Vitamin D Prevents Pancreatic Cancer-Induced Apoptosis Signaling of Inflammatory Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Isolation of Human Peripheral Blood Mononuclear Cells
2.3. Experimental Design
2.4. Immunoblot Analysis
2.5. Intracellular Calcium Flow Analysis
2.6. Cytokine Assay
2.7. T-Lymphocyte Proliferation Assay
2.8. Flow Cytometry
2.9. Statistical Analysis
3. Results
3.1. BxPC3, not BxPC3-SMAD4+ CM, Antagonizes Calcipotriol-Induced Intracellular Calcium Accumulation in PBMCs
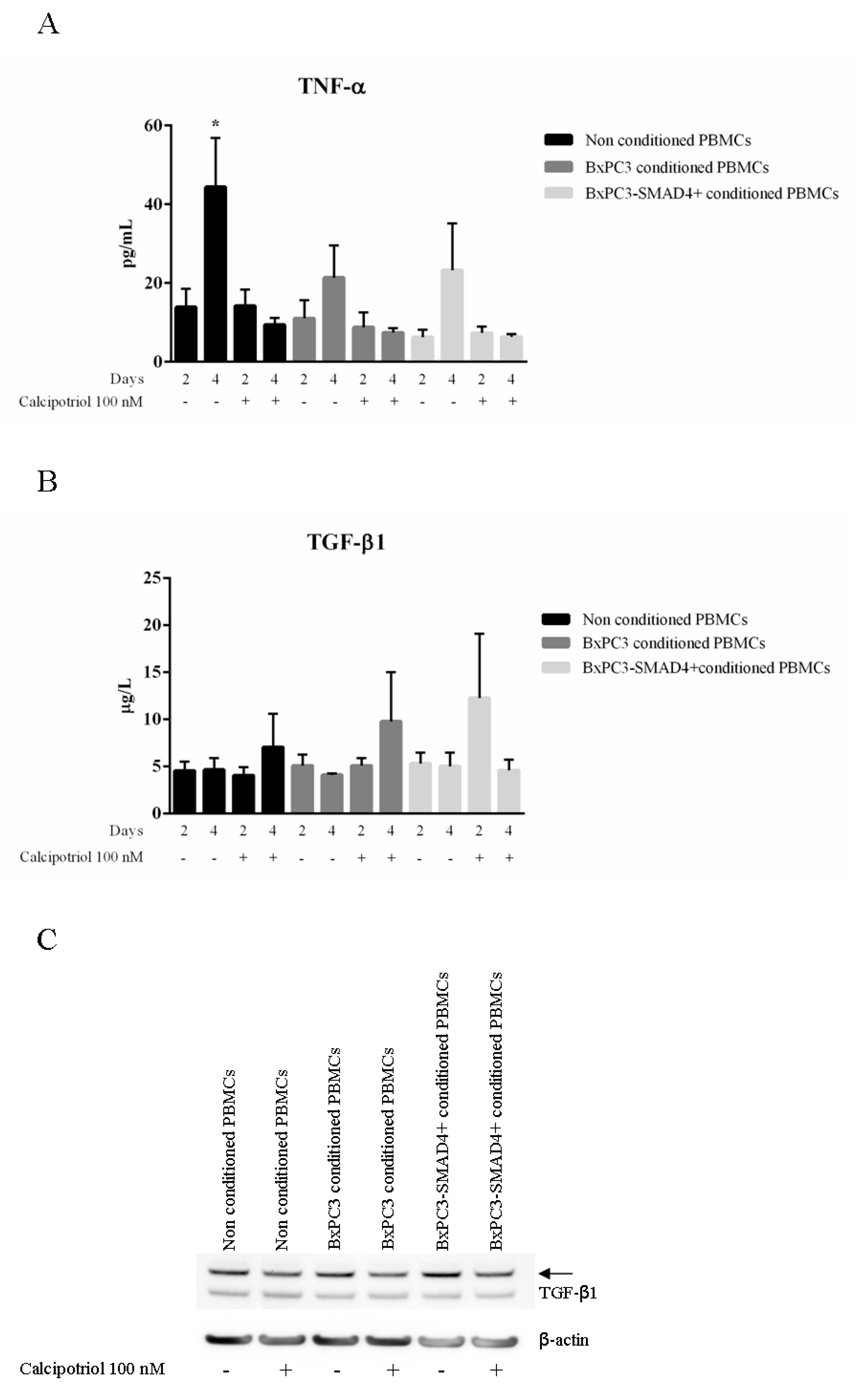
3.2. Calcipotriol Reduces PBMC Release of TNF-α But Does Not Antagonize PDAC-Induced Lymphocytes Proliferation
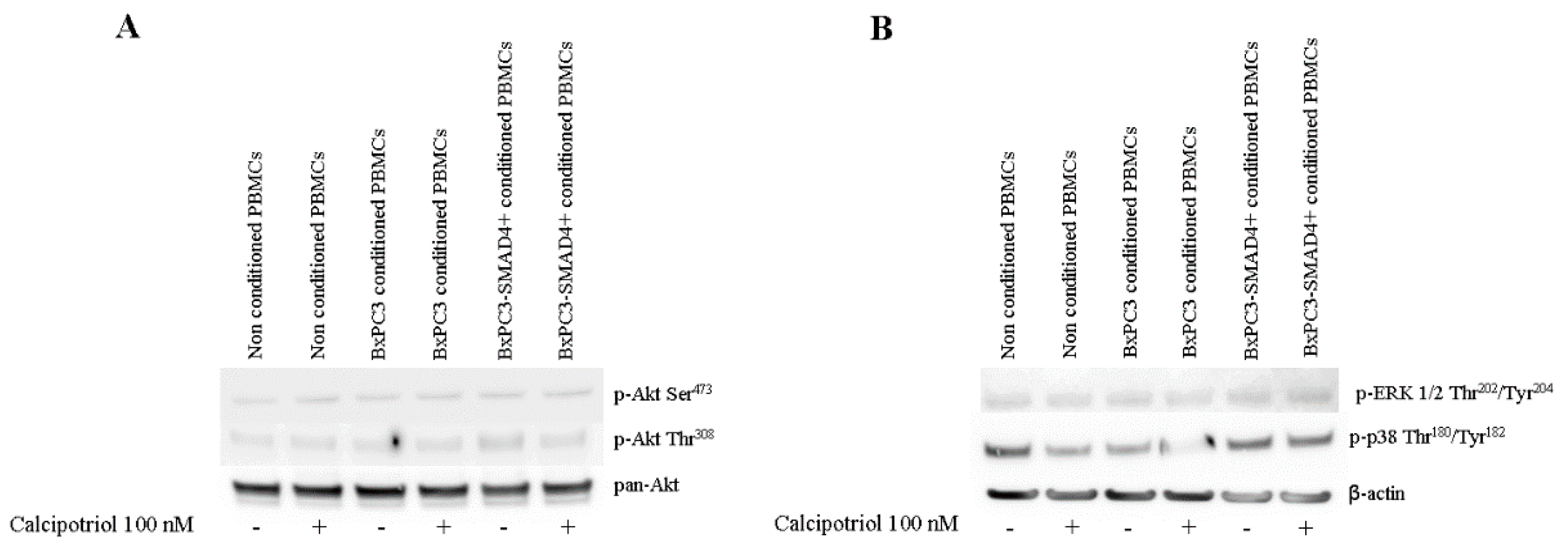
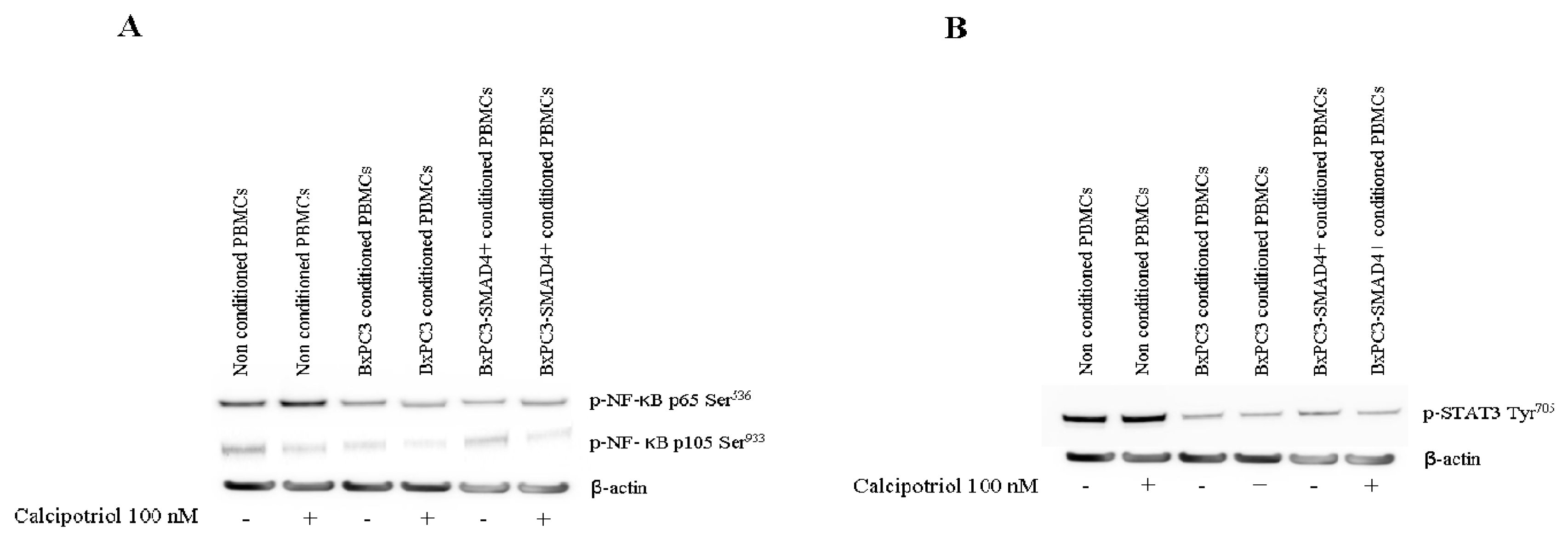
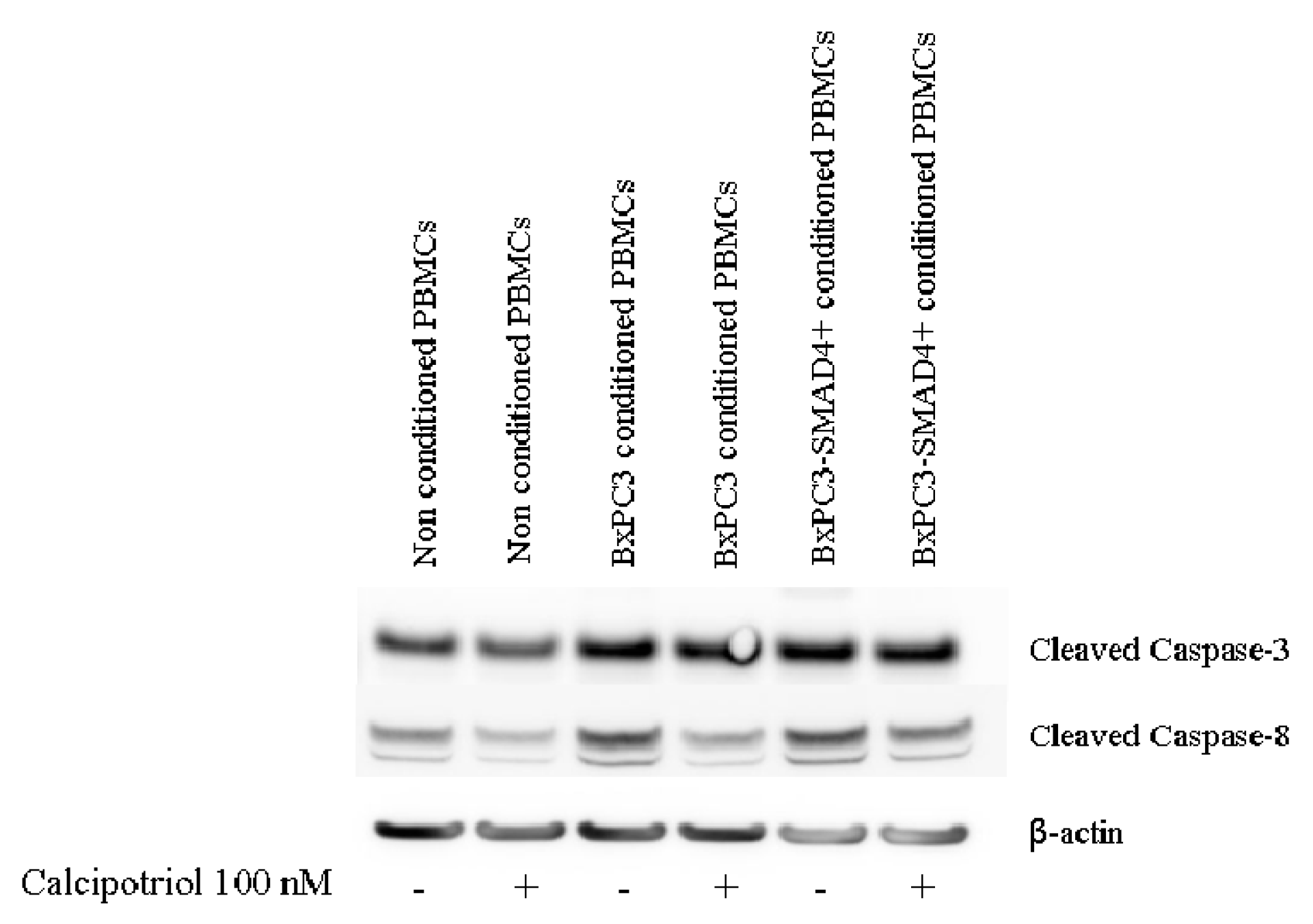
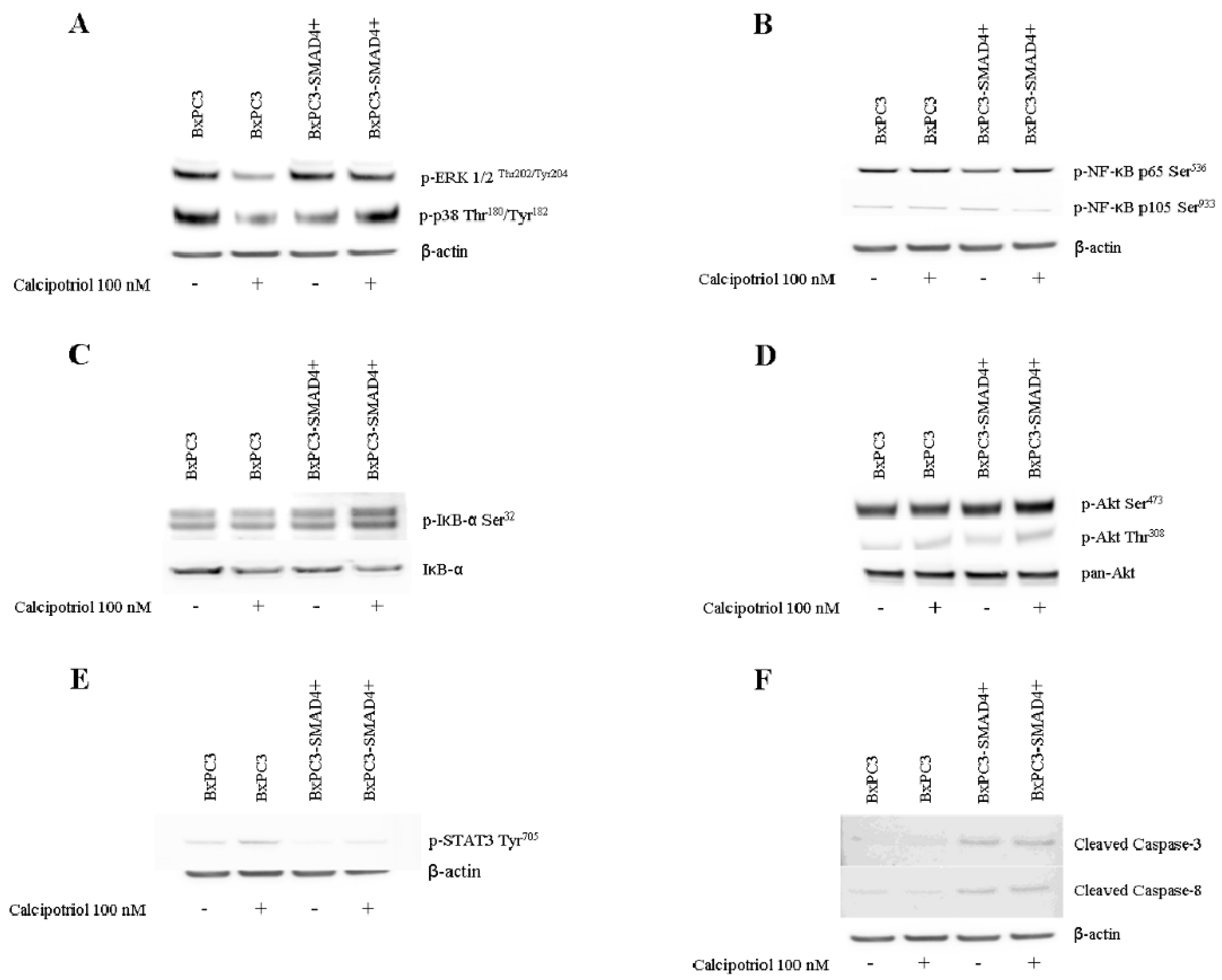
3.3. Effects of PDAC Conditioned Media and Calcipotriol on Proliferation, Inflammation, and Apoptosis Signaling Pathways in PBMCs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Masamune, A.; Watanabe, T.; Kikuta, K.; Shimosegawa, T. Roles of pancreatic stellate cells in pancreatic inflammation and fibrosis. Clin. Gastroenterol. Hepatol. 2009, 7, S48–S54. [Google Scholar] [CrossRef]
- Apte, M.V.; Pirola, R.C.; Wilson, J.S. Pancreatic stellate cells: A starring role in normal and diseased pancreas. Front. Physiol. 2012, 3, 344. [Google Scholar] [CrossRef]
- Sarantis, P.; Koustas, E.; Papadimitropoulou, A.; Papavassiliou, A.G.; Karamouzis, M.V. Pancreatic ductal adenocarcinoma: Treatment hurdles, tumor microenvironment and immunotherapy. World J. Gastrointest. Oncol. 2020, 12, 173–181. [Google Scholar] [CrossRef]
- Wu, X.; Hu, W.; Lu, L.; Zhao, Y.; Zhou, Y.; Xiao, Z.; Zhang, L.; Zhang, H.; Li, X.; Li, W.; et al. Repurposing vitamin D for treatment of human malignancies via targeting tumor microenvironment. Acta Pharm. Sin. B. 2019, 9, 203–219. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, M.; Risch, H.A.; Bosetti, C.; Anderson, K.E.; Petersen, G.M.; Bamlet, W.R.; Cotterchio, M.; Cleary, S.P.; Ibiebele, T.I.; La Vecchia, C.; et al. Pancreatic Cancer Case-Control Consortium(PanC4). Vitamin D and pancreatic cancer: A pooled analysis from the Pancreatic Cancer Case-Control Consortium. Ann. Oncol. 2016, 27, 208. [Google Scholar] [CrossRef]
- Zablotska, L.B.; Gong, Z.; Wang, F.; Holly, E.A.; Bracci, P.M. Vitamin D, calcium, andretinol intake, and pancreatic cancer in a population-based case-control study inthe San Francisco Bay area. Cancer Causes Control 2011, 22, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Skinner, H.G.; Michaud, D.S.; Giovannucci, E.; Willett, W.C.; Colditz, G.A.; Fuchs, C.S. Vitamin D intake and the risk for pancreatic cancer in two cohort studies. Cancer Epidemiol. Biomarkers Prev. 2006, 15, 1688–1695. [Google Scholar] [CrossRef] [PubMed]
- Genkinger, J.M.; Wang, M.; Li, R.; Albanes, D.; Anderson, K.E.; Bernstein, L.; van den Brandt, P.A.; English, D.R.; Freudenheim, J.L.; Fuchs, C.S.; et al. Dairy products and pancreatic cancer risk: A pooled analysis of 14 cohort studies. Ann. Oncol. 2014, 25, 1106–1115. [Google Scholar] [CrossRef]
- Wolpin, B.M.; Ng, K.; Bao, Y.; Kraft, P.; Stampfer, M.J.; Michaud, D.S.; Ma, J.; Buring, J.E.; Sesso, H.D.; Lee, I.M.; et al. Plasma 25-hydroxyvitamin D and risk of pancreatic cancer. Cancer Epidemiol. Biomarkers Prev. 2012, 21, 82–91. [Google Scholar] [CrossRef]
- Stolzenberg-Solomon, R.Z.; Jacobs, E.J.; Arslan, A.A.; Qi, D.; Patel, A.V.; Helzlsouer, K.J.; Weinstein, S.J.; McCullough, M.L.; Purdue, M.P.; Shu, X.O.; et al. Circulating25-hydroxyvitamin D and risk of pancreatic cancer: Cohort Consortium Vitamin D Pooling Project of Rarer Cancers. Am. J. Epidemiol. 2010, 172, 81–93. [Google Scholar] [CrossRef]
- Stolzenberg-Solomon, R.Z.; Hayes, R.B.; Horst, R.L.; Anderson, K.E.; Hollis, B.W.; Silverman, D.T. Serum vitamin D and risk of pancreatic cancer in the prostate, lung, colorectal, and ovarian screening trial. Cancer Res. 2009, 69, 1439–1447. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Qian, Z.R.; Babic, A.; Morales-Oyarvide, V.; Rubinson, D.A.; Kraft, P.; Ng, K.; Bao, Y.; Giovannucci, E.L.; Ogino, S.; et al. Prediagnostic Plasma 25-Hydroxyvitamin D and Pancreatic Cancer Survival. J. Clin. Oncol. 2016, 34, 2899–2905. [Google Scholar] [CrossRef] [PubMed]
- Barreto, S.G.; Neale, R.E. Vitamin D and pancreatic cancer. Cancer Lett. 2015, 368, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Mukai, Y.; Yamada, D.; Eguchi, H.; Iwagami, Y.; Asaoka, T.; Noda, T.; Kawamoto, K.; Gotoh, K.; Kobayashi, S.; Takeda, Y.; et al. Vitamin D Supplementation is a Promising Therapy for Pancreatic Ductal Adenocarcinoma in Conjunction with Current Chemoradiation Therapy. Ann. Surg. Oncol. 2018, 25, 1868–1879. [Google Scholar] [CrossRef]
- Sherman, M.H.; Yu, R.T.; Engle, D.D.; Ding, N.; Atkins, A.R.; Tiriac, H.; Collisson, E.A.; Connor, F.; Van Dyke, T.; Kozlov, S.; et al. Vitamin D receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell 2014, 159, 80–93. [Google Scholar] [CrossRef] [PubMed]
- Basso, D.; Gnatta, E.; Padoan, A.; Fogar, P.; Furlanello, S.; Aita, A.; Bozzato, D.; Zambon, C.F.; Arrigoni, G.; Frasson, C.; et al. PDAC-derived exosomes enrich the microenvironment in MDSCs in a SMAD4-dependent manner through a new calcium related axis. Oncotarget 2017, 8, 84928–84944. [Google Scholar] [CrossRef]
- Greco, E.; Basso, D.; Fadi, E.; Padoan, A.; Fogar, P.; Zambon, C.F.; Navaglia, F.; Bozzato, D.; Moz, S.; Pedrazzoli, S.; et al. Analogs of vitamin E epitomized by alpha-tocopheryl succinate for pancreatic cancer treatment: In vitro results induce caution for in vivo applications. Pancreas 2010, 39, 662–668. [Google Scholar] [CrossRef]
- Moz, S.; Basso, D.; Bozzato, D.; Galozzi, P.; Navaglia, F.; Negm, O.H.; Arrigoni, G.; Zambon, C.F.; Padoan, A.; Tighe, P.; et al. SMAD4 loss enables EGF, TGFβ1 and S100A8/A9 induced activation of critical pathways to invasion in human pancreatic adenocarcinoma cells. Oncotarget 2016, 7, 69927–69944. [Google Scholar] [CrossRef]
- Moz, S.; Lorenzin, M.; Ramonda, R.; Aneloni, V.; La Raja, M.; Plebani, M.; Basso, D. Emerging role of monocytes and their intracellular calcium pattern inspondyloarthritis. Clin. Chim. Acta. 2020, 500, 180–188. [Google Scholar] [CrossRef]
- Guo, S.; Deng, C.X. Effect of Stromal Cells in Tumor Microenvironment on Metastasis Initiation. Int. J. Biol. Sci. 2018, 14, 2083–2093. [Google Scholar] [CrossRef]
- Mao, Y.; Poschke, I.; Kiessling, R. Tumour-induced immune suppression: Role of inflammatory mediators released by myelomonocytic cells. J. Intern. Med. 2014, 276, 154–170. [Google Scholar] [CrossRef] [PubMed]
- Ricca, C.; Aillon, A.; Viano, M.; Bergandi, L.; Aldieri, E.; Silvagno, F. Vitamin D inhibits the epithelial-mesenchymal transition by a negative feedback regulation of TGF-β activity. J. Steroid Biochem. Mol. Biol. 2019, 187, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Neme, A.; Seuter, S.; Malinen, M.; Nurmi, T.; Tuomainen, T.P.; Virtanen, J.K.; Carlberg, C. In vivo transcriptome changes of human white blood cells in response to vitamin D. J. Steroid Biochem. Mol. Biol. 2019, 188, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Miyazono, K.; Katsuno, Y.; Koinuma, D.; Ehata, S.; Morikawa, M. Intracellular and extracellular TGF-b signaling in cancer: Some recent topics. Front. Med. 2018, 12, 387–411. [Google Scholar] [CrossRef] [PubMed]
- Rosen, C.J. Clinical practice. Vitamin D insufficiency. N. Engl. J. Med. 2011, 364, 248–254. [Google Scholar] [CrossRef]
- Li, Q.; Yang, G.; Feng, M.; Zheng, S.; Cao, Z.; Qiu, J.; You, L.; Zheng, L.; Hu, Y.; Zhang, T.; et al. NF-κB in pancreatic cancer: Its key role in chemoresistance. Cancer Lett. 2018, 421, 127–134. [Google Scholar] [CrossRef]
- Igney, F.H.; Krammer, P.H. Death and anti-death: Tumour resistance to apoptosis. Nat. Rev. Cancer 2002, 4, 277–288. [Google Scholar] [CrossRef]
- Carlberg, C. Vitamin D Signaling in the Context of Innate Immunity: Focus on Human Monocytes. Front. Immunol. 2019, 10, 2211. [Google Scholar] [CrossRef]
- Dong, H.P.; Holth, A.; Kleinberg, L.; Ruud, M.G.; Elstrand, M.B.; Tropé, C.G.; Davidson, B.; Risberg, B. Evaluation of cell surface expression of phosphatidylserine in ovarian carcinoma effusions using the annexin-V/7-AAD assay: Clinical relevance and comparison with other apoptosis parameters. Am. J. Clin. Pathol. 2009, 132, 756–762. [Google Scholar] [CrossRef]
- Balasubramanian, K.; Mirnikjoo, B.; Schroit, A.J. Regulated externalization of phosphatidylserine at the cell surface: Implications for apoptosis. J. Biol. Chem. 2007, 282, 18357–18364. [Google Scholar] [CrossRef]
- Ropolo, A.; Bagnes, C.I.; Molejon, M.I.; Lo Re, A.; Boggio, V.; Gonzalez, C.D.; Vaccaro, M.I. Chemotherapy and autophagy-mediated cell death in pancreatic cancer cells. Pancreatology 2012, 12, 1–7. [Google Scholar] [CrossRef] [PubMed]
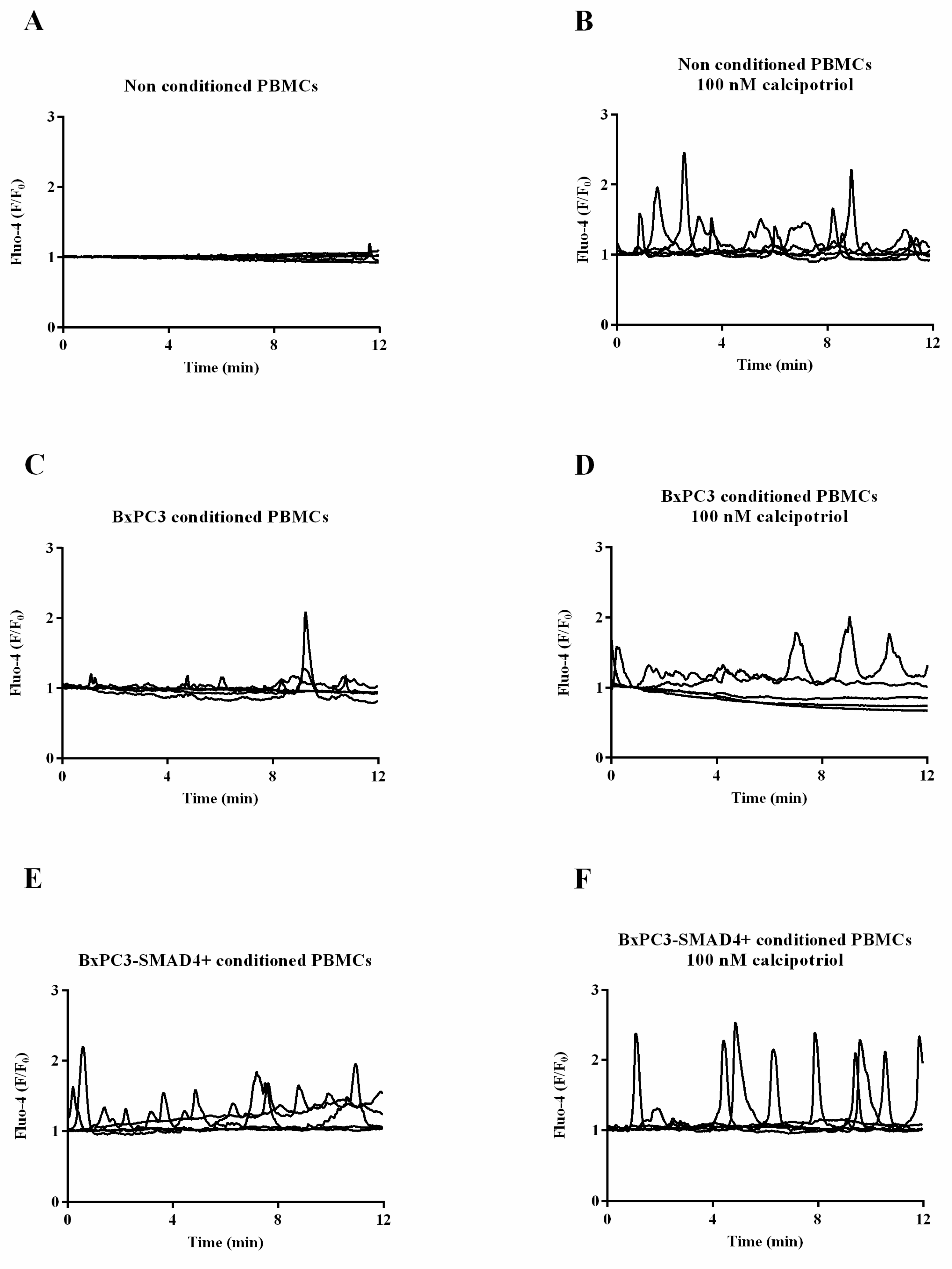

| Untreated PBMCs | Calcipotriol Treated PBMCs | ||||||
|---|---|---|---|---|---|---|---|
| Time | 2 days (n = 32) | 3 days (n = 58) | 4 days (n = 32) | 2 days (n = 52) | 3 days (n = 68) | 4 days (n = 30) | |
| Number of [Ca2+]i Peaks | Median | 2 | 1 | 1 # | 3 | 1 * | 1 * |
| 10th–90th percentiles | 1–4 | 1–4 | 1–1 | 1–12 | 1–4 | 1–2 | |
| Kruskal–Wallis test | p = 0.0001 | p = 0.0001 | |||||
| Whole [Ca2+]i Area | Median | 31 | 16 § | 34 | 14 | 16 | 13 |
| 10th–90th percentiles | 17–59 | 6–30 | 21–52 | 5–57 | 7–83 | 8–23 | |
| Kruskal–Wallis test | p = 0.0001 | p = 0.0966 | |||||
| Peak [Ca2+]i Area | Median | 17 | 11 * | 13 | 8 | 8 | 5 |
| 10th–90th percentiles | 5–41 | 3–20 | 7–17 | 2–25 | 1–54 | 3–10 | |
| Kruskal–Wallis test | p = 0.0099 | p = 0.1284 | |||||
| . | Non Conditioned PBMCs (n = 94) | BxPC3 Conditioned PBMCs (n = 52) | BxPC3-SMAD4+Conditioned PBMCs (n = 17) | |
|---|---|---|---|---|
| Number of [Ca2+]i Peaks | Median | 1 | 1 | 5 * |
| 10th-90th percentiles | 1–4 | 1–3 | 1–7 | |
| Kruskal–Wallis test | p = 0.0001 | |||
| Whole [Ca2+]i Area | Median | 17 | 21 | 8 * |
| 10th-90th percentiles | 5–45 | 10–80 | 5–20 | |
| Kruskal–Wallis test | p = 0.0001 | |||
| Peak [Ca2+]i Area | Median | 8 | 11 | 7 |
| 10th-90th percentiles | 1–27 | 3–34 | 3–11 | |
| Kruskal–Wallis test | p = 0.0348 | |||
| Non Conditioned PBMCs | BxPC3 Conditioned PBMCs | BxPC3-SMAD4+Conditioned PBMCs | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Calcipotriol Dosage | 0 nM (n = 94) | 10 nM (n = 26) | 100 nM (n = 61) | 1000 nM (n = 28) | 0 nM (n = 52) | 10 nM (n = 55) | 100 nM (n = 60) | 1000 nM (n = 17) | 0 nM (n = 17) | 10 nM (n = 42) | 100 nM (n = 27) | 1000 nM (n = 31) | |
| Number of [Ca2+]i Peaks | Median | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 5 | 1 # | 3 | 2 |
| 10th-90th percentiles | 1–4 | 1–3 | 1–4 | 1–3 | 1–3 | 1–4 | 1–4 | 1–4 | 1–7 | 1–4 | 1–6 | 1–5 | |
| Kruskal-Wallis test | p = 0.2814 | p = 0.9430 | p = 0.0001 | ||||||||||
| Whole [Ca2+]i Area | Median | 17 | 26 | 50 ** | 22 | 21 | 21 | 30 | 20 | 8 | 14 | 48 *** | 27 *** |
| 10th-90th percentiles | 5–45 | 11–102 | 11–110 | 11–78 | 10–80 | 10–63 | 11–174 | 11–156 | 5–20 | 6–33 | 12–171 | 12–96 | |
| Kruskal-Wallis test | p = 0.0001 | p = 0.0567 | p = 0.0001 | ||||||||||
| Peak [Ca2+]i Area | Median | 8 | 9 | 22 * | 14 | 11 | 9 | 16 | 16 | 7 | 7 | 29 *** | 23 *** |
| 10th-90thpercentiles | 1–27 | 1–94 | 5–64 | 1–42 | 3–34 | 2–20 | 2–82 | 4–92 | 3–11 | 3–24 | 8–77 | 7–77 | |
| Kruskal-Wallis test | p = 0.0001 | p = 0.0099 | p = 0.0001 | ||||||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moz, S.; Contran, N.; Facco, M.; Trimarco, V.; Plebani, M.; Basso, D. Vitamin D Prevents Pancreatic Cancer-Induced Apoptosis Signaling of Inflammatory Cells. Biomolecules 2020, 10, 1055. https://doi.org/10.3390/biom10071055
Moz S, Contran N, Facco M, Trimarco V, Plebani M, Basso D. Vitamin D Prevents Pancreatic Cancer-Induced Apoptosis Signaling of Inflammatory Cells. Biomolecules. 2020; 10(7):1055. https://doi.org/10.3390/biom10071055
Chicago/Turabian StyleMoz, Stefania, Nicole Contran, Monica Facco, Valentina Trimarco, Mario Plebani, and Daniela Basso. 2020. "Vitamin D Prevents Pancreatic Cancer-Induced Apoptosis Signaling of Inflammatory Cells" Biomolecules 10, no. 7: 1055. https://doi.org/10.3390/biom10071055
APA StyleMoz, S., Contran, N., Facco, M., Trimarco, V., Plebani, M., & Basso, D. (2020). Vitamin D Prevents Pancreatic Cancer-Induced Apoptosis Signaling of Inflammatory Cells. Biomolecules, 10(7), 1055. https://doi.org/10.3390/biom10071055







