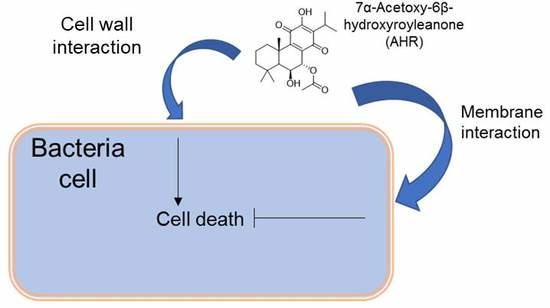
Unveiling the Mechanism of Action of 7α-acetoxy-6β-hydroxyroyleanone on an MRSA/VISA Strain: Membrane and Cell Wall Interactions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Extraction, Isolation and Purification of AHR
2.3. Liposome Preparation
2.4. Absorption Measurements
2.5. Strains and Growth Conditions
2.6. Synergy Studies
2.7. Bacterial Growth Curve
2.8. Effect of AHR on the Viability of CIP106760 Strain
2.9. Cell Leakage Assay
2.10. Membrane Interaction and Leakage Assay
2.11. Proton Leakage Assay
2.12. Cell Surface Charge
2.13. Lysis Assay
2.14. Analysis of Peptidoglycan Composition
2.15. Scanning Electron Microscopy (SEM) Analysis
3. Results and Discussion
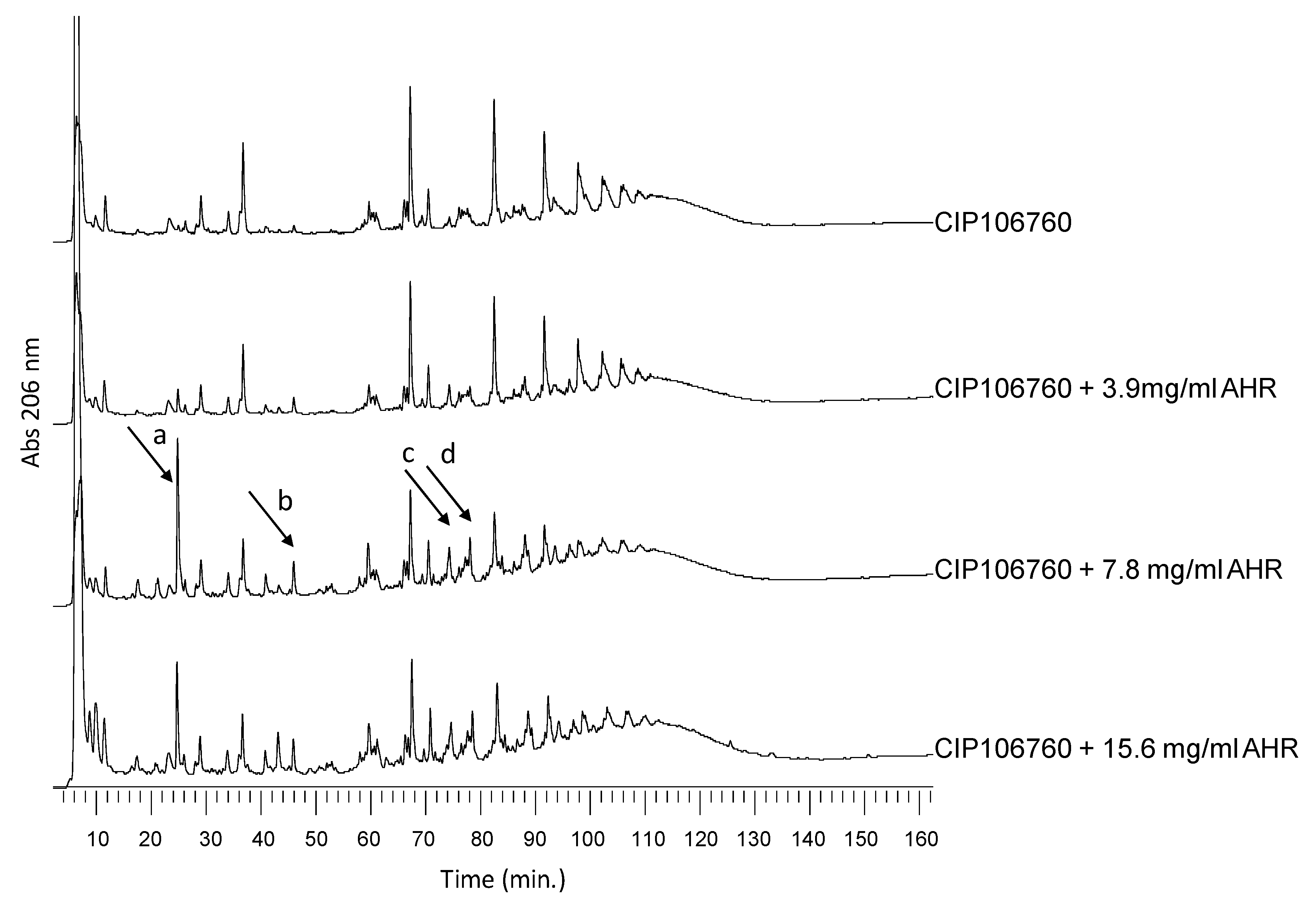
3.1. Extraction Optimization of AHR
3.2. AHR does not Provide a Synergistic Effect with Cell Wall Antibiotics
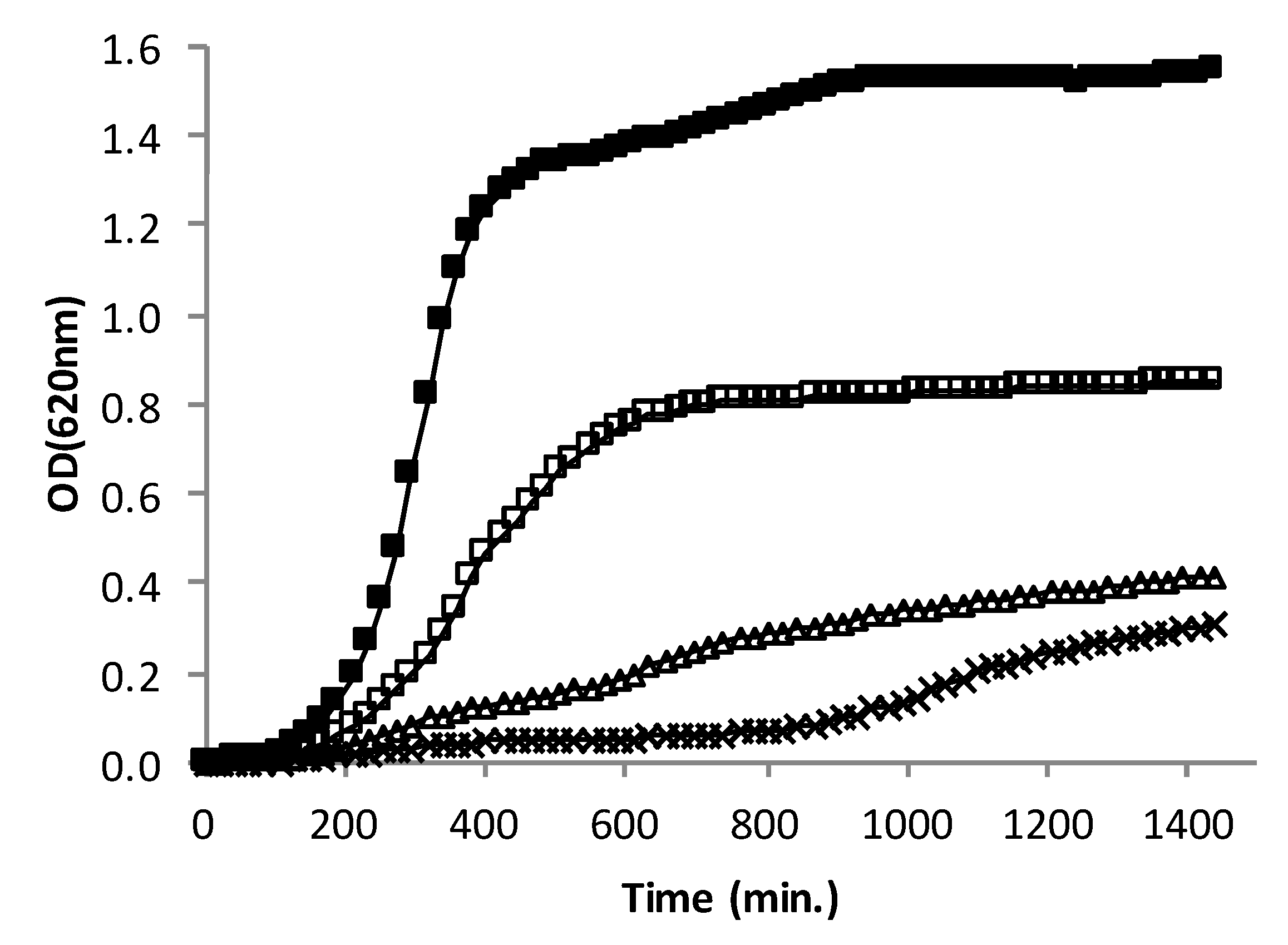
3.3. Effect of AHR on the Growth Rate of CIP106760 Strain
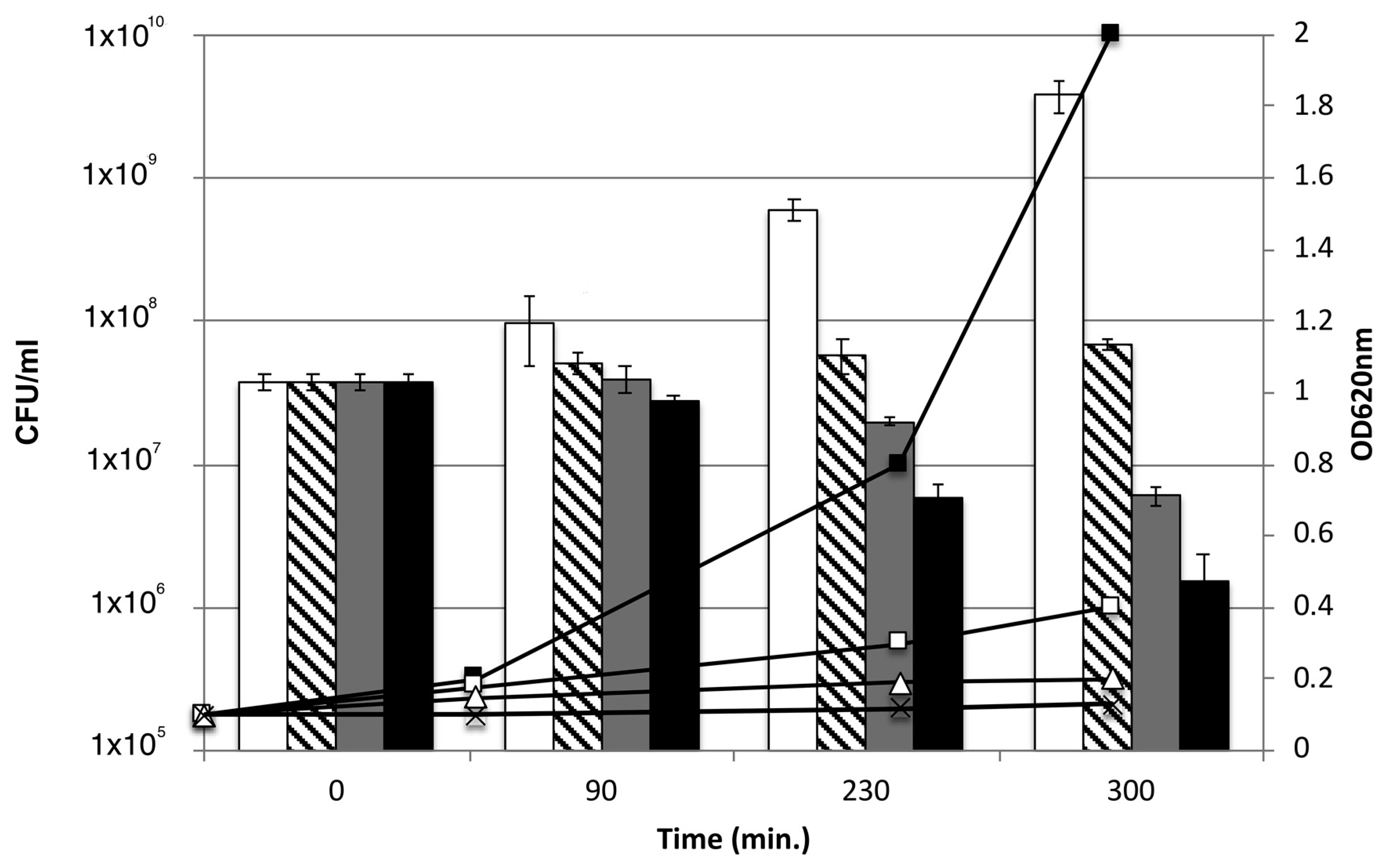
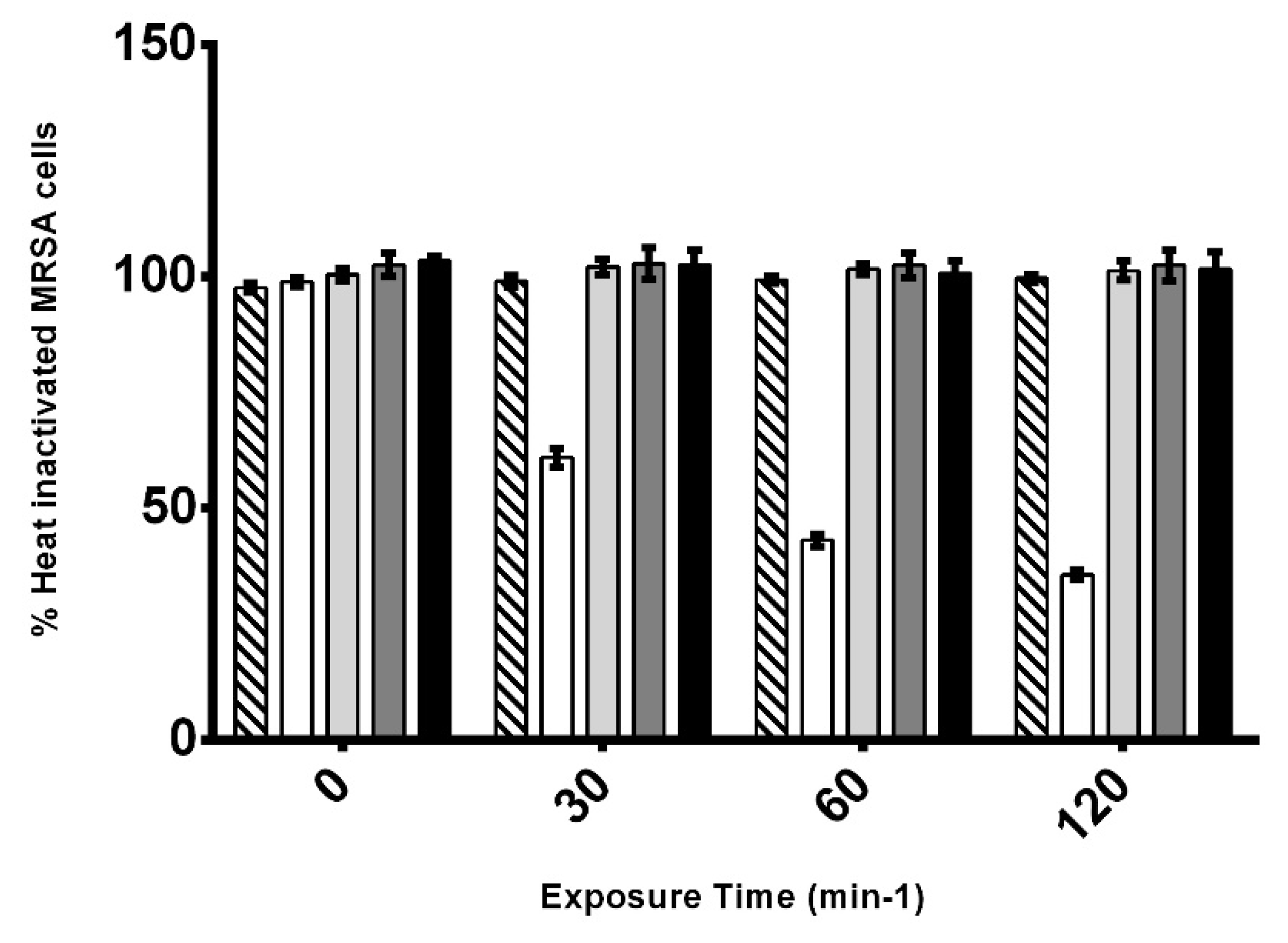
3.4. Effect of AHR on the Viability of CIP106760 Strain
3.5. Effect of AHR on the cell integrity of CIP106760 strain
3.6. Assessment of AHR Cell Wall Lytic Activity
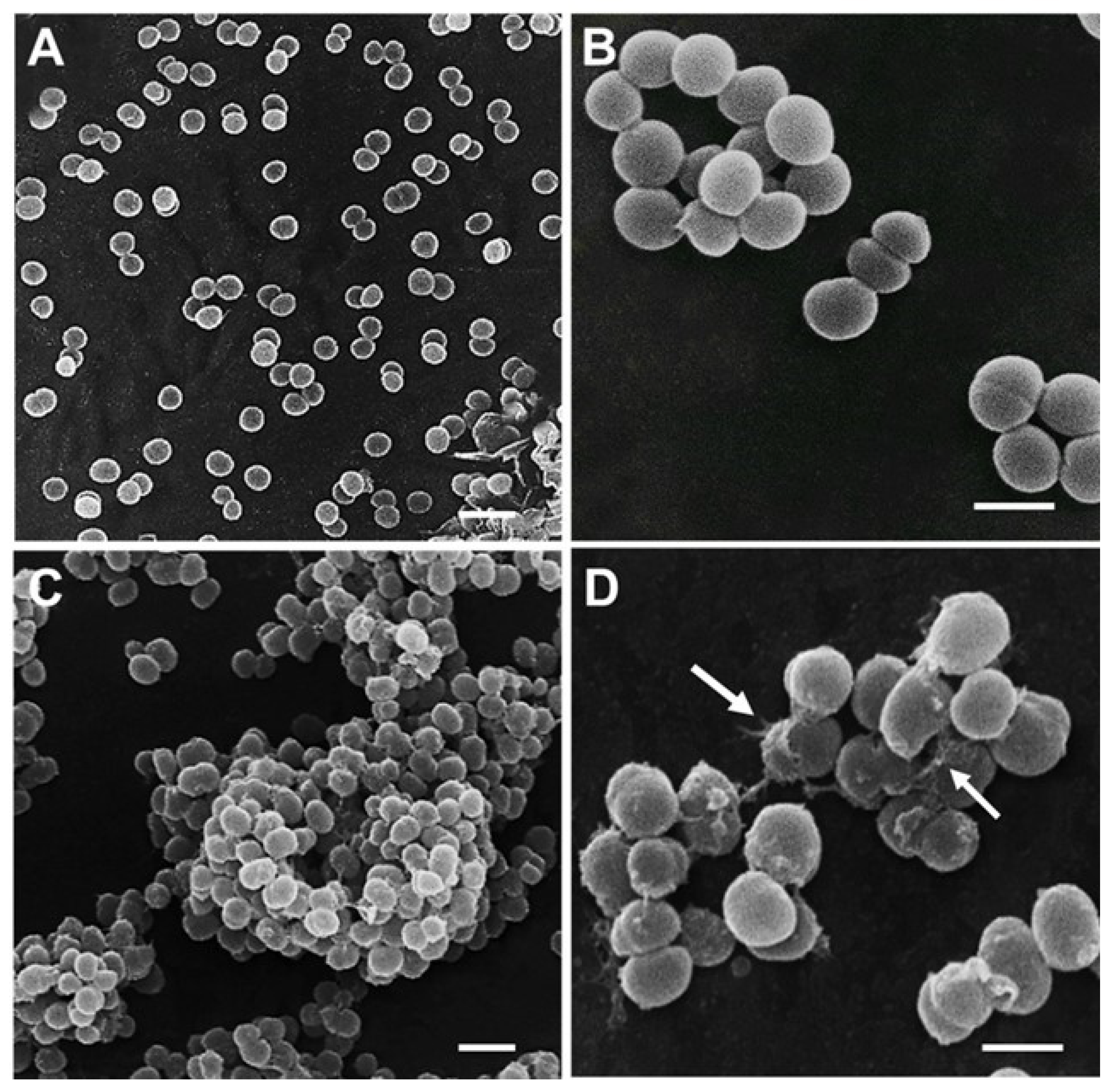
3.7. Cell Morphological Alterations upon Challenge with AHR
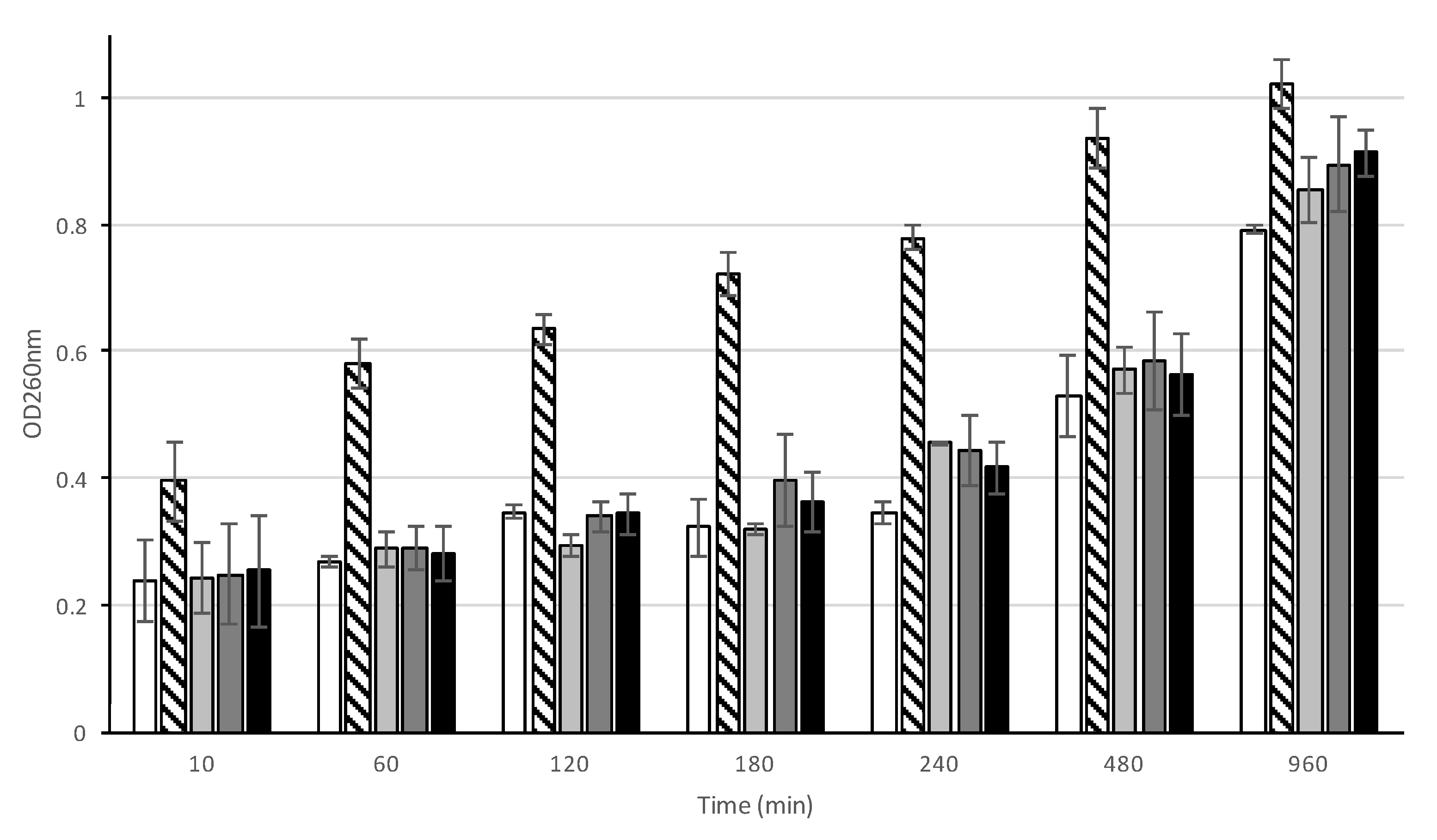
3.8. Effect of AHR on Cell Wall Synthesis and Peptidoglycan Composition
3.9. Assessment of AHR Effect on the Cell Surface Net Charge
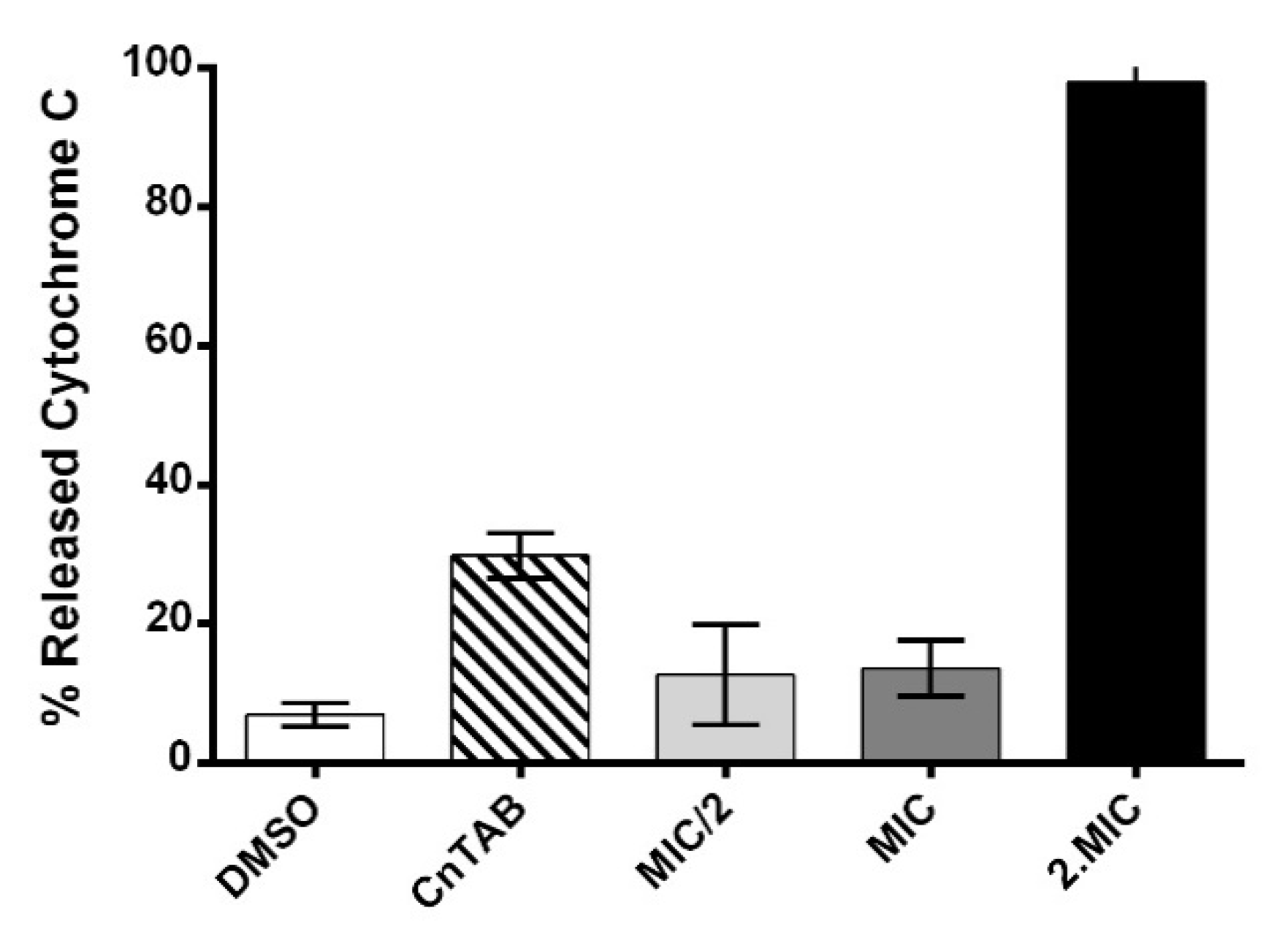
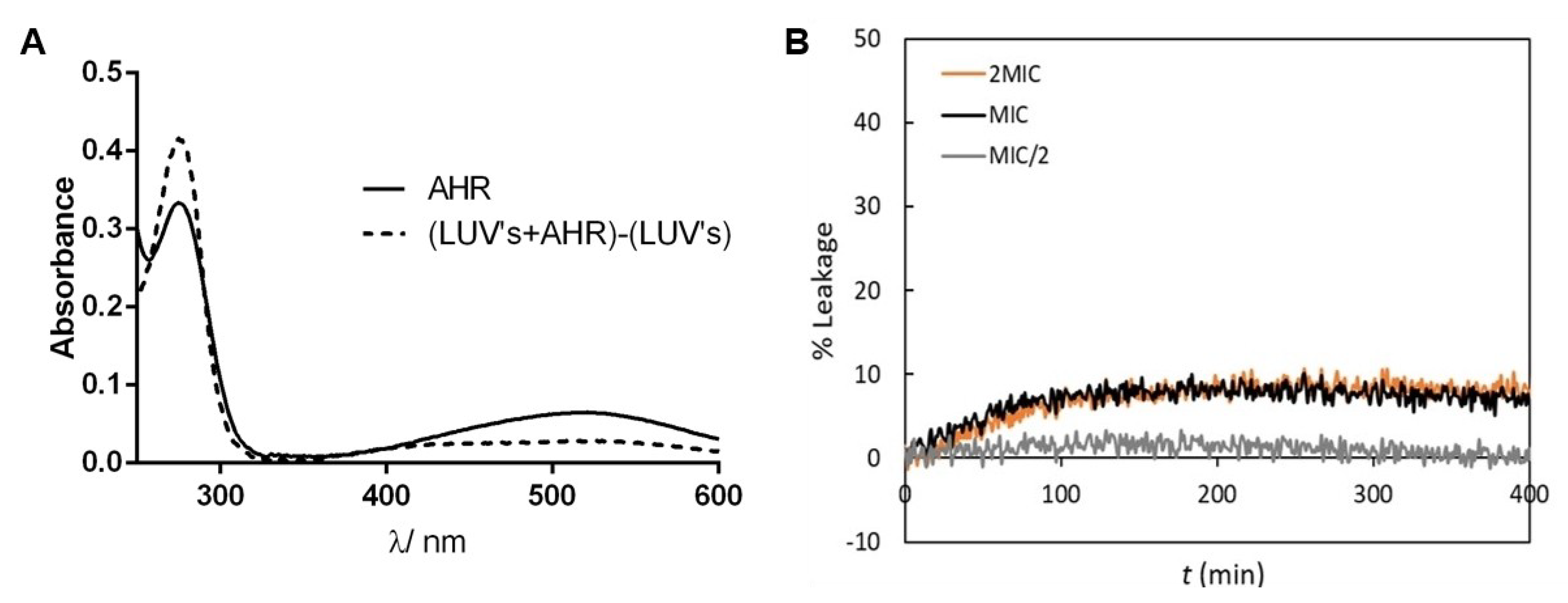
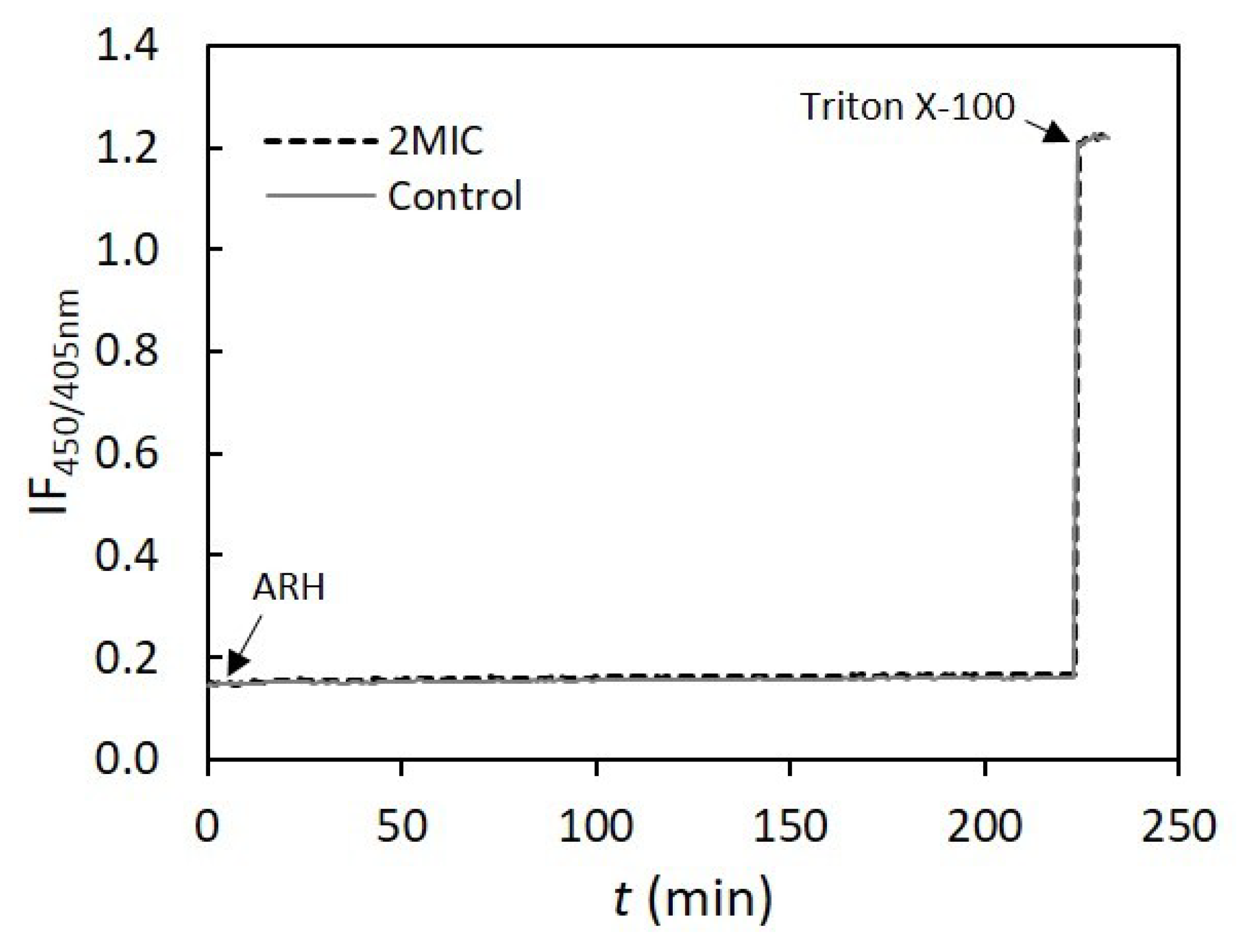
3.10. Assessment of AHR Interaction and Effect on Membrane Lipid Bilayers’ Permeability
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AHR | 7α-Acetoxy-6β-hydroxyroyleanone |
| CF | 5,6-carboxyfluorescein |
| DMSO | Dimethyl sulfoxide |
| cfu | Colony-forming unit |
| DOPC | 1,2-dioleoyl-sn-glycero-3-phosphocholine |
| EDTA | Ethylenediaminetetraacetic acid |
| GI50 | Concentration for 50% of Maximal Inhibition of Cell Growth |
| HCl | Hydrogen chloride |
| HPLC-DAD | High-pressure Liquid Chromatography-diode-array detection |
| HPTS | 8 hydroxypyrene-1,3,6-trisulfonic acid |
| LUVs | Large Unilamellar Vesicles |
| MH | Mueller-Hinton |
| MIC | Minimum Inhibitory Concentration |
| MRSA | Methicillin-Resistant Staphylococcus aureus |
| NaCl | Sodium chloride |
| OD | Optical Density |
| PBS | Phosphate Buffered Saline |
| POPC | 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine |
| PSM | N-palmitoyl-sphingomyelin |
| RP-HPLC | Reverse Phase- High-pressure Liquid Chromatography |
| SARSFP | Severe Acute Respiratory Syndrome-related coronavirus |
| SEM | Scanning Electron Microscopy |
| SCF | Supercritical fluid |
| UV | UltraViolet |
| VISA | Vancomycin-intermediate S. aureus. |
References
- Palacios, L.; Rosado, H.; Micol, V.; Rosato, A.E.; Bernal, P.; Arroyo, R.; Grounds, H.; Anderson, J.C.; Stabler, R.A.; Taylor, P.W. Staphylococcal phenotypes induced by naturally occurring and synthetic membrane-interactive polyphenolic β-lactam resistance modifiers. PLoS ONE 2014, 9, e93830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rijo, P.; Duarte, A.; Francisco, A.P.; Semedo-Lemsaddek, T.; Simões, M.F. In vitro Antimicrobial Activity of Royleanone Derivatives against Gram-Positive Bacterial Pathogens. Phyther. Res. 2014, 28, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Greay, S.J.; Hammer, K.A. Recent developments in the bioactivity of mono- and diterpenes: Anticancer and antimicrobial activity. Phytochem. Rev. 2015, 14, 1–6. [Google Scholar] [CrossRef]
- Ladeiras, D.; Monteiro, C.; Pereira, F.; Reis, C.; Afonso, C.; Rijo, P. Reactivity of Diterpenoid Quinones: Royleanones. Curr. Pharm. Des. 2016, 22, 1682–1714. [Google Scholar] [CrossRef] [PubMed]
- Gaspar-Marques, C.; Rijo, P.; Simões, M.F.; Duarte, M.A.; Rodriguez, B. Abietanes from Plectranthus grandidentatus and P. hereroensis against methicillin- and vancomycin-resistant bacteria. Phytomedicine 2006, 13, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Rijo, P. Phytochemical Study and Biological Activities of Diterpenes and Derivatives from Plectranthus Species; Universidade De Lisboa Faculdade De Farmácia: Lisbon, Portugal, 2011. [Google Scholar]
- Carreira, A.C.; de Almeida, R.F.M.; Silva, L.C. Development of lysosome-mimicking vesicles to study the effect of abnormal accumulation of sphingosine on membrane properties. Sci. Rep. 2017, 7, 3949. [Google Scholar] [CrossRef] [PubMed]
- Guillén, J.; de Almeida, R.F.M.; Prieto, M.; Villalaín, J. Structural and Dynamic Characterization of the Interaction of the Putative Fusion Peptide of the S2 SARS-CoV Virus Protein with Lipid Membranes. J. Phys. Chem. B 2008, 112, 6997–7007. [Google Scholar] [CrossRef] [PubMed]
- McClare, C.W. An accurate and convenient organic phosphorus assay. Anal. Biochem. 1971, 39, 527–530. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Springer: New Delhi, India, 2006. [Google Scholar]
- Grilo, I.R.; Ludovice, A.M.; Tomasz, A.; de Lencastre, H.; Sobral, R.G. The glucosaminidase domain of Atl-the major Staphylococcus aureus autolysin—Has DNA-binding activity. Microbiologyopen 2014, 3, 247–256. [Google Scholar] [CrossRef] [PubMed]
- De Jonge, B.L.; Chang, Y.; Gage, D.; Tomasz, A. Peptidoglycan composition of a highly methicillin-resistant Staphylococcus aureus strain. The role of penicillin binding protein 2A. J. Biol. Chem. 1992, 267, 11248–11254. [Google Scholar] [PubMed]
- Sobral, R.G.; Ludovice, A.M.; de Lencastre, H.; Tomasz, A. Role of murF in Cell Wall Biosynthesis: Isolation and Characterization of a murF Conditional Mutant of Staphylococcus aureus. J. Bacteriol. 2006, 188, 2543–2553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Figueiredo, T.A.; Sobral, R.G.; Ludovice, A.M.; de Almeida, J.M.F.; Bui, N.K.; Vollmer, W.; de Lencastre, H.; Tomasz, A. Identification of Genetic Determinants and Enzymes Involved with the Amidation of Glutamic Acid Residues in the Peptidoglycan of Staphylococcus aureus. PLoS Pathog. 2012, 8, e1002508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, F.; Yang, Y.; Yang, G.; Yu, L. Studies on antibacterial activity and antibacterial mechanism of a novel polysaccharide from Streptomyces virginia H03. Food Control 2010, 21, 1257–1262. [Google Scholar] [CrossRef]
- Mohammad, H.; Reddy, P.V.N.; Monteleone, D.; Mayhoub, A.S.; Cushman, M.; Hammac, G.K.; Seleem, M.N. Antibacterial Characterization of Novel Synthetic Thiazole Compounds against Methicillin-Resistant Staphylococcus pseudintermedius. PLoS ONE 2015, 10, e0130385. [Google Scholar] [CrossRef] [PubMed]
- Bayer, A.S.; Kupferwasser, L.I.; Brown, M.H.; Skurray, R.A.; Grkovic, S.; Jones, T.; Mukhopadhay, K.; Yeaman, M.R. Low-level resistance of Staphylococcus aureus to thrombin-induced platelet microbicidal protein 1 in vitro associated with qacA gene carriage is independent of multidrug efflux pump activity. Antimicrob. Agents Chemother. 2006, 50, 2448–2454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halder, S.; Yadav, K.K.; Sarkar, R.; Mukherjee, S.; Saha, P.; Haldar, S.; Karmakar, S.; Sen, T. Alteration of Zeta potential and membrane permeability in bacteria: A study with cationic agents. Springerplus 2015, 4, 672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bastos, A.E.P.; Marinho, H.S.; Cordeiro, A.M.; de Soure, A.M.; de Almeida, R.F.M. Biophysical properties of ergosterol-enriched lipid rafts in yeast and tools for their study: Characterization of ergosterol/phosphatidylcholine membranes with three fluorescent membrane probes. Chem. Phys. Lipids 2012, 165, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Ambroggio, E.E.; Separovic, F.; Bowie, J.H.; Fidelio, G.D.; Bagatolli, L.A. Direct Visualization of Membrane Leakage Induced by the Antibiotic Peptides: Maculatin, Citropin, and Aurein. Biophys. J. 2005, 89, 1874–1881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gray, D.A.; Wenzel, M. More than a pore: A current perspective on the in vivo mode of action of the lipopeptide antibiotic daptomycin. Antibiotics 2020, 9, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Figueiredo, T.A.; Ludovice, A.M.; Sobral, R.G. Contribution of peptidoglycan amidation to beta-lactam and lysozyme resistance in different genetic lineages of staphylococcus aureus. Microb. Drug Resist. 2014, 20, 238–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Extraction Method | AHR Index (µg mg−1 Extract) |
|---|---|
| Acetone maceration | 73.3 |
| Acetone ultrasonic-assisted | 52.0 |
| Supercritical fluid | 52.3 |
| Compound. | Zone Inhibition (mm). | |||
|---|---|---|---|---|
| DMSO | MET | AMP | VANC | |
| AHR | 21 ± 2 | 20 ± 2 | 20 ± 2 | 19 ± 3 |
| No AHR | 5 | 5 | 5 | 17 ± 1 |
| AHR Concentration | % ΔpH | |
|---|---|---|
| Low Cholesterol LUVs | High Cholesterol LUVs | |
| 15.6 mg/L (2MIC) | 5.1 ± 0.1 | 1.4 ± 0.2 |
| 7.8 mg/L (MIC) | 4.8 ± 0.3 | 1.5 ± 0.3 |
| 3.9 mg/L (MIC/2) | 4.6 ± 0.7 | 1.6 ± 0.2 |
| Control | 3.6 ± 0.2 | 1.3 ± 0.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, F.; Figueiredo, T.; de Almeida, R.F.M.; Antunes, C.A.C.; Garcia, C.; Reis, C.P.; Ascensão, L.; Sobral, R.G.; Rijo, P. Unveiling the Mechanism of Action of 7α-acetoxy-6β-hydroxyroyleanone on an MRSA/VISA Strain: Membrane and Cell Wall Interactions. Biomolecules 2020, 10, 983. https://doi.org/10.3390/biom10070983
Pereira F, Figueiredo T, de Almeida RFM, Antunes CAC, Garcia C, Reis CP, Ascensão L, Sobral RG, Rijo P. Unveiling the Mechanism of Action of 7α-acetoxy-6β-hydroxyroyleanone on an MRSA/VISA Strain: Membrane and Cell Wall Interactions. Biomolecules. 2020; 10(7):983. https://doi.org/10.3390/biom10070983
Chicago/Turabian StylePereira, Filipe, Teresa Figueiredo, Rodrigo F. M. de Almeida, Catarina A. C. Antunes, Catarina Garcia, Catarina P. Reis, Lia Ascensão, Rita G. Sobral, and Patricia Rijo. 2020. "Unveiling the Mechanism of Action of 7α-acetoxy-6β-hydroxyroyleanone on an MRSA/VISA Strain: Membrane and Cell Wall Interactions" Biomolecules 10, no. 7: 983. https://doi.org/10.3390/biom10070983
APA StylePereira, F., Figueiredo, T., de Almeida, R. F. M., Antunes, C. A. C., Garcia, C., Reis, C. P., Ascensão, L., Sobral, R. G., & Rijo, P. (2020). Unveiling the Mechanism of Action of 7α-acetoxy-6β-hydroxyroyleanone on an MRSA/VISA Strain: Membrane and Cell Wall Interactions. Biomolecules, 10(7), 983. https://doi.org/10.3390/biom10070983