Co-Delivery of Imiquimod and Curcumin by Nanoemugel for Improved Topical Delivery and Reduced Psoriasis-Like Skin Lesions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
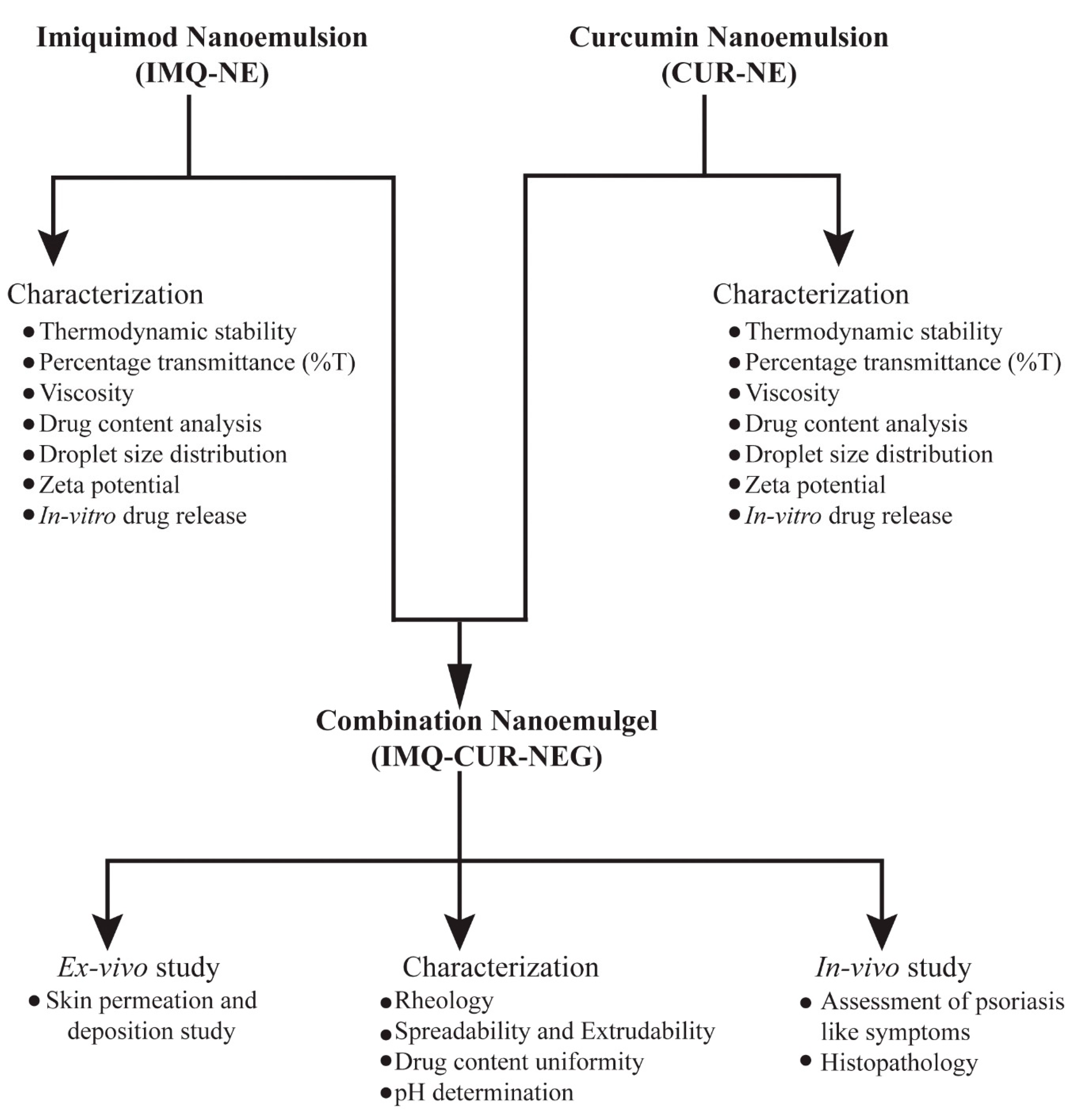
2.2. Formulation Design, Optimization and Characterization of Imiquimod Nanoemulsion
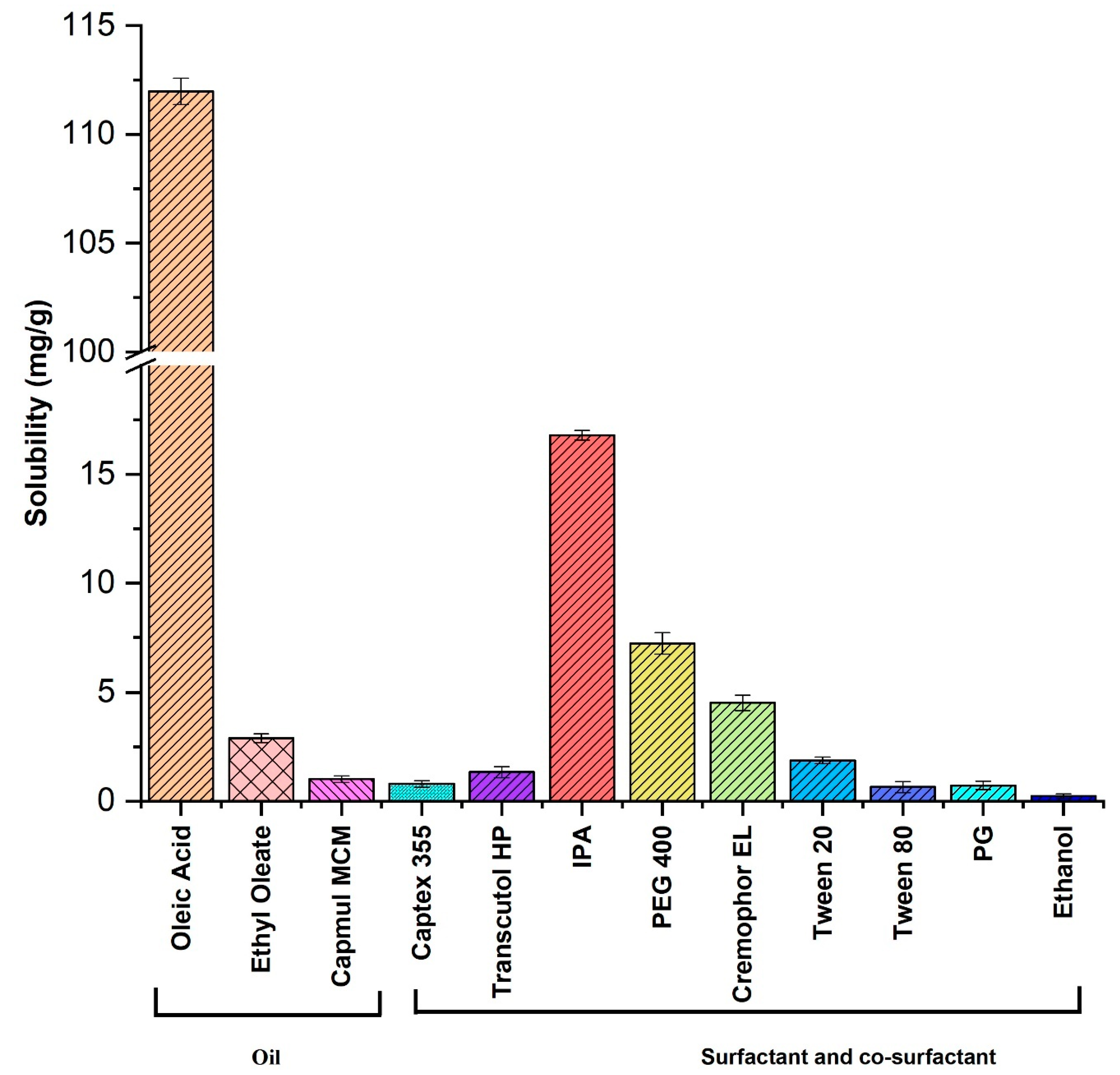
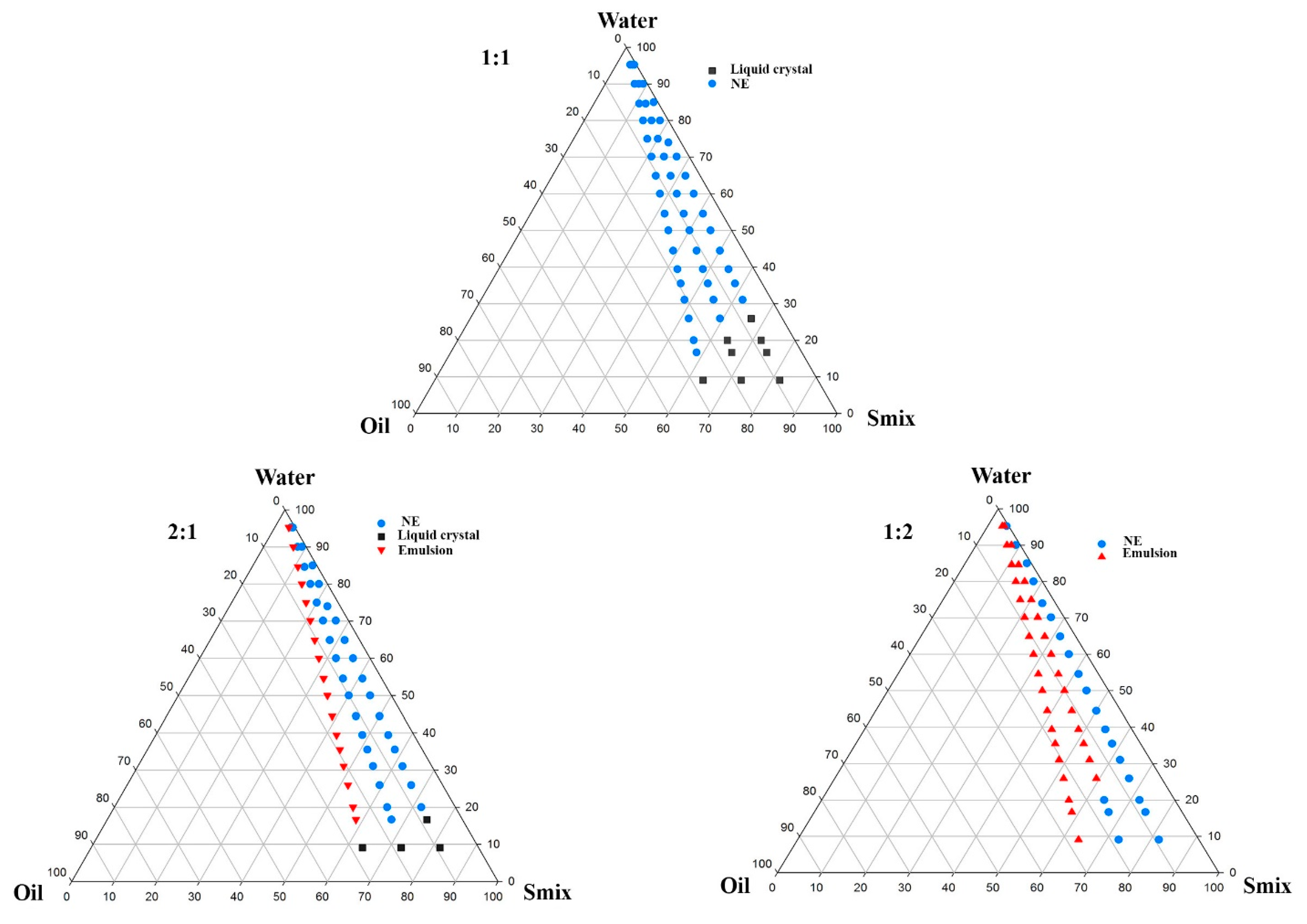
2.2.1. Solubility and Phase Behavior Study
2.2.2. Thermodynamic Stability
2.2.3. Percentage Transmittance (%T)
2.2.4. Viscosity
2.2.5. Drug Content Analysis
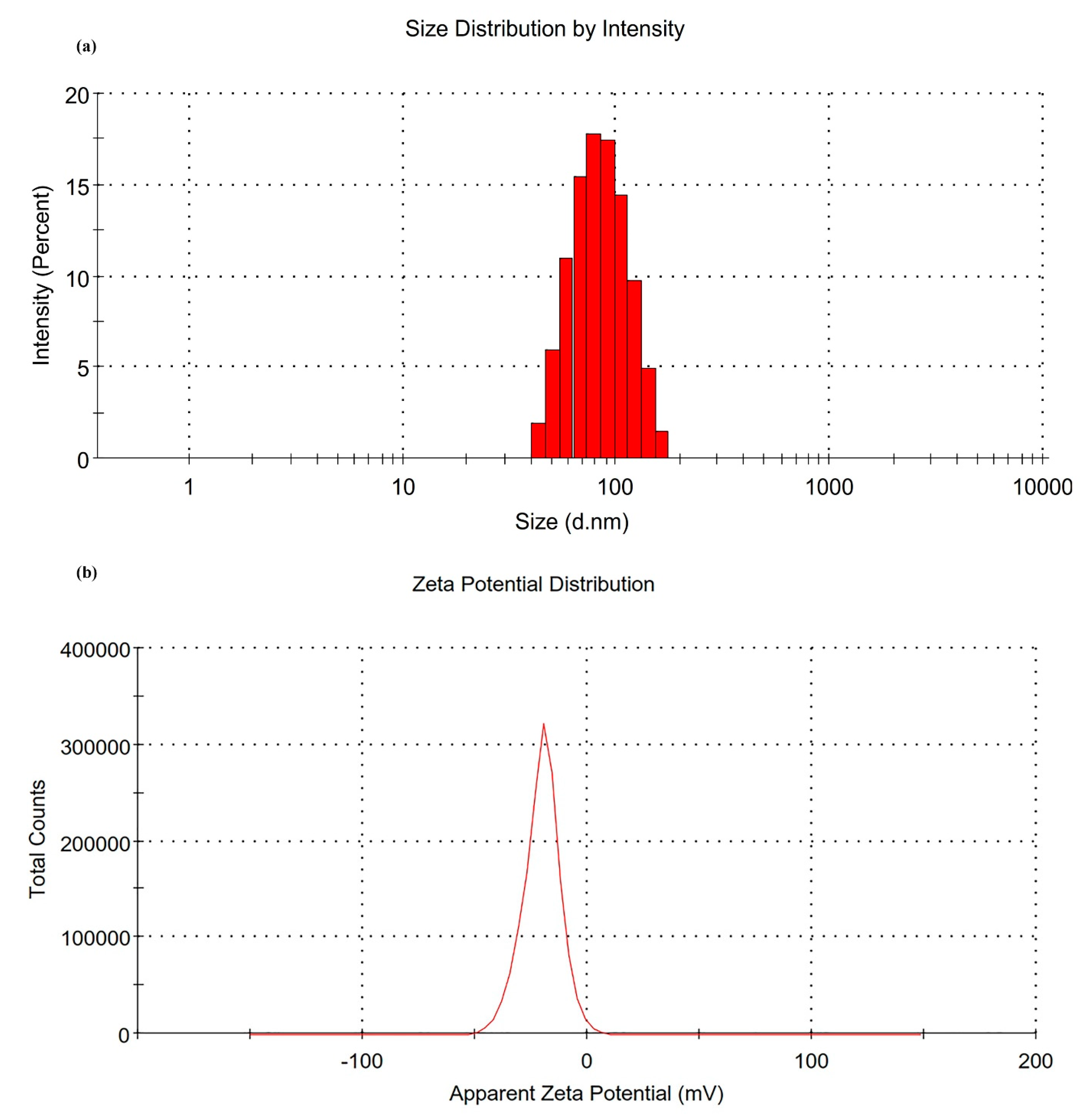
2.2.6. Analysis of Droplet Size Distribution and Zeta Potential
2.3. Preparation and Characterization of Curcumin Nanoemulsion
2.4. In Vitro Drug Release Study
2.5. Preparation and Characterization of Combination Nanoemulgel
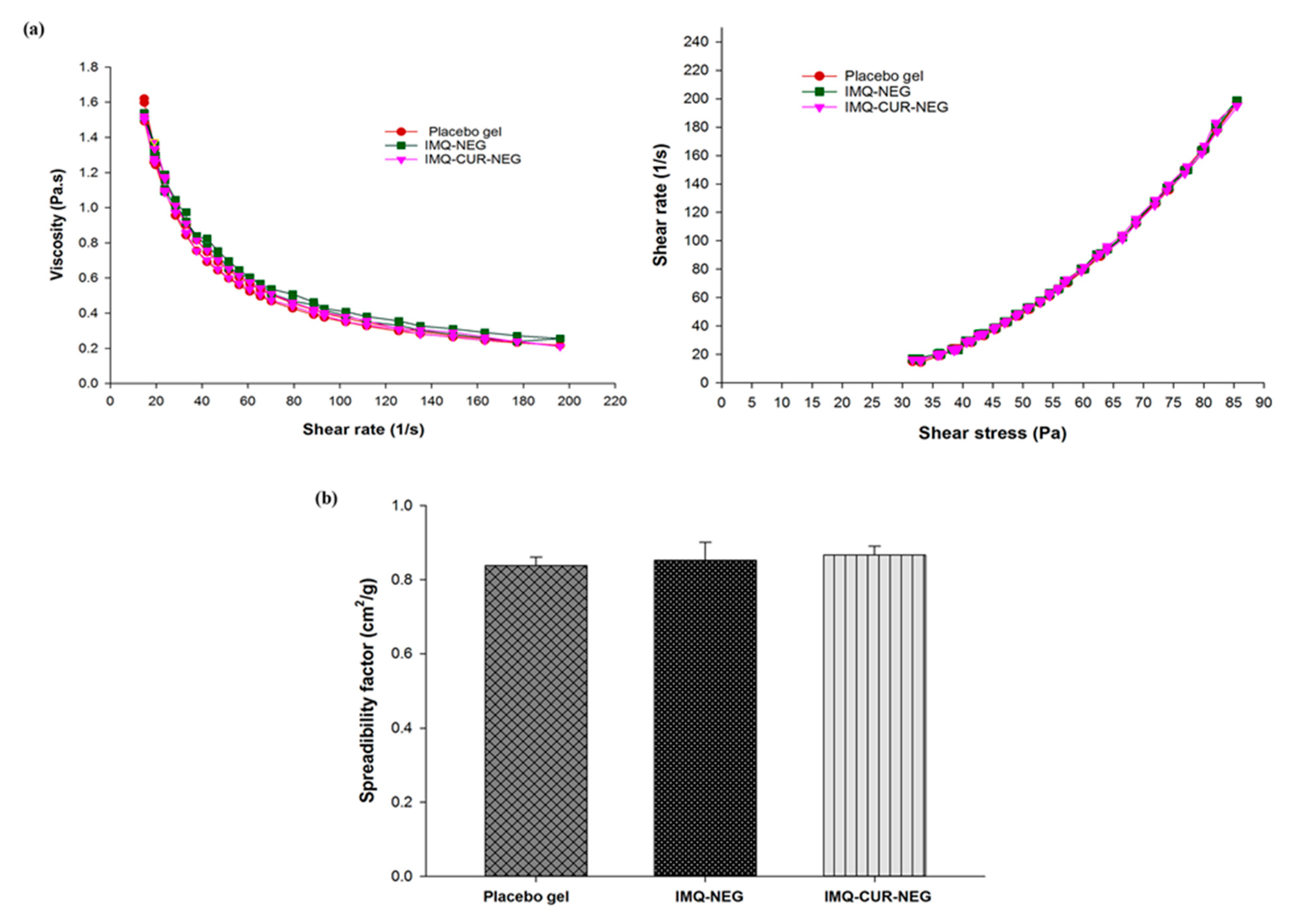
2.5.1. Rheology
2.5.2. Spreadability and Extrudability
2.5.3. Drug Content Uniformity
2.5.4. pH Determination
2.6. Ex Vivo Skin Permeation and Deposition Study
2.6.1. Preparation of Rat Skin
2.6.2. Drug Permeation and Deposition in the Skin
2.7. In Vivo Study
2.7.1. Animals
2.7.2. Assessment of Psoriasis-Like Symptoms
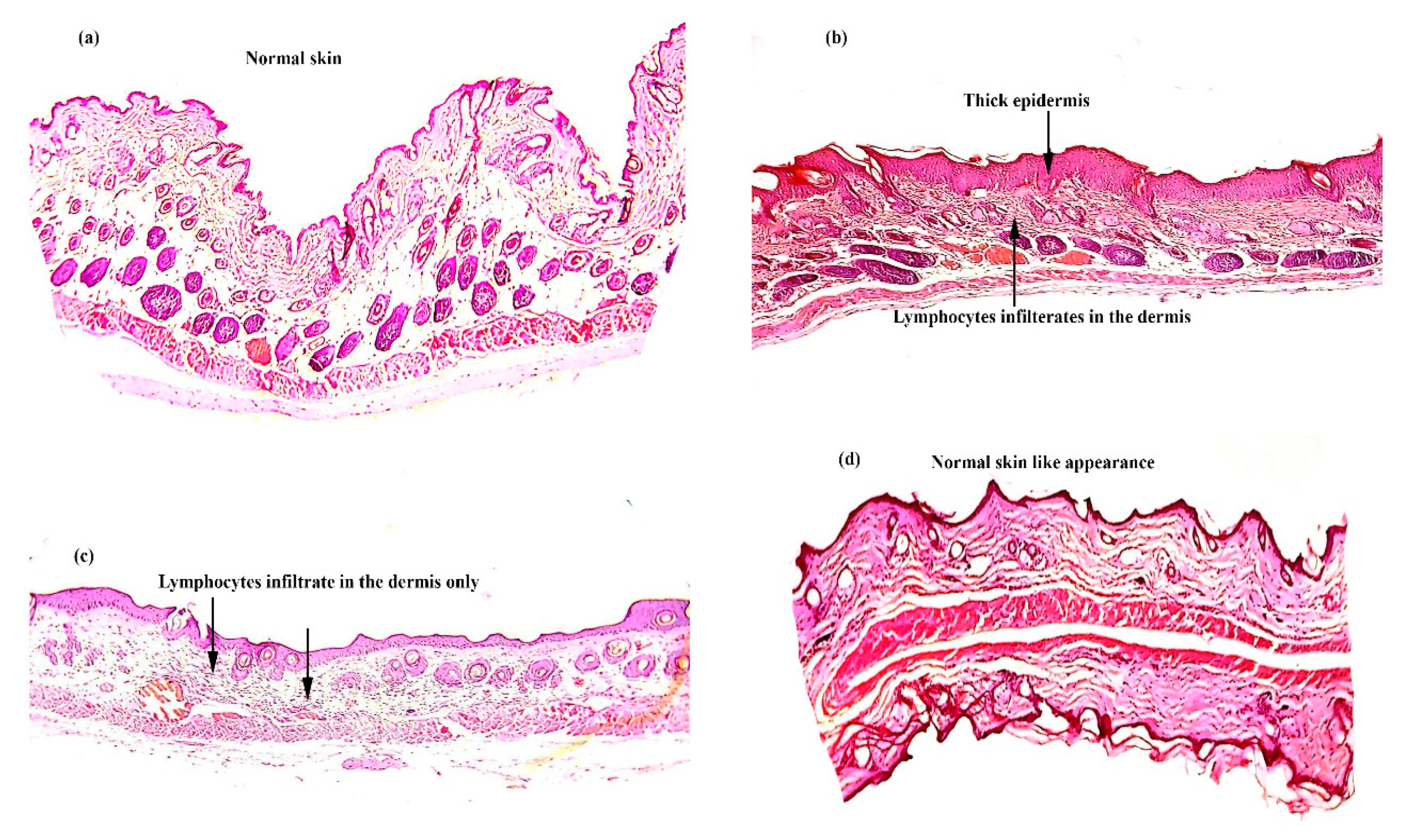
2.7.3. Histopathology
2.8. Statistical Analysis
3. Results and Discussion
3.1. Preparation and Characterization of IMQ-NE and CUR-NE
3.1.1. Solubility Study of IMQ-NE
3.1.2. Optimization of IMQ-NE Exploiting Phase Behavior Study
3.1.3. Characterization of IMQ-NE
3.1.4. Characterization of CUR-NE
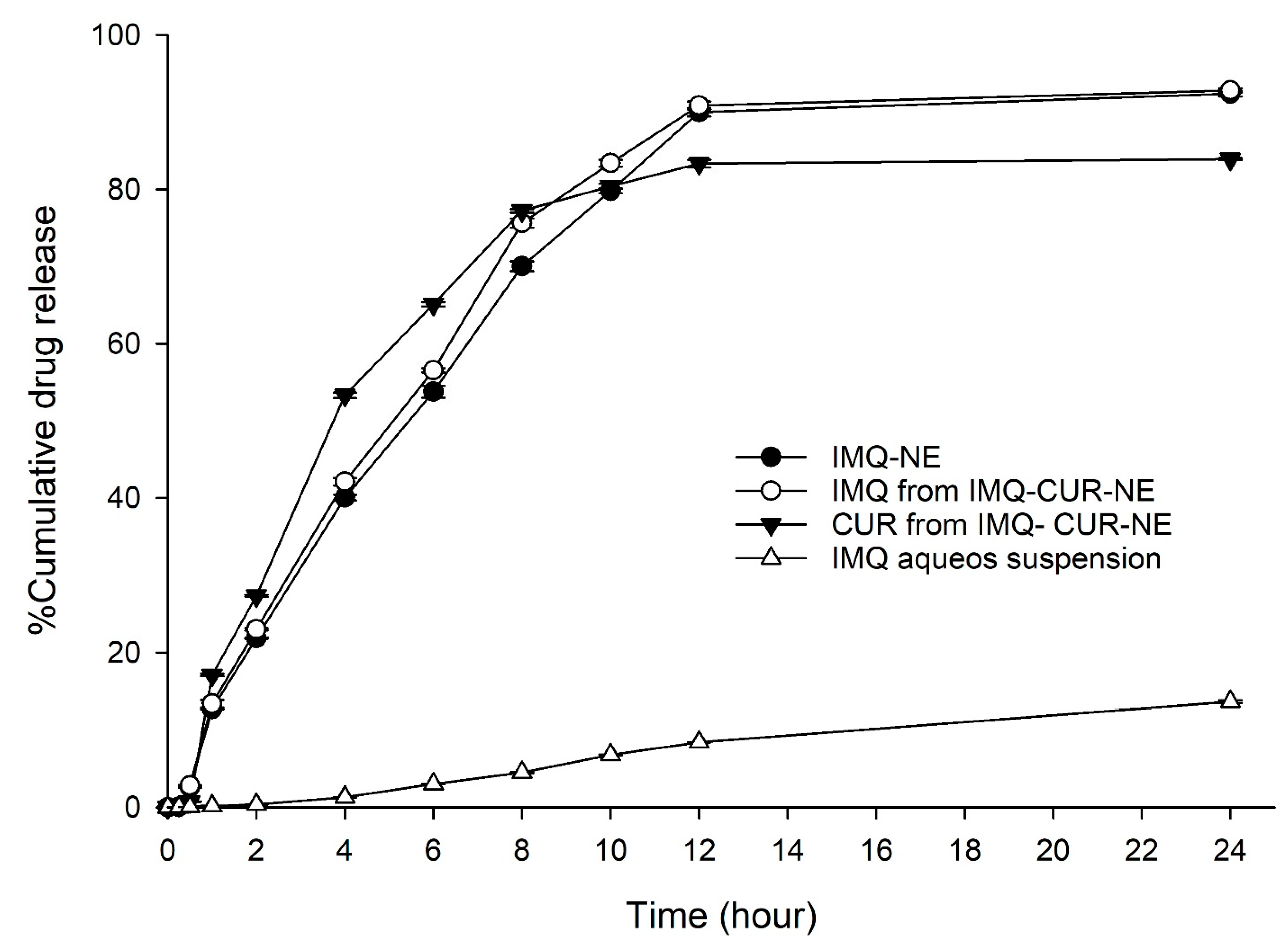
3.2. In Vitro Drug Release Study
3.3. Preparation and Characterization of IMQ-NEG and IMQ-CUR-NEG
3.4. Ex Vivo Skin Permeation and Deposition Study
3.5. In Vivo Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Venturini, C.G.; Bruinsmann, F.A.; Contri, R.V.; Fonseca, F.N.; Frank, L.A.; D’Amore, C.M.; Raffin, R.P.; Buffon, A.; Pohlmann, A.R.; Guterres, S.S. Co-encapsulation of imiquimod and copaiba oil in novel nanostructured systems: Promising formulations against skin carcinoma. Eur. J. Pharm. Sci. 2015, 79, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Telò, I.; Favero, E.D.; Cantù, L.; Frattini, N.; Pescina, S.; Padula, C.; Santi, P.; Sonvico, F.; Nicoli, S. Gel-like TPGS-Based Microemulsions for Imiquimod Dermal Delivery: Role of Mesostructure on the Uptake and Distribution into the Skin. Mol. Pharm. 2017, 14, 3281–3289. [Google Scholar] [CrossRef] [PubMed]
- Wagstaff, A.J.; Perry, C.M. Topical imiquimod: A review of its use in the management of anogenital warts, actinic keratoses, basal cell carcinoma and other skin lesions. Drugs 2007, 67, 2187–2210. [Google Scholar] [CrossRef] [PubMed]
- Micali, G.; Lacarrubba, F.; Dinotta, F.; Massimino, D.; Nasca, M.R. Treating skin cancer with topical cream. Expert Opin. Pharmacother. 2010, 11, 1515–1527. [Google Scholar] [CrossRef] [PubMed]
- IMIQUIMOD CREAM. Available online: https://www.bad.org.uk/shared/get-file.ashx?id=209&itemtype=document (accessed on 5 April 2020).
- Ahmad, M.Z.; Akhter, S.; Mohsin, N.; Abdel-Wahab, B.A.; Ahmad, J.; Warsi, M.H.; Rahman, M.; Mallick, N.; Ahmad, F.J. Transformation of curcumin from food additive to multifunctional medicine: Nanotechnology bridging the gap. Curr. Drug Discov. Technol. 2014, 11, 197–213. [Google Scholar] [CrossRef] [PubMed]
- Bashang, H.; Tamma, S. The use of curcumin as an effective adjuvant to cancer therapy: A short review. Biotechnol. Appl. Biochem. 2019. [Google Scholar] [CrossRef]
- Liu, Z.; Huang, P.; Law, S.; Tian, H.; Leung, W.; Xu, C. Preventive Effect of Curcumin Against Chemotherapy-Induced Side-Effects. Front. Pharmacol. 2018, 9, 1374. [Google Scholar] [CrossRef] [Green Version]
- Farokhzad, O.C.; Langer, R. Impact of nanotechnology on drug delivery. ACS Nano 2009, 3, 16–20. [Google Scholar] [CrossRef]
- Borgheti-Cardoso, L.N.; Viegas, J.S.R.; Silvestrini, A.V.P.; Caron, A.L.; Praça, F.G.; Kravicz, M.; Bentley, M. Nanotechnology approaches in the current therapy of skin cancer. Adv. Drug Deliv. Rev. 2020. [Google Scholar] [CrossRef]
- Contri, R.V.; Frank, L.A.; Kaiser, M.; Pohlmann, A.R.; Guterres, S.S. The use of nanoencapsulation to decrease human skin irritation caused by capsaicinoids. Int. J. Nanomed. 2014, 9, 951–962. [Google Scholar] [CrossRef] [Green Version]
- Pople, P.V.; Singh, K.K. Targeting tacrolimus to deeper layers of skin with improved safety for treatment of atopic dermatitis. Int. J. Pharm. 2010, 398, 165–178. [Google Scholar] [CrossRef]
- Fontana, M.C.; Rezer, J.F.; Coradini, K.; Leal, D.B.; Beck, R.C. Improved efficacy in the treatment of contact dermatitis in rats by a dermatological nanomedicine containing clobetasol propionate. Eur. J. Pharm. Biopharm. 2011, 79, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, J.; Gautam, A.; Komath, S.; Bano, M.; Garg, A.; Jain, K. Topical Nano-emulgel for Skin Disorders: Formulation Approach and Characterization. Recent Pat. Anti Infect. Drug Discov. 2019, 14, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Xi, J.; Chang, Q.; Chan, C.K.; Meng, Z.Y.; Wang, G.N.; Sun, J.B.; Wang, Y.T.; Tong, H.H.; Zheng, Y. Formulation development and bioavailability evaluation of a self-nanoemulsified drug delivery system of oleanolic acid. AAPS Pharmscitech 2009, 10, 172–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Chang, X.; Weng, T.; Zhao, X.; Gao, Z.; Yang, Y.; Xu, H.; Yang, X. A study of microemulsion systems for transdermal delivery of triptolide. J. Control Release 2004, 98, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Magalhaes, N.S.; Cave, G.; Seiller, M.; Benita, S. The stability and in vitro release kinetics of a clofibride emulsion. Int. J. Pharm. 1991, 76, 225–237. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, Y.; Feng, N.; Xu, J. Preparation and evaluation of self-microemulsifying drug delivery system of oridonin. Int. J. Pharm. 2008, 355, 269–276. [Google Scholar] [CrossRef]
- Jain, A.; Doppalapudi, S.; Domb, A.J.; Khan, W. Tacrolimus and curcumin co-loaded liposphere gel: Synergistic combination towards management of psoriasis. J. Control Release 2016, 243, 132–145. [Google Scholar] [CrossRef]
- Thomas, L.; Zakir, F.; Mirza, M.A.; Anwer, M.K.; Ahmad, F.J.; Iqbal, Z. Development of Curcumin loaded chitosan polymer based nanoemulsion gel: In vitro, ex vivo evaluation and in vivo wound healing studies. Int. J. Biol. Macromol. 2017, 101, 569–579. [Google Scholar] [CrossRef]
- Shakeel, F.; Baboota, S.; Ahuja, A.; Ali, J.; Aqil, M.; Shafiq, S. Nanoemulsions as vehicles for transdermal delivery of aceclofenac. AAPS Pharmscitech 2007, 8, E104. [Google Scholar] [CrossRef] [Green Version]
- Algahtani, M.S.; Ahmad, M.Z.; Ahmad, J. Nanoemulgel for Improved Topical Delivery of Retinyl Palmitate: Formulation Design and Stability Evaluation. Nanomaterials 2020, 10, 848. [Google Scholar] [CrossRef]
- Alvarado, H.L.; Abrego, G.; Souto, E.B.; Garduño-Ramirez, M.L.; Clares, B.; García, M.L.; Calpena, A.C. Nanoemulsions for dermal controlled release of oleanolic and ursolic acids: In vitro, ex vivo and in vivo characterization. Colloids Surf. B Biointerfaces 2015, 130, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Katiyar, S.S.; Kushwah, V.; Jain, S. Nanoemulsion loaded gel for topical co-delivery of clobitasol propionate and calcipotriol in psoriasis. Nanomedicine 2017, 13, 1473–1482. [Google Scholar] [CrossRef] [PubMed]
- Chollet, J.L.; Jozwiakowski, M.J.; Phares, K.R.; Reiter, M.J.; Roddy, P.J.; Schultz, H.J.; Ta, Q.V.; Tomai, M.A. Development of a topically active imiquimod formulation. Pharm. Dev. Technol. 1999, 4, 35–43. [Google Scholar] [CrossRef]
- Telò, I.; Pescina, S.; Padula, C.; Santi, P.; Nicoli, S. Mechanisms of imiquimod skin penetration. Int. J. Pharm. 2016, 511, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, J.; Mir, S.R.; Kohli, K.; Chuttani, K.; Mishra, A.K.; Panda, A.K.; Amin, S. Solid-nanoemulsion preconcentrate for oral delivery of paclitaxel: Formulation design, biodistribution, and γ scintigraphy imaging. BioMed Res. Int. 2014, 2014, 984756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jain, G.; Khar, R.K.; Ahmad, F.J. Theory and Practice of Physical Pharmacy-E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Teo, S.Y.; Lee, S.Y.; Ong, H.L.; Ong, C.L.; Gan, S.N.; Rathbone, M.J.; Coombes, A.G. Evaluation of biosourced alkyd nanoemulsions as drug carriers. J. Nanomater. 2015, 2015. [Google Scholar] [CrossRef] [Green Version]
- Fernández Campos, F.; Calpena Campmany, A.C.; Rodríguez Delgado, G.; López Serrano, O.; Clares Naveros, B. Development and characterization of a novel nystatin-loaded nanoemulsion for the buccal treatment of candidosis: Ultrastructural effects and release studies. J. Pharm. Sci. 2012, 101, 3739–3752. [Google Scholar] [CrossRef]
- Garduño-Ramírez, M.L.; Clares, B.; Domínguez-Villegas, V.; Peraire, C.; Ruiz, M.A.; García, M.L.; Calpena, A.C. Skin permeation of cacalol, cacalone and 6-epi-cacalone sesquiterpenes from a nanoemulsion. Nat. Prod. Commun. 2012, 7, 821–823. [Google Scholar] [CrossRef] [Green Version]
- Sobhani, H.; Tarighi, P.; Ostad, S.N.; Shafaati, A.; Nafissi-Varcheh, N.; Aboofazeli, R. Formulation Development and Toxicity Assessment of Triacetin Mediated Nanoemulsions as Novel Delivery Systems for Rapamycin. Iran. J. Pharm. Res. IJPR 2015, 14, 3–21. [Google Scholar]
- Bali, V.; Ali, M.; Ali, J. Nanocarrier for the enhanced bioavailability of a cardiovascular agent: In vitro, pharmacodynamic, pharmacokinetic and stability assessment. Int. J. Pharm. 2011, 403, 46–56. [Google Scholar] [CrossRef]
- Khurana, S.; Jain, N.K.; Bedi, P.M. Nanoemulsion based gel for transdermal delivery of meloxicam: Physico-chemical, mechanistic investigation. Life Sci. 2013, 92, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Rai, V.K.; Mishra, N.; Yadav, K.S.; Yadav, N.P. Nanoemulsion as pharmaceutical carrier for dermal and transdermal drug delivery: Formulation development, stability issues, basic considerations and applications. J. Control Release 2018, 270, 203–225. [Google Scholar] [CrossRef] [PubMed]
- Klang, V.; Matsko, N.B.; Valenta, C.; Hofer, F. Electron microscopy of nanoemulsions: An essential tool for characterisation and stability assessment. Micron 2012, 43, 85–103. [Google Scholar] [CrossRef] [PubMed]
- Algahtani, M.S.; Ahmad, M.Z.; Ahmad, J. Nanoemulsion loaded polymeric hydrogel for topical delivery of curcumin in psoriasis. J. Drug Deliv. Sci. Tec. 2020, in press. [Google Scholar] [CrossRef]
- Rodríguez-Burneo, N.; Busquets, M.A.; Estelrich, J. Magnetic Nanoemulsions: Comparison between Nanoemulsions Formed by Ultrasonication and by Spontaneous Emulsification. Nanomaterials 2017, 7, 190. [Google Scholar] [CrossRef] [Green Version]
- Sinko, P.J.; Singh, Y. Martin’s Physical Pharmacy and Pharmaceutical Sciences: Physical Chemical and Biopharmaceutical Principles in the Pharmaceutical Sciences; Walter Kluer: Alphen aan den Rijn, The Netherlands, 2011. [Google Scholar]
- Lee, C.H.; Moturi, V.; Lee, Y. Thixotropic property in pharmaceutical formulations. J. Control Release 2009, 136, 88–98. [Google Scholar] [CrossRef]
- Chen, M.X.; Alexander, K.S.; Baki, G. Formulation and Evaluation of Antibacterial Creams and Gels Containing Metal Ions for Topical Application. J. Pharm. 2016, 2016, 5754349. [Google Scholar] [CrossRef] [Green Version]
- Elmataeeshy, M.E.; Sokar, M.S.; Bahey-El-Din, M.; Shaker, D.S. Enhanced transdermal permeability of Terbinafine through novel nanoemulgel formulation; Development, in vitro and in vivo characterization. Future J. Pharm. Sci. 2018, 4, 18–28. [Google Scholar] [CrossRef]
- Sun, J.; Zhao, Y.; Hu, J. Curcumin inhibits imiquimod-induced psoriasis-like inflammation by inhibiting IL-1beta and IL-6 production in mice. PLoS ONE 2013, 8, e67078. [Google Scholar] [CrossRef]
- Batra, H.; Pawar, S.; Bahl, D. Curcumin in combination with anti-cancer drugs: A nanomedicine review. Pharmacol. Res. 2019, 139, 91–105. [Google Scholar] [CrossRef]
- Cullen, J.K.; Simmons, J.L.; Parsons, P.G.; Boyle, G.M. Topical treatments for skin cancer. Adv. Drug Deliv. Rev. 2019. [Google Scholar] [CrossRef] [PubMed]
- Lelli, D.; Pedone, C.; Sahebkar, A. Curcumin and treatment of melanoma: The potential role of microRNAs. Biomed. Pharmacother. 2017, 88, 832–834. [Google Scholar] [CrossRef] [PubMed]

| Formu-Lation | %Oil | %Smix | %Water | %T | η (cp) | %Drug Content | Mean Droplet Size (nm) | PDI | ζ (mv) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Tween 20 (%) | Trans-Cutol HP (%) | |||||||||
| NE1 | 15.0 | 18.33 | 36.67 | 30.0 | 93.18 ± 0.217 | 55.26 ± 0.81 | 99.73 ± 0.09 | 197.1 | 0.249 | −10.9 |
| NE2 | 10.0 | 20.0 | 40.0 | 30.0 | 98.51 ± 0.180 | 63.82 ± 0.62 | 99.30 ± 0.22 | 147.7 | 0.245 | −35.8 |
| NE3 | 15.0 | 36.67 | 18.33 | 30.0 | 91.07 ± 0.698 | 60.25 ± 0.64 | 99.83 ± 0.07 | 122.5 | 0.242 | −26.2 |
| NE4 | 10.0 | 40.0 | 20.0 | 30.0 | 98.88 ± 0.036 | 62.98 ± 0.65 | 99.24 ± 0.13 | 76.93 | 0.121 | −20.5 |
| Figure | Korsmeyer-Peppas Model | Zero-Order | First-Order | Higuchi Model | |
|---|---|---|---|---|---|
| r2 | n | r2 | r2 | r2 | |
| IMQ-NE | 0.9986 | 0.35 | 0.9863 | 0.9855 | 0.9707 |
| IMQ from IMQ-CUR-NE | 0.9963 | 0.344 | 0.9792 | 0.9800 | 0.9724 |
| CUR from IMQ-CUR-NE | 0.9934 | 0.290 | 0.9258 | 0.9843 | 0.9846 |
| IMQ aqueous suspension | 0.9258 | 0.48 | 0.7896 | 0.9807 | 0.8844 |
| Parameters | IMQ-NEG | IMQ-CUR-NEG | IMQ-CUR Gel |
|---|---|---|---|
| Spreadability factor (cm2/g) | 0.85 ± 0.04 | 0.87 ± 0.02 | - |
| Drug content uniformity(mg) | 99.8 ± 0.1 | 99.48 ± 0.01 | - |
| 98.85 ± 0.01 (CUR) | - | ||
| pH | 5.54 ± 0.03 | 5.51 ± 0.02 | - |
| Drug deposited in skin (µg/cm2) | 1205.190 ± 21.40 | 1367.646 ± 13.21 | 243.01 ± 5.90 |
| 5178.442 ± 22.23 (CUR) | 570.86 ± 12.01 (CUR) | ||
| Cumulative amount of drug permeated (µg) | 1.14 ± 0.01 | 1.34 ± 0.02 | 0.123 ± 0.01 |
| 989.333 ± 3.97 (CUR) | 226.853 ± 0.98 (CUR) | ||
| Jss (µg/cm2 .h) * | 0.042 ± 0.01 | 0.071 ± 0.01 | 0.004 ± 0.001 |
| 36.47 ± 0.01 (CUR) | 8.365 ± 0.03 (CUR) | ||
| Lag time (h) | 0.72 ± 0.016 | 0.934 ± 0.01 | 2.92 ± 0.02 |
| 0.668 ± 0.01 (CUR) | 1.70 ± 0.06 (CUR) | ||
| Permeability coefficient (Kp x 10-3) ** | 0.0047 | 0.0806 | 0.45 |
| 3.04 (CUR) | 0.7 (CUR) | ||
| ER *** | 9.54 ± 0.79 | 16.19 ± 1.36 | 1 |
| 4.36 ± 0.03 (CUR) | 1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Algahtani, M.S.; Ahmad, M.Z.; Nourein, I.H.; Ahmad, J. Co-Delivery of Imiquimod and Curcumin by Nanoemugel for Improved Topical Delivery and Reduced Psoriasis-Like Skin Lesions. Biomolecules 2020, 10, 968. https://doi.org/10.3390/biom10070968
Algahtani MS, Ahmad MZ, Nourein IH, Ahmad J. Co-Delivery of Imiquimod and Curcumin by Nanoemugel for Improved Topical Delivery and Reduced Psoriasis-Like Skin Lesions. Biomolecules. 2020; 10(7):968. https://doi.org/10.3390/biom10070968
Chicago/Turabian StyleAlgahtani, Mohammed S., Mohammad Zaki Ahmad, Ihab Hamed Nourein, and Javed Ahmad. 2020. "Co-Delivery of Imiquimod and Curcumin by Nanoemugel for Improved Topical Delivery and Reduced Psoriasis-Like Skin Lesions" Biomolecules 10, no. 7: 968. https://doi.org/10.3390/biom10070968
APA StyleAlgahtani, M. S., Ahmad, M. Z., Nourein, I. H., & Ahmad, J. (2020). Co-Delivery of Imiquimod and Curcumin by Nanoemugel for Improved Topical Delivery and Reduced Psoriasis-Like Skin Lesions. Biomolecules, 10(7), 968. https://doi.org/10.3390/biom10070968








