Validation of a Novel Zebrafish Model of Dengue Virus (DENV-3) Pathology Using the Pentaherbal Medicine Denguenil Vati
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Zebrafish Care and Maintenance
2.3. Propagation of Dengue Virus (Serotype, DENV-3) in Zebrafish
2.4. Blood Collection Harvesting Serum from Carrier Zebrafish
2.5. Dengue Viral Infection Study Design
2.6. Preparation of Zebrafish Test Feed and Dosing
2.7. Harvesting Liver Tissue and Caudal Fins for Histopathology
2.8. RNA Isolation and Gene Expression Analysis
- DENV-3:
- Forward 5′-CGGGAAAACCGTCTATCAATATGC-3′Reverse 5′-TGAGAATCTCTTCGCCAACTGTG-3′
- CCL3:
- Forward 5′-CCGCGGATCCGACGATTTA-3′Reverse 5′-AATGACTCCAGGCAGAGTGC-3′
- ANG2:
- Forward 5′-TATTTGTGAGGTTTTCCGTTCCCATCGGGCT-3′Reverse 5′-AGAGGACTATGAGAAGTCGGCTCCTCGGATCAT-3′.
2.9. Complete Blood Count
2.10. Hematoxylin and Eosin (H&E) Staining, Imaging, and Cell Counting
2.11. Cell Culture
2.12. Cell Viability Assay
2.13. Reactive Oxygen Species (ROS) Measurement
2.14. Malondialdehyde (MDA) Estimation
2.15. Preparation of Denguenil Sample for HPLC
2.16. Statistical Analysis
3. Results
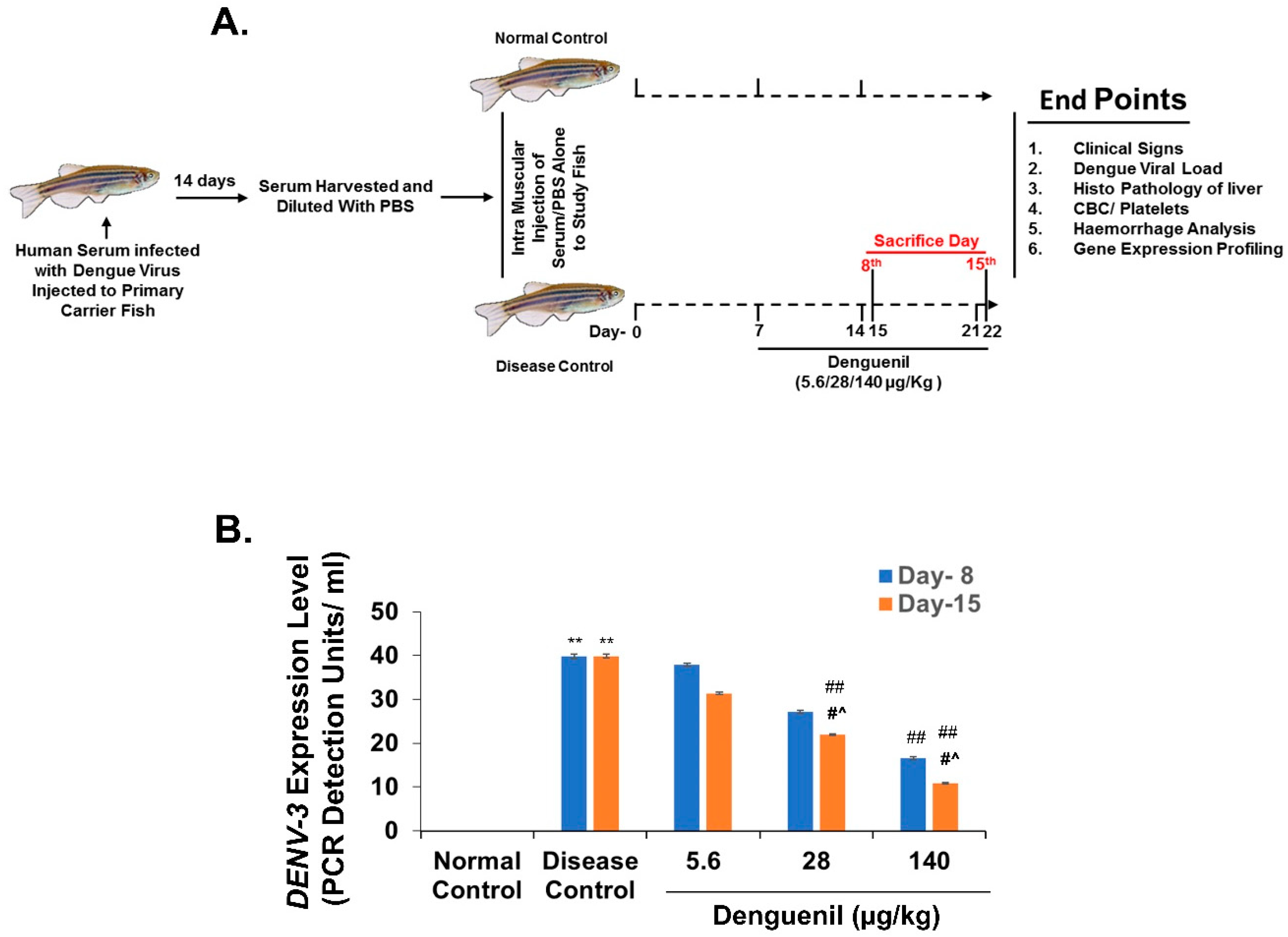
3.1. Study Design for Translational Dosing
3.2. Denguenil Inhibited DENV-3 Viral Transcript Copy Numbers
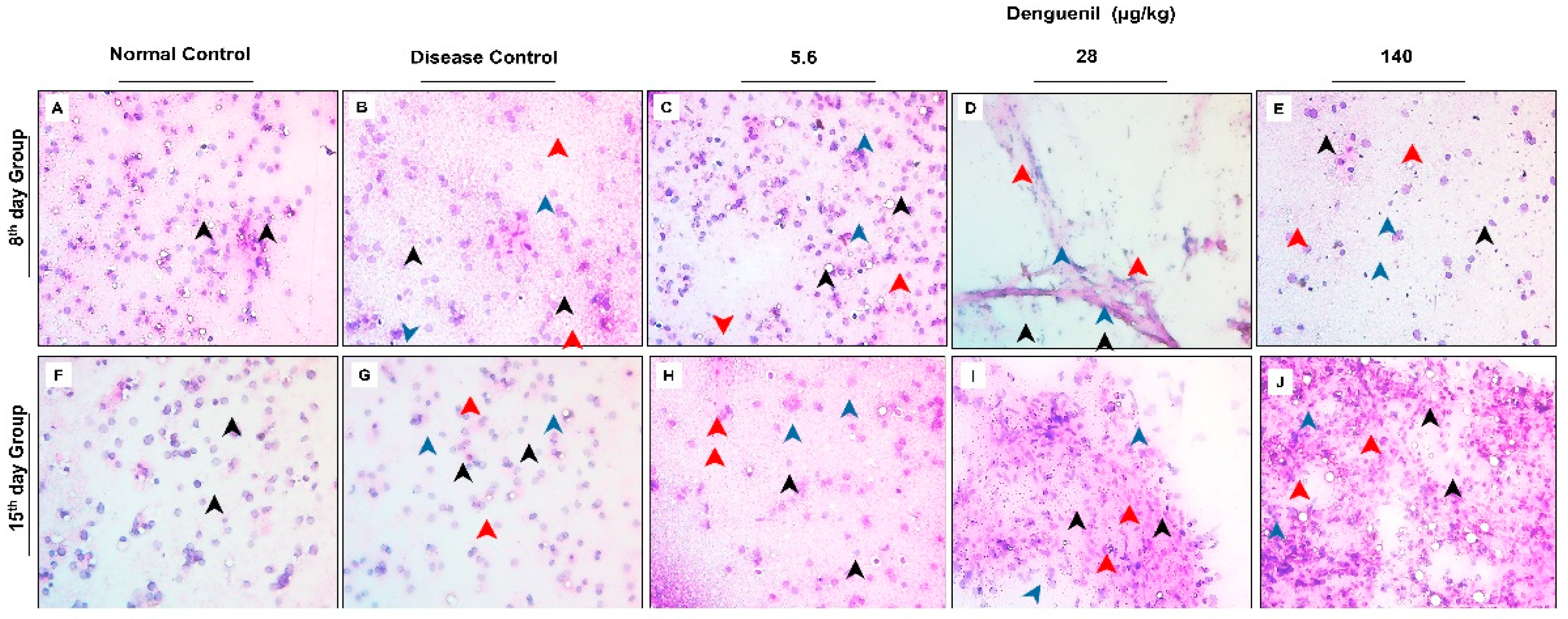
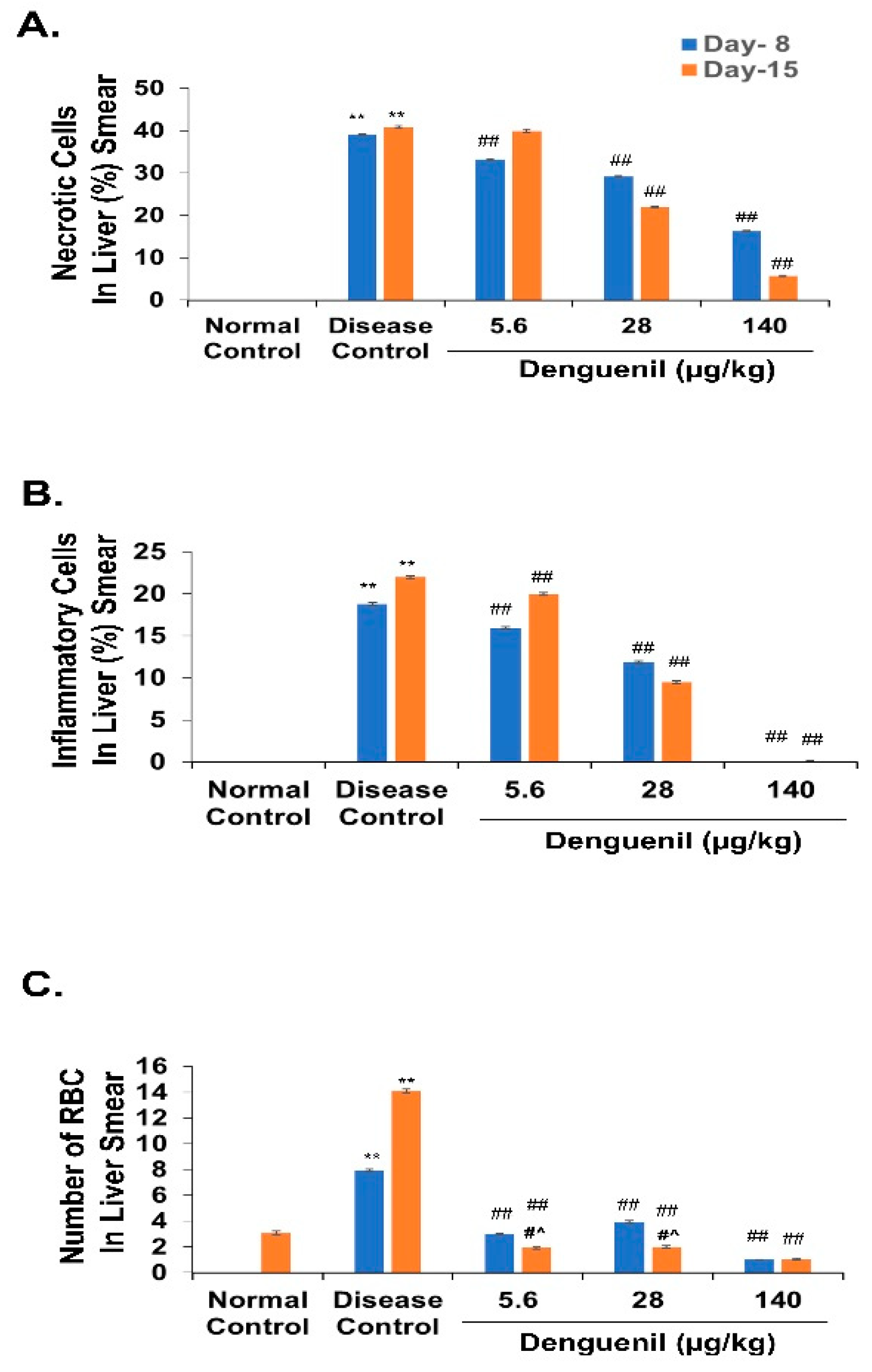
3.3. Denguenil Inhibited Dengue Virus Induced Hepatocyte Necrosis
3.4. Denguenil Inhibited Dengue-Virus-Induced Liver Inflammation
3.5. Denguenil Attenuated Dengue-Virus-Induced RBC Infiltration into Liver
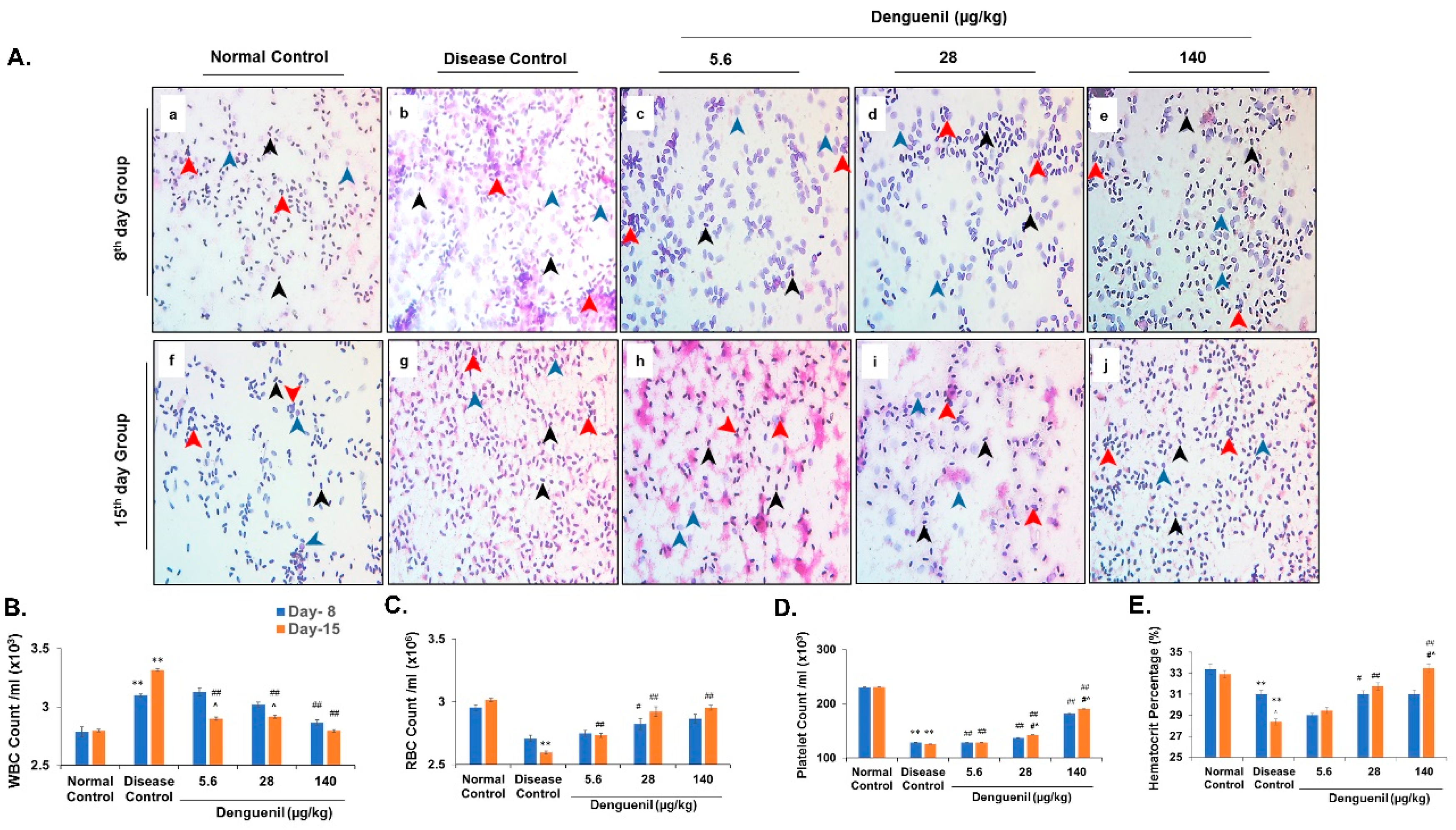
3.6. Dengue-Virus-Infected Zebrafish Blood Phenotyping Identified Protective Effects of Denguenil
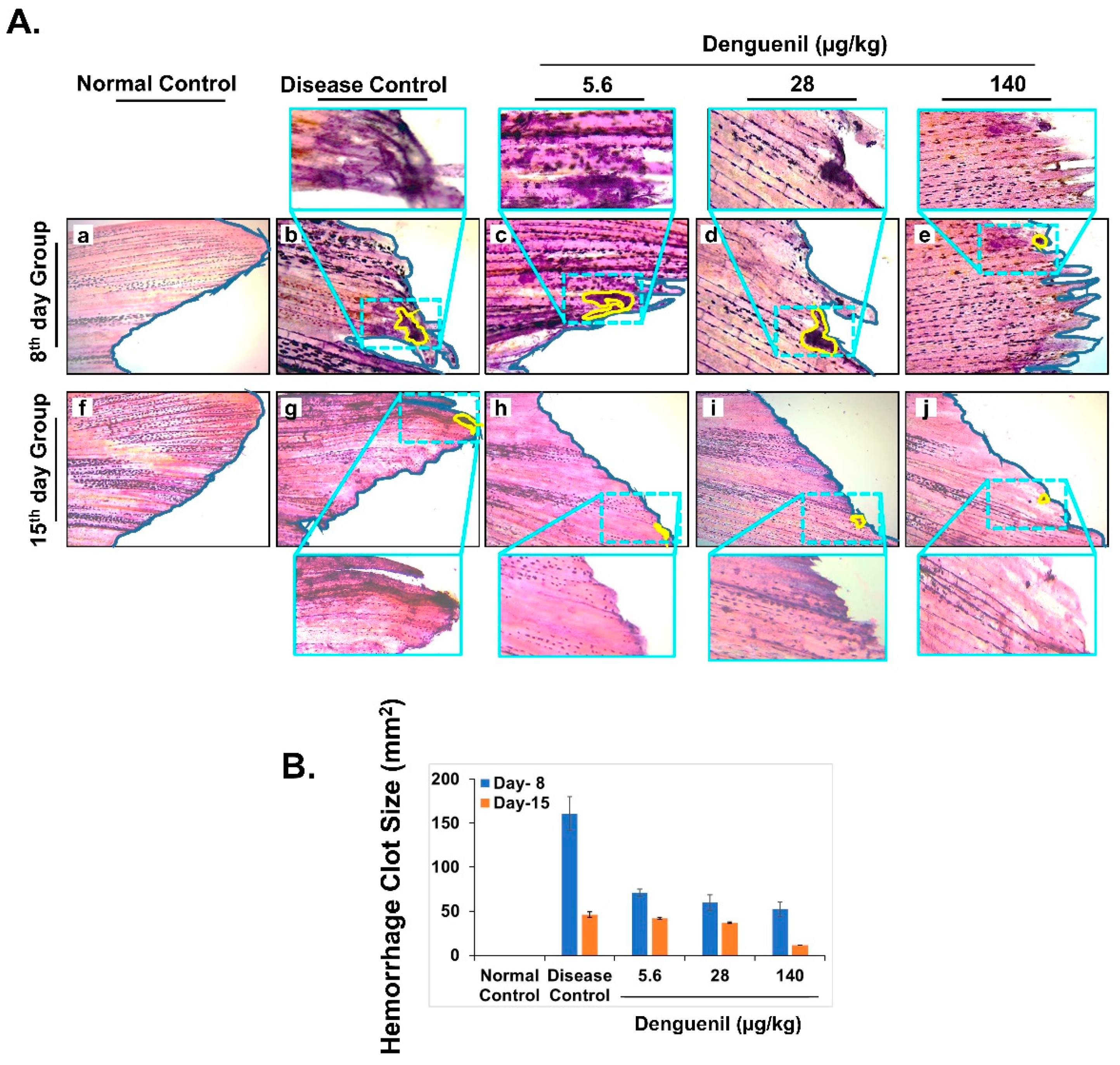
3.7. Denguenil Attenuated Dengue-Virus-Induced Hemorrhage
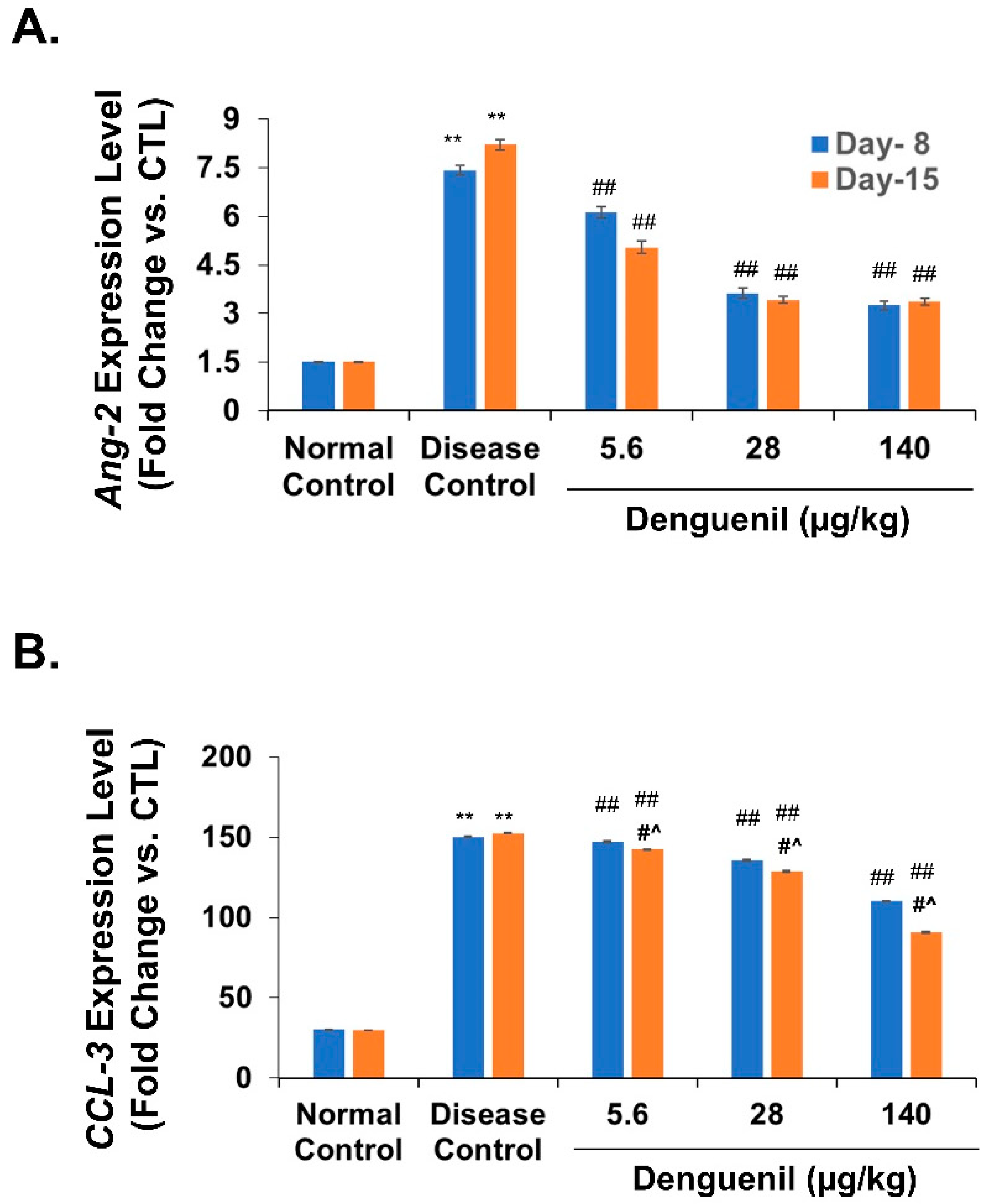
3.8. Denguenil Normalized Expression of Endothelial Apoptotic Marker, Angiopoietin-2
3.9. Denguenil Inhibited Upregulation of the CCL3 Gene
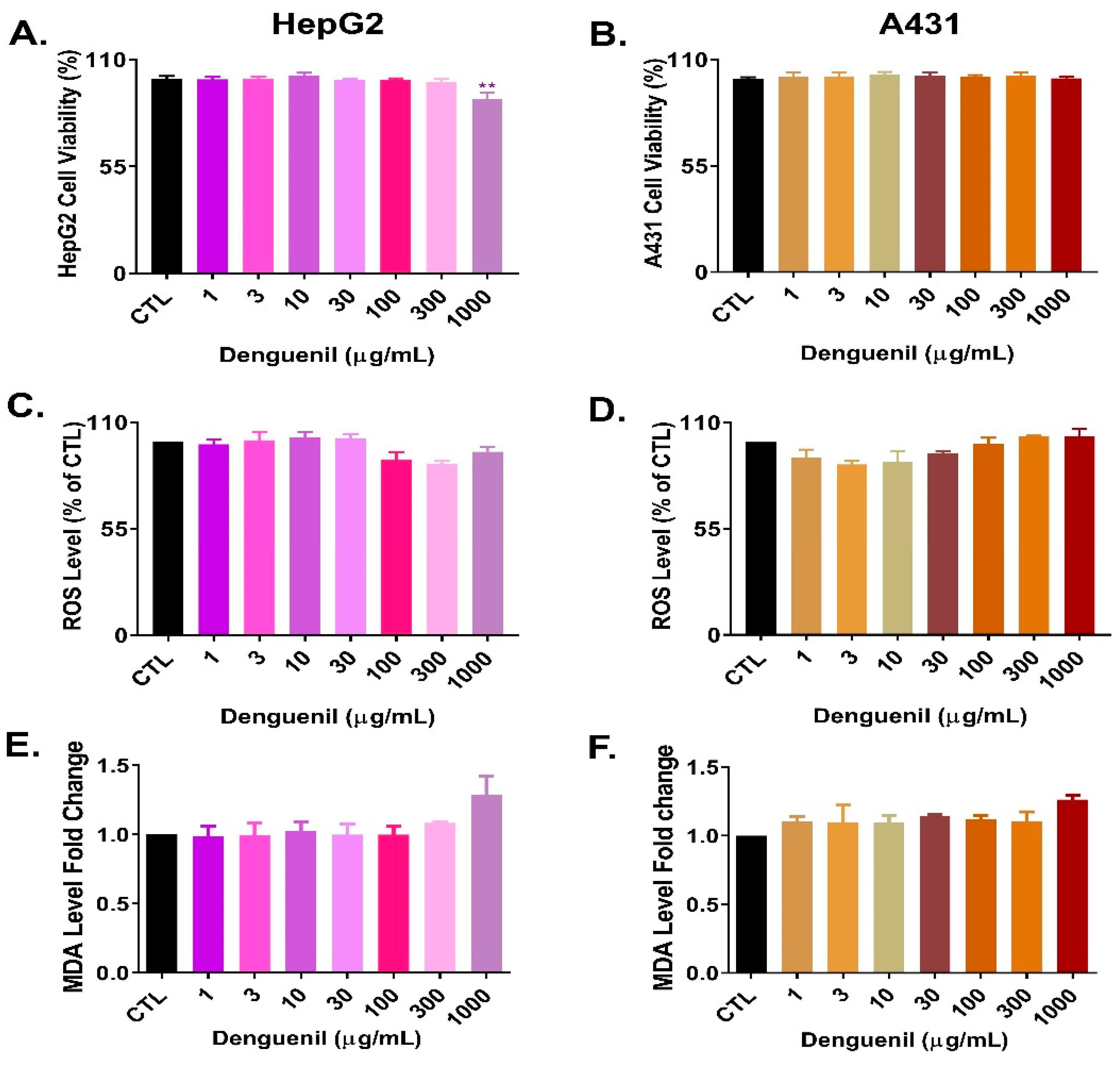
3.10. Cytosafety of Denguenil in Human Cell Lines
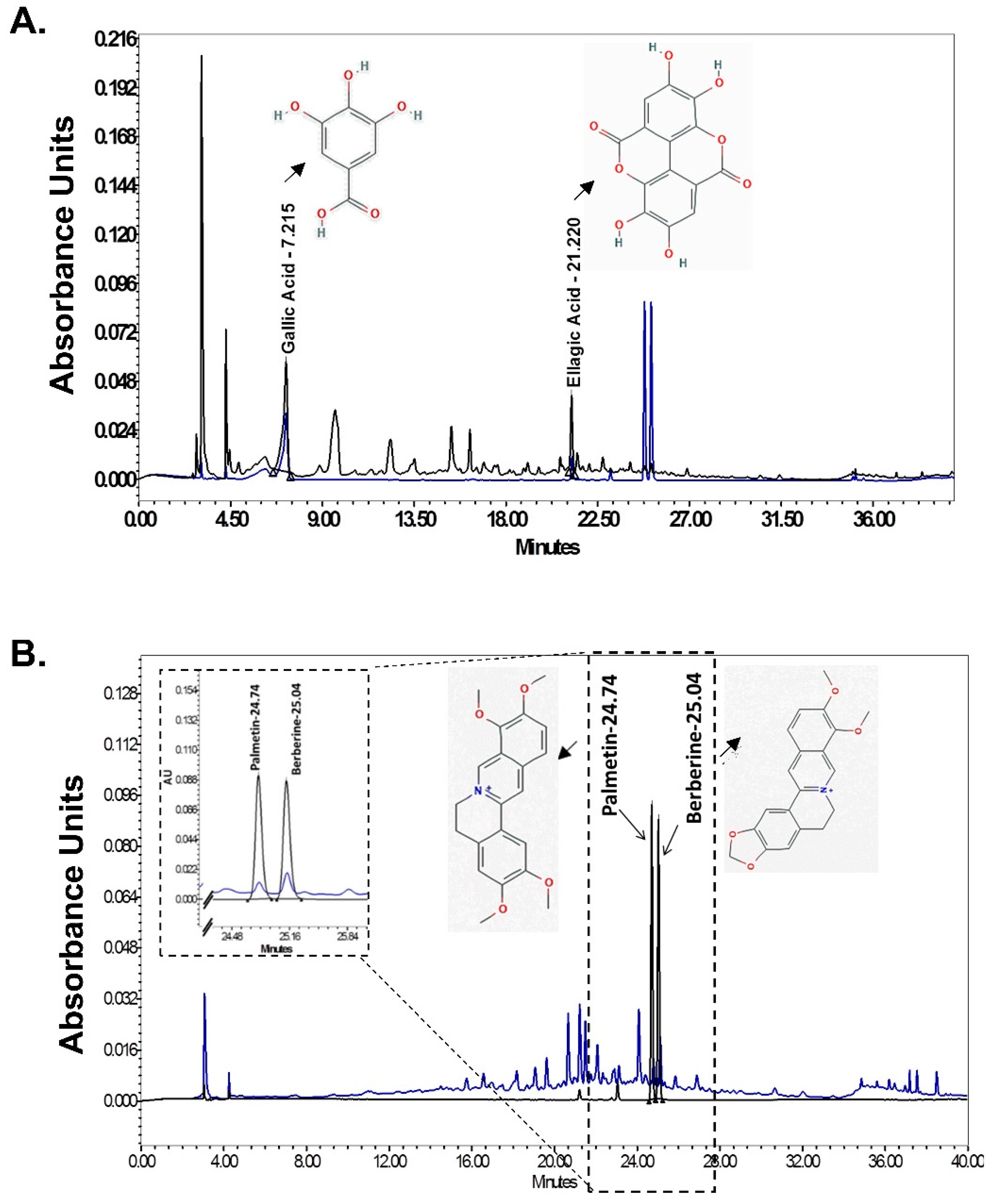
3.11. HPLC Analysis of Denguenil
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO. Global Strategy for Dengue Prevention and Control 2012–2020. In World Health Organiszation; WHO: Geneva, Switzerland, 2012; p. 43. ISBN 978-92-4-150403-4. [Google Scholar]
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention National Center for Emerging and Zoonotic Infectious Diseases. Available online: https://www.cdc.gov/dengue/about/index.html (accessed on 5 May 2020).
- Brady, O.J.; Gething, P.W.; Bhatt, S.; Messina, J.P.; Brownstein, J.S.; Hoen, A.G.; Moyes, C.L.; Farlow, A.W.; Scott, T.W.; Hay, S.I. Refining the Global Spatial Limits of Dengue Virus Transmission by Evidence-Based Consensus. PLoS Negl. Trop. Dis. 2012, 6, e1760. [Google Scholar] [CrossRef] [PubMed]
- Guzman, M.G.; Harris, E. Dengue. Lancet 2015, 385, 453–465. [Google Scholar] [CrossRef]
- Rothman, A.L. Immunity to dengue virus: A tale of original antigenic sin and tropical cytokine storms. Nat. Rev. Immunol. 2011, 11, 532–543. [Google Scholar] [CrossRef] [PubMed]
- Sayce, A.C.; Miller, J.L.; Zitzmann, N. Targeting a host process as an antiviral approach against dengue virus. Trends Microbiol. 2010, 18, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Halstead, S.B. Dengue. Lancet 2007, 370, 1644–1652. [Google Scholar] [CrossRef]
- WHO. Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control 2009; WHO: Geneva, Switzerland, 2013. [Google Scholar]
- Noisakran, S.; Onlamoon, N.; Songprakhon, P.; Hsiao, H.M.; Chokephaibulkit, K.; Perng, G.C. Cells in dengue virus infection in vivo. Adv. Virol. 2010, 2010, 164878. [Google Scholar] [CrossRef] [PubMed]
- Bhamarapravati, N. Hemostatic Defects in Dengue Hemorrhagic Fever. Rev. Infect. Dis. 1989, 11, 826–829. [Google Scholar] [CrossRef]
- Green, A.M.; Beatty, P.R.; Hadjilaou, A.; Harris, E. Innate immunity to dengue virus infection and subversion of antiviral responses. J. Mol. Biol. 2014, 426, 1148–1160. [Google Scholar] [CrossRef]
- Gagnon, S.J.; Ennis, F.A.; Rothman, A.L. Bystander target cell lysis and cytokine production by dengue virus-specific human CD4(+) cytotoxic T-lymphocyte clones. J. Virol. 1999, 73, 3623–3629. [Google Scholar] [CrossRef]
- Tolfvenstam, T.; Lindblom, A.; Schreiber, M.J.; Ling, L.; Chow, A.; Ooi, E.E.; Hibberd, M.L. Characterization of early host responses in adults with dengue disease. BMC Infect. Dis. 2011, 11, 209. [Google Scholar] [CrossRef] [PubMed]
- Marchette, N.J.; Halstead, S.B.; Falkler, W.A.; Stenhouse, A.; Nash, D. Studies on the pathogenesis of dengue infection in monkeys. III. sequential distribution of virus in primary and heterologous infections. J. Infect. Dis. 1973, 128, 23–30. [Google Scholar] [CrossRef]
- Halstead, S.B.; Shotwell, H.; Casals, J. Studies on the pathogenesis of dengue infection in monkeys. I. clinical laboratory responses to primary infection. J. Infect. Dis. 1973, 128, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Onlamoon, N.; Noisakran, S.; Hsiao, H.M.; Duncan, A.; Villinger, F.; Ansari, A.A.; Perng, G.C. Dengue virus—Induced hemorrhage in a nonhuman primate model. Blood 2010, 115, 1823–1834. [Google Scholar] [CrossRef]
- Raut, C.G.; Deolankar, R.P.; Kolhapure, R.M.; Goverdhan, M.K. Susceptibility of laboratory-bred rodent to the experimental infection with dengue virus type 2. Acta Virol. 1996, 40, 146. [Google Scholar]
- Paes, M.V.; Pinhão, A.T.; Barreto, D.F.; Costa, S.M.; Oliveira, M.P.; Nogueira, A.C.; Takiya, C.M.; Farias-Filho, J.C.; Schatzmayr, H.G.; Alves, A.M.B.; et al. Liver injury and viremia in mice infected with dengue-2 virus. Virology 2005, 338, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Zompi, S.; Harris, E. Animal models of dengue virus infection. Viruses 2012, 4, 62–82. [Google Scholar] [CrossRef] [PubMed]
- Krishnakumar, V.; Durairajan, S.S.K.; Alagarasu, K.; Li, M.; Dash, A.P. Recent updates on mouse models for human immunodeficiency, influenza, and dengue viral infections. Viruses 2019, 11, 252. [Google Scholar] [CrossRef] [PubMed]
- Levraud, J.P.; Palha, N.; Langevin, C.; Boudinot, P. Through the looking glass: Witnessing host-virus interplay in zebrafish. Trends Microbiol. 2014, 22, 490–497. [Google Scholar] [CrossRef]
- Varela, M.; Figueras, A.; Novoa, B. Modelling viral infections using zebrafish: Innate immune response and antiviral research. Antiviral Res. 2017, 139, 59–68. [Google Scholar] [CrossRef]
- Vaz, R.L.; Outeiro, T.F.; Ferreira, J.J. Zebrafish as an animal model for drug discovery in Parkinson’s disease and other movement disorders: A systematic review. Front. Neurol. 2018, 9, 347. [Google Scholar] [CrossRef]
- Rissone, A.; Burgess, S.M. Rare genetic blood disease modeling in zebrafish. Front. Genet. 2018, 9, 348. [Google Scholar] [CrossRef]
- Chan, C.Y.; Ooi, E.E. Dengue: An update on treatment options. Future Microbiol. 2015, 10, 2017–2031. [Google Scholar] [CrossRef] [PubMed]
- Rothman, A.L.; Ennis, F.A. Dengue Vaccine: The Need, the Challenges, and Progress. J. Infect. Dis. 2016, 214, 825–827. [Google Scholar] [CrossRef]
- Lin, L.T.; Hsu, W.C.; Lin, C.C. Antiviral natural products and herbal medicines. J. Tradit. Complement. Med. 2014, 4, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.L.; Edrada-Ebel, R.; Quinn, R.J. The re-emergence of natural products for drug discovery in the genomics era. Nat. Rev. Drug Discov. 2015, 14, 111–129. [Google Scholar] [CrossRef] [PubMed]
- Hert, J.; Irwin, J.J.; Laggner, C.; Keiser, M.J.; Shoichet, B.K. Quantifying biogenic bias in screening libraries. Nat. Chem. Biol. 2009, 5, 479–483. [Google Scholar] [CrossRef]
- Zandi, K.; Teoh, B.T.; Sam, S.S.; Wong, P.F.; Mustafa, M.R.; AbuBakar, S. Novel antiviral activity of baicalein against dengue virus. BMC Complement. Altern. Med. 2012, 12, 214. [Google Scholar] [CrossRef]
- Frabasile, S.; Koishi, A.C.; Kuczera, D.; Silveira, G.F.; Verri, W.A.; Dos Santos, C.N.D.; Bordignon, J. The citrus flavanone naringenin impairs dengue virus replication in human cells. Sci. Rep. 2017, 7, 41864. [Google Scholar] [CrossRef]
- Lin, L.T.; Chen, T.Y.; Lin, S.C.; Chung, C.Y.; Lin, T.C.; Wang, G.H.; Anderson, R.; Lin, C.C.; Richardson, C.D. Broad-spectrum antiviral activity of chebulagic acid and punicalagin against viruses that use glycosaminoglycans for entry. BMC Microbiol. 2013, 13, 187. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Ducharme, N.A.; Reif, D.M.; Gustafsson, J.A.; Bondesson, M. Comparison of toxicity values across zebrafish early life stages and mammalian studies: Implications for chemical testing. Reprod. Toxicol. 2015, 55, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Seol, I.; Son, C. Interpretation of Animal Dose and Human Equivalent Dose for Drug Development. J. Korean Orient. Med. 2010, 31, 1–7. [Google Scholar]
- Scholz, A.; Plate, K.H.; Reiss, Y. Angiopoietin-2: A multifaceted cytokine that functions in both angiogenesis and inflammation. Ann. N. Y. Acad. Sci. 2015, 1347, 45–51. [Google Scholar] [CrossRef]
- Taylor, K.L.; Grant, N.J.; Temperley, N.D.; Patton, E.E. Small molecule screening in zebrafish: An in vivo approach to identifying new chemical tools and drug leads. Cell Commun. Signal. 2010, 8, 11. [Google Scholar] [CrossRef] [PubMed]
- Bency, B.J.; Helen, P.A.M. In silico identification of dengue inhibitors in Giloy (Tinospora cordifolia) and Papaya. J. Emerg. Technol. Innov. Res. 2018, 5, 506–511. [Google Scholar]
- Eshun, K.; He, Q. Aloe Vera: A Valuable Ingredient for the Food, Pharmaceutical and Cosmetic Industries—A Review. Crit. Rev. Food Sci. Nutr. 2004, 44, 91–96. [Google Scholar] [CrossRef]
- Chandan, B.K.; Saxena, A.K.; Shukla, S.; Sharma, N.; Gupta, D.K.; Suri, K.A.; Suri, J.; Bhadauria, M.; Singh, B. Hepatoprotective potential of Aloe barbadensis Mill. against carbon tetrachloride induced hepatotoxicity. J. Ethnopharmacol. 2007, 111, 560–566. [Google Scholar] [CrossRef]
- Sharma, N.; Mishra, K.P.; Chanda, S.; Bhardwaj, V.; Tanwar, H.; Ganju, L.; Kumar, B.; Singh, S.B. Evaluation of anti-dengue activity of Carica papaya aqueous leaf extract and its role in platelet augmentation. Arch. Virol. 2019, 164, 1095–1110. [Google Scholar] [CrossRef]
- Dharmarathna, S.L.C.A.; Wickramasinghe, S.; Waduge, R.N.; Rajapakse, R.P.V.J.; Kularatne, S.A.M. Does Carica papaya leaf-extract increase the platelet count? An experimental study in a murine model. Asian Pac. J. Trop. Biomed. 2013, 3, 720–724. [Google Scholar] [CrossRef]
- Zhao, F.; Pang, W.; Zhang, Z.; Zhao, J.; Wang, X.; Liu, Y.; Wang, X.; Feng, Z.; Zhang, Y.; Sun, W.; et al. Pomegranate extract and exercise provide additive benefits on improvement of immune function by inhibiting inflammation and oxidative stress in high-fat-diet-induced obesity rats. J. Nutr. Biochem. 2016, 32, 20–28. [Google Scholar] [CrossRef]
- Houston, D.M.J.; Bugert, J.J.; Denyer, S.P.; Heard, C.M. Potentiated virucidal activity of pomegranate rind extract (PRE) and punicalagin against Herpes simplex virus (HSV) when coadministered with zinc (II) ions, and antiviral activity of PRE against HSV and aciclovir-resistant HSV. PLoS ONE 2017, 12, e0179291. [Google Scholar] [CrossRef]
- Neurath, A.R.; Strick, N.; Li, Y.Y.; Debnath, A.K. Punica granatum (pomegranate) juice provides an HIV-1 entry inhibitor and candidate topical microbicide. Ann. N. Y. Acad. Sci. 2005, 1056, 311–327. [Google Scholar] [CrossRef] [PubMed]
- Pattanayak, P.; Behera, P.; Das, D.; Panda, S. Ocimum sanctum Linn. A reservoir plant for therapeutic applications: An overview. Pharmacogn. Rev. 2010, 4, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.I.C.; Ling, A.P.K.; Koh, R.Y.; Chye, S.M.; Voon, K.G.L. Screening of anti-dengue activity in methanolic extracts of medicinal plants. BMC Complement. Altern. Med. 2012, 12, 3. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.; Du, R.; Anantpadma, M.; Schafer, A.; Hou, L.; Tian, J.; Davey, R.A.; Cheng, H.; Rong, L. Identification of ellagic acid from plant rhodiola rosea l. as an anti-ebola virus entry inhibitor. Viruses 2018, 10, 152. [Google Scholar] [CrossRef]
- Kratz, J.M.; Andrighetti-Fröhner, C.R.; Kolling, D.J.; Leal, P.C.; Cirne-Santos, C.C.; Yunes, R.A.; Nunes, R.J.; Trybala, E.; Bergström, T.; Frugulhetti, I.C.P.P.; et al. Anti-HSV-1 and anti-HIV-1 activity of gallic acid and pentyl gallate. Mem. Inst. Oswaldo Cruz 2008, 103, 437–442. [Google Scholar] [CrossRef]
- Choi, H.J.; Song, J.H.; Bhatt, L.R.; Baek, S.H. Anti-human rhinovirus activity of gallic acid possessing antioxidant capacity. Phyther. Res. 2010, 24, 1292–1296. [Google Scholar] [CrossRef]
- Li, H.L.; Han, T.; Liu, R.H.; Zhang, C.; Chen, H.S.; Zhang, W.D. Alkaloids from Corydalis saxicola and their anti-hepatitis B virus activity. Chem. Biodivers. 2008, 5, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Zou, K.; Li, Z.; Zhang, Y.; Zhang, H.Y.; Li, B.; Zhu, W.L.; Shi, J.Y.; Jia, Q.; Li, Y.M. Advances in the study of berberine and its derivatives: A focus on anti-inflammatory and anti-tumor effects in the digestive system. Acta Pharmacol. Sin. 2017, 38, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Varghese, F.S.; Kaukinen, P.; Gläsker, S.; Bespalov, M.; Hanski, L.; Wennerberg, K.; Kümmerer, B.M.; Ahola, T. Discovery of berberine, abamectin and ivermectin as antivirals against chikungunya and other alphaviruses. Antiviral Res. 2016, 126, 117–124. [Google Scholar] [CrossRef] [PubMed]
- De Paula, S.O.; Fonseca, B.A.L.D. Dengue: A review of the laboratory tests a clinician must know to achieve a correct diagnosis. Braz. J. Infect. Dis. 2004, 8, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Srichaikul, T.; Nimmannitya, S. Haematology in dengue and dengue haemorrhagic fever. Bailliere’s Best Pract. Res. Clin. Haematol. 2000, 13, 261–276. [Google Scholar] [CrossRef] [PubMed]
- Bierman, H.R.; Nelson, E.R. Hematodepressive Virus Diseases of Thailand. Ann. Intern. Med. 1965, 62, 867–884. [Google Scholar] [CrossRef]
- Bente, D.A.; Rico-Hesse, R. Models of dengue virus infection. Drug Discov. Today Dis. Model. 2006, 3, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Seneviratne, S.L.; Malavige, G.N.; de Silva, H.J. Pathogenesis of liver involvement during dengue viral infections. Trans. R. Soc. Trop. Med. Hyg. 2006, 100, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Sreekanth, G.P.; Chuncharunee, A.; Cheunsuchon, B.; Noisakran, S.; Yenchitsomanus, P.T.; Limjindaporn, T. JNK1/2 inhibitor reduces dengue virus-induced liver injury. Antiviral Res. 2017, 141, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Sreekanth, G.P.; Chuncharunee, A.; Sirimontaporn, A.; Panaampon, J.; Srisawat, C.; Morchang, A.; Malakar, S.; Thuwajit, P.; Kooptiwut, S.; Suttitheptumrong, A.; et al. Role of ERK1/2 signaling in dengue virus-induced liver injury. Virus Res. 2014, 188, 15–26. [Google Scholar] [CrossRef]
- Sreekanth, G.P.; Chuncharunee, A.; Sirimontaporn, A.; Panaampon, J.; Noisakran, S.; Yenchitsomanus, P.T.; Limjindaporn, T. SB203580 modulates p38 MAPK signaling and Dengue virus-induced liver injury by reducing MAPKAPK2, HSP27, and ATF2 phosphorylation. PLoS ONE 2016, 11, e0149486. [Google Scholar] [CrossRef]
- Huerre, M.R.; Lan, N.T.; Marianneau, P.; Hue, N.B.; Khun, H.; Hung, N.T.; Khen, N.T.; Drouet, M.T.; Huong, V.T.Q.; Buisson, Y.; et al. Liver histopathology and biological correlates in five cases of fatal dengue fever in Vietnamese children. Virchows Arch. 2001, 438, 107–115. [Google Scholar] [CrossRef]
- Nguyen, N.M.; Tran, C.N.B.; Phung, L.K.; Duong, K.T.H.; Huynh, H.L.A.; Farrar, J.; Nguyen, Q.T.H.; Tran, H.T.; Nguyen, C.V.V.; Merson, L.; et al. A randomized, double-blind placebo controlled trial of balapiravir, a polymerase inhibitor, in Adult dengue patients. J. Infect. Dis. 2013, 207, 1442–1450. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Chen, Y.L.; Schul, W.; Wang, Q.Y.; Gu, F.; Duraiswamy, J.; Kondreddi, R.R.; Niyomrattanakit, P.; Lakshminarayana, S.B.; Goh, A.; et al. An adenosine nucleoside inhibitor of dengue virus. Proc. Natl. Acad. Sci. USA 2009, 106, 20435–20439. [Google Scholar] [CrossRef] [PubMed]
- Gu, B.; Liu, C.; Lin-Goerke, J.; Maley, D.R.; Gutshall, L.L.; Feltenberger, C.A.; Del Vecchio, A.M. The RNA Helicase and Nucleotide Triphosphatase Activities of the Bovine Viral Diarrhea Virus NS3 Protein Are Essential for Viral Replication. J. Virol. 2000, 74, 1794–1800. [Google Scholar] [CrossRef] [PubMed]
- Phong, W.Y.; Moreland, N.J.; Lim, S.P.; Wen, D.; Paradkar, P.N.; Vasudevan, S.G. Dengue protease activity: The structural integrity and interaction of NS2B with NS3 protease and its potential as a drug target. Biosci. Rep. 2011, 31, 399–409. [Google Scholar] [CrossRef]
- Leangpibul, P.; Thongcharoen, P. Monograph on dengue/dengue haemorrhagic fever. In World Health Organization. No. Regional Publication No. 22; WHO Regional Office for South-East Asia: New Delhi, India, 1993; pp. 62–71. [Google Scholar]
- Lam, P.K.; Ngoc, T.V.; Thu Thuy, T.T.; Hong Van, N.T.; Nhu Thuy, T.T.; Hoai Tam, D.T.; Dung, N.M.; Hanh Tien, N.T.; Thanh Kieu, N.T.; Simmons, C.; et al. The value of daily platelet counts for predicting dengue shock syndrome: Results from a prospective observational study of 2301 Vietnamese children with dengue. PLoS Negl. Trop. Dis. 2017, 11, e0005498. [Google Scholar] [CrossRef]
- Dewi, B.E.; Takasaki, T.; Kurane, I. In vitro assessment of human endothelial cell permeability: Effects of inflammatory cytokines and dengue virus infection. J. Virol. Methods 2004, 121, 171–180. [Google Scholar] [CrossRef]
- Mapalagamage, M.; Handunnetti, S.M.; Wickremasinghe, A.R.; Premawansa, G.; Thillainathan, S.; Fernando, T.; Kanapathippillai, K.; de Silva, A.D.; Premawansa, S. High levels of serum angiopoietin 2 and angiopoietin 2/1 ratio at the critical stage of dengue hemorrhagic fever in patients and association with clinical and biochemical parameters. J. Clin. Microbiol. 2020, 58. [Google Scholar] [CrossRef]
- Malavige, G.N.; Ogg, G.S. Pathogenesis of vascular leak in dengue virus infection. Immunology 2017, 151, 261–269. [Google Scholar] [CrossRef]
- John, D.V.; Lin, Y.S.; Perng, G.C. Biomarkers of severe dengue disease—A review. J. Biomed. Sci. 2015, 22, 83. [Google Scholar] [CrossRef]
- Nag, S.; Papneja, T.; Venugopalan, R.; Stewart, D.J. Increased angiopoietin2 expression is associated with endothelial apoptosis and blood-brain barrier breakdown. Lab. Investig. 2005, 85, 1189–1198. [Google Scholar] [CrossRef]
- Soukaloun, D. Dengue infection in Lao PDR. Southeast. Asian J. Trop. Med. Public Health 2014, 45, 113–119. [Google Scholar]
- Guabiraba, R.; Marques, R.E.; Besnard, A.G.; Fagundes, C.T.; Souza, D.G.; Ryffel, B.; Teixeira, M.M. Role of the Chemokine Receptors CCR1, CCR2 and CCR4 in the Pathogenesis of Experimental Dengue Infection in Mice. PLoS ONE 2010, 5, e15680. [Google Scholar] [CrossRef]
- Lok, S.M.; Ng, M.L.; Aaskov, J. MIP-1α and MIP-1β induction by dengue virus. J. Med. Virol. 2001, 65, 324–330. [Google Scholar] [CrossRef]
- Rothman, A.L. Immunology and Immunopathogenesis of Dengue Disease. Adv. Virus Res. 2003, 60, 397–419. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Altamirano, M.M.B.; Romano, M.; Legorreta-Herrera, M.; Sánchez-García, F.J.; Colston, M.J. Gene expression in human macrophages infected with dengue virus serotype-2. Scand. J. Immunol. 2004, 60, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Ocazionez, R.E.; Meneses, R.; Torres, F.Á.; Stashenko, E. Virucidal activity of Colombian Lippia essential oils on dengue virus replication in vitro. Mem. Inst. Oswaldo Cruz 2010, 105, 304–309. [Google Scholar] [CrossRef]
- Kurokawa, M.; Shimizu, T.; Watanabe, W.; Shiraki, K. Development of New Antiviral Agents from Natural Products. Open Antimicrob. Agents J. 2010, 2, 49–57. [Google Scholar] [CrossRef]
- Zandi, K.; Teoh, B.T.; Sam, S.S.; Wong, P.F.; Mustafa, M.; Abubakar, S. Antiviral activity of four types of bioflavonoid against dengue virus type-2. Virol. J. 2011, 8, 560. [Google Scholar] [CrossRef]
- Low, J.S.Y.; Wu, K.X.; Chen, K.C.; Ng, M.M.L.; Chu, J.J.H. Narasin, a novel antiviral compound that blocks dengue virus protein expression. Antivir. Ther. 2011, 16, 1203–1218. [Google Scholar] [CrossRef]
| Course of Dengue Illness | Zebrafish Aetiology | Human Dengue Endpoints | ||
|---|---|---|---|---|
| Disease Initiation | Critical Progression | Recovery by Denguenil Vati | ||
| Phenotypic Changes | Hypoactivity and Body discolouration | Vascular Hemorrhage of blood vessels in the caudal extremities | Caudal tip with no fringes | Lethargy, Restlessness, Endothelial dysfunction, Vascular leak and Hemorrhage development |
| Pathological Changes | RBC infiltration | Hepatocyte necrosis | Normal hepatocytes and Reduction in RBC infiltration | Hepatocellular necrosis and Cellular infiltration |
| Molecular Changes | Dengue biomarker DENV-3 expression | No expression | DENV transcript expression | |
| Expression of Ang-2 | Low expression of Ang-2 | Elevated Ang-2 expression | ||
| Increased CCL3 expression | Low CCL3 expression | Elevated CCL3 levels associated with disease severity | ||
| Blood Counts | Decline in RBC count Increase in WBC count Decline in Platelet count | Restoration of Blood count for RBC- 3 × 106 WBC- 2.8 × 103 Platelet- 191 × 103 | Anemia Elevated lymphocytes Decrease in platelet counts | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balkrishna, A.; Solleti, S.K.; Verma, S.; Varshney, A. Validation of a Novel Zebrafish Model of Dengue Virus (DENV-3) Pathology Using the Pentaherbal Medicine Denguenil Vati. Biomolecules 2020, 10, 971. https://doi.org/10.3390/biom10070971
Balkrishna A, Solleti SK, Verma S, Varshney A. Validation of a Novel Zebrafish Model of Dengue Virus (DENV-3) Pathology Using the Pentaherbal Medicine Denguenil Vati. Biomolecules. 2020; 10(7):971. https://doi.org/10.3390/biom10070971
Chicago/Turabian StyleBalkrishna, Acharya, Siva Kumar Solleti, Sudeep Verma, and Anurag Varshney. 2020. "Validation of a Novel Zebrafish Model of Dengue Virus (DENV-3) Pathology Using the Pentaherbal Medicine Denguenil Vati" Biomolecules 10, no. 7: 971. https://doi.org/10.3390/biom10070971
APA StyleBalkrishna, A., Solleti, S. K., Verma, S., & Varshney, A. (2020). Validation of a Novel Zebrafish Model of Dengue Virus (DENV-3) Pathology Using the Pentaherbal Medicine Denguenil Vati. Biomolecules, 10(7), 971. https://doi.org/10.3390/biom10070971





