Ampicillin/Sulbactam Treatment Modulates NMDA Receptor NR2B Subunit and Attenuates Neuroinflammation and Alcohol Intake in Male High Alcohol Drinking Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Drinking Protocol
2.2. Brain Dissection
2.3. Western Blot Analyses
2.4. Statistical Analyses
2.4.1. Drinking-Solution Data
2.4.2. Western Blot Data
3. Results
3.1. Effects of AMP/SUL (0, 100 or 200 mg/kg) on Fluid and Food Intake as Well as Body Weight
3.1.1. Effects of AMP/SUL (0 or 100 or 200 mg/kg) on 24 h Ethanol Intake (g/kg/day)
3.1.2. Effects of AMP/SUL (0 or 100 or 200 mg/kg) on 24 h Water Intake (ml/day)
3.1.3. Effects of AMP/SUL (0 or 100 or 200 mg/kg) on Average Body Weight (mL/day)
3.1.4. Effects of AMP/SUL (0 or 100 or 200 mg/kg) on Food Intake (g/day)
3.2. Effects of AMP/SUL (0 or 100 or 200 mg/kg) on the Expression of GLT-1 in the AcbCo and AcbSh of Chronically Ethanol Drinking HAD1 Rats
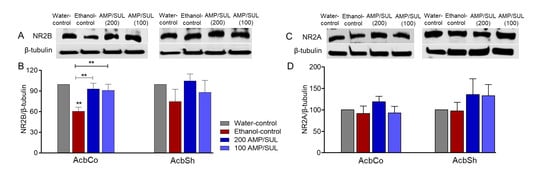
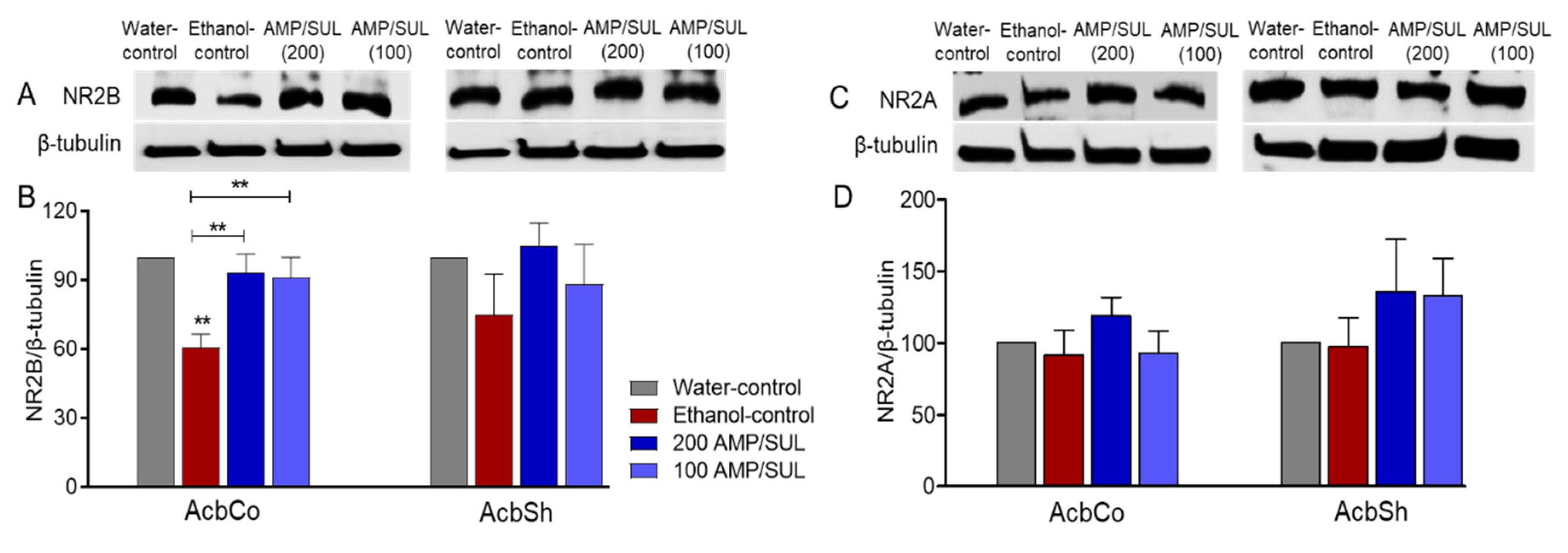
3.3. Effects of AMP/SUL (0 or 100 or 200 mg/kg) on the Expression of NR2B and NR2A in the AcbCo and AcbSh of Chronically Ethanol Drinking HAD1 Rats
3.4. Effects of AMP/SUL (0 or 100 or 200 mg/kg) on the Expression of HMGB1 and RAGE in the AcbCo and AcbSh of Chronically Ethanol Drinking HAD1 Rats
3.5. Effects of AMP/SUL (0 or 100 or 200 mg/kg) on the Expression of TNF-α in the AcbCo and AcbSh of Chronically Ethanol Drinking HAD1 Rats
3.6. Effects of AMP/SUL (0 or 200 mg/kg) on the Expression of mGluR5 in the AcbCo and AcbSh of Chronically Ethanol Drinking HAD1 Rats
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. Global Status Report on Alcohol and Health 2018; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Goodwani, S.; Rao, P.S.; Bell, R.L.; Sari, Y. Amoxicillin and amoxicillin/clavulanate reduce ethanol intake and increase GLT-1 expression as well as AKT phosphorylation in mesocorticolimbic regions. Brain Res. 2015, 1622, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Das, S.C.; Yamamoto, B.K.; Hristov, A.M.; Sari, Y. Ceftriaxone attenuates ethanol drinking and restores extracellular glutamate concentration through normalization of GLT-1 in nucleus accumbens of male alcohol-preferring rats. Neuropharmacology 2015, 97, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.M.; Rodd, Z.A.; Engleman, E.A.; Bailey, J.A.; Lahiri, D.K.; McBride, W.J. Alcohol drinking and deprivation alter basal extracellular glutamate concentrations and clearance in the mesolimbic system of alcohol-preferring (P) rats. Addict. Biol. 2013, 18, 297–306. [Google Scholar] [CrossRef]
- Griffin , W.C., III; Haun, H.L.; Hazelbaker, C.L.; Ramachandra, V.S.; Becker, H.C. Increased extracellular glutamate in the nucleus accumbens promotes excessive ethanol drinking in ethanol dependent mice. Neuropsychopharmacology 2014, 39, 707–717. [Google Scholar] [CrossRef] [PubMed]
- Fiorenza, A.M.; Shnitko, T.A.; Sullivan, K.M.; Vemuru, S.R.; Gomez-A, A.; Esaki, J.Y.; Boettiger, C.A.; Da Cunha, C.; Robinson, D.L. Ethanol exposure history and alcoholic reward differentially alter dopamine release in the nucleus accumbens to a reward-predictive cue. Alcohol. Clin. Exp. Res. 2018, 42, 1051–1061. [Google Scholar] [CrossRef]
- Eisenhardt, M.; Leixner, S.; Luján, R.; Spanagel, R.; Bilbao, A. Glutamate receptors within the mesolimbic dopamine system mediate alcohol relapse behavior. J. Neurosci. 2015, 35, 15523–15538. [Google Scholar] [CrossRef]
- Obara, I.; Bell, R.L.; Goulding, S.P.; Reyes, C.M.; Larson, L.A.; Ary, A.W.; Truitt, W.A.; Szumlinski, K.K. Differential effects of chronic ethanol consumption and withdrawal on homer/glutamate receptor expression in subregions of the accumbens and amygdala of P rats. Alcohol. Clin. Exp. Res. 2009, 33, 1924–1934. [Google Scholar] [CrossRef]
- Chaudhri, N.; Sahuque, L.L.; Schairer, W.W.; Janak, P.H. Separable roles of the nucleus accumbens core and shell in context-and cue-induced alcohol-seeking. Neuropsychopharmacology 2010, 35, 783–791. [Google Scholar] [CrossRef]
- Melendez, R.I.; Hicks, M.P.; Cagle, S.S.; Kalivas, P.W. Ethanol exposure decreases glutamate uptake in the nucleus accumbens. Alcohol. Clin. Exp. Res. 2005, 29, 326–333. [Google Scholar] [CrossRef]
- Rao, P.S.; Goodwani, S.; Bell, R.L.; Wei, Y.; Boddu, S.H.; Sari, Y. Effects of ampicillin, cefazolin and cefoperazone treatments on GLT-1 expressions in the mesocorticolimbic system and ethanol intake in alcohol-preferring rats. Neuroscience 2015, 295, 164–174. [Google Scholar] [CrossRef]
- Sari, Y.; Toalston, J.E.; Rao, P.S.; Bell, R.L. Effects of ceftriaxone on ethanol, nicotine or sucrose intake by alcohol-preferring (P) rats and its association with GLT-1 expression. Neuroscience 2016, 326, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.; Saternos, H.; Goodwani, S.; Sari, Y. Effects of ceftriaxone on GLT1 isoforms, xCT and associated signaling pathways in P rats exposed to ethanol. Psychopharmacology 2015, 232, 2333–2342. [Google Scholar] [CrossRef] [PubMed]
- Sari, Y.; Sakai, M.; Weedman, J.M.; Rebec, G.V.; Bell, R.L. Ceftriaxone, a beta-lactam antibiotic, reduces ethanol consumption in alcohol-preferring rats. Alcohol Alcohol. 2011, 46, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Hakami, A.Y.; Sari, Y. β-Lactamase inhibitor, clavulanic acid, attenuates ethanol intake and increases glial glutamate transporters expression in alcohol preferring rats. Neurosci. Lett. 2017, 657, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; John, J.; Langford, D.; Walker, E.; Ward, S.; Rawls, S.M. Clavulanic acid enhances glutamate transporter subtype I (GLT-1) expression and decreases reinforcing efficacy of cocaine in mice. Amino Acids 2016, 48, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Cebers, G.; Cebere, A.; Kovács, A.D.; Högberg, H.; Moreira, T.; Liljequist, S. Increased ambient glutamate concentration alters the expression of NMDA receptor subunits in cerebellar granule neurons. Neurochem. Int. 2001, 39, 151–160. [Google Scholar] [CrossRef]
- Kaur, B.; Prakash, A. Ceftriaxone attenuates glutamate-mediated neuro-inflammation and restores BDNF in MPTP model of Parkinson’s disease in rats. Pathophysiology 2017, 24, 71–79. [Google Scholar] [CrossRef]
- Meyer, P.J.; Phillips, T.J. Bivalent effects of MK-801 on ethanol-induced sensitization do not parallel its effects on ethanol-induced tolerance. Behav. Neurosci. 2003, 117, 641. [Google Scholar] [CrossRef][Green Version]
- Boyce-Rustay, J.M.; Cunningham, C.L. The role of NMDA receptor binding sites in ethanol place conditioning. Behav. Neurosci. 2004, 118, 822. [Google Scholar] [CrossRef]
- Bienkowski, P.; Krzascik, P.; Koros, E.; Kostowski, W.; Scinska, A.; Danysz, W. Effects of a novel uncompetitive NMDA receptor antagonist, MRZ 2/579 on ethanol self-administration and ethanol withdrawal seizures in the rat. Eur. J. Pharmacol. 2001, 413, 81–89. [Google Scholar] [CrossRef]
- Jeanblanc, J.; Coune, F.; Botia, B.; Naassila, M. Brain-derived neurotrophic factor mediates the suppression of alcohol self-administration by memantine. Addict. Biol. 2014, 19, 758–769. [Google Scholar] [CrossRef] [PubMed]
- Hicklin, T.R.; Wu, P.H.; Radcliffe, R.A.; Freund, R.K.; Goebel-Goody, S.M.; Correa, P.R.; Proctor, W.R.; Lombroso, P.J.; Browning, M.D. Alcohol inhibition of the NMDA receptor function, long-term potentiation, and fear learning requires striatal-enriched protein tyrosine phosphatase. Proc. Natl. Acad. Sci. USA 2011, 108, 6650–6655. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Carnicella, S.; Phamluong, K.; Jeanblanc, J.; Ronesi, J.A.; Chaudhri, N.; Janak, P.H.; Lovinger, D.M.; Ron, D. Ethanol induces long-term facilitation of NR2B-NMDA receptor activity in the dorsal striatum: Implications for alcohol drinking behavior. J. Neurosci. 2007, 27, 3593–3602. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lanfranco, M.F.; Gibb, S.L.; Yowell, Q.V.; Carnicella, S.; Ron, D. Long-lasting adaptations of the NR2B-containing NMDA receptors in the dorsomedial striatum play a crucial role in alcohol consumption and relapse. J. Neurosci. 2010, 30, 10187–10198. [Google Scholar] [CrossRef]
- Floyd, D.W.; Jung, K.-Y.; McCool, B.A. Chronic ethanol ingestion facilitates N-methyl-D-aspartate receptor function and expression in rat lateral/basolateral amygdala neurons. J. Pharmacol. Exp. Ther. 2003, 307, 1020–1029. [Google Scholar] [CrossRef]
- Cozzoli, D.K.; Goulding, S.P.; Zhang, P.W.; Xiao, B.; Hu, J.-H.; Ary, A.W.; Obara, I.; Rahn, A.; Abou-Ziab, H.; Tyrrel, B.; et al. Binge drinking upregulates accumbens mGluR5–Homer2–PI3K signaling: Functional implications for alcoholism. J. Neurosci. 2009, 29, 8655–8668. [Google Scholar] [CrossRef]
- Pian, J.P.; Criado, J.R.; Milner, R.; Ehlers, C.L. N-methyl-D-aspartate receptor subunit expression in adult and adolescent brain following chronic ethanol exposure. Neuroscience 2010, 170, 645–654. [Google Scholar] [CrossRef]
- Goebel, D.J.; Poosch, M.S. NMDA receptor subunit gene expression in the rat brain: A quantitative analysis of endogenous mRNA levels of NR1Com, NR2A, NR2B, NR2C, NR2D and NR3A. Mol. Brain Res. 1999, 69, 164–170. [Google Scholar] [CrossRef]
- Chu, B.; Anantharam, V.; Treistman, S.N. Ethanol inhibition of recombinant heteromeric NMDA channels in the presence and absence of modulators. J. Neurochem. 1995, 65, 140–148. [Google Scholar] [CrossRef]
- Chang, Y.C.; Kim, H.-W.; Rapoport, S.I.; Rao, J.S. Chronic NMDA administration increases neuroinflammatory markers in rat frontal cortex: Cross-talk between excitotoxicity and neuroinflammation. Neurochem. Res. 2008, 33, 2318–2323. [Google Scholar] [CrossRef]
- Zou, J.; Crews, F.T. Glutamate/NMDA excitotoxicity and HMGB1/TLR4 neuroimmune toxicity converge as components of neurodegeneration. Aims Mol. Sci. 2015, 2, 77–100. [Google Scholar] [CrossRef]
- Sil, S.; Ghosh, T.; Ghosh, R. NMDA receptor is involved in neuroinflammation in intracerebroventricular colchicine-injected rats. J. Immunotoxicol. 2016, 13, 474–489. [Google Scholar] [CrossRef] [PubMed]
- Goodwani, S.; Saternos, H.; Alasmari, F.; Sari, Y. Metabotropic and ionotropic glutamate receptors as potential targets for the treatment of alcohol use disorder. Neurosci. Biobehav. Rev. 2017, 77, 14–31. [Google Scholar] [CrossRef] [PubMed]
- Olive, M.F. Metabotropic glutamate receptor ligands as potential therapeutics for addiction. Curr. Drug Abus. Rev. 2009, 2, 83–98. [Google Scholar] [CrossRef]
- Bäckström, P.; Bachteler, D.; Koch, S.; Hyytiä, P.; Spanagel, R. mGluR5 antagonist MPEP reduces ethanol-seeking and relapse behavior. Neuropsychopharmacology 2004, 29, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Sung, K.-W.; Choi, S.; Lovinger, D.M. Activation of group I mGluRs is necessary for induction of long-term depression at striatal synapses. J. Neurophysiol. 2001, 86, 2405–2412. [Google Scholar] [CrossRef]
- Nandi, A.; Valentine, H.; McCaul, M.; Wong, D. Glutamatergic abnormalities in a rodent model of alcohol abuse. J. Nucl. Med. 2016, 57, 1866a. [Google Scholar]
- de Laat, B.; Weerasekera, A.; Leurquin-Sterk, G.; Gsell, W.; Bormans, G.; Himmelreich, U.; Casteels, C.; Van Laere, K. Effects of alcohol exposure on the glutamatergic system: A combined longitudinal 18F-FPEB and 1H-MRS study in rats. Addict. Biol. 2019, 24, 696–706. [Google Scholar] [CrossRef]
- Byrnes, K.R.; Stoica, B.; Loane, D.J.; Riccio, A.; Davis, M.I.; Faden, A.I. Metabotropic glutamate receptor 5 activation inhibits microglial associated inflammation and neurotoxicity. Glia 2009, 57, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Yam Nath Paudel, M.; Shaikh, F.; Chakraborti, A.; Kumari, Y.; Aledo-Serrano, Á.; Aleksovska, K.; Alvim, M.K.M.; Othman, I. HMGB1: A common biomarker and potential target for TBI, neuroinflammation, epilepsy, and cognitive dysfunction. Front. Mol. Neurosci. 2018, 12, 628. [Google Scholar] [CrossRef]
- You, W.-C.; Wang, C.-x.; Pan, Y.-x.; Zhang, X.; Zhou, X.-M.; Zhang, X.-S.; Shi, J.-X.; Zhou, M.-L. Activation of nuclear factor-κB in the brain after experimental subarachnoid hemorrhage and its potential role in delayed brain injury. PLoS ONE 2013, 8, e60290. [Google Scholar] [CrossRef]
- Orio, L.; Alen, F.; Pavón, F.J.; Serrano, A.; García-Bueno, B. Oleoylethanolamide, neuroinflammation and alcohol abuse. Front. Mol. Neurosci. 2018, 11, 490. [Google Scholar] [CrossRef] [PubMed]
- Ureña-Peralta, J.; Alfonso-Loeches, S.; Cuesta-Diaz, C.; García-García, F.; Guerri, C. Deep sequencing and miRNA profiles in alcohol-induced neuroinflammation and the TLR4 response in mice cerebral cortex. Sci. Rep. 2018, 8, 15913. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Lizarbe, S.; Pascual, M.; Guerri, C. Critical role of TLR4 response in the activation of microglia induced by ethanol. J. Immunol. 2009, 183, 4733–4744. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; He, J.; Hanes, R.N.; Pluzarev, O.; Hong, J.-S.; Crews, F.T. Increased systemic and brain cytokine production and neuroinflammation by endotoxin following ethanol treatment. J. Neuroinflamm. 2008, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.Y.; Crews, F.T. Release of neuronal HMGB1 by ethanol through decreased HDAC activity activates brain neuroimmune signaling. PLoS ONE 2014, 9, e87915. [Google Scholar] [CrossRef] [PubMed]
- Antón, M.; Rodríguez-González, A.; Rodríguez-Rojo, I.C.; Pastor, A.; Correas, Á.; Serrano, A.; Ballesta, A.; Alén, F.; Gómez de Heras, R.; de la Torre, R. Increased plasma oleoylethanolamide and palmitoleoylethanolamide levels correlate with inflammatory changes in alcohol binge drinkers: The case of HMGB1 in women. Addict. Biol. 2018, 23, 1242–1250. [Google Scholar] [CrossRef]
- Coleman, L.G.; Zou, J.; Crews, F.T. Microglial-derived miRNA let-7 and HMGB1 contribute to ethanol-induced neurotoxicity via TLR7. J. Neuroinflammation 2017, 14, 22. [Google Scholar] [CrossRef]
- Mayer, S.; Harris, B.R.; Gibson, D.A.; Blanchard, J.A.; Prendergast, M.A.; Holley, R.C.; Littleton, J. Acamprosate, MK-801, and ifenprodil inhibit neurotoxicity and calcium entry induced by ethanol withdrawal in organotypic slice cultures from neonatal rat hippocampus. Alcohol. Clin. Exp. Res. 2002, 26, 1468–1478. [Google Scholar] [CrossRef]
- Foulds, G.; McBride, T.; Knirsch, A.; Rodriguez, W.; Khan, W. Penetration of sulbactam and ampicillin into cerebrospinal fluid of infants and young children with meningitis. Antimicrob. Agents Chemother. 1987, 31, 1703–1705. [Google Scholar] [CrossRef]
- Hammad, A.M.; Alasmari, F.; Althobaiti, Y.S.; Sari, Y. Modulatory effects of Ampicillin/Sulbactam on glial glutamate transporters and metabotropic glutamate receptor 1 as well as reinstatement to cocaine-seeking behavior. Behav. Brain Res. 2017, 332, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Hakami, A.Y.; Alshehri, F.S.; Sari, Y. β-lactams modulate astroglial glutamate transporters and attenuate dependence to CP 55,940, a CB1 receptor agonist, in rat model. Behav. Brain Res. 2019, 359, 709–718. [Google Scholar] [CrossRef]
- Rothstein, J.D.; Patel, S.; Regan, M.R.; Haenggeli, C.; Huang, Y.H.; Bergles, D.E.; Jin, L.; Hoberg, M.D.; Vidensky, S.; Chung, D.S.; et al. β-Lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature 2005, 433, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Bell, R.L.; Hauser, S.R.; Liang, T.; Sari, Y.; Maldonado-Devincci, A.; Rodd, Z.A. Rat animal models for screening medications to treat alcohol use disorders. Neuropharmacology 2017, 122, 201–243. [Google Scholar] [CrossRef] [PubMed]
- Bell, R.L.; Sable, H.J.; Colombo, G.; Hyytia, P.; Rodd, Z.A.; Lumeng, L. Animal models for medications development targeting alcohol abuse using selectively bred rat lines: Neurobiological and pharmacological validity. Pharmacol. Biochem. Behav. 2012, 103, 119–155. [Google Scholar] [CrossRef] [PubMed]
- McBride, W.J.; Rodd, Z.A.; Bell, R.L.; Lumeng, L.; Li, T.K. The alcohol-preferring (P) and high-alcohol-drinking (HAD) rats--animal models of alcoholism. Alcohol 2014, 48, 209–215. [Google Scholar] [CrossRef]
- Murphy, J.M.; Stewart, R.B.; Bell, R.L.; Badia-Elder, N.E.; Carr, L.G.; McBride, W.J.; Lumeng, L.; Li, T.-K. Phenotypic and genotypic characterization of the Indiana University rat lines selectively bred for high and low alcohol preference. Behav. Genet. 2002, 32, 363–388. [Google Scholar] [CrossRef]
- Hammad, A.M.; Sari, Y. Effects of Cocaine Exposure on Astrocytic Glutamate Transporters and Relapse-Like Ethanol-Drinking Behavior in Male Alcohol-Preferring Rats. Alcohol Alcohol. 2020, 55, 254–263. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates in Stereotaxic Coordinates, 6th ed.; Academic Press: San Diego, CA, USA, 2007. [Google Scholar]
- Alasmari, F.; Alexander, L.E.C.; Nelson, J.A.; Schiefer, I.T.; Breen, E.; Drummond, C.A.; Sari, Y. Effects of chronic inhalation of electronic cigarettes containing nicotine on glial glutamate transporters and α-7 nicotinic acetylcholine receptor in female CD-1 mice. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2017, 77, 1–8. [Google Scholar] [CrossRef]
- Li, J.; Olinger, A.; Dassow, M.; Abel, M. Up-regulation of GABA B receptor mRNA and protein in the hippocampus of cocaine-and lidocaine-kindled rats. Neuroscience 2003, 118, 451–462. [Google Scholar] [CrossRef]
- Raval, A.P.; Dave, K.R.; Mochly-Rosen, D.; Sick, T.J.; Pérez-Pinzón, M.A. εPKC is required for the induction of tolerance by ischemic and NMDA-mediated preconditioning in the organotypic hippocampal slice. J. Neurosci. 2003, 23, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Tan, Y. Nerve growth factor augments neuronal responsiveness to noradrenaline in cultured dorsal root ganglion neurons of rats. Neuroscience 2011, 193, 72–79. [Google Scholar] [CrossRef]
- Devoto, V.P.; Bogetti, M.E.; de Plazas, S.F. Developmental and hypoxia-induced cell death share common ultrastructural and biochemical apoptotic features in the central nervous system. Neuroscience 2013, 252, 190–200. [Google Scholar] [CrossRef]
- Alasmari, F.; Bell, R.L.; Rao, P.S.S.; Hammad, A.M.; Sari, Y. Peri-adolescent drinking of ethanol and/or nicotine modulates astroglial glutamate transporters and metabotropic glutamate receptor-1 in female alcohol-preferring rats. Pharmacol. Biochem. Behav. 2018, 170, 44–55. [Google Scholar] [CrossRef]
- Koehler, D.; Shah, Z.A.; Williams, F.E. The GSK3β inhibitor, TDZD-8, rescues cognition in a zebrafish model of okadaic acid-induced Alzheimer’s disease. Neurochem. Int. 2019, 122, 31–37. [Google Scholar] [CrossRef]
- Cui, X.; Li, L.; Hu, Y.-Y.; Ren, S.; Zhang, M.; Li, W.-B. Sulbactam plays neuronal protective effect against brain ischemia via upregulating GLT1 in rats. Mol. Neurobiol. 2015, 51, 1322–1333. [Google Scholar] [CrossRef]
- Alhaddad, H.; Kim, N.T.; Aal-Aaboda, M.; Althobaiti, Y.S.; Leighton, J.; Boddu, S.H.; Wei, Y.; Sari, Y. Effects of MS-153 on chronic ethanol consumption and GLT1 modulation of glutamate levels in male alcohol-preferring rats. Front Behav. Neurosci. 2014, 8, 366. [Google Scholar] [CrossRef] [PubMed]
- Althobaiti, Y.S.; Alshehri, F.S.; Hakami, A.Y.; Hammad, A.M.; Sari, Y. Effects of Clavulanic Acid Treatment on Reinstatement to Methamphetamine, Glial Glutamate Transporters, and mGluR 2/3 Expression in P Rats Exposed to Ethanol. J. Mol. Neurosci. 2019, 67, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Wang, W.; Wang, F.; Hu, Z.-L.; Xiao, J.-L.; Yang, S.; Zhang, J.; Peng, X.-Z.; Wang, J.-H.; Chen, J.-G. The stability of NR2B in the nucleus accumbens controls behavioral and synaptic adaptations to chronic stress. Biol. Psychiatry 2013, 74, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.A.; Goto, Y. Chronic stress modulation of prefrontal cortical NMDA receptor expression disrupts limbic structure–prefrontal cortex interaction. Eur. J. Neurosci. 2011, 34, 426–436. [Google Scholar] [CrossRef]
- Qin, L.; Crews, F.T. Chronic ethanol increases systemic TLR3 agonist-induced neuroinflammation and neurodegeneration. J. Neuroinflamm. 2012, 9, 130. [Google Scholar] [CrossRef]
- Montesinos, J.; Gil, A.; Guerri, C. Nalmefene prevents alcohol-induced neuroinflammation and alcohol drinking preference in adolescent female mice: Role of TLR4. Alcohol. Clin. Exp. Res. 2017, 41, 1257–1270. [Google Scholar] [CrossRef] [PubMed]
- Lippai, D.; Bala, S.; Petrasek, J.; Csak, T.; Levin, I.; Kurt-Jones, E.A.; Szabo, G. Alcohol-induced IL-1β in the brain is mediated by NLRP3/ASC inflammasome activation that amplifies neuroinflammation. J. Leukoc. Biol. 2013, 94, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Lowe, P.P.; Gyongyosi, B.; Satishchandran, A.; Iracheta-Vellve, A.; Cho, Y.; Ambade, A.; Szabo, G. Reduced gut microbiome protects from alcohol-induced neuroinflammation and alters intestinal and brain inflammasome expression. J. Neuroinflamm. 2018, 15, 298. [Google Scholar] [CrossRef] [PubMed]
- Laird, M.D.; Shields, J.S.; Sukumari-Ramesh, S.; Kimbler, D.E.; Fessler, R.D.; Shakir, B.; Youssef, P.; Yanasak, N.; Vender, J.R.; Dhandapani, K.M. High mobility group box protein-1 promotes cerebral edema after traumatic brain injury via activation of toll-like receptor 4. Glia 2014, 62, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Lowe, P.P.; Gyongyosi, B.; Satishchandran, A.; Iracheta-Vellve, A.; Ambade, A.; Kodys, K.; Catalano, D.; Ward, D.V.; Szabo, G. Alcohol-related changes in the intestinal microbiome influence neutrophil infiltration, inflammation and steatosis in early alcoholic hepatitis in mice. PLoS ONE 2017, 12, e0174554. [Google Scholar]
- Zhang, Z.-y.; Sun, B.-l.; Liu, J.-k.; Yang, M.-f.; Li, D.-w.; Fang, J.; Zhang, S.; Yuan, Q.-l.; Huang, S.-l. Activation of mGluR5 attenuates microglial activation and neuronal apoptosis in early brain injury after experimental subarachnoid hemorrhage in rats. Neurochem. Res. 2015, 40, 1121–1132. [Google Scholar] [CrossRef]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alasmari, F.; Alhaddad, H.; Wong, W.; Bell, R.L.; Sari, Y. Ampicillin/Sulbactam Treatment Modulates NMDA Receptor NR2B Subunit and Attenuates Neuroinflammation and Alcohol Intake in Male High Alcohol Drinking Rats. Biomolecules 2020, 10, 1030. https://doi.org/10.3390/biom10071030
Alasmari F, Alhaddad H, Wong W, Bell RL, Sari Y. Ampicillin/Sulbactam Treatment Modulates NMDA Receptor NR2B Subunit and Attenuates Neuroinflammation and Alcohol Intake in Male High Alcohol Drinking Rats. Biomolecules. 2020; 10(7):1030. https://doi.org/10.3390/biom10071030
Chicago/Turabian StyleAlasmari, Fawaz, Hasan Alhaddad, Woonyen Wong, Richard L. Bell, and Youssef Sari. 2020. "Ampicillin/Sulbactam Treatment Modulates NMDA Receptor NR2B Subunit and Attenuates Neuroinflammation and Alcohol Intake in Male High Alcohol Drinking Rats" Biomolecules 10, no. 7: 1030. https://doi.org/10.3390/biom10071030
APA StyleAlasmari, F., Alhaddad, H., Wong, W., Bell, R. L., & Sari, Y. (2020). Ampicillin/Sulbactam Treatment Modulates NMDA Receptor NR2B Subunit and Attenuates Neuroinflammation and Alcohol Intake in Male High Alcohol Drinking Rats. Biomolecules, 10(7), 1030. https://doi.org/10.3390/biom10071030