Glutamate-Gated NMDA Receptors: Insights into the Function and Signaling in the Kidney
Abstract
1. Introduction
2. NMDA Receptor: Structure, Distribution and Functionality in the Kidney
2.1. NMDAR Subunit Composition
2.2. Distribution of NMDAR in the Kidney
2.3. Functional Properties of NMDAR in the Kidney
3. Distinctive Physiological and Pathophysiological Roles of NMDAR in the Kidney
3.1. Physiological Role of NMDAR in Renal Hemodynamics and Glomerular Filtration
3.2. Pathological Role of NMDAR in Different Renal Conditions
3.2.1. Role of NMDAR in Renal Fibrosis
3.2.2. Role of NMDAR in Secondary Hyperparathyroidism in CKD
3.2.3. Role of NMDAR in Acute Kidney Injury
3.2.4. Role of NMDAR in Glomerular Disorders
3.2.5. Role of NMDAR in Nephrotoxic Renal Failure
4. NMDAR-Mediated Signaling Pathways in the Kidney
4.1. Role of NMDAR in TGF-β1 Signaling Pathway
4.2. Role of NMDAR in Antioxidant Response
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Haddad, J.J. N-methyl-d-aspartate (NMDA) and the regulation of mitogen-activated protein kinase (MAPK) signaling pathways: A revolving neurochemical axis for therapeutic intervention? Prog. Neurobiol. 2005, 77, 252–282. [Google Scholar] [CrossRef] [PubMed]
- Monaghan, D.T.; Bridges, R.J.; Cotman, C.W. The excitatory amino acid receptors: Their classes, pharmacology, and distinct properties in the function of the central nervous system. Annu. Rev. Pharmacol. Toxicol. 1989, 29, 365–402. [Google Scholar] [CrossRef] [PubMed]
- Vyklicky, V.; Korinek, M.; Smejkalova, T.; Balik, A.; Krausova, B.; Kaniakova, M.; Lichnerova, K.; Cerny, J.; Krusek, J.; Dittert, I.; et al. Structure, function, and pharmacology of NMDA receptor channels. Physiol. Res. 2014, 63 (Suppl 1), S191–S203. [Google Scholar]
- Hollmann, M.; O’Shea-Greenfield, A.; Rogers, S.W.; Heinemann, S. Cloning by functional expression of a member of the glutamate receptor family. Nature 1989, 342, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Mayer, M.L. The Challenge of interpreting glutamate-receptor ion-channel structures. Biophys. J. 2017, 113, 2143–2151. [Google Scholar] [CrossRef]
- Hansen, K.B.; Yi, F.; Perszyk, R.E.; Furukawa, H.; Wollmuth, L.P.; Gibb, A.J.; Traynelis, S.F. Structure, function, and allosteric modulation of NMDA receptors. J. Gen. Physiol. 2018, 150, 1081–1105. [Google Scholar] [CrossRef]
- Traynelis, S.F.; Wollmuth, L.P.; McBain, C.J.; Menniti, F.S.; Vance, K.M.; Ogden, K.K.; Hansen, K.B.; Yuan, H.; Myers, S.J.; Dingledine, R. Glutamate receptor ion channels: Structure, regulation, and function. Pharmacol. Rev. 2010, 62, 405–496. [Google Scholar] [CrossRef]
- Cull-Candy, S.G.; Brickley, S.G.; Misra, C.; Feldmeyer, D.; Momiyama, A.; Farrant, M. NMDA receptor diversity in the cerebellum: Identification of subunits contributing to functional receptors. Neuropharmacology 1998, 37, 1369–1380. [Google Scholar] [CrossRef]
- Van Dongen, A.M. (Ed.) Biology of the NMDA Receptor; Frontiers in Neuroscience; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2009; Chapter 13. Available online: https://www.ncbi.nlm.nih.gov/pubmed/21204408 (accessed on 14 July 2020).
- Scheetz, A.J.; Constantine-Paton, M. Modulation of NMDA receptor function: Implications for vertebrate neural development. FASEB J. 1994, 8, 745–752. [Google Scholar] [CrossRef]
- Guttmann, R.P.; Sokol, S.; Baker, D.L.; Simpkins, K.L.; Dong, Y.; Lynch, D.R. Proteolysis of the N-methyl-d-aspartate receptor by calpain in situ. J. Pharmacol. Exp. Ther. 2002, 302, 1023–1030. [Google Scholar] [CrossRef]
- Hardingham, G.E.; Bading, H. The Yin and Yang of NMDA receptor signalling. Trends Neurosci. 2003, 26, 81–89. [Google Scholar] [CrossRef]
- Hardingham, G.E. Coupling of the NMDA receptor to neuroprotective and neurodestructive events. Biochem. Soc. Trans. 2009, 37, 1147–1160. [Google Scholar] [CrossRef]
- Mayer, M.L.; Armstrong, N. Structure and function of glutamate receptor ion channels. Annu. Rev. Physiol. 2004, 66, 161–181. [Google Scholar] [CrossRef] [PubMed]
- Lipton, S.A.; Rosenberg, P.A. Excitatory amino acids as a final common pathway for neurologic disorders. N. Engl. J. Med. 1994, 330, 613–622. [Google Scholar] [PubMed]
- Miglio, G.; Dianzani, C.; Fallarini, S.; Fantozzi, R.; Lombardi, G. Stimulation of N-methyl-d-aspartate receptors modulates Jurkat T cell growth and adhesion to fibronectin. Biochem. Biophys. Res. Commun. 2007, 361, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Nahm, W.K.; Philpot, B.D.; Adams, M.M.; Badiavas, E.V.; Zhou, L.H.; Butmarc, J.; Bear, M.F.; Falanga, V. Significance of N-methyl-d-aspartate (NMDA) receptor-mediated signaling in human keratinocytes. J. Cell Physiol. 2004, 200, 309–317. [Google Scholar] [CrossRef]
- Mentaverri, R.; Kamel, S.; Wattel, A.; Prouillet, C.; Sevenet, N.; Petit, J.P.; Tordjmann, T.; Brazier, M. Regulation of bone resorption and osteoclast survival by nitric oxide: Possible involvement of NMDA-receptor. J. Cell. Biochem. 2003, 88, 1145–1156. [Google Scholar] [CrossRef]
- Rakic, P.; Komuro, H. The role of receptor/channel activity in neuronal cell migration. J. Neurobiol. 1995, 26, 299–315. [Google Scholar] [CrossRef] [PubMed]
- Shorte, S.L. N-methyl-d-aspartate evokes rapid net depolymerization of filamentous actin in cultured rat cerebellar granule cells. J. Neurophysiol. 1997, 78, 1135–1143. [Google Scholar] [CrossRef][Green Version]
- Itzstein, C.; Espinosa, L.; Delmas, P.D.; Chenu, C. Specific antagonists of NMDA receptors prevent osteoclast sealing zone formation required for bone resorption. Biochem. Biophys. Res. Commun. 2000, 268, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Parisi, E.; Almaden, Y.; Ibarz, M.; Panizo, S.; Cardus, A.; Rodriguez, M.; Fernandez, E.; Valdivielso, J.M. N-methyl-d-aspartate receptors are expressed in rat parathyroid gland and regulate PTH secretion. Am. J. Physiol. Ren. Physiol. 2009, 296, F1291–F1296. [Google Scholar] [CrossRef]
- Bozic, M.; Valdivielso, J.M. The potential of targeting NMDA receptors outside the CNS. Expert Opin. Ther. Targets 2015, 19, 399–413. [Google Scholar] [CrossRef] [PubMed]
- Bellone, C.; Nicoll, R.A. Rapid bidirectional switching of synaptic NMDA receptors. Neuron 2007, 55, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Paoletti, P.; Neyton, J. NMDA receptor subunits: Function and pharmacology. Curr. Opin. Pharmacol. 2007, 7, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Rebola, N.; Srikumar, B.N.; Mulle, C. Activity-dependent synaptic plasticity of NMDA receptors. J. Physiol. 2010, 588, 93–99. [Google Scholar] [CrossRef]
- Luo, J.; Wang, Y.; Yasuda, R.P.; Dunah, A.W.; Wolfe, B.B. The majority of N-methyl-d-aspartate receptor complexes in adult rat cerebral cortex contain at least three different subunits (NR1/NR2A/NR2B). Mol. Pharmacol. 1997, 51, 79–86. [Google Scholar] [CrossRef]
- Chazot, P.L. The NMDA receptor NR2B subunit: A valid therapeutic target for multiple CNS pathologies. Curr. Med. Chem. 2004, 11, 389–396. [Google Scholar] [CrossRef]
- Ishii, T.; Moriyoshi, K.; Sugihara, H.; Sakurada, K.; Kadotani, H.; Yokoi, M.; Akazawa, C.; Shigemoto, R.; Mizuno, N.; Masu, M. Molecular characterization of the family of the N-methyl-d-aspartate receptor subunits. J. Biol. Chem. 1993, 268, 2836–2843. [Google Scholar] [PubMed]
- Meguro, H.; Mori, H.; Araki, K.; Kushiya, E.; Kutsuwada, T.; Yamazaki, M.; Kumanishi, T.; Arakawa, M.; Sakimura, K.; Mishina, M. Functional characterization of a heteromeric NMDA receptor channel expressed from cloned cDNAs. Nature 1992, 357, 70–74. [Google Scholar] [CrossRef]
- Nakanishi, S. Molecular diversity of glutamate receptors and implications for brain function. Science 1992, 258, 597–603. [Google Scholar] [CrossRef]
- Ciabarra, A.M.; Sullivan, J.M.; Gahn, L.G.; Pecht, G.; Heinemann, S.; Sevarino, K.A. Cloning and characterization of chi-1: A developmentally regulated member of a novel class of the ionotropic glutamate receptor family. J. Neurosci. 1995, 15, 6498–6508. [Google Scholar] [CrossRef]
- Lynch, D.R.; Guttmann, R.P. NMDA receptor pharmacology: Perspectives from molecular biology. Curr. Drug Targets. 2001, 2, 215–231. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Sasaki, Y.F.; Rothe, T.; Premkumar, L.S.; Takasu, M.; Crandall, J.E.; Dikkes, P.; Conner, D.A.; Rayudu, P.V.; Cheung, W.; et al. Increased NMDA current and spine density in mice lacking the NMDA receptor subunit NR3A. Nature 1998, 393, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Paoletti, P.; Bellone, C.; Zhou, Q. NMDA receptor subunit diversity: Impact on receptor properties, synaptic plasticity and disease. Nat. Rev. Neurosci. 2013, 14, 383–400. [Google Scholar] [CrossRef] [PubMed]
- Al-Hallaq, R.A.; Jarabek, B.R.; Fu, Z.; Vicini, S.; Wolfe, B.B.; Yasuda, R.P. Association of NR3A with the N-methyl-d-aspartate receptor NR1 and NR2 subunits. Mol. Pharmacol. 2002, 62, 1119–1127. [Google Scholar] [CrossRef]
- Ulbrich, M.H.; Isacoff, E.Y. Rules of engagement for NMDA receptor subunits. Proc. Natl. Acad. Sci. USA 2008, 105, 14163–14168. [Google Scholar] [CrossRef]
- Chatterton, J.E.; Awobuluyi, M.; Premkumar, L.S.; Takahashi, H.; Talantova, M.; Shin, Y.; Cui, J.; Tu, S.; Sevarino, K.A.; Nakanishi, N.; et al. Excitatory glycine receptors containing the NR3 family of NMDA receptor subunits. Nature 2002, 415, 793–798. [Google Scholar] [CrossRef]
- Cavara, N.A.; Orth, A.; Hollmann, M. Effects of NR1 splicing on NR1/NR3B-type excitatory glycine receptors. BMC Neurisci. 2009, 10, 32. [Google Scholar] [CrossRef]
- Nishi, M.; Hinds, H.; Lu, H.P.; Kawata, M.; Hayashi, Y. Motoneuron-specific expression of NR3B, a novel NMDA-type glutamate receptor subunit that works in a dominant-negative manner. J. Neurosci. 2001, 21, RC185. [Google Scholar] [CrossRef]
- Matsuda, K.; Fletcher, M.; Kamiya, Y.; Yuzaki, M. Specific assembly with the NMDA receptor 3B subunit controls surface expression and calcium permeability of NMDA receptors. J. Neurosci. 2003, 23, 10064–10073. [Google Scholar] [CrossRef]
- Perez-Otano, I.; Ehlers, M.D. Learning from NMDA receptor trafficking: Clues to the development and maturation of glutamatergic synapses. Neurosignals 2004, 13, 175–189. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.B.; Chang, S.; Xu, P.; Miao, M.; Wu, H.; Zhang, Y.; Zhang, T.; Wang, H.; Zhang, J.; Xie, C.; et al. Structural basis of the proton sensitivity of human GluN1-GluN2A NMDA receptors. Cell Rep. 2018, 25, 3582–3590.e3584. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, T.; Morita, K.; Kinjo, K.; Tsujimoto, A. Stimulation of catecholamine release from isolated adrenal glands by some amino acids. Jpn. J. Pharmacol. 1982, 32, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Leung, J.C.; Travis, B.R.; Verlander, J.W.; Sandhu, S.K.; Yang, S.G.; Zea, A.H.; Weiner, I.D.; Silverstein, D.M. Expression and developmental regulation of the NMDA receptor subunits in the kidney and cardiovascular system. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002, 283, R964–R971. [Google Scholar] [CrossRef] [PubMed]
- Morhenn, V.B.; Waleh, N.S.; Mansbridge, J.N.; Unson, D.; Zolotorev, A.; Cline, P.; Toll, L. Evidence for an NMDA receptor subunit in human keratinocytes and rat cardiocytes. Eur. J. Pharmacol. 1994, 268, 409–414. [Google Scholar] [CrossRef]
- Patton, A.J.; Genever, P.G.; Birch, M.A.; Suva, L.J.; Skerry, T.M. Expression of an N-methyl-d-aspartate-type receptor by human and rat osteoblasts and osteoclasts suggests a novel glutamate signaling pathway in bone. Bone 1998, 22, 645–649. [Google Scholar] [CrossRef]
- Seeber, S.; Becker, K.; Rau, T.; Eschenhagen, T.; Becker, C.M.; Herkert, M. Transient expression of NMDA receptor subunit NR2B in the developing rat heart. J. Neurochem. 2000, 75, 2472–2477. [Google Scholar] [CrossRef] [PubMed]
- McGee, M.A.; Abdel-Rahman, A.A. Enhanced vascular neuronal Nitric-Oxide Synthase-Derived Nitric-Oxide production underlies the pressor response caused by peripheral n-methyl-d-aspartate receptor activation in conscious rats. J. Pharmacol. Exp. Ther. 2012, 342, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Deng, A.; Valdivielso, J.M.; Munger, K.A.; Blantz, R.C.; Thomson, S.C. Vasodilatory N-methyl-d-aspartate receptors are constitutively expressed in rat kidney. J. Am. Soc. Nephrol. 2002, 13, 1381–1384. [Google Scholar] [CrossRef]
- Gill, S.S.; Pulido, O.M.; Mueller, R.W.; McGuire, P.F. Molecular and immunochemical characterization of the ionotropic glutamate receptors in the rat heart. Brain Res. Bull. 1998, 46, 429–434. [Google Scholar] [CrossRef]
- Chen, H.J.; Fitzgerald, R.; Brown, A.T.; Qureshi, I.; Breckenridge, J.; Kazi, R.; Wang, Y.F.; Wu, Y.M.; Zhang, X.J.; Mukunyadzi, P.; et al. Identification of a homocysteine receptor in the peripheral endothelium and its role in proliferation. J. Vasc. Surg. 2005, 41, 853–860. [Google Scholar] [CrossRef]
- Gill, S.S.; Pulido, O.M. Glutamate receptors in peripheral tissues: Current knowledge, future research, and implications for toxicology. Toxicol. Pathol. 2001, 29, 208–223. [Google Scholar] [CrossRef]
- Lin, Y.J.; Bovetto, S.; Carver, J.M.; Giordano, T. Cloning of the cDNA for the human NMDA receptor NR2C subunit and its expression in the central nervous system and periphery. Brain Res. Mol. Brain Res. 1996, 43, 57–64. [Google Scholar] [CrossRef]
- Gonzalez-Cadavid, N.F.; Ryndin, I.; Vernet, D.; Magee, T.R.; Rajfer, J. Presence of NMDA receptor subunits in the male lower urogenital tract. J. Androl. 2000, 21, 566–578. [Google Scholar] [PubMed]
- Ma, M.C.; Huang, H.S.; Chen, Y.S.; Lee, S.H. Mechanosensitive N-Methyl-D-Aspartate receptors contribute to sensory activation in the rat renal pelvis. Hypertension 2008, 52, 938–944. [Google Scholar] [CrossRef]
- Parisi, E.; Bozic, M.; Ibarz, M.; Panizo, S.; Valcheva, P.; Coll, B.; Fernandez, E.; Valdivielso, J. Sustained activation of renal N-methyl-d-aspartate receptors decreases vitamin D synthesis: A possible role for glutamate on the onset of secondary HPT. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E825–E831. [Google Scholar] [CrossRef]
- Bozic, M.; de Rooij, J.; Parisi, E.; Ortega, M.; Fernandez, E.; Valdivielso, J. Glutamatergic signaling maintains the epithelial phenotype of proximal tubular cells. J. Am. Soc. Nephrol. 2011, 22, 1099–1111. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yi, F.; Xia, M.; Boini, K.M.; Zhu, Q.; Laperle, L.A.; Abais, J.M.; Brimson, C.A.; Li, P.L. NMDA Receptor-Mediated activation of NADPH Oxidase and glomerulosclerosis in hyperhomocysteinemic rats. Antioxid. Redox Signal. 2010, 13, 975–986. [Google Scholar] [CrossRef]
- Roshanravan, H.; Kim, E.Y.; Dryer, S.E. NMDA Receptors as potential therapeutic targets in diabetic nephropathy: Increased renal nmda receptor subunit expression in akita mice and reduced nephropathy following sustained treatment with memantine or MK-801. Diabetes 2016, 65, 3139–3150. [Google Scholar] [CrossRef]
- Anderson, M.; Suh, J.M.; Kim, E.Y.; Dryer, S.E. Functional NMDA receptors with atypical properties are expressed in podocytes. Am. J. Physiol. Cell Physiol. 2011, 300, C22–C32. [Google Scholar] [CrossRef]
- Giardino, L.; Armelloni, S.; Corbelli, A.; Mattinzoli, D.; Zennaro, C.; Guerrot, D.; Tourrel, F.; Ikehata, M.; Li, M.; Berra, S.; et al. Podocyte glutamatergic signaling contributes to the function of the glomerular filtration barrier. J. Am. Soc. Nephrol. 2009, 20, 1929–1940. [Google Scholar] [CrossRef] [PubMed]
- Sproul, A.D.; Steele, S.L.; Thai, T.L.; Yu, S.P.; Klein, J.D.; Sands, J.M.; Bell, P.D. N-Methyl-D-Aspartate Receptor Subunit NR3a Expression and function in principal cells of the collecting duct. Am. J. Physiol. Ren. Physiol. 2011, 301, F44–F54. [Google Scholar] [CrossRef] [PubMed]
- Deng, A.; Thomson, S.C. Renal NMDA receptors independently stimulate proximal reabsorption and glomerular filtration. Am. J. Physiol. Ren. Physiol. 2009, 296, F976–F982. [Google Scholar] [CrossRef] [PubMed]
- Dingledine, R.; Borges, K.; Bowie, D.; Traynelis, S.F. The glutamate receptor ion channels. Pharmacol. Rev. 1999, 51, 7–61. [Google Scholar] [PubMed]
- van de Poll, M.C.; Soeters, P.B.; Deutz, N.E.; Fearon, K.C.; Dejong, C.H. Renal metabolism of amino acids: Its role in interorgan amino acid exchange. Am. J. Clin. Nutr. 2004, 79, 185–197. [Google Scholar] [CrossRef]
- Du, J.; Li, X.H.; Li, Y.J. Glutamate in peripheral organs: Biology and pharmacology. Eur. J. Pharmacol. 2016, 784, 42–48. [Google Scholar] [CrossRef]
- Divino Filho, J.C.; Hazel, S.J.; Fürst, P.; Bergström, J.; Hall, K. Glutamate concentration in plasma, erythrocyte and muscle in relation to plasma levels of insulin-like growth factor (IGF)-I, IGF binding protein-1 and insulin in patients on haemodialysis. J. Endocrinol. 1998, 156, 519–527. [Google Scholar] [CrossRef]
- Zhou, Y.; Qiu, L.; Xiao, Q.; Wang, Y.; Meng, X.; Xu, R.; Wang, S.; Na, R. Obesity and diabetes related plasma amino acid alterations. Clin. Biochem. 2013, 46, 1447–1452. [Google Scholar] [CrossRef]
- Tizianello, A.; De Ferrari, G.; Garibotto, G.; Gurreri, G.; Robaudo, C. Renal metabolism of amino acids and ammonia in subjects with normal renal function and in patients with chronic renal insufficiency. J. Clin. Investig. 1980, 65, 1162–1173. [Google Scholar] [CrossRef]
- Hediger, M.A. Glutamate transporters in kidney and brain. Am. J. Physiol. 1999, 277, F487–F492. [Google Scholar] [CrossRef]
- Welbourne, T.C.; Matthews, J.C. Glutamate transporters and renal function. Am. J. Physiol. 1999, 277, F501–F505. [Google Scholar]
- Silbernagl, S. The renal handling of amino acids and oligopeptides. Physiol. Rev. 1988, 68, 911–1007. [Google Scholar] [CrossRef]
- Xue, H.; Field, C.J. New role of glutamate as an immunoregulator via glutamate receptors and transporters. Front. Biosci. (Sch. Ed.) 2011, 1, 1007–1020. [Google Scholar] [CrossRef]
- Hinoi, E.; Yoneda, Y. Possible involvement of glutamatergic signaling machineries in pathophysiology of rheumatoid arthritis. J. Pharmacol. Sci. 2011, 116, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Evans, R.H.; Francis, A.A.; Watkins, J.C. Selective antagonism by Mg2+ of amino acid-induced depolarization of spinal neurones. Experientia 1977, 33, 489–491. [Google Scholar] [CrossRef] [PubMed]
- Nowak, L.; Bregestovski, P.; Ascher, P.; Herbet, A.; Prochiantz, A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature 1984, 307, 462–465. [Google Scholar] [CrossRef] [PubMed]
- Mayer, M.L.; Westbrook, G.L.; Guthrie, P.B. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature 1984, 309, 261–263. [Google Scholar] [CrossRef]
- Monyer, H.; Burnashev, N.; Laurie, D.J.; Sakmann, B.; Seeburg, P.H. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron 1994, 12, 529–540. [Google Scholar] [CrossRef]
- Kampa, B.M.; Clements, J.; Jonas, P.; Stuart, G.J. Kinetics of Mg2+ unblock of NMDA receptors: Implications for spike-timing dependent synaptic plasticity. J. Physiol. 2004, 556, 337–345. [Google Scholar] [CrossRef]
- Blantz, R.C.; Deng, A.; Miracle, C.M.; Thomson, S.C. Regulation of kidney function and metabolism: A question of supply and demand. Trans. Am. Clin. Climatol. Assoc. 2007, 118, 23–43. [Google Scholar]
- Bozic, M.; Valdivielso, J.M. Functional Distribution and Regulation of the NMDAR in the Kidney, Heart and Parathyroid Gland. In The Nmda Receptors; Humana Press: Cham, Switzerland, 2017; Volume 30, pp. 51–68. Available online: https://link.springer.com/chapter/10.1007/978-3-319-49795-2_3 (accessed on 14 July 2020).
- Bądzyńska, B.; Zakrocka, I.; Sadowski, J.; Turski, W.A.; Kompanowska-Jezierska, E. Effects of systemic administration of kynurenic acid and glycine on renal haemodynamics and excretion in normotensive and spontaneously hypertensive rats. Eur. J. Pharmacol. 2014, 743, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Slomowitz, L.A.; Gabbai, F.B.; Khang, S.J.; Satriano, J.; Thareau, S.; Deng, A.H.; Thomson, S.C.; Blantz, R.C.; Munger, K.A. Protein intake regulates the vasodilatory function of the kidney and NMDA receptor expression. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 287, R1184–R1189. [Google Scholar] [CrossRef] [PubMed]
- Zakrocka, I.; Targowska-Duda, K.M.; Wnorowski, A.; Kocki, T.; Jóźwiak, K.; Turski, W.A. Angiotensin II type 1 receptor blockers decrease kynurenic acid production in rat kidney in vitro. Naunyn-Schmiedebergs Arch. Pharmacol. 2019, 392, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Bellisle, F.; France, B. Experimental studies of food choices and palatability responses in European subjects exposed to the Umami taste. Asia Pac. J. Clin. Nutr. 2008, 17 (Suppl 1), 376–379. [Google Scholar]
- Mahieu, S.; Klug, M.; Millen, N.; Fabro, A.; Benmelej, A.; Contini, M.e.C. Monosodium glutamate intake affect the function of the kidney through NMDA receptor. Life Sci. 2016, 149, 114–119. [Google Scholar] [CrossRef]
- Frokiaer, J. Collecting duct expression of N-methyl-d-aspartate receptor subtype NR3a regulates urinary concentrating capacity. Am. J. Physiol. Ren. Physiol. 2011, 301, F42–F43. [Google Scholar] [CrossRef]
- Franke, W.W.; Schmid, E.; Osborn, M.; Weber, K. Different intermediate-sized filaments distinguished by immunofluorescence microscopy. Proc. Natl. Acad. Sci. USA 1978, 75, 5034–5038. [Google Scholar] [CrossRef]
- Yang, C.C.; Chien, C.T.; Wu, M.H.; Ma, M.C.; Chen, C.F. NMDA receptor blocker ameliorates ischemia-reperfusion-induced renal dysfunction in rat kidneys. Am. J. Physiol. Ren. Physiol. 2008, 294, F1433–F1440. [Google Scholar] [CrossRef]
- Pundir, M.; Arora, S.; Kaur, T.; Singh, R.; Singh, A.P. Effect of modulating the allosteric sites of N-methyl-d-aspartate receptors in ischemia-reperfusion induced acute kidney injury. J. Surg. Res. 2013, 183, 668–677. [Google Scholar] [CrossRef]
- Arora, S.; Kaur, T.; Kaur, A.; Singh, A.P. Glycine aggravates ischemia reperfusion-induced acute kidney injury through N-Methyl-D-Aspartate receptor activation in rats. Mol. Cell. Biochem. 2014, 393, 123–131. [Google Scholar] [CrossRef]
- Singh, A.P.; Singh, N.; Bedi, P.M. Pioglitazone ameliorates renal ischemia reperfusion injury through NMDA receptor antagonism in rats. Mol. Cell. Biochem. 2016, 417, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.P.; Singh, N.; Bedi, P.M.S. Estradiol mitigates ischemia reperfusion-induced acute renal failure through NMDA receptor antagonism in rats. Mol. Cell. Biochem. 2017, 434, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Kaur, T.; Singh, B.; Pathak, D.; Singh Buttar, H.; Pal Singh, A. Curcumin alleviates ischemia reperfusion-induced acute kidney injury through NMDA receptor antagonism in rats. Ren. Fail. 2016, 38, 1462–1467. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.S.; Hung, S.F.; Huang, H.S.; Ma, M.C. Blockade of the N-Methyl-D-Aspartate glutamate receptor ameliorates lipopolysaccharide-induced renal insufficiency. PLoS ONE 2015, 10, e0132204. [Google Scholar] [CrossRef]
- Ying, J.; Wu, J.; Zhang, Y.; Han, Y.; Qian, X.; Yang, Q.; Chen, Y.; Zhu, H. Ligustrazine suppresses renal NMDAR1 and caspase-3 expressions in a mouse model of sepsis-associated acute kidney injury. Mol. Cell. Biochem. 2020, 464, 73–81. [Google Scholar] [CrossRef]
- Kim, E.Y.; Anderson, M.; Dryer, S.E. Sustained activation of N-Methyl-D-Aspartate receptors in podoctyes leads to oxidative stress, mobilization of transient receptor potential canonical 6 channels, nuclear factor of activated t cells activation, and apoptotic cell death. Mol. Pharmacol. 2012, 82, 728–737. [Google Scholar] [CrossRef]
- Kundu, S.; Pushpakumar, S.B.; Tyagi, A.; Coley, D.; Sen, U. Hydrogen sulfide deficiency and diabetic renal remodeling: Role of matrix metalloproteinase-9. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E1365–E1378. [Google Scholar] [CrossRef]
- Kundu, S.; Pushpakumar, S.; Sen, U. MMP-9- and NMDA receptor-mediated mechanism of diabetic renovascular remodeling and kidney dysfunction: Hydrogen sulfide is a key modulator. Nitric Oxide 2015, 46, 172–185. [Google Scholar] [CrossRef]
- Shen, J.; Wang, R.; He, Z.; Huang, H.; He, X.; Zhou, J.; Yan, Y.; Shen, S.; Shao, X.; Shen, X.; et al. NMDA receptors participate in the progression of diabetic kidney disease by decreasing Cdc42-GTP activation in podocytes. J. Pathol. 2016, 240, 149–160. [Google Scholar] [CrossRef]
- Leung, J.C.; Marphis, T.; Craver, R.D.; Silverstein, D.M. Altered NMDA receptor expression in renal toxicity: Protection with a receptor antagonist. Kidney Int. 2004, 66, 167–176. [Google Scholar] [CrossRef]
- Leung, J.C.; Ragland, N.; Marphis, T.; Silverstein, D.M. NMDA Agonists and antagonists induce renal culture cell toxicity. Med. Chem. 2008, 4, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Chen, J.; Ding, F.; Chou, X.; Zhang, X.; Wan, Y.; Hu, J.; Wu, Q. Activation of the N-methyl-d-aspartate receptor is involved in glyphosate-induced renal proximal tubule cell apoptosis. J. Appl. Toxicol. 2019, 39, 1096–1107. [Google Scholar] [CrossRef]
- Cauli, O.; González-Usano, A.; Cabrera-Pastor, A.; Gimenez-Garzó, C.; López-Larrubia, P.; Ruiz-Sauri, A.; Hernández-Rabaza, V.; Duszczyk, M.; Malek, M.; Lazarewicz, J.W.; et al. Blocking NMDA receptors delays death in rats with acute liver failure by dual protective mechanisms in kidney and brain. Neuromolecular Med. 2014, 16, 360–375. [Google Scholar] [CrossRef] [PubMed]
- Basile, A.S.; Huang, J.M.; Xie, C.; Webster, D.; Berlin, C.; Skolnick, P. N-methyl-d-aspartate antagonists limit aminoglycoside antibiotic-induced hearing loss. Nat. Med. 1996, 2, 1338–1343. [Google Scholar] [CrossRef] [PubMed]
- Puel, J.L.; Ladrech, S.; Chabert, R.; Pujol, R.; Eybalin, M. Electrophysiological evidence for the presence of nmda receptors in the guinea-pig cochlea. Hear. Res. 1991, 51, 255–264. [Google Scholar] [CrossRef]
- Segal, J.A.; Harris, B.D.; Kustova, Y.; Basile, A.; Skolnick, P. Aminoglycoside neurotoxicity involves NMDA receptor activation. Brain Res. 1999, 815, 270–277. [Google Scholar] [CrossRef]
- Jiang, Q.; Gu, Z.; Zhang, G.; Jing, G. Diphosphorylation and involvement of extracellular signal-regulated kinases (ERK1/2) in glutamate-induced apoptotic-like death in cultured rat cortical neurons. Brain Res. 2000, 857, 71–77. [Google Scholar] [CrossRef]
- Chandler, L.J.; Sutton, G.; Dorairaj, N.R.; Norwood, D. N-methyl D-aspartate receptor-mediated bidirectional control of extracellular signal-regulated kinase activity in cortical neuronal cultures. J. Biol. Chem. 2001, 276, 2627–2636. [Google Scholar] [CrossRef]
- Kim, M.J.; Dunah, A.W.; Wang, Y.T.; Sheng, M. Differential roles of of NR2A- and NR2B-containing NMDA receptors in Ras-ERK signaling and AMPA receptor trafficking. Neuron 2005, 46, 745–760. [Google Scholar] [CrossRef]
- Ivanov, A.; Pellegrino, C.; Rama, S.; Dumalska, I.; Salyha, Y.; Ben Ari, Y.; Medina, I. Opposing role of synaptic and extrasynaptic NMDA receptors in regulation of the extracellular signal-regulated kinases (ERK) activity in cultured rat hippocampal neurons. J. Physiol. 2006, 572, 789–798. [Google Scholar] [CrossRef]
- Hetman, M.; Kharebava, G. Survival signaling pathways activated by NMDA receptors. Curr. Top. Med. Chem. 2006, 6, 787–799. [Google Scholar] [CrossRef] [PubMed]
- Hardingham, G.E. 2B synaptic or extrasynaptic determines signalling from the NMDA receptor. J. Physiol. 2006, 572, 614–615. [Google Scholar] [CrossRef]
- Jan, C.R.; Ho, C.M.; Wu, S.N.; Tseng, C.J. Mechanism of rise and decay of thapsigargin-evoked calcium signals in MDCK cells. Life Sci. 1999, 64, 259–267. [Google Scholar] [CrossRef]
- Boldyrev, A.A.; Carpenter, D.O.; Huentelman, M.J.; Peters, C.M.; Johnson, P. Sources of reactive oxygen species production in excitotoxin- stimulated cerebellar granule cells. Biochem. Biophys. Res. Commun. 1999, 256, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Lafon-Cazal, M.; Pietri, S.; Culcasi, M.; Bockaert, J. NMDA-dependent superoxide production and neurotoxicity. Nature 1993, 364, 535–537. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Ou, Z.; Grotta, J.C.; Waxham, N.; Aronowski, J. Peroxisome-proliferator-activated receptor-gamma (PPARgamma) activation protects neurons from NMDA excitotoxicity. Brain Res. 2006, 1073–1074, 460–469. [Google Scholar] [CrossRef]
- Uryu, S.; Harada, J.; Hisamoto, M.; Oda, T. Troglitazone inhibits both post-glutamate neurotoxicity and low-potassium-induced apoptosis in cerebellar granule neurons. Brain Res. 2002, 924, 229–236. [Google Scholar] [CrossRef]
- Aoun, P.; Simpkins, J.W.; Agarwal, N. Role of PPAR-gamma ligands in neuroprotection against glutamate-induced cytotoxicity in retinal ganglion cells. Investig. Ophthalmol. Vis. Sci. 2003, 44, 2999–3004. [Google Scholar] [CrossRef]
- Szaroma, W.; Dziubek, K.; Kapusta, E. Effect of N-methyl-D-aspartic acid on activity of superoxide dismutase, catalase, glutathione peroxidase and reduced glutathione level in selected organs of the mouse. Acta Physiol. Hung. 2014, 101, 377–387. [Google Scholar] [CrossRef]
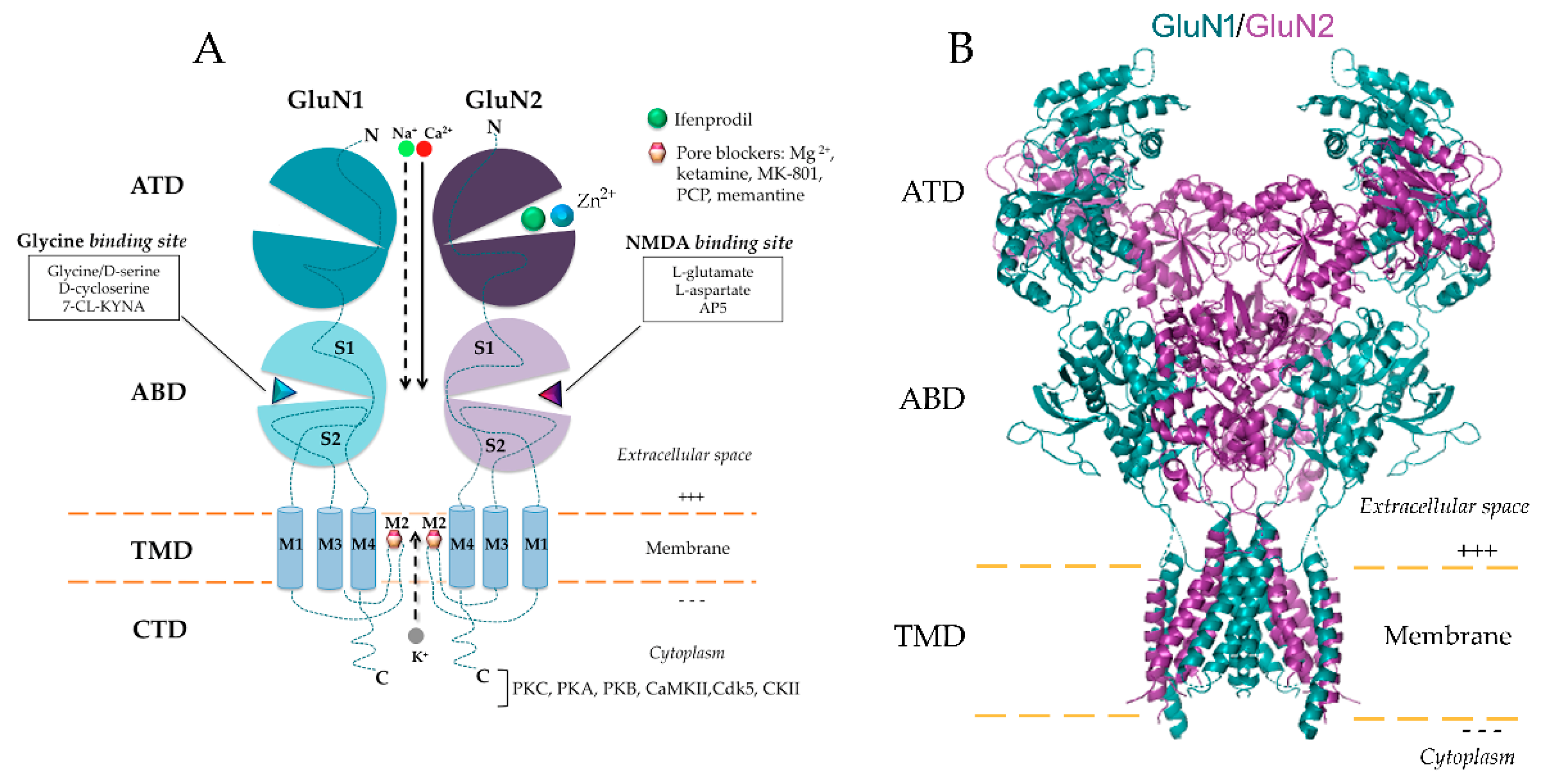
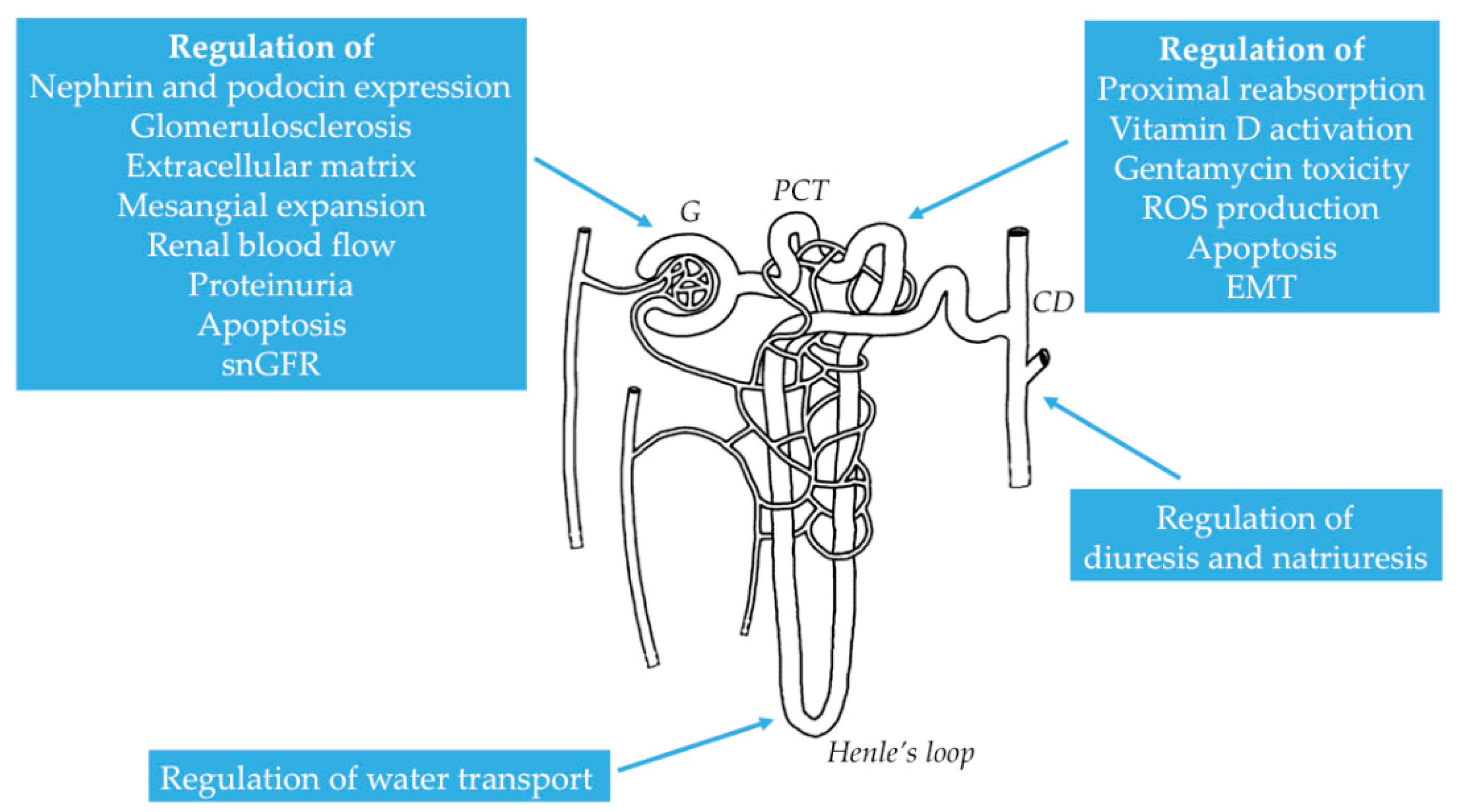
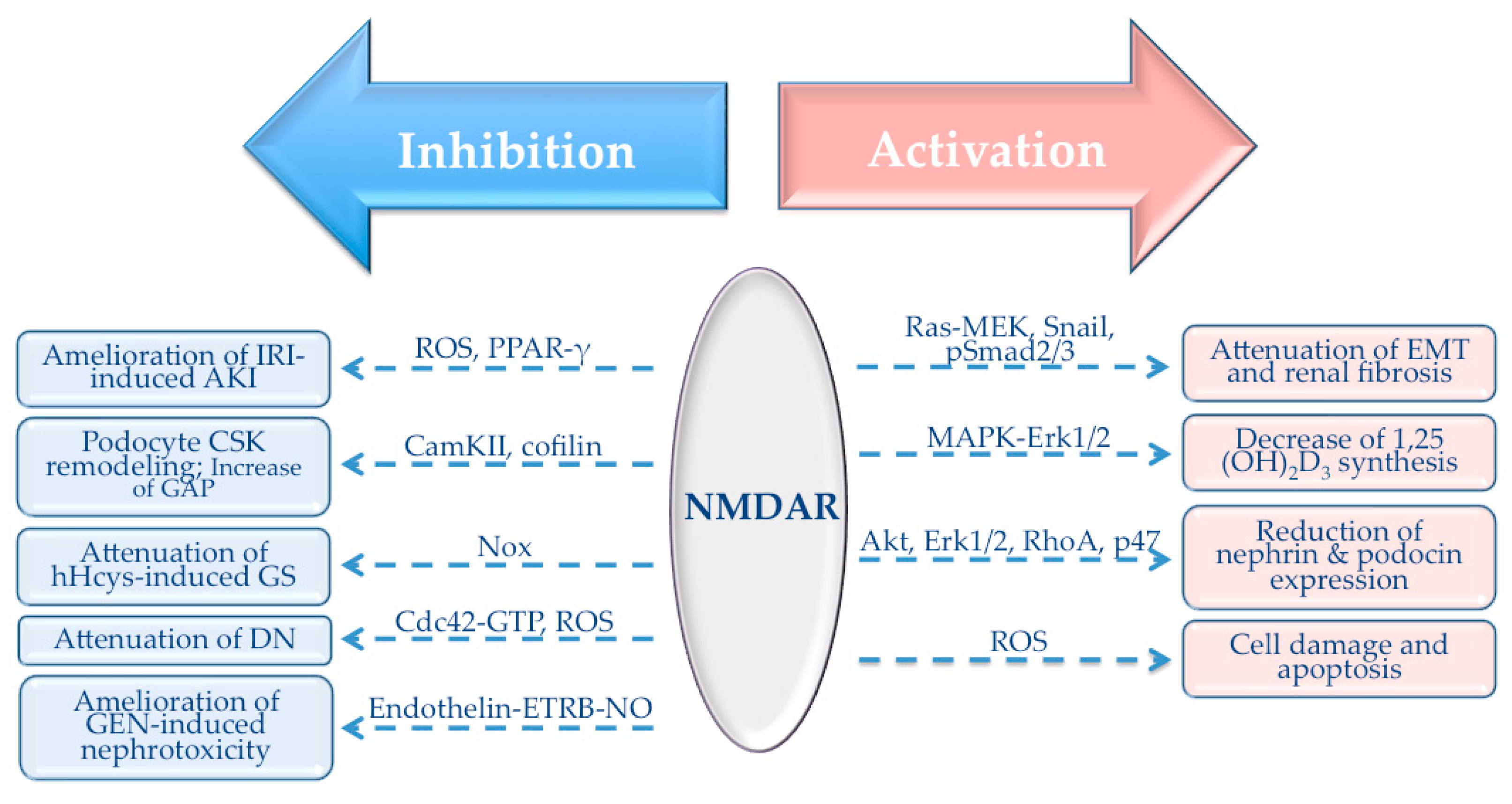
| NMDAR Subunit | Tissue/Cell Type | Reference |
|---|---|---|
| GluN1 | HK-2, Kidney (medulla, cortex), tubules, glomeruli, podocytes, OKs, MDCKs, IMCDs, LLC-PK1 | [45,57,58,59,60,61,62,63,64] |
| GluN2A | HK-2, Glomeruli, podocytes | [57,59,60] |
| GluN2B | Kidney cortex, HK-2, podocytes | [57,58,60] |
| GluN2C | Kidney (medulla, cortex), MDCKs, HK-2, OKs, IMCDs, LLC-PK1, podocytes | [45,57,58,60,63] |
| GluN2D | Kidney cortex, HK-2, podocytes | [57,58,60] |
| GluN3A | Kidney, IMCDs | [63] |
| GluN3B | Kidney, IMCDs | [63] |
| Pharmacological Modulator | Mode of Action | Relation Function/Disease | Signaling Pathway | Reference |
|---|---|---|---|---|
| MK-801, 5,7-DCKA | Inhibition | Renal vasoconstriction | [50] | |
| Glycine | Activation | Increased RBF, diuresis, natriuresis | [83] | |
| KYNA | Inhibition | Antihypertensive action | [83] | |
| MK-801 | Inhibition | Reduction of SNGFR | [64] | |
| MSG | Activation | Increase of GFR and tubular reabsorption of Na, K | [87] | |
| NMDA | Activation | Attenuation of EMT and renal fibrosis | Ras-MEK, Snail, pSmad2/3 | [58] |
| NMDA | Activation | Decrease of 1,25(OH)2D3 synthesis | MAPK-Erk1/2 | [57] |
| NMDA | Activation | Decrease of GFR | [90] | |
| D-AP5, KYNA, KET, MgSO4, PGZ, CUR, E2 | Inhibition | Amelioration of IRI-induced AKI | ROS, PPAR-γ | [90,91,92,93,94,95] |
| MK-801 | Inhibition | Attenuation of LPS-induced endotoxemia | [96] | |
| Ligustrazine | Inhibition | Attenuation of experimental sepsis-associated AKI | [97] | |
| Nor-KA, MK-801 | Inhibition | Podocyte CSK remodeling; Increase of GAP | CaMKII, cofilin | [62] |
| NMDA | Activation | Reduction of nephrin and podocin expression; apoptosis | Akt, Erk1/2, RhoA, ROS, p47 | [61,98] |
| MK-801 | Inhibition | Attenuation of hHcys-induced GS | Nox | [59] |
| MK-801, H2S, memantine | Inhibition | Attenuation of DN | Cdc42-GTP, ROS | [60,99,100,101] |
| MK-801 | Inhibition | Amelioration of GEN-induced nephrotoxicity | Endothelin-ETRB-NO | [102] |
| Glutamate/MK-801, GLY | Activation/Inhibition | Cell damage and apoptosis | ROS | [103,104] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valdivielso, J.M.; Eritja, À.; Caus, M.; Bozic, M. Glutamate-Gated NMDA Receptors: Insights into the Function and Signaling in the Kidney. Biomolecules 2020, 10, 1051. https://doi.org/10.3390/biom10071051
Valdivielso JM, Eritja À, Caus M, Bozic M. Glutamate-Gated NMDA Receptors: Insights into the Function and Signaling in the Kidney. Biomolecules. 2020; 10(7):1051. https://doi.org/10.3390/biom10071051
Chicago/Turabian StyleValdivielso, José M., Àuria Eritja, Maite Caus, and Milica Bozic. 2020. "Glutamate-Gated NMDA Receptors: Insights into the Function and Signaling in the Kidney" Biomolecules 10, no. 7: 1051. https://doi.org/10.3390/biom10071051
APA StyleValdivielso, J. M., Eritja, À., Caus, M., & Bozic, M. (2020). Glutamate-Gated NMDA Receptors: Insights into the Function and Signaling in the Kidney. Biomolecules, 10(7), 1051. https://doi.org/10.3390/biom10071051







