ProAKAP4 as Novel Molecular Marker of Sperm Quality in Ram: An Integrative Study in Fresh, Cooled and Cryopreserved Sperm
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Sample Collection
2.3. Experimental Groups and Sample Treatments
2.4. Sperm Motility and Kinetic Parameters
2.5. Multiparametric Flow Cytometry Analyses
2.6. RedoxSYS Analysis
2.7. ProAKAP4 ELISA Assay
2.8. Statistical Analysis
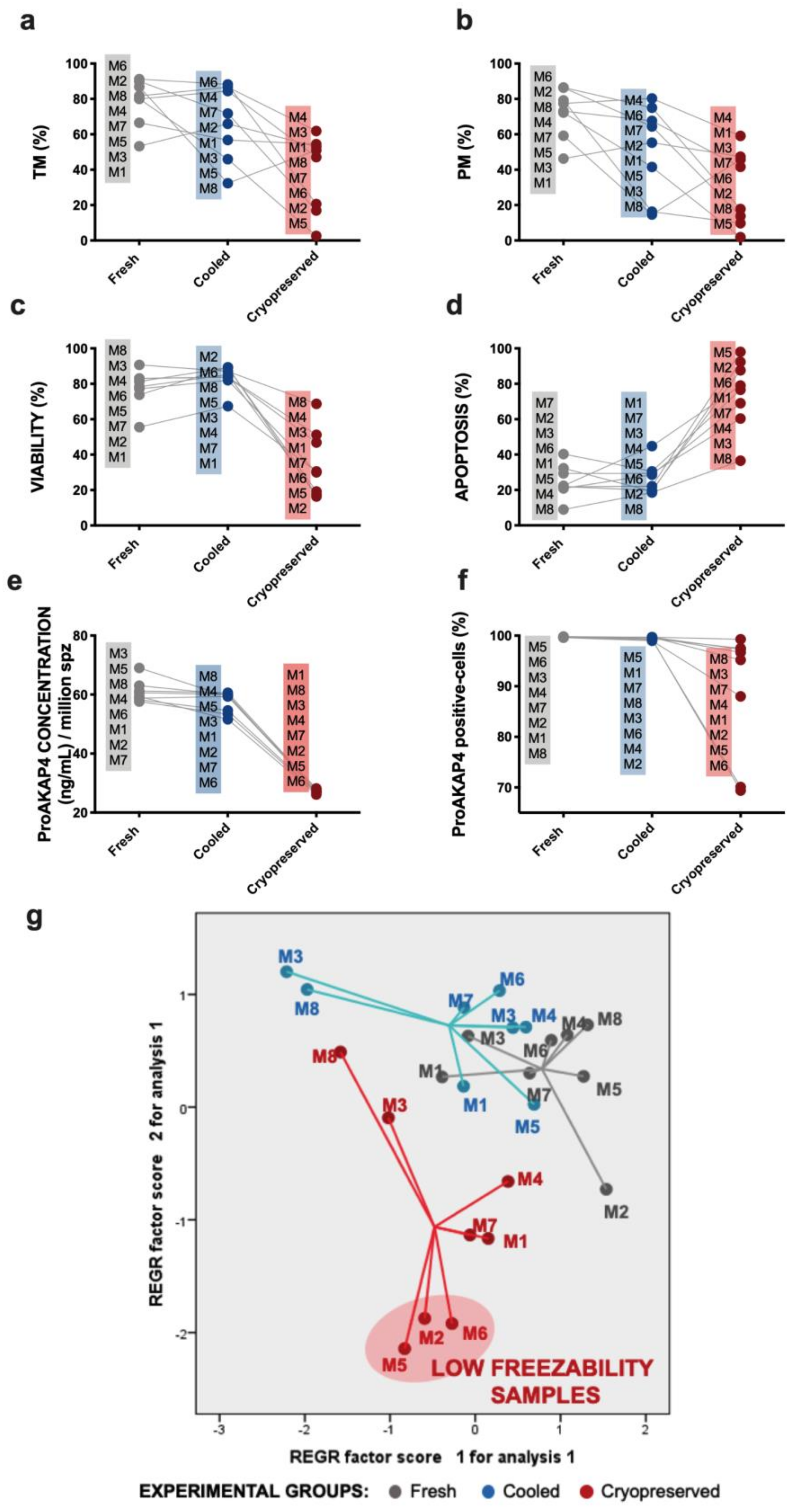
3. Results
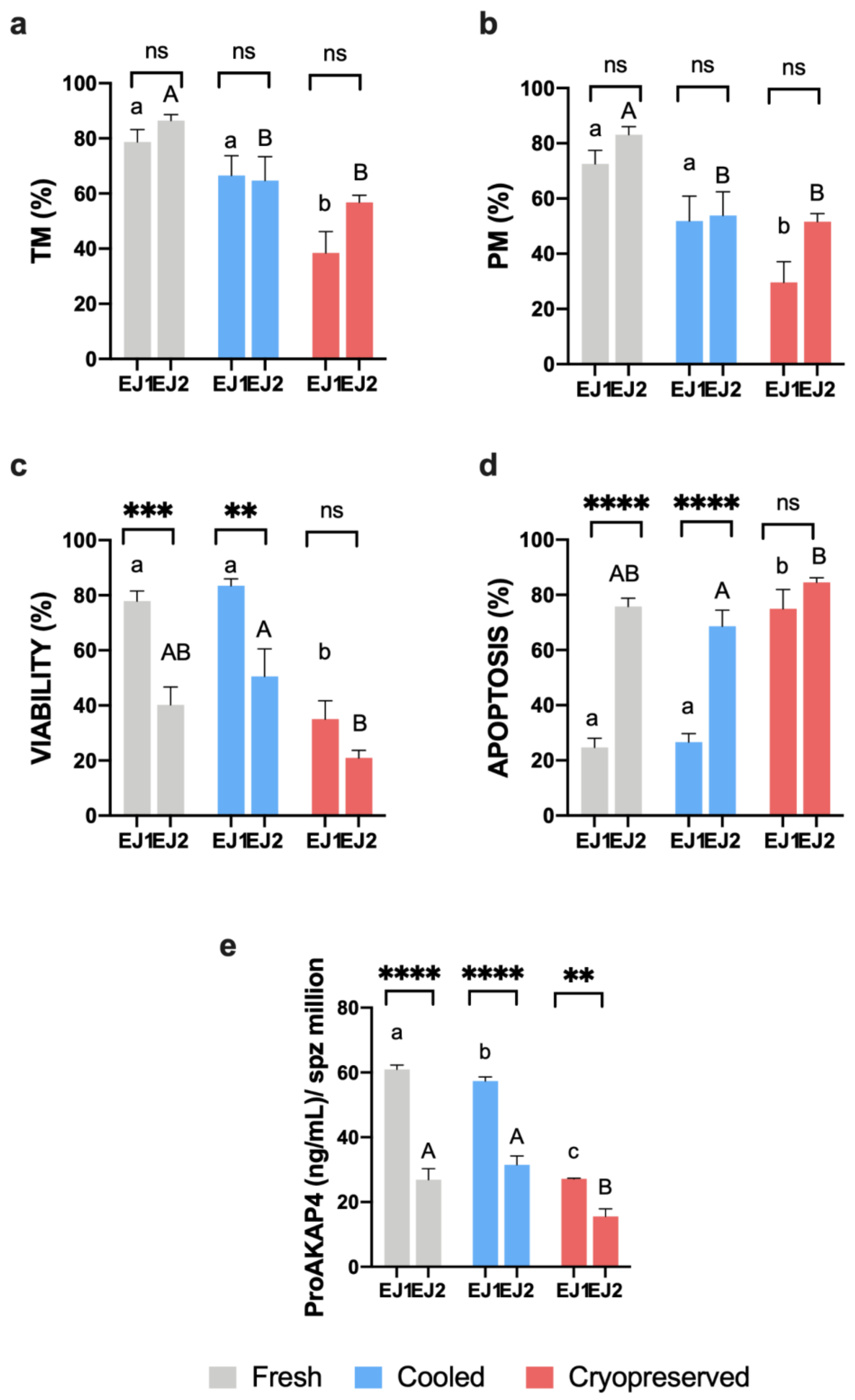
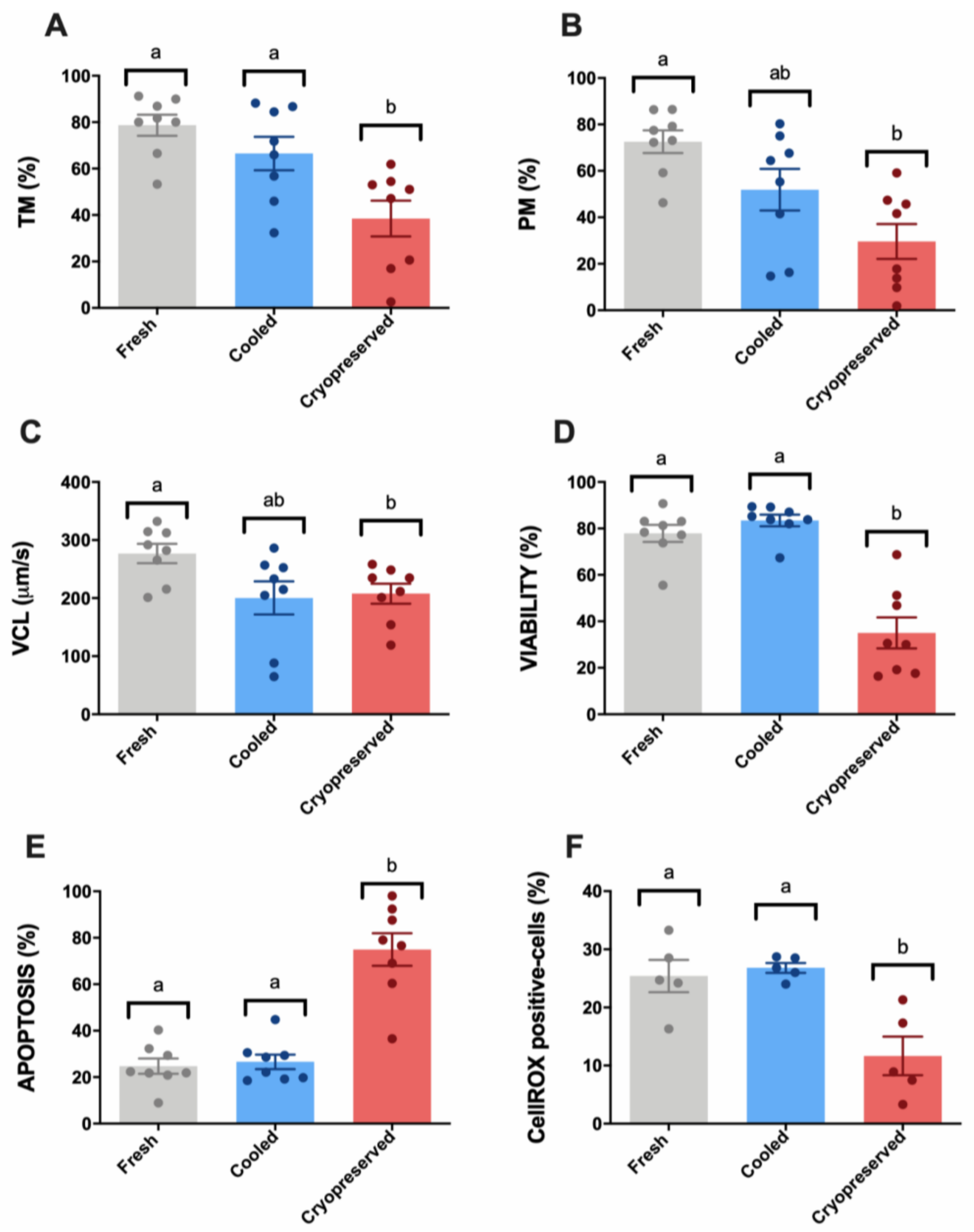
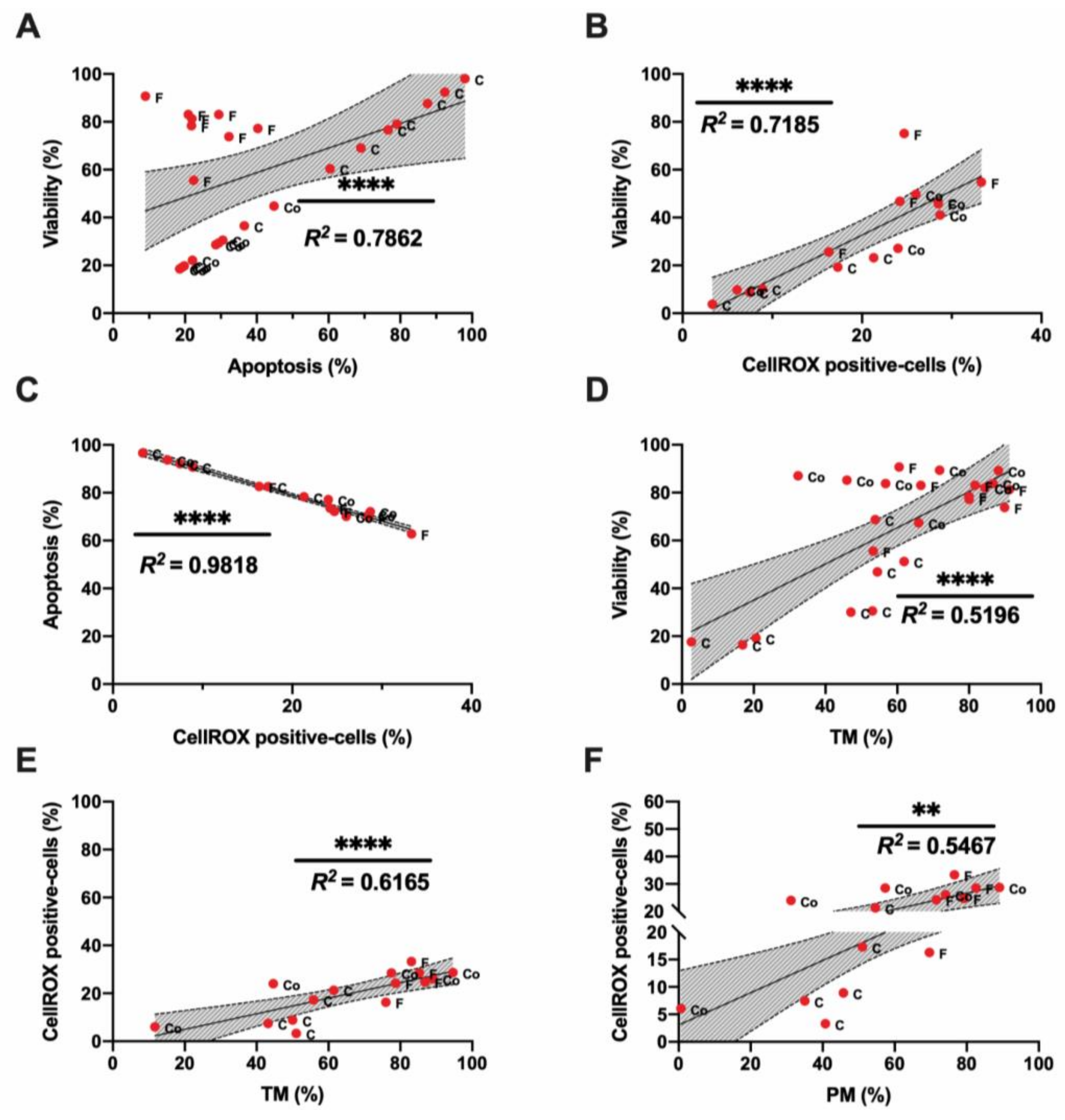
3.1. Sperm Motility and Multiparametric Flow Cytometry Analyses
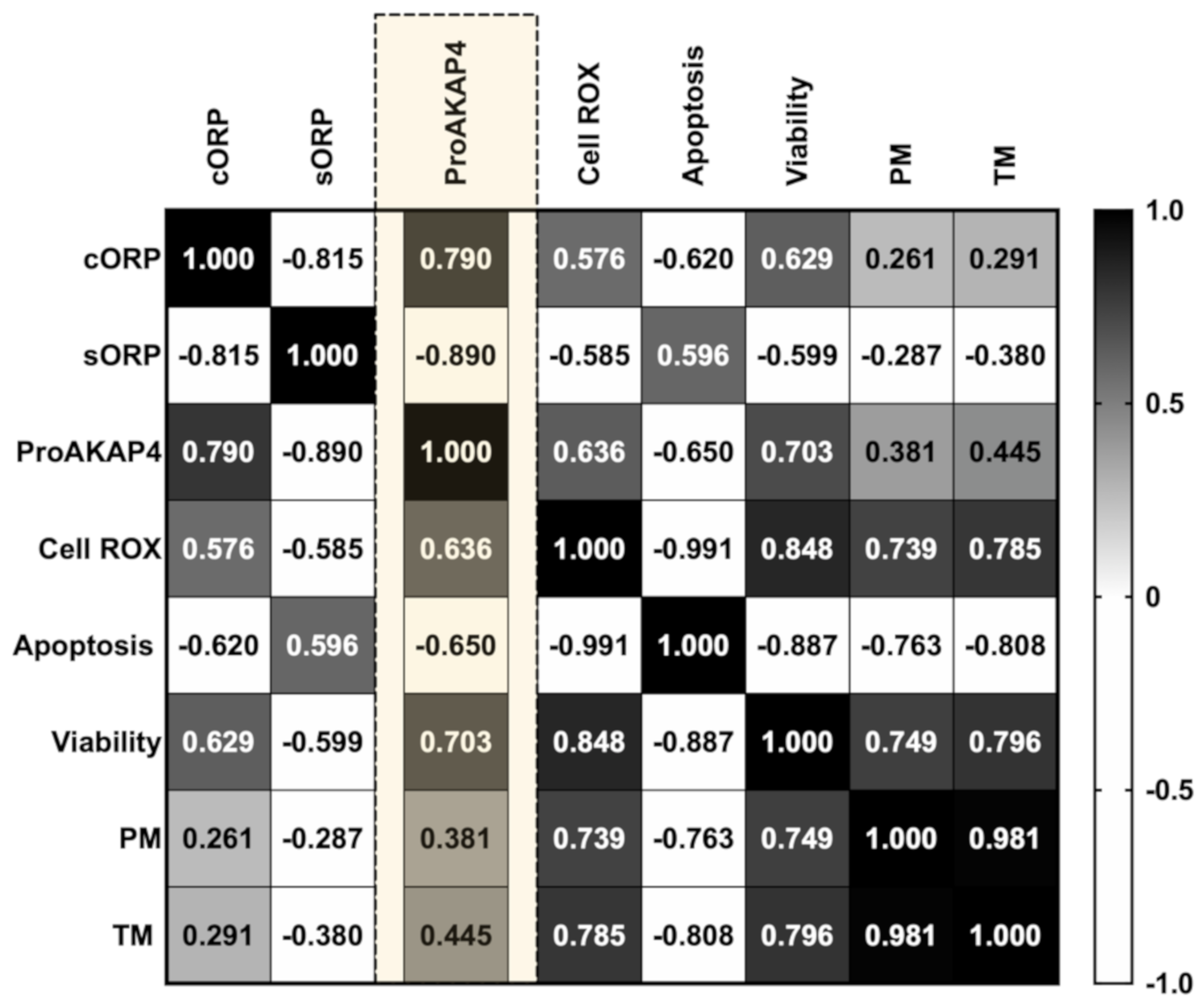
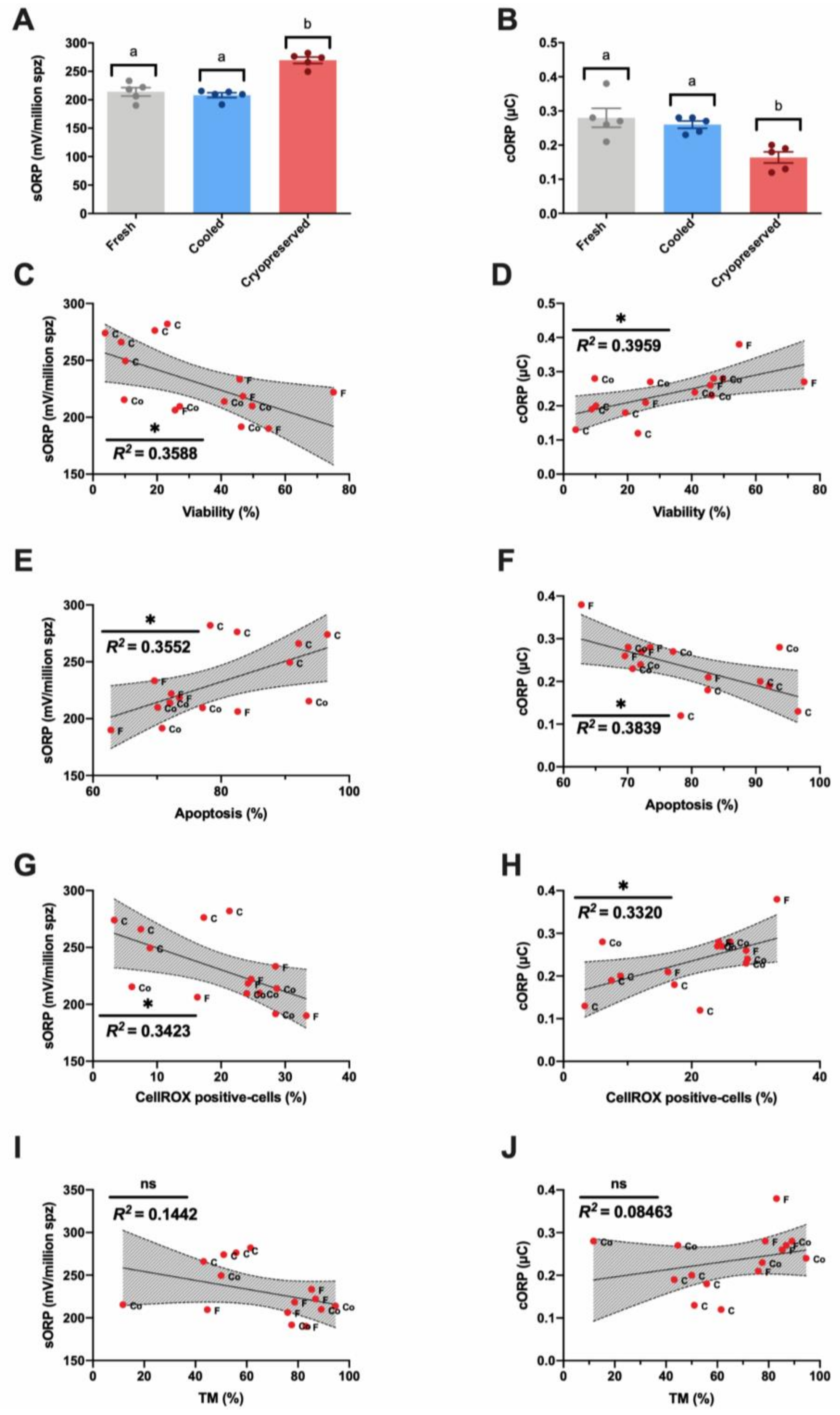
3.2. Novel Sperm Quality Markers: Oxidation Reduction Potential
3.3. Novel Sperm Quality Markers: ProAKAP4 Expression and Quantification
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- O’Hara, L.; Hanrahan, J.P.P.; Richardson, L.; Donovan, A.; Fair, S.; Evans, A.C.O.; Lonergan, P. Effect of storage duration, storage temperature, and diluent on the viability and fertility of fresh ram sperm. Theriogenology 2010, 73, 541–549. [Google Scholar] [CrossRef]
- Kukovics, S.; Gyoker, E.; Nemeth, T.; Gergatz, E. Artificial Insemination of Sheep-Possibilities, Realities and Techniques at the Farm Level. In Artificial Insemination in Farm Animals; InTech: London, UK, 2011. [Google Scholar]
- Faigl, V.; Vass, N.; Jávor, A.; Kulcsár, M.; Solti, L.; Amiridis, G.; Cseh, S. Artificial insemination of small ruminants—A review. Acta Vet. Hung. 2012, 60, 115–129. [Google Scholar] [CrossRef]
- Gibbons, A.E.; Fernandez, J.; Bruno-Galarraga, M.M.; Spinelli, M.V.; Cueto, M.I. Technical recommendations for artificial insemination in sheep. Anim. Reprod. 2019, 16, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Anel, L.; Alvarez, M.; Martinez-Pastor, F.; Garcia-Macias, V.; Anel, E.; de Paz, P. Improvement Strategies in Ovine Artificial Insemination. Reprod. Domest. Anim. 2006, 41, 30–42. [Google Scholar] [CrossRef]
- Anel, L.; De Paz, P.; Álvarez, M.; Chamorro, C.A.; Boixo, J.C.; Manso, A.; González, M.; Kaabi, M.; Anel, E. Field and in vitro assay of three methods for freezing ram semen. Theriogenology 2003, 60, 1293–1308. [Google Scholar] [CrossRef]
- Alvarez, M.; Anel-Lopez, L.; Boixo, J.C.; Chamorro, C.; Neila-Montero, M.; Montes-Garrido, R.; de Paz, P.; Anel, L. Current challenges in sheep artificial insemination: A particular insight. Reprod. Domest. Anim. 2019, 54, 32–40. [Google Scholar] [CrossRef]
- Masoudi, R.; Zare Shahneh, A.; Towhidi, A.; Kohram, H.; Akbarisharif, A.; Sharafi, M. Fertility response of artificial insemination methods in sheep with fresh and frozen-thawed semen. Cryobiology 2017, 74, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Hiwasa, M.; Kohno, H.; Togari, T.; Okabe, K.; Fukui, Y. Fertility after Different Artificial Insemination Methods Using a Synthetic Semen Extender in Sheep. J. Reprod. Dev. 2009, 55, 50–54. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mata-Campuzano, M.; Soleilhavoup, C.; Tsikis, G.; Martinez-Pastor, F.; de Graaf, S.P.; Druart, X. Motility of liquid stored ram spermatozoa is altered by dilution rate independent of seminal plasma concentration. Anim. Reprod. Sci. 2015, 162, 31–36. [Google Scholar] [CrossRef]
- Petrunkina, A.M.; Waberski, D.; Günzel-Apel, A.R.; Töpfer-Petersen, E. Determinants of sperm quality and fertility in domestic species. Reproduction 2007, 134, 3–17. [Google Scholar] [CrossRef]
- Palomar Rios, A.; Molina Botella, I. Sperm parameters that play a major role in the assessment of semen quality after cryopreservation. J. Assist. Reprod. Genet. 2017, 34, 1271–1276. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Pastor, F.; Garcia-Macias, V.; Alvarez, M.; Herraez, P.; Anel, L.; de Paz, P. Sperm Subpopulations in Iberian Red Deer Epididymal Sperm and Their Changes Through the Cryopreservation Process1. Biol. Reprod. 2005, 72, 316–327. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Martínez, H. Can We Increase the Estimative Value of Semen Assessment?*. Reprod. Domest. Anim. 2006, 41, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Ramón, M.; Pérez-Guzmán, M.D.; Jiménez-Rabadán, P.; Esteso, M.C.; García-Álvarez, O.; Maroto-Morales, A.; Anel-López, L.; Soler, A.J.; Fernández-Santos, M.R.; Garde, J.J. Sperm Cell Population Dynamics in Ram Semen during the Cryopreservation Process. PLoS ONE 2013, 8, e59189. [Google Scholar] [CrossRef]
- Thurston, L.M.; Watson, P.F.; Mileham, A.J.; Holt, W.V. Morphologically distinct sperm subpopulations defined by Fourier shape descriptors in fresh ejaculates correlate with variation in boar semen quality following cryopreservation. J. Androl. 2018, 22, 382–394. [Google Scholar]
- Barroso, G.; Alvarez, A.; Valdespin, C. Sperm Flow Cytometry: Beyond Human Fertilization and Embryo Development. In Flow Cytometry—Select Topics; InTech: London, UK, 2016. [Google Scholar]
- Marchiani, S.; Tamburrino, L.; Olivito, B.; Betti, L.; Azzari, C.; Forti, G.; Baldi, E.; Muratori, M. Characterization and sorting of flow cytometric populations in human semen. Andrology 2014, 2, 394–401. [Google Scholar] [CrossRef]
- Love, C.C.; Thompson, J.A.; Brinsko, S.P.; Rigby, S.L.; Blanchard, T.L.; Lowry, V.K.; Varner, D.D. Relationship between stallion sperm motility and viability as detected by two fluorescence staining techniques using flow cytometry. Theriogenology 2003, 60, 1127–1138. [Google Scholar] [CrossRef]
- Barrier Battut, I.; Kempfer, A.; Becker, J.; Lebailly, L.; Camugli, S.; Chevrier, L. Development of a new fertility prediction model for stallion semen, including flow cytometry. Theriogenology 2016, 86, 1111–1131. [Google Scholar] [CrossRef] [PubMed]
- Peña, F.J.; Ball, B.A.; Squires, E.L. A New Method for Evaluating Stallion Sperm Viability and Mitochondrial Membrane Potential in Fixed Semen Samples. Cytom. Part B Clin. Cytom. 2018, 94, 302–311. [Google Scholar] [CrossRef]
- Torres, M.A.; Díaz, R.; Boguen, R.; Martins, S.M.M.K.; Ravagnani, G.M.; Leal, D.F.; de Lima Oliveira, M.; Muro, B.B.D.; Parra, B.M.; Meirelles, F.V.; et al. Novel Flow Cytometry Analyses of Boar Sperm Viability: Can the Addition of Whole Sperm-Rich Fraction Seminal Plasma to Frozen-Thawed Boar Sperm Affect It? PLoS ONE 2016, 11, e0160988. [Google Scholar] [CrossRef]
- Boe-Hansen, G.B. An update on boar semen assessments by flow cytometry and CASA. Theriogenology 2019, 137, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Pastor, F.; Mata-Campuzano, M.; Álvarez-Rodríguez, M.; Álvarez, M.; Anel, L.; De Paz, P. Probes and Techniques for Sperm Evaluation by Flow Cytometry. Reprod. Domest. Anim. 2010, 45, 67–78. [Google Scholar] [CrossRef]
- Hossain, M.S.; Johannisson, A.; Wallgren, M.; Nagy, S.; Siqueira, A.P.; Rodriguez-Martinez, H. Flow cytometry for the assessment of animal sperm integrity and functionality: State of the art. Asian J. Androl. 2011, 13, 406–419. [Google Scholar] [CrossRef]
- Aitken, R.J.; Baker, M.A. Causes and consequences of apoptosis in spermatozoa; contributions to infertility and impacts on development. Int. J. Dev. Biol. 2013, 57, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Wagner, H.; Cheng, J.W.; Ko, E.Y. Role of reactive oxygen species in male infertility: An updated review of literature. Arab J. Urol. 2018, 16, 35–43. [Google Scholar] [CrossRef]
- Peña, F.J.; O’Flaherty, C.; Ortiz Rodríguez, J.M.; Martín Cano, F.E.; Gaitskell-Phillips, G.L.; Gil, M.C.; Ferrusola, C.O. Redox regulation and oxidative stress: The particular case of the stallion spermatozoa. Antioxidants 2019, 8, 567. [Google Scholar] [CrossRef]
- Gibb, Z.; Lambourne, S.R.; Aitken, R.J. The Paradoxical Relationship Between Stallion Fertility and Oxidative Stress1. Biol. Reprod. 2014, 91. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Majzoub, A.; Agarwal, A. Oxidative stress and sperm function: A systematic review on evaluation and management. Arab J. Urol. 2019, 17, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Roychoudhury, S.; Bjugstad, K.B.; Cho, C.L. Oxidation-reduction potential of semen: What is its role in the treatment of male infertility? Ther. Adv. Urol. 2016, 8, 302–318. [Google Scholar] [CrossRef]
- Agarwal, A.; Gupta, S.; Sharma, R. Oxidation–Reduction Potential Measurement in Ejaculated Semen Samples. In Andrological Evaluation of Male Infertility; Springer International Publishing: Cham, Switzerland, 2016; pp. 165–170. [Google Scholar]
- Stagos, D.; Goutzourelas, N.; Bar-Or, D.; Ntontou, A.M.; Bella, E.; Becker, A.T.; Statiri, A.; Kafantaris, I.; Kouretas, D. Application of a new oxidation-reduction potential assessment method in strenuous exercise-induced oxidative stress. Redox Rep. 2015, 20, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Rodriguez, J.M.; da Silva, C.B.; Masot, J.; Redondo, E.; Gazquez, A.; Tapia, J.A.; Gil, C.; Ortega-Ferrusola, C.; Peña, F.J. Rosiglitazone in the thawing medium improves mitochondrial function in stallion spermatozoa through regulating Akt phosphorylation and reduction of caspase 3. PLoS ONE 2019, 14, e0211994. [Google Scholar] [CrossRef] [PubMed]
- Alahmar, A.T. Role of Oxidative Stress in Male Infertility: An Updated Review. J. Hum. Reprod. Sci. 2019, 12, 4–18. [Google Scholar] [CrossRef] [PubMed]
- Ryu, D.Y.; Song, W.H.; Pang, W.K.; Yoon, S.J.; Rahman, M.S.; Pang, M.G. Freezability biomarkers in bull epididymal spermatozoa. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Marti, E.; Marti, J.I.; Muiño-Blanco, T.; Cebrián-Pérez, J.A. Effect of the cryopreservation process on the activity and immunolocalization of antioxidant enzymes in ram spermatozoa. J. Androl. 2008, 29, 459–467. [Google Scholar] [CrossRef]
- Sarsaifi, K.; Haron, A.W.; Vejayan, J.; Yusoff, R.; Hani, H.; Omar, M.A.; Hong, L.W.; Yimer, N.; Ying Ju, T.; Othman, A.M. Two-dimensional polyacrylamide gel electrophoresis of Bali bull (Bos javanicus) seminal plasma proteins and their relationship with semen quality. Theriogenology 2015, 84, 956–968. [Google Scholar] [CrossRef] [PubMed]
- Ugur, M.R.; Saber Abdelrahman, A.; Evans, H.C.; Gilmore, A.A.; Hitit, M.; Arifiantini, R.I.; Purwantara, B.; Kaya, A.; Memili, E. Advances in Cryopreservation of Bull Sperm. Front. Vet. Sci. 2019, 6, 268. [Google Scholar] [CrossRef]
- Wang, P.; Wang, Y.F.; Wang, H.; Wang, C.W.; Zan, L.-S.; Hu, J.H.; Li, Q.W.; Jia, Y.H.; Ma, G.J. HSP90 expression correlation with the freezing resistance of bull sperm. Zygote 2014, 22, 239–245. [Google Scholar] [CrossRef]
- Holt, W.V.; Del Valle, I.; Fazeli, A. Heat shock protein A8 stabilizes the bull sperm plasma membrane during cryopreservation: Effects of breed, protein concentration, and mode of use. Theriogenology 2015, 84, 693–701. [Google Scholar] [CrossRef]
- Mostek, A.; Dietrich, M.A.; Słowińska, M.; Ciereszko, A. Cryopreservation of bull semen is associated with carbonylation of sperm proteins. Theriogenology 2017, 92, 95–102. [Google Scholar] [CrossRef]
- Einspanier, R.; Krause, I.; Calvete, J.J.; Töfper-Petersen, E.; Klostermeyer, H.; Karg, H. Bovine seminal plasma ASFP: Localization of disulfide bridges and detection of three different isoelectric forms. FEBS Lett. 1994, 344, 61–64. [Google Scholar] [CrossRef]
- Peris-Frau, P.; Martín-Maestro, A.; Iniesta-Cuerda, M.; Sánchez-Ajofrín, I.; Mateos-Hernández, L.; Garde, J.J.; Villar, M.; Soler, A.J. Freezing-thawing procedures remodel the proteome of ram sperm before and after in vitro capacitation. Int. J. Mol. Sci. 2019, 20, 4596. [Google Scholar] [CrossRef]
- Peris-Frau, P.; Soler, A.J.; Iniesta-Cuerda, M.; Martín-Maestro, A.; Sánchez-Ajofrín, I.; Medina-Chávez, D.A.; Fernández-Santos, M.R.; García-Álvarez, O.; Maroto-Morales, A.; Montoro, V.; et al. Sperm Cryodamage in Ruminants: Understanding the Molecular Changes Induced by the Cryopreservation Process to Optimize Sperm Quality. Int. J. Mol. Sci. 2020, 21, 2781. [Google Scholar] [CrossRef]
- Fang, X.; Huang, L.-L.L.; Xu, J.; Ma, C.-Q.Q.; Chen, Z.-H.H.; Zhang, Z.; Liao, C.-H.H.; Zheng, S.-X.X.; Huang, P.; Xu, W.-M.M.; et al. Proteomics and single-cell RNA analysis of Akap4-knockout mice model confirm indispensable role of Akap4 in spermatogenesis. Dev. Biol. 2019, 454, 118–127. [Google Scholar] [CrossRef]
- Blommaert, D.; Sergeant, N.; Delehedde, M.; Jouy, N.; Mitchell, V.; Franck, T.; Donnay, I.; Lejeune, J.P.P.; Serteyn, D. Expression, localization, and concentration of A-kinase anchor protein 4 (AKAP4) and its precursor (proAKAP4) in equine semen: Promising marker correlated to the total and progressive motility in thawed spermatozoa. Theriogenology 2019, 131, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Kwon, W.-S.; Oh, S.-A.; Kim, Y.-J.; Rahman, M.S.; Park, Y.-J.; Pang, M.-G. Proteomic approaches for profiling negative fertility markers in inferior boar spermatozoa. Sci. Rep. 2015, 5, 13821. [Google Scholar] [CrossRef] [PubMed]
- Rahamim Ben-Navi, L.; Almog, T.; Yao, Z.; Seger, R.; Naor, Z. A-Kinase Anchoring Protein 4 (AKAP4) is an ERK1/2 substrate and a switch molecule between cAMP/PKA and PKC/ERK1/2 in human spermatozoa. Sci. Rep. 2016, 6, 37922. [Google Scholar] [CrossRef]
- Nixon, B.; Bernstein, I.R.; Cafe, S.L.; Delehedde, M.; Sergeant, N.; Anderson, A.L.; Trigg, N.A.; Eamens, A.L.; Lord, T.; Dun, M.D.; et al. A Kinase Anchor Protein 4 Is Vulnerable to Oxidative Adduction in Male Germ Cells. Front. Cell Dev. Biol. 2019, 7, 319. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Somanath, P.R.; Chakrabarti, R.; Eddy, E.M.; Vijayaraghavan, S. Changes in Intracellular Distribution and Activity of Protein Phosphatase PP1γ2 and Its Regulating Proteins in Spermatozoa Lacking AKAP41. Biol. Reprod. 2005, 72, 384–392. [Google Scholar] [CrossRef]
- Pereira, R.; Sá, R.; Barros, A.; Sousa, M. Major regulatory mechanisms involved in sperm motility. Asian J. Androl. 2015, 19, 5. [Google Scholar] [CrossRef] [PubMed]
- Carrera, A.; Moos, J.; Ning, X.P.; Gerton, G.L.; Tesarik, J.; Kopf, G.S.; Moss, S.B. Regulation of Protein Tyrosine Phosphorylation in Human Sperm by a Calcium/Calmodulin-Dependent Mechanism: Identification of A Kinase Anchor Proteins as Major Substrates for Tyrosine Phosphorylation. Dev. Biol. 1996, 180, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Delehedde, M.; Briand-Amirat, L.; Bencharif, D.; Delehedde, M. The Sperm Specific Protein Proakap4 as an Innovative Marker to Evaluate Sperm Quality and Fertility. J. Dairy Vet. Sci. 2019, 11, 1–7. [Google Scholar] [CrossRef]
- Peddinti, D.; Nanduri, B.; Kaya, A.; Feugang, J.M.; Burgess, S.C.; Memili, E. Comprehensive proteomic analysis of bovine spermatozoa of varying fertility rates and identification of biomarkers associated with fertility. BMC Syst. Biol. 2008, 2, 19. [Google Scholar] [CrossRef]
- Soggiu, A.; Piras, C.; Hussein, H.A.; De Canio, M.; Gaviraghi, A.; Galli, A.; Urbani, A.; Bonizzi, L.; Roncada, P. Unravelling the bull fertility proteome. Mol. Biosyst. 2013, 9, 1188. [Google Scholar] [CrossRef] [PubMed]
- Légaré, C.; Akintayo, A.; Blondin, P.; Calvo, E.; Sullivan, R. Impact of male fertility status on the transcriptome of the bovine epididymis. MHR Basic Sci. Reprod. Med. 2017, 23, 355–369. [Google Scholar] [CrossRef] [PubMed]
- Ruelle, I.; Seregeant, N.; Bencharif, D.; Charreaux, F.; Thorin, C.; Michaud, S.; Dordas-Perpinyà, M.; Jouy, N.; Audry, S.; Maurage, C.; et al. 145 ProAKAP4 concentrations in semen as a predictive tool of bull fertility: A preliminary study. Reprod. Fertil. Dev. 2020, 32, 199. [Google Scholar] [CrossRef]
- Mata-Campuzano, M.; Álvarez-Rodríguez, M.; Álvarez, M.; Anel, L.; de Paz, P.; Garde, J.; Martínez-Pastor, F. Effect of Several Antioxidants on Thawed Ram Spermatozoa Submitted to 37 °C up to Four Hours. Reprod. Domest. Anim. 2012, 47, 907–914. [Google Scholar] [CrossRef]
- Donovan, A.; Hanrahan, J.P.; Kummen, E.; Duffy, P.; Boland, M.P. Fertility in the ewe following cervical insemination with fresh or frozen-thawed semen at a natural or synchronised oestrus. Anim. Reprod. Sci. 2004, 84, 359–368. [Google Scholar] [CrossRef]
- Kershaw, C.M.; Khalid, M.; McGowan, M.R.; Ingram, K.; Leethongdee, S.; Wax, G.; Scaramuzzi, R.J. The anatomy of the sheep cervix and its influence on the transcervical passage of an inseminating pipette into the uterine lumen. Theriogenology 2005, 64, 1225–1235. [Google Scholar] [CrossRef]
- Donnelly, E.T.; McClure, N.; Lewis, S.E. Glutathione and hypotaurine in vitro: Effects on human sperm motility, DNA integrity and production of reactive oxygen species. Mutagenesis 2000, 15, 61–68. [Google Scholar] [CrossRef]
- Aprioku, J.S. Pharmacology of free radicals and the impact of reactive oxygen species on the testis. J. Reprod. Infertil. 2013, 14, 158–172. [Google Scholar]
- Agarwal, A.; Qiu, E.; Sharma, R. Laboratory assessment of oxidative stress in semen. Arab J. Urol. 2018, 16, 77–86. [Google Scholar] [CrossRef]
- Palmieri, B.; Sblendorio, V. Current Status of Measuring Oxidative Stress; Humana Press: Totowa, NJ, USA, 2010; pp. 3–17. [Google Scholar]
- Ortiz-Rodriguez, J.M.; Martín-Cano, F.E.; Ortega-Ferrusola, C.; Masot, J.; Redondo, E.; Gázquez, A.; Gil, M.C.; Aparicio, I.M.; Rojo-Domínguez, P.; Tapia, J.A.; et al. The incorporation of cystine by the soluble carrier family 7 member 11 (SLC7A11) is a component of the redox regulatory mechanism in stallion spermatozoa†. Biol. Reprod. 2019, 101, 208–222. [Google Scholar] [CrossRef] [PubMed]
- Davila, M.P.; Muñoz, P.M.; Tapia, J.A.; Ferrusola, C.O.; Da Silva, C.C.B.; Peña, F.J.; Plaza Davila, M.; Martin Muñoz, P.; Tapia, J.A.; Ortega Ferrusola, C.; et al. Inhibition of mitochondrial complex I leads to decreased motility and membrane integrity related to increased hydrogen peroxide and reduced ATP production, while the inhibition of glycolysis has less impact on sperm motility. PLoS ONE 2015, 10, e0138777. [Google Scholar] [CrossRef]
- Peña, F.J.; Ortega Ferrusola, C.; Martín Muñoz, P. New flow cytometry approaches in equine andrology. Theriogenology 2016, 86, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Bromfield, E.G.; Aitken, R.J.; Anderson, A.L.; McLaughlin, E.A.; Nixon, B. The impact of oxidative stress on chaperone-mediated human sperm–egg interaction. Hum. Reprod. 2015, 30, 2597–2613. [Google Scholar] [CrossRef] [PubMed]
- Redgrove, K.A.; Nixon, B.; Baker, M.A.; Hetherington, L.; Baker, G.; Liu, D.-Y.; Aitken, R.J. The Molecular Chaperone HSPA2 Plays a Key Role in Regulating the Expression of Sperm Surface Receptors That Mediate Sperm-Egg Recognition. PLoS ONE 2012, 7, e50851. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
F. Riesco, M.; Anel-Lopez, L.; Neila-Montero, M.; Palacin-Martinez, C.; Montes-Garrido, R.; Alvarez, M.; de Paz, P.; Anel, L. ProAKAP4 as Novel Molecular Marker of Sperm Quality in Ram: An Integrative Study in Fresh, Cooled and Cryopreserved Sperm. Biomolecules 2020, 10, 1046. https://doi.org/10.3390/biom10071046
F. Riesco M, Anel-Lopez L, Neila-Montero M, Palacin-Martinez C, Montes-Garrido R, Alvarez M, de Paz P, Anel L. ProAKAP4 as Novel Molecular Marker of Sperm Quality in Ram: An Integrative Study in Fresh, Cooled and Cryopreserved Sperm. Biomolecules. 2020; 10(7):1046. https://doi.org/10.3390/biom10071046
Chicago/Turabian StyleF. Riesco, Marta, Luis Anel-Lopez, Marta Neila-Montero, Cristina Palacin-Martinez, Rafael Montes-Garrido, Mercedes Alvarez, Paulino de Paz, and Luis Anel. 2020. "ProAKAP4 as Novel Molecular Marker of Sperm Quality in Ram: An Integrative Study in Fresh, Cooled and Cryopreserved Sperm" Biomolecules 10, no. 7: 1046. https://doi.org/10.3390/biom10071046
APA StyleF. Riesco, M., Anel-Lopez, L., Neila-Montero, M., Palacin-Martinez, C., Montes-Garrido, R., Alvarez, M., de Paz, P., & Anel, L. (2020). ProAKAP4 as Novel Molecular Marker of Sperm Quality in Ram: An Integrative Study in Fresh, Cooled and Cryopreserved Sperm. Biomolecules, 10(7), 1046. https://doi.org/10.3390/biom10071046





