Cell-Adhesion Properties of β-Subunits in the Regulation of Cardiomyocyte Sodium Channels
Abstract
1. Introduction
1.1. The Nav Channel α-Subunit
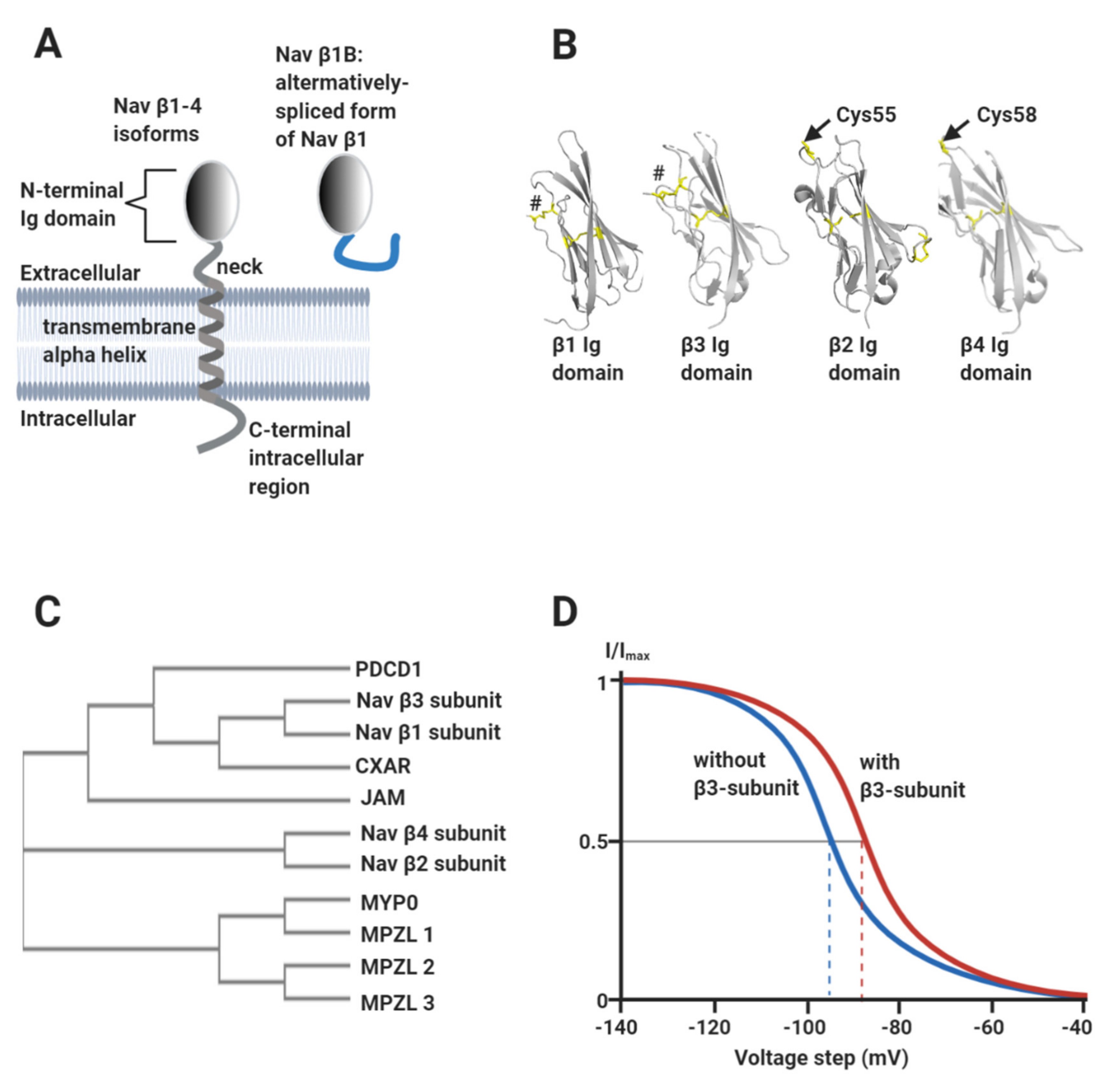
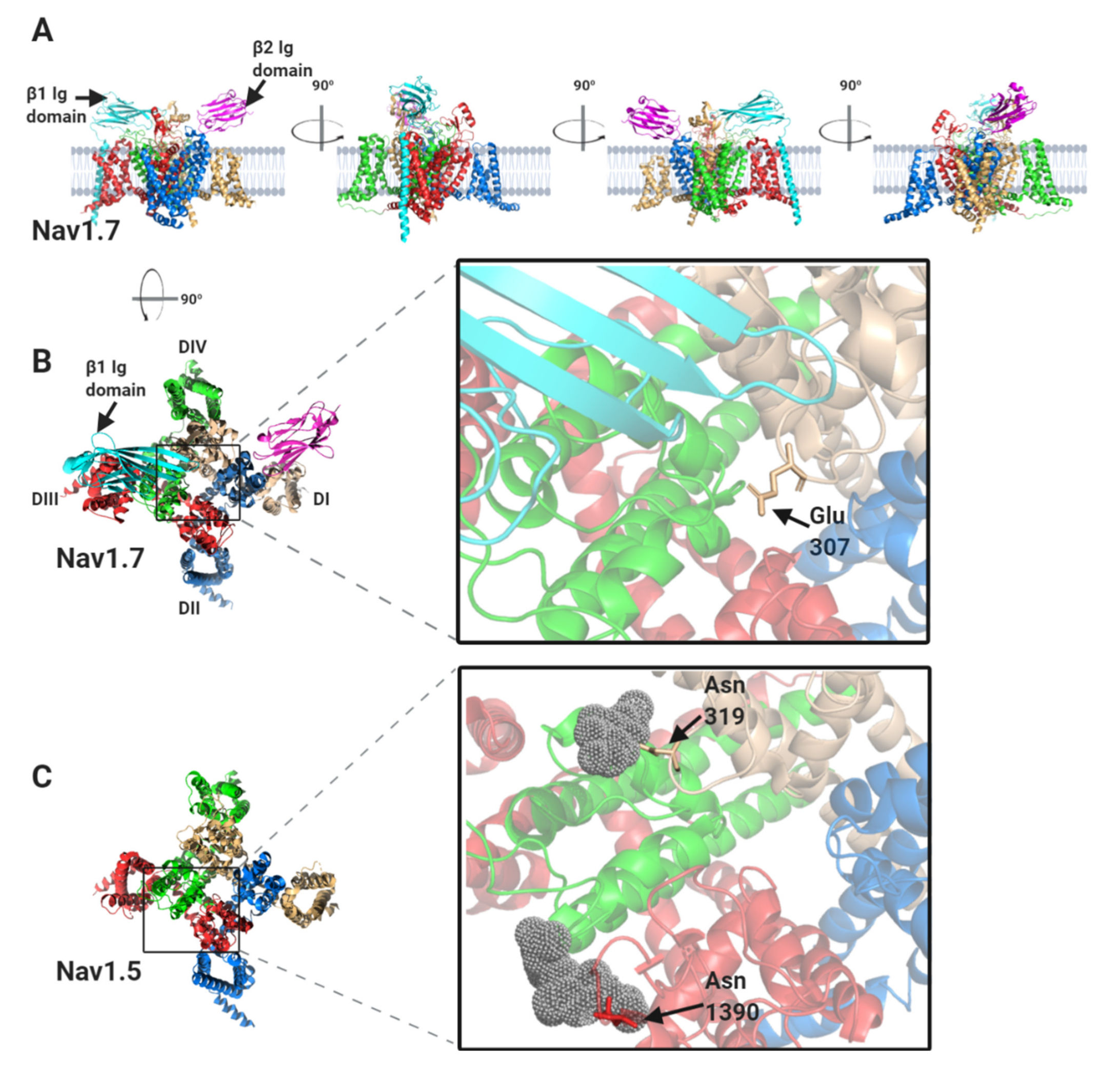
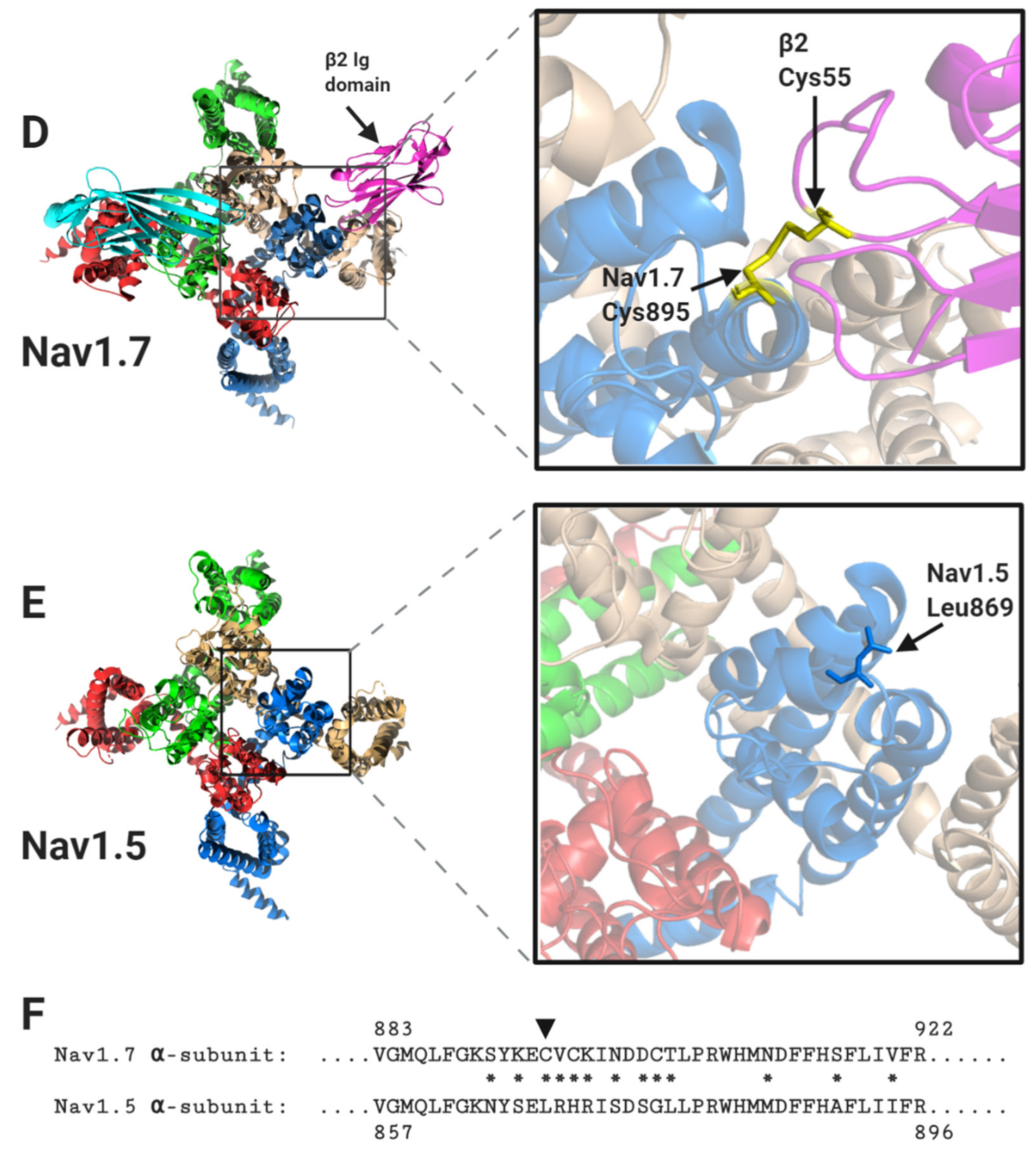
1.2. The Nav Channel β-Subunits and Their Binding Sites on the α-Subunits
2. The Nav Channel β-Subunits as Cell-Adhesion Molecules
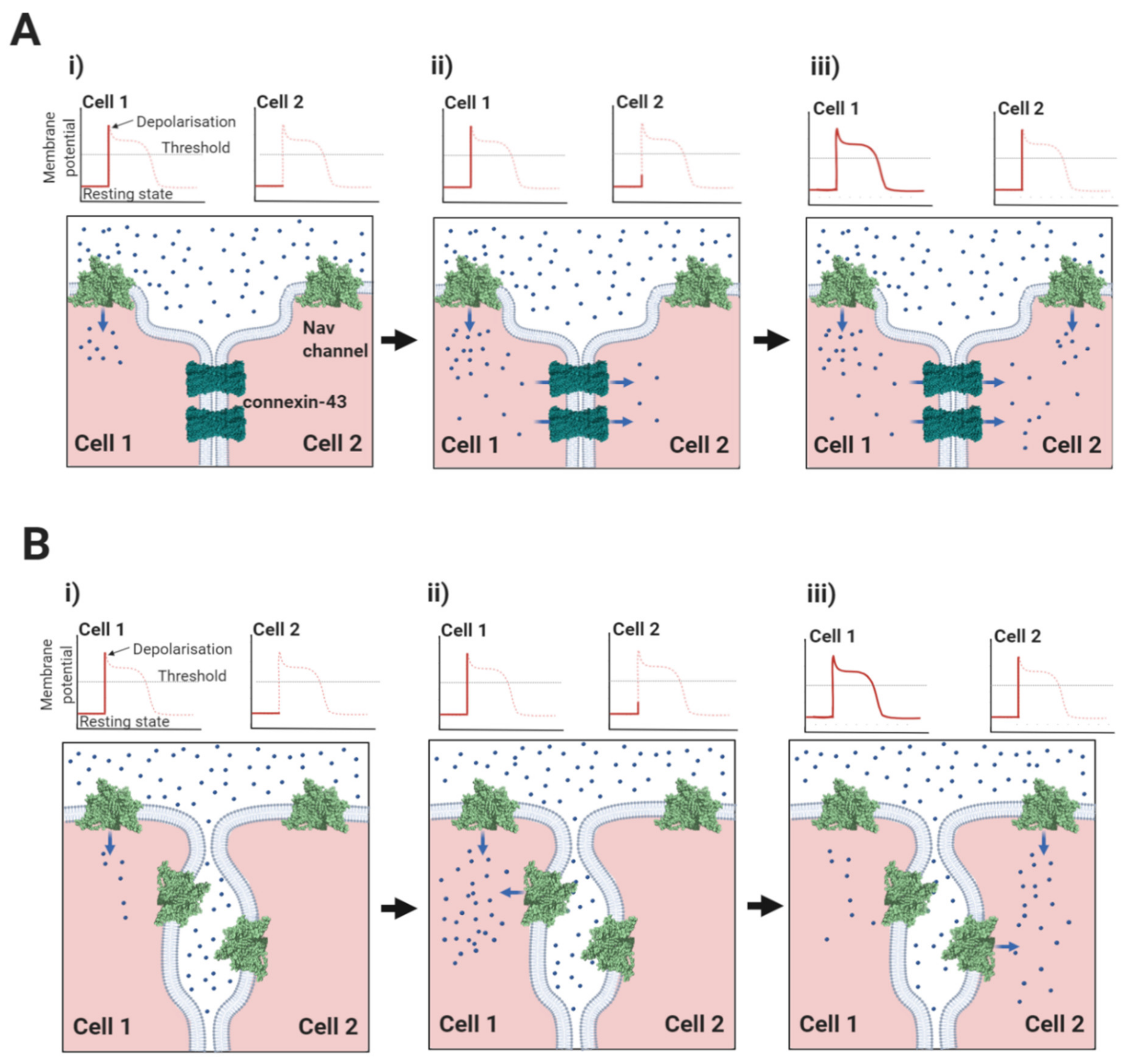
2.1. The Nav1.5-Associated β1-Subunit as a CAM at the Intercalated Disc: Its Role in Ephaptic Conduction
2.2. Do Other Nav Channel β-Subunits Facilitate Ephaptic Conduction?
2.3. Nav Channels and β-Subunits on the Lateral Membrane: A Role in Mechanosensing?
2.4. Nav β-Subunits and Neuronal Channels in the T-Tubules
3. Conclusions and Unsolved Problems
3.1. Evolutionary Relationship between Nav β-Subunits and Other CAMs
3.2. The Biophysics of Nav β-Subunit Cell-Adhesion
3.3. The Role of N-Linked Glycosylation
3.4. Ephaptic Conduction in the Heart and Elsewhere
3.5. Clinical Implications
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sweeney, H.L.; Hammers, D.W. Muscle Contraction. Cold Spring Harb. Perspect. Biol. 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Cortada, E.; Brugada, R.; Verges, M. Trafficking and Function of the Voltage-Gated Sodium Channel beta2 Subunit. Biomolecules 2019, 9, 604. [Google Scholar] [CrossRef] [PubMed]
- DeMarco, K.R.; Clancy, C.E. Cardiac Na Channels: Structure to Function. Curr. Top. Membr. 2016, 78, 287–311. [Google Scholar] [CrossRef] [PubMed]
- Veeraraghavan, R.; Gyorke, S.; Radwanski, P.B. Neuronal sodium channels: Emerging components of the nano-machinery of cardiac calcium cycling. J. Physiol. 2017, 595, 3823–3834. [Google Scholar] [CrossRef]
- Maier, S.K.; Westenbroek, R.E.; McCormick, K.A.; Curtis, R.; Scheuer, T.; Catterall, W.A. Distinct subcellular localization of different sodium channel alpha and beta subunits in single ventricular myocytes from mouse heart. Circulation 2004, 109, 1421–1427. [Google Scholar] [CrossRef] [PubMed]
- Fraser, J.A.; Huang, C.L.; Pedersen, T.H. Relationships between resting conductances, excitability, and t-system ionic homeostasis in skeletal muscle. J. Gen. Physiol. 2011, 138, 95–116. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, T.H.; Huang, C.L.-H.; Fraser, J.A. An analysis of the relationships between subthreshold electrical properties and excitability in skeletal muscle. J. Gen. Physiol. 2011, 138, 73–93. [Google Scholar] [CrossRef]
- Ahern, C.A.; Payandeh, J.; Bosmans, F.; Chanda, B. The hitchhiker’s guide to the voltage-gated sodium channel galaxy. J. Gen. Physiol. 2016, 147, 1–24. [Google Scholar] [CrossRef]
- Nishino, A.; Okamura, Y. Evolutionary History of Voltage-Gated Sodium Channels. Handb. Exp. Pharmacol. 2018, 246, 3–32. [Google Scholar] [CrossRef]
- Yan, Z.; Zhou, Q.; Wang, L.; Wu, J.; Zhao, Y.; Huang, G.; Peng, W.; Shen, H.; Lei, J.; Yan, N. Structure of the Nav1.4-beta1 Complex from Electric Eel. Cell 2017, 170, 470–482.e11. [Google Scholar] [CrossRef]
- Pan, X.; Li, Z.; Zhou, Q.; Shen, H.; Wu, K.; Huang, X.; Chen, J.; Zhang, J.; Zhu, X.; Lei, J.; et al. Structure of the human voltage-gated sodium channel Nav1.4 in complex with beta1. Science 2018, 362. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Liu, D.; Wu, K.; Lei, J.; Yan, N. Structures of human Nav1.7 channel in complex with auxiliary subunits and animal toxins. Science 2019, 363, 1303–1308. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Li, Z.; Huang, X.; Huang, G.; Gao, S.; Shen, H.; Liu, L.; Lei, J.; Yan, N. Molecular basis for pore blockade of human Na(+) channel Nav1.2 by the mu-conotoxin KIIIA. Science 2019, 363, 1309–1313. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Shi, H.; Tonggu, L.; Gamal El-Din, T.M.; Lenaeus, M.J.; Zhao, Y.; Yoshioka, C.; Zheng, N.; Catterall, W.A. Structure of the Cardiac Sodium Channel. Cell 2020, 180, 122–134.e110. [Google Scholar] [CrossRef]
- Edokobi, N.; Isom, L.L. Voltage-Gated Sodium Channel beta1/beta1B Subunits Regulate Cardiac Physiology and Pathophysiology. Front. Physiol. 2018, 9, 351. [Google Scholar] [CrossRef]
- Brackenbury, W.J.; Isom, L.L. Na Channel beta Subunits: Overachievers of the Ion Channel Family. Front. Pharmacol. 2011, 2, 53. [Google Scholar] [CrossRef]
- Namadurai, S.; Yereddi, N.R.; Cusdin, F.S.; Huang, C.L.; Chirgadze, D.Y.; Jackson, A.P. A new look at sodium channel beta subunits. Open Biol. 2015, 5, 140192. [Google Scholar] [CrossRef]
- Patino, G.A.; Isom, L.L. Electrophysiology and beyond: Multiple roles of Na+ channel beta subunits in development and disease. Neurosci. Lett. 2010, 486, 53–59. [Google Scholar] [CrossRef]
- Salvage, S.C.; Zhu, W.; Habib, Z.F.; Hwang, S.S.; Irons, J.R.; Huang, C.L.H.; Silva, J.R.; Jackson, A.P. Gating control of the cardiac sodium channel Nav1.5 by its beta3-subunit involves distinct roles for a transmembrane glutamic acid and the extracellular domain. J. Biol. Chem. 2019. [Google Scholar] [CrossRef]
- Salvage, S.C.; Rees, J.S.; McStea, A.; Hirsch, M.; Wang, L.; Tynan, C.J.; Reed, M.W.; Irons, J.R.; Butler, R.; Thompson, A.J.; et al. Supramolecular clustering of the cardiac sodium channel Nav1.5 in HEK293F cells, with and without the auxiliary beta3-subunit. FASEB J. 2020, 34, 3537–3553. [Google Scholar] [CrossRef]
- Zhu, W.; Voelker, T.L.; Varga, Z.; Schubert, A.R.; Nerbonne, J.M.; Silva, J.R. Mechanisms of noncovalent beta subunit regulation of NaV channel gating. J. Gen. Physiol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Hakim, P.; Gurung, I.S.; Pedersen, T.H.; Thresher, R.; Brice, N.; Lawrence, J.; Grace, A.A.; Huang, C.L. Scn3b knockout mice exhibit abnormal ventricular electrophysiological properties. Prog. Biophys. Mol. Biol. 2008, 98, 251–266. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.H.; Lenkowski, P.W.; Lee, H.C.; Mounsey, J.P.; Patel, M.K. Modulation of Na(v)1.5 by beta1- and beta3-subunit co-expression in mammalian cells. Pflug. Arch. 2005, 449, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Calhoun, J.D.; Zhang, Y.; Lopez-Santiago, L.; Zhou, N.; Davis, T.H.; Salzer, J.L.; Isom, L.L. Identification of the cysteine residue responsible for disulfide linkage of Na+ channel alpha and beta2 subunits. J. Biol. Chem. 2012, 287, 39061–39069. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.H.; Westenbroek, R.E.; Silos-Santiago, I.; McCormick, K.A.; Lawson, D.; Ge, P.; Ferriera, H.; Lilly, J.; DiStefano, P.S.; Catterall, W.A.; et al. Sodium channel beta4, a new disulfide-linked auxiliary subunit with similarity to beta2. J. Neurosci. 2003, 23, 7577–7585. [Google Scholar] [CrossRef]
- Gilchrist, J.; Das, S.; Van Petegem, F.; Bosmans, F. Crystallographic insights into sodium-channel modulation by the beta4 subunit. Proc. Natl. Acad. Sci. USA 2013, 110, E5016–E5024. [Google Scholar] [CrossRef]
- Das, S.; Gilchrist, J.; Bosmans, F.; Van Petegem, F. Binary architecture of the Nav1.2-beta2 signaling complex. eLife 2016, 5. [Google Scholar] [CrossRef]
- Molinarolo, S.; Granata, D.; Carnevale, V.; Ahern, C.A. Mining Protein Evolution for Insights into Mechanisms of Voltage-Dependent Sodium Channel Auxiliary Subunits. Handb. Exp. Pharmacol. 2018, 246, 33–49. [Google Scholar] [CrossRef]
- Kusano, K.; Thomas, T.N.; Fujiwara, K. Phosphorylation and localization of protein-zero related (PZR) in cultured endothelial cells. Endothelium 2008, 15, 127–136. [Google Scholar] [CrossRef][Green Version]
- Chopra, S.S.; Watanabe, H.; Zhong, T.P.; Roden, D.M. Molecular cloning and analysis of zebrafish voltage-gated sodium channel beta subunit genes: Implications for the evolution of electrical signaling in vertebrates. BMC Evol. Biol. 2007, 7, 113. [Google Scholar] [CrossRef]
- McEwen, D.P.; Isom, L.L. Heterophilic interactions of sodium channel beta1 subunits with axonal and glial cell adhesion molecules. J. Biol. Chem. 2004, 279, 52744–52752. [Google Scholar] [CrossRef] [PubMed]
- Jansson, K.H.; Castillo, D.G.; Morris, J.W.; Boggs, M.E.; Czymmek, K.J.; Adams, E.L.; Schramm, L.P.; Sikes, R.A. Identification of beta-2 as a key cell adhesion molecule in PCa cell neurotropic behavior: A novel ex vivo and biophysical approach. PLoS ONE 2014, 9, e98408. [Google Scholar] [CrossRef] [PubMed]
- Namadurai, S.; Balasuriya, D.; Rajappa, R.; Wiemhofer, M.; Stott, K.; Klingauf, J.; Edwardson, J.M.; Chirgadze, D.Y.; Jackson, A.P. Crystal structure and molecular imaging of the Nav channel beta3 subunit indicates a trimeric assembly. J. Biol. Chem. 2014, 289, 10797–10811. [Google Scholar] [CrossRef] [PubMed]
- Ratcliffe, C.F.; Westenbroek, R.E.; Curtis, R.; Catterall, W.A. Sodium channel beta1 and beta3 subunits associate with neurofascin through their extracellular immunoglobulin-like domain. J. Cell Biol. 2001, 154, 427–434. [Google Scholar] [CrossRef]
- Shimizu, H.; Miyazaki, H.; Ohsawa, N.; Shoji, S.; Ishizuka-Katsura, Y.; Tosaki, A.; Oyama, F.; Terada, T.; Sakamoto, K.; Shirouzu, M.; et al. Structure-based site-directed photo-crosslinking analyses of multimeric cell-adhesive interactions of voltage-gated sodium channel beta subunits. Sci. Rep. 2016, 6, 26618. [Google Scholar] [CrossRef]
- Isom, L.L. The role of sodium channels in cell adhesion. Front. Biosci. 2002, 7, 12–23. [Google Scholar] [CrossRef]
- Manring, H.R.; Dorn, L.E.; Ex-Willey, A.; Accornero, F.; Ackermann, M.A. At the heart of inter- and intracellular signaling: The intercalated disc. Biophys. Rev. 2018, 10, 961–971. [Google Scholar] [CrossRef]
- Li, Y.; Merkel, C.D.; Zeng, X.; Heier, J.A.; Cantrell, P.S.; Sun, M.; Stolz, D.B.; Watkins, S.C.; Yates, N.A.; Kwiatkowski, A.V. The N-cadherin interactome in primary cardiomyocytes as defined using quantitative proximity proteomics. J. Cell Sci. 2019, 132. [Google Scholar] [CrossRef]
- Schinner, C.; Erber, B.M.; Yeruva, S.; Waschke, J. Regulation of cardiac myocyte cohesion and gap junctions via desmosomal adhesion. Acta Physiol. 2019, 226, e13242. [Google Scholar] [CrossRef]
- Kleber, A.G.; Saffitz, J.E. Role of the intercalated disc in cardiac propagation and arrhythmogenesis. Front. Physiol. 2014, 5, 404. [Google Scholar] [CrossRef]
- Vozzi, C.; Dupont, E.; Coppen, S.R.; Yeh, H.I.; Severs, N.J. Chamber-related differences in connexin expression in the human heart. J. Mol. Cell Cardiol. 1999, 31, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Goodenough, D.A.; Goliger, J.A.; Paul, D.L. Connexins, connexons, and intercellular communication. Annu. Rev. Biochem. 1996, 65, 475–502. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, G.S.; Valiunas, V.; Brink, P.R. Selective permeability of gap junction channels. Biochim. Biophys. Acta 2004, 1662, 96–101. [Google Scholar] [CrossRef]
- Zhang, Q.; Bai, X.; Liu, Y.; Wang, K.; Shen, B.; Sun, X. Current Concepts and Perspectives on Connexin43: A Mini Review. Curr. Protein Pept. Sci. 2018, 19, 1049–1057. [Google Scholar] [CrossRef]
- Kleber, A.G.; Rudy, Y. Basic mechanisms of cardiac impulse propagation and associated arrhythmias. Physiol. Rev. 2004, 84, 431–488. [Google Scholar] [CrossRef] [PubMed]
- Rohr, S. Role of gap junctions in the propagation of the cardiac action potential. Cardiovasc. Res. 2004, 62, 309–322. [Google Scholar] [CrossRef]
- Rhett, J.M.; Veeraraghavan, R.; Poelzing, S.; Gourdie, R.G. The perinexus: Sign-post on the path to a new model of cardiac conduction? Trends Cardiovasc. Med. 2013, 23, 222–228. [Google Scholar] [CrossRef]
- Rhett, J.M.; Gourdie, R.G. The perinexus: A new feature of Cx43 gap junction organization. Heart Rhythm. 2012, 9, 619–623. [Google Scholar] [CrossRef]
- Rhett, J.M.; Ongstad, E.L.; Jourdan, J.; Gourdie, R.G. Cx43 associates with Na(v)1.5 in the cardiomyocyte perinexus. J. Membr. Biol. 2012, 245, 411–422. [Google Scholar] [CrossRef]
- Hunter, A.W.; Barker, R.J.; Zhu, C.; Gourdie, R.G. Zonula occludens-1 alters connexin43 gap junction size and organization by influencing channel accretion. Mol. Biol. Cell 2005, 16, 5686–5698. [Google Scholar] [CrossRef]
- Meadows, L.S.; Isom, L.L. Sodium channels as macromolecular complexes: Implications for inherited arrhythmia syndromes. Cardiovasc. Res. 2005, 67, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Sorgen, P.L.; Trease, A.J.; Spagnol, G.; Delmar, M.; Nielsen, M.S. Protein(-)Protein Interactions with Connexin 43: Regulation and Function. Int. J. Mol. Sci. 2018, 19, 1428. [Google Scholar] [CrossRef] [PubMed]
- Makara, M.A.; Curran, J.; Little, S.C.; Musa, H.; Polina, I.; Smith, S.A.; Wright, P.J.; Unudurthi, S.D.; Snyder, J.; Bennett, V.; et al. Ankyrin-G coordinates intercalated disc signaling platform to regulate cardiac excitability In Vivo. Circ. Res. 2014, 115, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Clatot, J.; Hoshi, M.; Wan, X.; Liu, H.; Jain, A.; Shinlapawittayatorn, K.; Marionneau, C.; Ficker, E.; Ha, T.; Deschenes, I. Voltage-gated sodium channels assemble and gate as dimers. Nat. Commun. 2017, 8, 2077. [Google Scholar] [CrossRef]
- Mori, Y.; Fishman, G.I.; Peskin, C.S. Ephaptic conduction in a cardiac strand model with 3D electrodiffusion. Proc. Natl. Acad. Sci. USA 2008, 105, 6463–6468. [Google Scholar] [CrossRef]
- Lin, J.; Keener, J.P. Modeling electrical activity of myocardial cells incorporating the effects of ephaptic coupling. Proc. Natl. Acad. Sci. USA 2010, 107, 20935–20940. [Google Scholar] [CrossRef]
- Hichri, E.; Abriel, H.; Kucera, J.P. Distribution of cardiac sodium channels in clusters potentiates ephaptic interactions in the intercalated disc. J. Physiol. 2018, 596, 563–589. [Google Scholar] [CrossRef]
- Sperelakis, N. An electric field mechanism for transmission of excitation between myocardial cells. Circ. Res. 2002, 91, 985–987. [Google Scholar] [CrossRef] [PubMed]
- Veeraraghavan, R.; Lin, J.; Keener, J.P.; Gourdie, R.; Poelzing, S. Potassium channels in the Cx43 gap junction perinexus modulate ephaptic coupling: An experimental and modeling study. Pflug. Arch. 2016, 468, 1651–1661. [Google Scholar] [CrossRef]
- Willis, B.C.; Ponce-Balbuena, D.; Jalife, J. Protein assemblies of sodium and inward rectifier potassium channels control cardiac excitability and arrhythmogenesis. Am. J. Physiol. Heart Circ. Physiol. 2015, 308, H1463–H1473. [Google Scholar] [CrossRef]
- Abriel, H.; Rougier, J.S.; Jalife, J. Ion channel macromolecular complexes in cardiomyocytes: Roles in sudden cardiac death. Circ. Res. 2015, 116, 1971–1988. [Google Scholar] [CrossRef] [PubMed]
- Milstein, M.L.; Musa, H.; Balbuena, D.P.; Anumonwo, J.M.; Auerbach, D.S.; Furspan, P.B.; Hou, L.; Hu, B.; Schumacher, S.M.; Vaidyanathan, R.; et al. Dynamic reciprocity of sodium and potassium channel expression in a macromolecular complex controls cardiac excitability and arrhythmia. Proc. Natl. Acad. Sci. USA 2012, 109, E2134–E2143. [Google Scholar] [CrossRef] [PubMed]
- Lopatin, A.N.; Nichols, C.G. Inward rectifiers in the heart: An update on I(K1). J. Mol. Cell Cardiol. 2001, 33, 625–638. [Google Scholar] [CrossRef] [PubMed]
- Burstein, B.; Nattel, S. Atrial fibrosis: Mechanisms and clinical relevance in atrial fibrillation. J. Am. Coll. Cardiol. 2008, 51, 802–809. [Google Scholar] [CrossRef] [PubMed]
- Jeevaratnam, K.; Poh Tee, S.; Zhang, Y.; Rewbury, R.; Guzadhur, L.; Duehmke, R.; Grace, A.A.; Lei, M.; Huang, C.L. Delayed conduction and its implications in murine Scn5a(+/−) hearts: Independent and interacting effects of genotype, age, and sex. Pflug. Arch. 2011, 461, 29–44. [Google Scholar] [CrossRef]
- Veeraraghavan, R.; Hoeker, G.S.; Alvarez-Laviada, A.; Hoagland, D.; Wan, X.; King, D.R.; Sanchez-Alonso, J.; Chen, C.; Jourdan, J.; Isom, L.L.; et al. The adhesion function of the sodium channel beta subunit (beta1) contributes to cardiac action potential propagation. eLife 2018, 7. [Google Scholar] [CrossRef]
- Malhotra, J.D.; Kazen-Gillespie, K.; Hortsch, M.; Isom, L.L. Sodium channel beta subunits mediate homophilic cell adhesion and recruit ankyrin to points of cell-cell contact. J. Biol. Chem. 2000, 275, 11383–11388. [Google Scholar] [CrossRef]
- Raisch, T.B.; Yanoff, M.S.; Larsen, T.R.; Farooqui, M.A.; King, D.R.; Veeraraghavan, R.; Gourdie, R.G.; Baker, J.W.; Arnold, W.S.; AlMahameed, S.T.; et al. Intercalated Disk Extracellular Nanodomain Expansion in Patients With Atrial Fibrillation. Front. Physiol. 2018, 9, 398. [Google Scholar] [CrossRef]
- Watanabe, H.; Koopmann, T.T.; Le Scouarnec, S.; Yang, T.; Ingram, C.R.; Schott, J.J.; Demolombe, S.; Probst, V.; Anselme, F.; Escande, D.; et al. Sodium channel beta1 subunit mutations associated with Brugada syndrome and cardiac conduction disease in humans. J. Clin. Investig. 2008, 118, 2260–2268. [Google Scholar] [CrossRef]
- Watanabe, H.; Darbar, D.; Kaiser, D.W.; Jiramongkolchai, K.; Chopra, S.; Donahue, B.S.; Kannankeril, P.J.; Roden, D.M. Mutations in sodium channel beta1- and beta2-subunits associated with atrial fibrillation. Circ. Arrhythm Electrophysiol. 2009, 2, 268–275. [Google Scholar] [CrossRef]
- Kobirumaki-Shimozawa, F.; Nakanishi, T.; Shimozawa, T.; Terui, T.; Oyama, K.; Li, J.; Louch, W.E.; Ishiwata, S.; Fukuda, N. Real-Time In Vivo Imaging of Mouse Left Ventricle Reveals Fluctuating Movements of the Intercalated Discs. Nanomaterials 2020, 10, 532. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, J.D.; Koopmann, M.C.; Kazen-Gillespie, K.A.; Fettman, N.; Hortsch, M.; Isom, L.L. Structural requirements for interaction of sodium channel beta 1 subunits with ankyrin. J. Biol. Chem. 2002, 277, 26681–26688. [Google Scholar] [CrossRef] [PubMed]
- Brackenbury, W.J.; Djamgoz, M.B.; Isom, L.L. An emerging role for voltage-gated Na+ channels in cellular migration: Regulation of central nervous system development and potentiation of invasive cancers. Neuroscientist 2008, 14, 571–583. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, J.D.; Thyagarajan, V.; Chen, C.; Isom, L.L. Tyrosine-phosphorylated and nonphosphorylated sodium channel beta1 subunits are differentially localized in cardiac myocytes. J. Biol. Chem. 2004, 279, 40748–40754. [Google Scholar] [CrossRef]
- Hu, D.; Barajas-Martinez, H.; Medeiros-Domingo, A.; Crotti, L.; Veltmann, C.; Schimpf, R.; Urrutia, J.; Alday, A.; Casis, O.; Pfeiffer, R.; et al. A novel rare variant in SCN1Bb linked to Brugada syndrome and SIDS by combined modulation of Na(v)1.5 and K(v)4.3 channel currents. Heart Rhythm. 2012, 9, 760–769. [Google Scholar] [CrossRef]
- Yereddi, N.R.; Cusdin, F.S.; Namadurai, S.; Packman, L.C.; Monie, T.P.; Slavny, P.; Clare, J.J.; Powell, A.J.; Jackson, A.P. The immunoglobulin domain of the sodium channel beta3 subunit contains a surface-localized disulfide bond that is required for homophilic binding. FASEB J. 2013, 27, 568–580. [Google Scholar] [CrossRef]
- McEwen, D.P.; Chen, C.; Meadows, L.S.; Lopez-Santiago, L.; Isom, L.L. The voltage-gated Na+ channel beta3 subunit does not mediate trans homophilic cell adhesion or associate with the cell adhesion molecule contactin. Neurosci. Lett. 2009, 462, 272–275. [Google Scholar] [CrossRef][Green Version]
- Dhar Malhotra, J.; Chen, C.; Rivolta, I.; Abriel, H.; Malhotra, R.; Mattei, L.N.; Brosius, F.C.; Kass, R.S.; Isom, L.L. Characterization of sodium channel alpha- and beta-subunits in rat and mouse cardiac myocytes. Circulation 2001, 103, 1303–1310. [Google Scholar] [CrossRef]
- Shimizu, H.; Tosaki, A.; Ohsawa, N.; Ishizuka-Katsura, Y.; Shoji, S.; Miyazaki, H.; Oyama, F.; Terada, T.; Shirouzu, M.; Sekine, S.I.; et al. Parallel homodimer structures of the extracellular domains of the voltage-gated sodium channel beta4 subunit explain its role in cell-cell adhesion. J. Biol. Chem. 2017, 292, 13428–13440. [Google Scholar] [CrossRef]
- Shapiro, L.; Fannon, A.M.; Kwong, P.D.; Thompson, A.; Lehmann, M.S.; Grubel, G.; Legrand, J.F.; Als-Nielsen, J.; Colman, D.R.; Hendrickson, W.A. Structural basis of cell-cell adhesion by cadherins. Nature 1995, 374, 327–337. [Google Scholar] [CrossRef]
- Harrison, O.J.; Brasch, J.; Lasso, G.; Katsamba, P.S.; Ahlsen, G.; Honig, B.; Shapiro, L. Structural basis of adhesive binding by desmocollins and desmogleins. Proc. Natl. Acad. Sci. USA 2016, 113, 7160–7165. [Google Scholar] [CrossRef] [PubMed]
- Brasch, J.; Harrison, O.J.; Honig, B.; Shapiro, L. Thinking outside the cell: How cadherins drive adhesion. Trends Cell Biol. 2012, 22, 299–310. [Google Scholar] [CrossRef]
- Medeiros-Domingo, A.; Kaku, T.; Tester, D.J.; Iturralde-Torres, P.; Itty, A.; Ye, B.; Valdivia, C.; Ueda, K.; Canizales-Quinteros, S.; Tusie-Luna, M.T.; et al. SCN4B-encoded sodium channel beta4 subunit in congenital long-QT syndrome. Circulation 2007, 116, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Yarbrough, T.L.; Lu, T.; Lee, H.C.; Shibata, E.F. Localization of cardiac sodium channels in caveolin-rich membrane domains: Regulation of sodium current amplitude. Circ. Res. 2002, 90, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Maguy, A.; Hebert, T.E.; Nattel, S. Involvement of lipid rafts and caveolae in cardiac ion channel function. Cardiovasc. Res. 2006, 69, 798–807. [Google Scholar] [CrossRef] [PubMed]
- Sanon, V.P.; Sawaki, D.; Mjaatvedt, C.H.; Jourdan-Le Saux, C. Myocardial tissue caveolae. Compr. Physiol. 2015, 5, 871–886. [Google Scholar] [CrossRef]
- Aicart-Ramos, C.; Valero, R.A.; Rodriguez-Crespo, I. Protein palmitoylation and subcellular trafficking. Biochim. Biophys. Acta 2011, 1808, 2981–2994. [Google Scholar] [CrossRef]
- Bouza, A.A.; Philippe, J.M.; Edokobi, N.; Pinsky, A.M.; Offord, J.; Calhoun, J.D.; Lopez-Floran, M.; Lopez-Santiago, L.F.; Jenkins, P.M.; Isom, L.L. Sodium channel beta1 subunits are post-translationally modified by tyrosine phosphorylation, S-palmitoylation, and regulated intramembrane proteolysis. J. Biol. Chem. 2020. [Google Scholar] [CrossRef]
- Alday, A.; Urrutia, J.; Gallego, M.; Casis, O. alpha1-adrenoceptors regulate only the caveolae-located subpopulation of cardiac K(V)4 channels. Channels (Austin) 2010, 4, 168–178. [Google Scholar] [CrossRef][Green Version]
- Vaidyanathan, R.; Reilly, L.; Eckhardt, L.L. Caveolin-3 Microdomain: Arrhythmia Implications for Potassium Inward Rectifier and Cardiac Sodium Channel. Front. Physiol. 2018, 9, 1548. [Google Scholar] [CrossRef]
- Marionneau, C.; Carrasquillo, Y.; Norris, A.J.; Townsend, R.R.; Isom, L.L.; Link, A.J.; Nerbonne, J.M. The sodium channel accessory subunit Navbeta1 regulates neuronal excitability through modulation of repolarizing voltage-gated K(+) channels. J. Neurosci. 2012, 32, 5716–5727. [Google Scholar] [CrossRef] [PubMed]
- Dabiri, B.E.; Lee, H.; Parker, K.K. A potential role for integrin signaling in mechanoelectrical feedback. Prog. Biophys. Mol. Biol. 2012, 110, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Kawabe, J.; Okumura, S.; Lee, M.C.; Sadoshima, J.; Ishikawa, Y. Translocation of caveolin regulates stretch-induced ERK activity in vascular smooth muscle cells. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, H1845–H1852. [Google Scholar] [CrossRef]
- Israeli-Rosenberg, S.; Chen, C.; Li, R.; Deussen, D.N.; Niesman, I.R.; Okada, H.; Patel, H.H.; Roth, D.M.; Ross, R.S. Caveolin modulates integrin function and mechanical activation in the cardiomyocyte. FASEB J. 2015, 29, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.J.; Wu, X.; Nurkiewicz, T.R.; Kawasaki, J.; Gui, P.; Hill, M.A.; Wilson, E. Regulation of ion channels by integrins. Cell Biochem. Biophys. 2002, 36, 41–66. [Google Scholar] [CrossRef]
- Tyan, L.; Foell, J.D.; Vincent, K.P.; Woon, M.T.; Mesquitta, W.T.; Lang, D.; Best, J.M.; Ackerman, M.J.; McCulloch, A.D.; Glukhov, A.V.; et al. Long QT syndrome caveolin-3 mutations differentially modulate Kv 4 and Cav 1.2 channels to contribute to action potential prolongation. J. Physiol. 2019, 597, 1531–1551. [Google Scholar] [CrossRef] [PubMed]
- Vatta, M.; Ackerman, M.J.; Ye, B.; Makielski, J.C.; Ughanze, E.E.; Taylor, E.W.; Tester, D.J.; Balijepalli, R.C.; Foell, J.D.; Li, Z.; et al. Mutant caveolin-3 induces persistent late sodium current and is associated with long-QT syndrome. Circulation 2006, 114, 2104–2112. [Google Scholar] [CrossRef]
- Echarri, A.; Del Pozo, M.A. Caveolae—mechanosensitive membrane invaginations linked to actin filaments. J. Cell Sci. 2015, 128, 2747–2758. [Google Scholar] [CrossRef]
- Kohl, P.; Cooper, P.J.; Holloway, H. Effects of acute ventricular volume manipulation on In Situ cardiomyocyte cell membrane configuration. Prog. Biophys. Mol. Biol. 2003, 82, 221–227. [Google Scholar] [CrossRef]
- Sinha, B.; Koster, D.; Ruez, R.; Gonnord, P.; Bastiani, M.; Abankwa, D.; Stan, R.V.; Butler-Browne, G.; Vedie, B.; Johannes, L.; et al. Cells respond to mechanical stress by rapid disassembly of caveolae. Cell 2011, 144, 402–413. [Google Scholar] [CrossRef]
- Wary, K.K.; Mariotti, A.; Zurzolo, C.; Giancotti, F.G. A requirement for caveolin-1 and associated kinase Fyn in integrin signaling and anchorage-dependent cell growth. Cell 1998, 94, 625–634. [Google Scholar] [CrossRef]
- Ahern, C.A.; Zhang, J.F.; Wookalis, M.J.; Horn, R. Modulation of the cardiac sodium channel NaV1.5 by Fyn, a Src family tyrosine kinase. Circ. Res. 2005, 96, 991–998. [Google Scholar] [CrossRef]
- Beyder, A.; Rae, J.L.; Bernard, C.; Strege, P.R.; Sachs, F.; Farrugia, G. Mechanosensitivity of Nav1.5, a voltage-sensitive sodium channel. J. Physiol. 2010, 588, 4969–4985. [Google Scholar] [CrossRef] [PubMed]
- Morris, C.E.; Juranka, P.F. Nav channel mechanosensitivity: Activation and inactivation accelerate reversibly with stretch. Biophys. J. 2007, 93, 822–833. [Google Scholar] [CrossRef]
- Maroni, M.; Korner, J.; Schuttler, J.; Winner, B.; Lampert, A.; Eberhardt, E. beta1 and beta3 subunits amplify mechanosensitivity of the cardiac voltage-gated sodium channel Nav1.5. Pflug. Arch. 2019, 471, 1481–1492. [Google Scholar] [CrossRef] [PubMed]
- Koivumaki, J.T.; Clark, R.B.; Belke, D.; Kondo, C.; Fedak, P.W.; Maleckar, M.M.; Giles, W.R. Na(+) current expression in human atrial myofibroblasts: Identity and functional roles. Front. Physiol. 2014, 5, 275. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nattel, S. Electrical coupling between cardiomyocytes and fibroblasts: Experimental testing of a challenging and important concept. Cardiovasc. Res. 2018, 114, 349–352. [Google Scholar] [CrossRef] [PubMed]
- Rog-Zielinska, E.A.; Kong, C.H.T.; Zgierski-Johnston, C.M.; Verkade, P.; Mantell, J.; Cannell, M.B.; Kohl, P. Species differences in the morphology of transverse tubule openings in cardiomyocytes. Europace 2018, 20, iii120–iii124. [Google Scholar] [CrossRef]
- Kostin, S.; Scholz, D.; Shimada, T.; Maeno, Y.; Mollnau, H.; Hein, S.; Schaper, J. The internal and external protein scaffold of the T-tubular system in cardiomyocytes. Cell Tissue Res. 1998, 294, 449–460. [Google Scholar] [CrossRef]
- Lin, X.; O’Malley, H.; Chen, C.; Auerbach, D.; Foster, M.; Shekhar, A.; Zhang, M.; Coetzee, W.; Jalife, J.; Fishman, G.I.; et al. Scn1b deletion leads to increased tetrodotoxin-sensitive sodium current, altered intracellular calcium homeostasis and arrhythmias in murine hearts. J. Physiol. 2015, 593, 1389–1407. [Google Scholar] [CrossRef]
- Abriel, H. Cardiac sodium channel Na(v)1.5 and interacting proteins: Physiology and pathophysiology. J. Mol. Cell Cardiol. 2010, 48, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Kitazawa, M.; Kubo, Y.; Nakajo, K. The stoichiometry and biophysical properties of the Kv4 potassium channel complex with K+ channel-interacting protein (KChIP) subunits are variable, depending on the relative expression level. J. Biol. Chem. 2014, 289, 17597–17609. [Google Scholar] [CrossRef] [PubMed]
- Doolittle, R.F. The multiplicity of domains in proteins. Annu. Rev. Biochem. 1995, 64, 287–314. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.J.; Zhao, R. Purification and cloning of PZR, a binding protein and putative physiological substrate of tyrosine phosphatase SHP-2. J. Biol. Chem. 1998, 273, 29367–29372. [Google Scholar] [CrossRef]
- Wang, L.; Nomura, Y.; Du, Y.; Dong, K. Differential effects of TipE and a TipE-homologous protein on modulation of gating properties of sodium channels from Drosophila melanogaster. PLoS ONE 2013, 8, e67551. [Google Scholar] [CrossRef]
- Muhamed, I.; Chowdhury, F.; Maruthamuthu, V. Biophysical Tools to Study Cellular Mechanotransduction. Bioengineering 2017, 4, 12. [Google Scholar] [CrossRef]
- Ohtsubo, K.; Marth, J.D. Glycosylation in cellular mechanisms of health and disease. Cell 2006, 126, 855–867. [Google Scholar] [CrossRef]
- Cortada, E.; Brugada, R.; Verges, M. N-Glycosylation of the voltage-gated sodium channel beta2 subunit is required for efficient trafficking of NaV1.5/beta2 to the plasma membrane. J. Biol. Chem. 2019, 294, 16123–16140. [Google Scholar] [CrossRef]
- Johnson, D.; Montpetit, M.L.; Stocker, P.J.; Bennett, E.S. The sialic acid component of the beta1 subunit modulates voltage-gated sodium channel function. J. Biol. Chem. 2004, 279, 44303–44310. [Google Scholar] [CrossRef]
- Veeraraghavan, R.; Gourdie, R.G.; Poelzing, S. Mechanisms of cardiac conduction: A history of revisions. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H619–H627. [Google Scholar] [CrossRef]
- Scheffer, I.E.; Harkin, L.A.; Grinton, B.E.; Dibbens, L.M.; Turner, S.J.; Zielinski, M.A.; Xu, R.; Jackson, G.; Adams, J.; Connellan, M.; et al. Temporal lobe epilepsy and GEFS+ phenotypes associated with SCN1B mutations. Brain 2007, 130, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wang, S.C.; Li, D.; Li, T.; Yang, H.P.; Wang, L.; Wang, Y.F.; Parpura, V. Role of Connexin 36 in Autoregulation of Oxytocin Neuronal Activity in Rat Supraoptic Nucleus. ASN Neuro. 2019, 11, 1759091419843762. [Google Scholar] [CrossRef] [PubMed]
- Micevych, P.E.; Popper, P.; Hatton, G.I. Connexin 32 mRNA levels in the rat supraoptic nucleus: Up-regulation prior to parturition and during lactation. Neuroendocrinology 1996, 63, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Furshpan, E.J.; Furukawa, T. Intracellular and extracellular responses of the several regions of the Mauthner cell of the goldfish. J. Neurophysiol. 1962, 25, 732–771. [Google Scholar] [CrossRef]
- Ter Keurs, H.E.; Zhang, Y.M.; Davidoff, A.W.; Boyden, P.A.; Wakayama, Y.; Miura, M. Damage induced arrhythmias: Mechanisms and implications. Can. J. Physiol. Pharmacol. 2001, 79, 73–81. [Google Scholar] [CrossRef]
- Brackenbury, W.J.; Isom, L.L. Voltage-gated Na+ channels: Potential for beta subunits as therapeutic targets. Expert Opin. Ther. Targets 2008, 12, 1191–1203. [Google Scholar] [CrossRef]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salvage, S.C.; Huang, C.L.-H.; Jackson, A.P. Cell-Adhesion Properties of β-Subunits in the Regulation of Cardiomyocyte Sodium Channels. Biomolecules 2020, 10, 989. https://doi.org/10.3390/biom10070989
Salvage SC, Huang CL-H, Jackson AP. Cell-Adhesion Properties of β-Subunits in the Regulation of Cardiomyocyte Sodium Channels. Biomolecules. 2020; 10(7):989. https://doi.org/10.3390/biom10070989
Chicago/Turabian StyleSalvage, Samantha C., Christopher L.-H. Huang, and Antony P. Jackson. 2020. "Cell-Adhesion Properties of β-Subunits in the Regulation of Cardiomyocyte Sodium Channels" Biomolecules 10, no. 7: 989. https://doi.org/10.3390/biom10070989
APA StyleSalvage, S. C., Huang, C. L.-H., & Jackson, A. P. (2020). Cell-Adhesion Properties of β-Subunits in the Regulation of Cardiomyocyte Sodium Channels. Biomolecules, 10(7), 989. https://doi.org/10.3390/biom10070989





