 | ||
| Hemolytic uremic syndrome | 2 (4%), 0 (0%) | 7 (9%), 4 (5%) |
| Capillary leak syndrome | 8 (16%), 0 (0%) | 7 (9%), 2 (3%) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kreitman, R.J.; Pastan, I. Development of Recombinant Immunotoxins for Hairy Cell Leukemia. Biomolecules 2020, 10, 1140. https://doi.org/10.3390/biom10081140
Kreitman RJ, Pastan I. Development of Recombinant Immunotoxins for Hairy Cell Leukemia. Biomolecules. 2020; 10(8):1140. https://doi.org/10.3390/biom10081140
Chicago/Turabian StyleKreitman, Robert J., and Ira Pastan. 2020. "Development of Recombinant Immunotoxins for Hairy Cell Leukemia" Biomolecules 10, no. 8: 1140. https://doi.org/10.3390/biom10081140
APA StyleKreitman, R. J., & Pastan, I. (2020). Development of Recombinant Immunotoxins for Hairy Cell Leukemia. Biomolecules, 10(8), 1140. https://doi.org/10.3390/biom10081140






