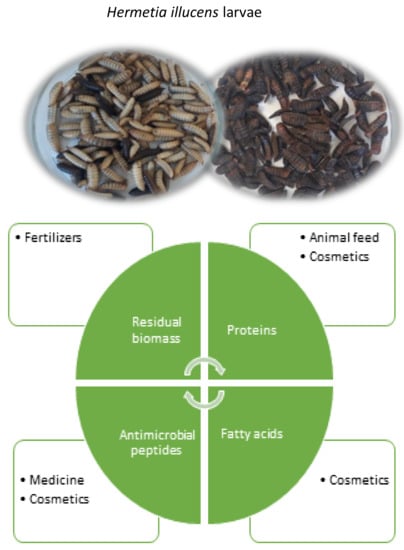
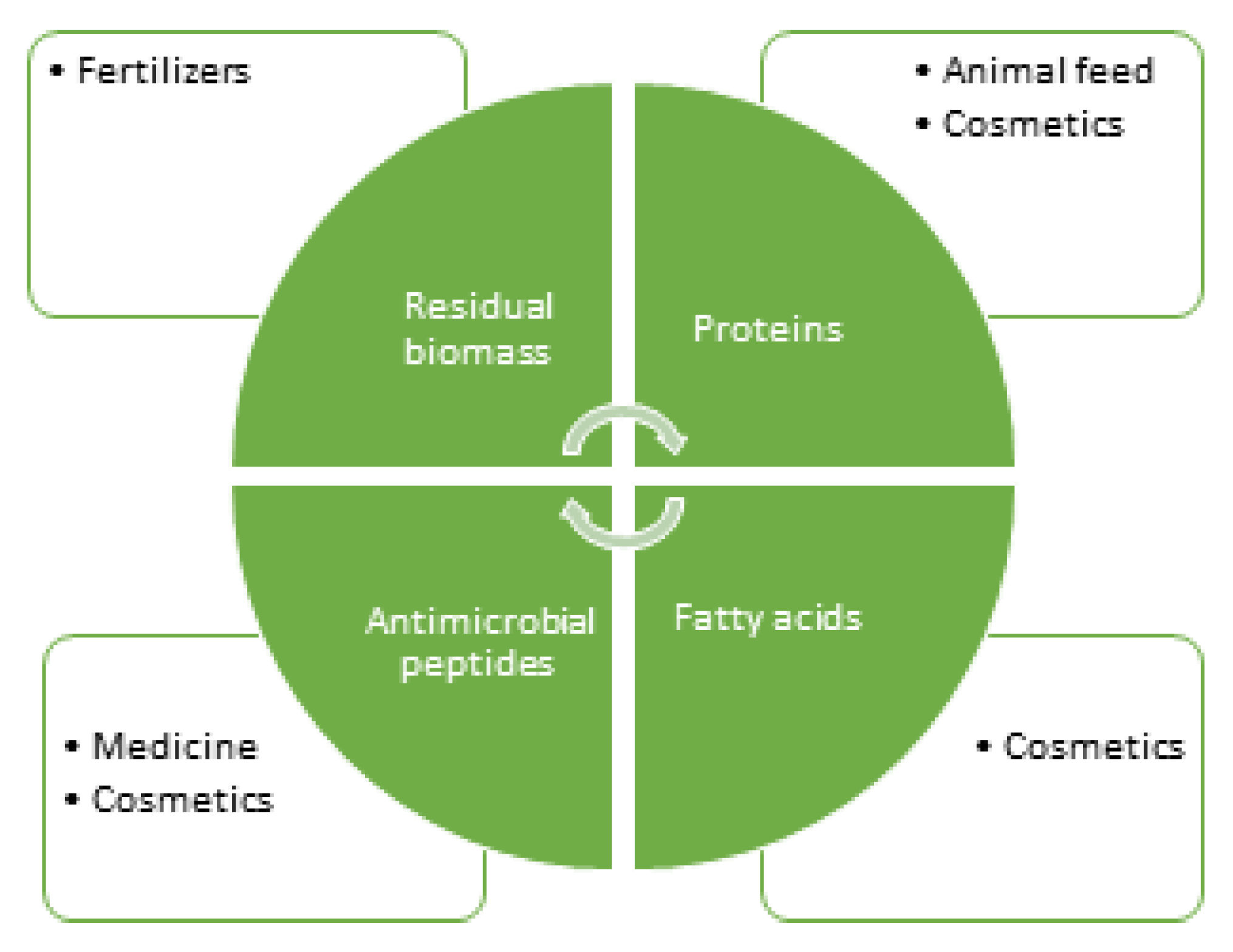
Bioactive Compounds from Hermetia Illucens Larvae as Natural Ingredients for Cosmetic Application
Abstract
1. Introduction
2. Hermetia Lllucens Life Cycle and Rearing Process
3. Extraction Methods
4. H. illucens Larvae Bioactives Composition and Cosmetic Applications
4.1. Proteins and Peptides
4.2. Lipids and Fatty Acids
4.3. Polysaccharides
5. Conclusions
Funding
Conflicts of Interest
References
- Teixeira Filho, N.P. Devoradores de lixo: Aspectos biológicos, produtivos e nutricionais da mosca soldado Hermetia illucens (L., 1758) (Díptera; Stratiomyidae) em resíduos sólidos orgânicos em Manaus, AM. Master’s Thesis, Universidade Federal do Amazonas, Manaus, Brazil, 2018. [Google Scholar]
- Cragg, G.M.; Newman, D.J. Natural products: A continuing source of novel drug leads. Biochim. Biophys. Acta 2013, 1830, 3670–3695. [Google Scholar] [CrossRef] [PubMed]
- Van Huis, A. Potential of Insects as Food and Feed in Assuring Food Security. Annu. Rev. Entomol. 2012, 58, 563–583. [Google Scholar] [CrossRef] [PubMed]
- Oonincx, D.G.A.B.; van Itterbeeck, J.; Heetkamp, M.J.W.; van den Brand, H.; van Loon, J.J.A.; van Huis, A. An exploration on greenhouse gas and ammonia production by insect species suitable for animal or human consumption. PLoS ONE 2010, 5, e14445. [Google Scholar] [CrossRef] [PubMed]
- Verheyen, G.R.; Ooms, T.; Vogels, L.; Vreysen, S.; Bovy, A.; Van Miert, S.; Meersman, F. Insects as an Alternative Source for the Production of Fats for Cosmetics. J. Cosmet. Sci. 2018, 69, 187–202. [Google Scholar]
- Van Huis, A.; van Itterbeeck, J.; Klunder, H.; Mertens, E.; Halloran, A.; Muir, G.; Vantomme, P. Future prospects for food and feed security. Food Agric. Organ. United Nations 2013, 171, 178. [Google Scholar]
- Tomberlin, J.K.; Sheppard, D.C.; Joyce, J.A. Selected Life-History Traits of Black Soldier Flies (Diptera: Stratiomyidae) Reared on Three Artificial Diets. Ann. Entomol. Soc. Am. 2002, 95, 379–386. [Google Scholar] [CrossRef]
- Devic, E.; Fahmi, M.R. Biology of Hermetia illucens. In Technical Handbook of Domestication and Production of diptera Black Soldier Fly (BSF) Hermetia illucens, Stratiomyidae, 1st ed.; Caruso, D., Devic, E., Wayan Subamia, I., Talamond, P., Baras, E., Eds.; PT Penerbit IPB Press: Jakarta, Indonesia, 2013; pp. 3–10. [Google Scholar]
- Diener, S.; Zurbrügg, C.; Tockner, K. Conversion of organic material by black soldier fly larvae: Establishing optimal feeding rates. Waste Manag. Res. 2009, 27, 603–610. [Google Scholar] [CrossRef]
- Ferrarezi, R.; Cannella, L.; Nassef, A.; Bailey, D. Annual Report 2006: Alternative Sources of Food for Aquaponics in the U.S. Virgin Islands: A Case Study with Black Soldier Flies; University of the Virgin Islands: Charlotte Amalie West, St. Thomas, US Virgin Islands, 2016. [Google Scholar]
- Nguyen, T.T.X.; Tomberlin, J.K.; VanLaerhoven, S. Influence of resources on Hermetia illucens (Diptera: Stratiomyidae) larval development. J. Med. Entomol. 2017, 50, 898–906. [Google Scholar] [CrossRef]
- Tomberlin, J.K.; Adler, P.H.; Myers, H.M. Development of the Black Soldier Fly (Diptera: Stratiomyidae) in Relation to Temperature: Table 1. Environ. Entomol. 2009, 38, 930–934. [Google Scholar] [CrossRef]
- Sheppard, D.C.; Tomberlin, J.K.; Joyce, J.A.; Kiser, B.C.; Sumner, S.M. Rearing Methods for the Black Soldier Fly (Diptera: Stratiomyidae). J. Med. Entomol. 2002, 39, 695–698. [Google Scholar] [CrossRef]
- Spranghers, T.; Ottoboni, M.; Klootwijk, C.; Ovyn, A.; Deboosere, S.; De Meulenaer, B.; Michiels, J.; Eeckhout, M.; De Clercq, P.; De Smet, S. Nutritional composition of black soldier fly (Hermetia illucens) prepupae reared on different organic waste substrates. J. Sci. Food Agric. 2017, 97, 2594–2600. [Google Scholar] [CrossRef]
- Meneguz, M.; Schiavone, A.; Gai, F.; Dama, A.; Lussiana, C.; Renna, M.; Gasco, L. Effect of rearing substrate on growth performance, waste reduction efficiency and chemical composition of black soldier fly (Hermetia illucens) larvae. J. Sci. Food Agric. 2018, 98, 5776–5784. [Google Scholar] [CrossRef]
- Lalander, C.; Diener, S.; Zurbrügg, C.; Vinnerås, B. Effects of feedstock on larval development and process efficiency in waste treatment with black soldier fly (Hermetia illucens). J. Clean. Prod. 2019, 208, 211–219. [Google Scholar] [CrossRef]
- Liland, N.S.; Biancarosa, I.; Araujo, P.; Biemans, D.; Bruckner, C.G.; Waagbø, R.; Torstensen, B.E.; Lock, E.J. Modulation of nutrient composition of black soldier fly (Hermetia illucens) larvae by feeding seaweed-enriched media. PLoS ONE 2017, 12, e0183188. [Google Scholar] [CrossRef] [PubMed]
- Tzompa-Sosa, D.; Yi, L.; Valenberg, H.J.F.; Boekel, M.; Lakemond, C. Insect lipid profile: Aqueous versus organic solvent-based extraction methods. Food Res. Int. 2014, 62, 1087–1094. [Google Scholar] [CrossRef]
- Xiaoming, C.; Ying, F.; Hong, Z.; Zhiyong, C. Review of the nutritive value of edible insects. In Forest Insects as Food: Humans Bite Back; Durst, P.B., Johnson, D.V., Leslie, R.N., Shono, K., Eds.; Food and Agricultural Organization (FAO) of the United Nations: Bangkok, Thailand, 2010; p. 85. [Google Scholar]
- Yi, L.; Lakemond, C.M.M.; Sagis, L.M.C.; Eisner-Schadler, V.; van Huis, A.; van Boekel, M.A.J.S. Extraction and characterisation of protein fractions from five insect species. Food Chem. 2013, 141, 3341–3348. [Google Scholar] [CrossRef]
- Janssen, R.H.; Vincken, J.P.; Van Den Broek, L.A.M.; Fogliano, V.; Lakemond, C.M.M. Nitrogen-to-Protein Conversion Factors for Three Edible Insects: Tenebrio molitor, Alphitobius diaperinus, and Hermetia illucens. J. Agric. Food Chem. 2017, 65, 2275–2278. [Google Scholar] [CrossRef] [PubMed]
- Newton, G.L.; Booram, C.V.; Barker, R.W.; Hale, O.M. Dried Hermetia illucens Larvae Meal as a Supplement for Swine. J. Anim. Sci. 1977, 44, 395–400. [Google Scholar] [CrossRef]
- Bosch, G.; Zhang, S.; Oonincx, D.G.A.B.; Hendriks, W.H. Protein quality of insects as potential ingredients for dog and cat foods. J. Nutr. Sci. 2014, 3, e29. [Google Scholar] [CrossRef]
- Rabani, V.; Cheatsazan, H.; Davani, S. Proteomics and Lipidomics of Black Soldier Fly (Diptera: Stratiomyidae) and Blow Fly (Diptera: Calliphoridae) Larvae. J. Insect Sci. 2019, 19, 29. [Google Scholar] [CrossRef]
- Renault, D.; Bouchereau, A.; Delettre, Y.R.; Hervant, F.; Vernon, P. Changes in free amino acids in Alphitobius diaperinus (Coleoptera: Tenebrionidae) during thermal and food stress. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2006, 143, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.; Fitzpatrick, M.P.; Dierenfeld, E.S. Nutrient composition of selected whole invertebrates. Zoo Biol. 1998, 17, 123–134. [Google Scholar] [CrossRef]
- Ramos-Elorduy, J. Insects: A sustainable source of food? Ecol. Food Nutr. 1997, 36, 247–276. [Google Scholar] [CrossRef]
- Bukkens, S.G.F. The nutritional value of edible insects. Ecol. Food Nutr. 1997, 36, 287–319. [Google Scholar] [CrossRef]
- Marcó, A.; Rubio, R.; Compañó, R.; Casals, I. Comparison of the Kjeldahl method and a combustion method for total nitrogen determination in animal feed. Talanta 2002, 57, 1019–1026. [Google Scholar] [CrossRef]
- Lynch, J.M.; Barbano, D.M. Kjeldahl nitrogen analysis as a reference method for protein determination in dairy products. J. AOAC Int. 1999, 82, 1389–1398. [Google Scholar] [CrossRef]
- Ribeiro, P.D.A. Implementação de análise de nitrogênio total em solo pelo método de Dumas. In Milho e Sorgo-Documentos; Embrapa: Brasília, Brazil, 2010; p. 26. [Google Scholar]
- Sánchez-Muros, M.J.; Barroso, F.G.; Manzano-Agugliaro, F. Insect meal as renewable source of food for animal feeding: A review. J. Clean. Prod. 2014, 65, 16–27. [Google Scholar] [CrossRef]
- Aguilar-Toalá, J.E.; Hernández-Mendoza, A.; González-Córdova, A.F.; Vallejo-Cordoba, B.; Liceaga, A.M. Potential role of natural bioactive peptides for development of cosmeceutical skin products. Peptides 2019, 122, 170170. [Google Scholar] [CrossRef]
- Aranaz, I.; Acosta, N.; Civera, C.; Elorza, B.; Mingo, J.; Castro, C.; Gandía, M.D.L.L.; Caballero, A.H. Cosmetics and cosmeceutical applications of chitin, chitosan and their derivatives. Polymers 2018, 20, 213. [Google Scholar] [CrossRef]
- Wang, Y.S.; Shelomi, M. Review of Black Soldier Fly (Hermetia illucens) as Animal Feed and Human Food. Foods 2017, 6, 91. [Google Scholar] [CrossRef]
- Finke, M.D.; Oonincx, D. Insects as Food for Insectivores. In Mass Production of Beneficial Organisms; Morales-Ramos, J.A., Rojas, M.G., Shapiro-Ilan, D.I., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 583–616. [Google Scholar]
- Kunz, R.I.; Brancalhão, R.M.C.; Ribeiro, L.D.F.C.; Natali, M.R.M. Silkworm Sericin: Properties and Biomedical Applications. Biomed. Res. Int. 2016, 2016, 1–19. [Google Scholar] [CrossRef]
- Kouřimská, L.; Adámková, A. Nutritional and sensory quality of edible insects. NFS J. 2016, 4, 22–26. [Google Scholar] [CrossRef]
- Rumpold, B.A.; Schlüter, O.K. Nutritional composition and safety aspects of edible insects. Mol. Nutr. Food Res. 2013, 57, 802–823. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, Y.H.; Yamada, M.; Lin, L.L.; Grice, J.E.; Roberts, M.S.; Raphael, A.P.; Benson, H.E.; Prow, T.W. Microneedle enhanced delivery of cosmeceutically relevant peptides in human skin. PLoS ONE 2014, 9, e101956. [Google Scholar] [CrossRef]
- Fields, K.; Falla, T.J.; Rodan, K.; Bush, L. Bioactive peptides: Signaling the future. J. Cosmet. Dermatol. 2009, 8, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Avila Rodríguez, M.I.; Rodríguez Barroso, L.G.; Sánchez, M.L. Collagen: A review on its sources and potential cosmetic applications. J. Cosmet. Dermatol. 2018, 17, 20–26. [Google Scholar] [CrossRef]
- Burnett, C.L.; Heldreth, B.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; et al. Safety Assessment of α-Amino Acids as Used in Cosmetics. Int. J. Toxicol. 2013, 32, 41S–64S. [Google Scholar] [CrossRef]
- Rebello, T. Produtos orgânicos naturais. In Guia de produtos cosméticos; Rebello, T., Ed.; Senac: São Paulo, Brazil, 2019; p. 320. [Google Scholar]
- Muller, A.; Wolf, D.; Gutzeit, H.O. The black soldier fly, Hermetia illucens—A promising source for sustainable production of proteins, lipids and bioactive substances. Z. Naturforsch. C. 2017, 72, 351–363. [Google Scholar] [CrossRef]
- Finke, M.D. Complete Nutrient Content of Four Species of Feeder Insects. Zoo Biol. 2013, 32, 27–36. [Google Scholar] [CrossRef]
- Alencar-Silva, T.; Braga, M.C.; Santana, G.O.S.; Saldanha-Araujo, F.; Pogue, R.; Dias, S.C.; Franco, O.L.; Carvalho, J.L. Breaking the frontiers of cosmetology with antimicrobial peptides. Biotechnol. Adv. 2018, 36, 2019–2031. [Google Scholar] [CrossRef]
- Vogel, H.; Müller, A.; Heckel, D.G.; Gutzeit, H.; Vilcinskas, A. Nutritional immunology: Diversification and diet-dependent expression of antimicrobial peptides in the black soldier fly Hermetia illucens. Dev. Comp. Immunol. 2018, 78, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Park, S.I.; Chang, B.S.; Yoe, S.M. Detection of antimicrobial substances from larvae of the black soldier fly, Hermetia illucens (Diptera: Stratiomyidae). Entomol. Res. 2014, 44, 58–64. [Google Scholar] [CrossRef]
- Ushakova, N.; Dontsov, A.; Sakina, N.; Bastrakov, A.; Ostrovsky, M. Antioxidative Properties of Melanins and Ommochromes from Black Soldier Fly Hermetia illucens. Biomolecules 2019, 9, 408. [Google Scholar] [CrossRef] [PubMed]
- Rahnamaeian, M.; Vilcinskas, A. Short antimicrobial peptides as cosmetic ingredients to deter dermatological pathogens. Appl. Microbiol. Biotechnol. 2015, 99, 8847–8855. [Google Scholar] [CrossRef]
- Craig Sheppard, D.; Larry Newton, G.; Thompson, S.A.; Savage, S. A value added manure management system using the black soldier fly. Bioresour. Technol. 1994, 50, 275–279. [Google Scholar] [CrossRef]
- Li, S.; Ji, H.; Zhang, B.; Tian, J.; Zhou, J.; Yu, H. Influence of black soldier fly (Hermetia illucens) larvae oil on growth performance, body composition, tissue fatty acid composition and lipid deposition in juvenile Jian carp (Cyprinus carpio var. Jian). Aquaculture 2016. [Google Scholar] [CrossRef]
- Ushakova, N.A.; Brodskii, E.S.; Kovalenko, A.A.; Bastrakov, A.I.; Kozlova, A.A.; Pavlov, D.S. Characteristics of lipid fractions of larvae of the black soldier fly Hermetia illucens. Dokl. Biochem. Biophys. 2016, 468, 209–212. [Google Scholar] [CrossRef]
- Caligiani, A.; Marseglia, A.; Sorci, A.; Bonzanini, F.; Lolli, V.; Maistrello, L.; Sforza, S. Influence of the killing method of the black soldier fly on its lipid composition. Food Res. Int. 2019. [Google Scholar] [CrossRef]
- Anzaku, A.A.; Akyala, J.I.; Juliet, A.; Obianuju, E.C. Antibacterial Activity of Lauric Acid on Some Selected Clinical Isolates. Ann. Clin. Lab. Res. 2017, 4, 3173–3177. [Google Scholar] [CrossRef]
- Rabasco Alvarez, A.M.; González Rodríguez, M.L. Lipids in pharmaceutical and cosmetic preparations. Grasas y Aceites 2000, 51, 74–96. [Google Scholar] [CrossRef]
- Kim, E.J.; Kim, M.K.; Jin, X.J.; Oh, J.H.; Kim, J.E.; Chung, J.H. Skin aging and photoaging alter fatty acids composition, including 11,14,17-eicosatrienoic acid, in the epidermis of human skin. J. Korean Med. Sci. 2010, 25, 980–983. [Google Scholar] [CrossRef] [PubMed]
- Mank, V.; Polonska, T. Use of natural oils as bioactive ingredients of cosmetic products. Ukr. Food J. 2016, 5, 281–289. [Google Scholar] [CrossRef]
- Sanders, T.A.B. Introduction: The Role of Fats in Human Diet. In Functional Dietary Lipids; Elsevier: Amsterdam, The Netherlands, 2016; pp. 1–20. [Google Scholar]
- Rustan, A.C.; Drevon, C.A. Fatty acids: Structures and properties. In Encyclopedia of Life Sciences; Wyley on Line Library: Hoboken, NJ, USA, 2001; pp. 1–7. [Google Scholar]
- Dutta, P.K.; Duta, J.; Tripathi, V.S. Chitin and Chitosan: Chemistry, properties and applications. J. Sci. Ind. Res. 2004, 63, 20–31. [Google Scholar]
- Le, Y.; Anand, S.C.; Horrocks, A.R. Recent developments in fibres and materials for wound management. Indian J. Fibre Text. Res. 1997, 22, 337–347. [Google Scholar]
- Lee, C.G.; Da Silva, C.A.; Lee, J.Y.; Hartl, D.; Elias, J.A. Chitin regulation of immune responses: An old molecule with new roles. Curr. Opin. Immunol. 2008, 20, 684–689. [Google Scholar] [CrossRef] [PubMed]
- Muzzarelli, R.A.A. Chitins and chitosans as immunoadjuvants and non-allergenic drug carriers. Mar. Drugs 2010, 8, 292–312. [Google Scholar] [CrossRef] [PubMed]
- Aranaz, I.; Mengibar, M.; Harris, R.; Panos, I.; Miralles, B.; Acosta, N.; Galed, G.; Heras, A. Functional Characterization of Chitin and Chitosan. Curr. Chem. Biol. 2009, 3, 203–230. [Google Scholar]
- Ngo, D.H.; Kim, S.K. Antioxidant effects of chitin, chitosan, and their derivatives. In Advances in Food and Nutrition Research; Elsevier: Amsterdam, The Netherlands, 2014; Volume 73, pp. 15–31. [Google Scholar]
- Senevirathne, M.; Ahn, C.B.; Kim, S.K.; Je, J.Y. Cosmeceutical Applications of Chitosan and Its Derivatives. In Marine Cosmeceuticals: Trends and Prospects; Kim, S.K., Ed.; CRC Press: Boca Raton, FL, USA, 2011; p. 169. [Google Scholar]
- Vinsova, J.; Vavrikova, E. Chitosan Derivatives with Antimicrobial, Antitumour and Antioxidant Activities—A Review. Curr. Pharm. Des. 2011, 17, 3596–3607. [Google Scholar] [CrossRef]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef]
- Chung, Y.C.; Chen, C.Y. Antibacterial characteristics and activity of acid-soluble chitosan. Bioresour. Technol. 2008, 99, 2806–2814. [Google Scholar] [CrossRef]


| Substrate Type | Larvae Composition | References | ||
|---|---|---|---|---|
| Crude Protein | Lipids | Chitin | ||
| 1. Chicken feed | 412 (0.6) a | 336 (0.4) a | 62 (2.8) a | [14] |
| 2. Vegetable waste | 399 (0.2) a | 371 (1.1) a | 57 (1.8) a | |
| 3. Restaurant waste | 431 (0.6) a | 386 (2.3) a | 67 (1.3) a | |
| 4. Biogas digestate | 422 (1.4) a | 218 (0.5) a | 56 (1.5) a | |
| 5. Poultry feed | 39.6 ± 0.2 b | - | - | [16] |
| 6. Food waste | 39.2 ± 2.5 b | - | - | |
| 7. Fruits and vegetables | 41.3 ± 1.0 b | - | - | |
| 8. Poultry manure | 41.6 ± 1.5 b | - | - | |
| 9. Fruit waste | 307.5 ± 10.2 d | 10.04 c | 56.0 ± 3.9 d | [15] |
| 10. Winery by-product | 344.3 ± 7.6 d | 73.57 c | 52.9 ± 9.2 d | |
| 11. Brewery by-product | 529.6 ± 5.2 d | 82.47 c | 14.2 ± 6.0 d | |
| 13. Seaweed (Ascophyllum nodosum) | 41.3 ± 1.1 e | 22.2 ± 0.2 f | - | [17] |
| Amino acids | Substrates | ||||
|---|---|---|---|---|---|
| Chicken Feed a [14] | Organic Waste b [45] | Cattle Manure c [21] | Human Faeces d [16] | Undefined e [46] | |
| Alanine | 25.2 | 6.2 | 3.7 | 65.3 | 12.2 |
| Arginine | 20.3 | 6.2 | 2.2 | 51.0 | 12.3 |
| Aspartic acid | 37.8 | 10.3 | 4.6 | 90.0 | 16.5 |
| Cysteine | 2.5 | 0.5 | 0.1 | 6.7 | 1.02 |
| Glutamic acid | 41.9 | 12.2 | 3.8 | 105.9 | 19.7 |
| Glycine | 22.6 | 5.4 | 2.9 | 59.2 | 9.14 |
| Histidine | 13.6 | 4.8 | 1.9 | 33.0 | 5.94 |
| Isoleucine | 17.2 | 4.8 | 2.0 | 46.7 | 7.62 |
| Leucine | 28.6 | 7.7 | 3.5 | 70.0 | 12.1 |
| Lysine | 23.4 | 7.4 | 3.4 | 61.7 | 11.9 |
| Methionine | 7.6 | 0.6 | 0.9 | 18.5 | 3.37 |
| Phenylalanine | 17 | 6.2 | 2.2 | 44.2 | 7.56 |
| Proline | 22.5 | 6.2 | 3.3 | 57.9 | 10.2 |
| Serine | 16.6 | 4.1 | 0.1 | 43.9 | 7.02 |
| Threonine | 16.4 | 4.5 | 0.6 | 37.6 | 6.82 |
| Tryptophan | Not analyzed | Not analyzed | 0.2 | 16.9 | 3.00 |
| Tyrosine | 6.7 | 6.0 | 2.5 | 108.8 | 12.1 |
| Valine | 24.1 | 6.7 | 3.4 | 65.9 | 12.9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almeida, C.; Rijo, P.; Rosado, C. Bioactive Compounds from Hermetia Illucens Larvae as Natural Ingredients for Cosmetic Application. Biomolecules 2020, 10, 976. https://doi.org/10.3390/biom10070976
Almeida C, Rijo P, Rosado C. Bioactive Compounds from Hermetia Illucens Larvae as Natural Ingredients for Cosmetic Application. Biomolecules. 2020; 10(7):976. https://doi.org/10.3390/biom10070976
Chicago/Turabian StyleAlmeida, Cíntia, Patrícia Rijo, and Catarina Rosado. 2020. "Bioactive Compounds from Hermetia Illucens Larvae as Natural Ingredients for Cosmetic Application" Biomolecules 10, no. 7: 976. https://doi.org/10.3390/biom10070976
APA StyleAlmeida, C., Rijo, P., & Rosado, C. (2020). Bioactive Compounds from Hermetia Illucens Larvae as Natural Ingredients for Cosmetic Application. Biomolecules, 10(7), 976. https://doi.org/10.3390/biom10070976