The Regulatory Role of IL-10 in Neurodegenerative Diseases
Abstract
1. Introduction
2. Biological Activity of IL-10
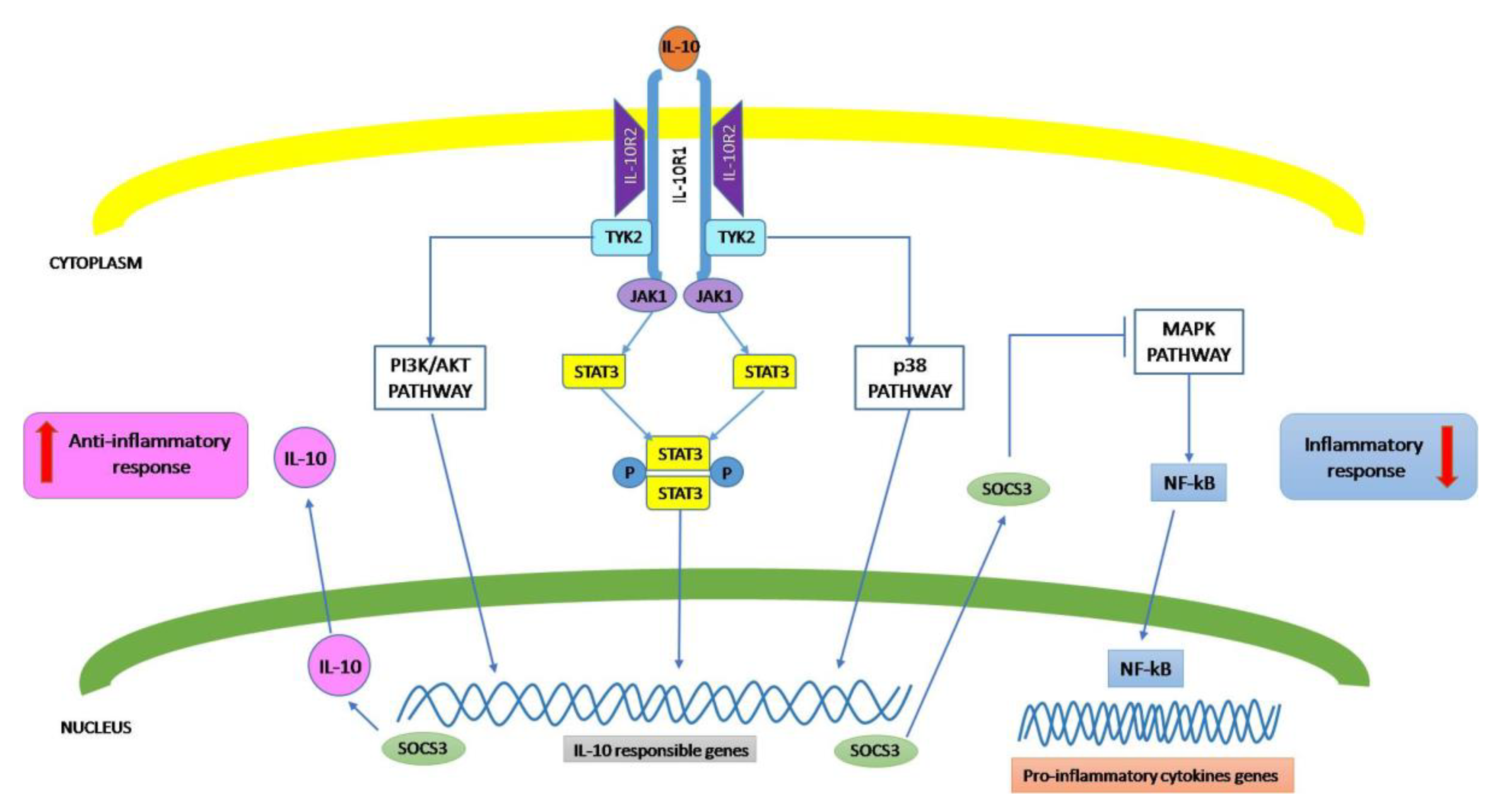
3. Signal Transduction Pathway of IL-10
4. IL-10 in the Brain
5. IL-10 in Brain Diseases
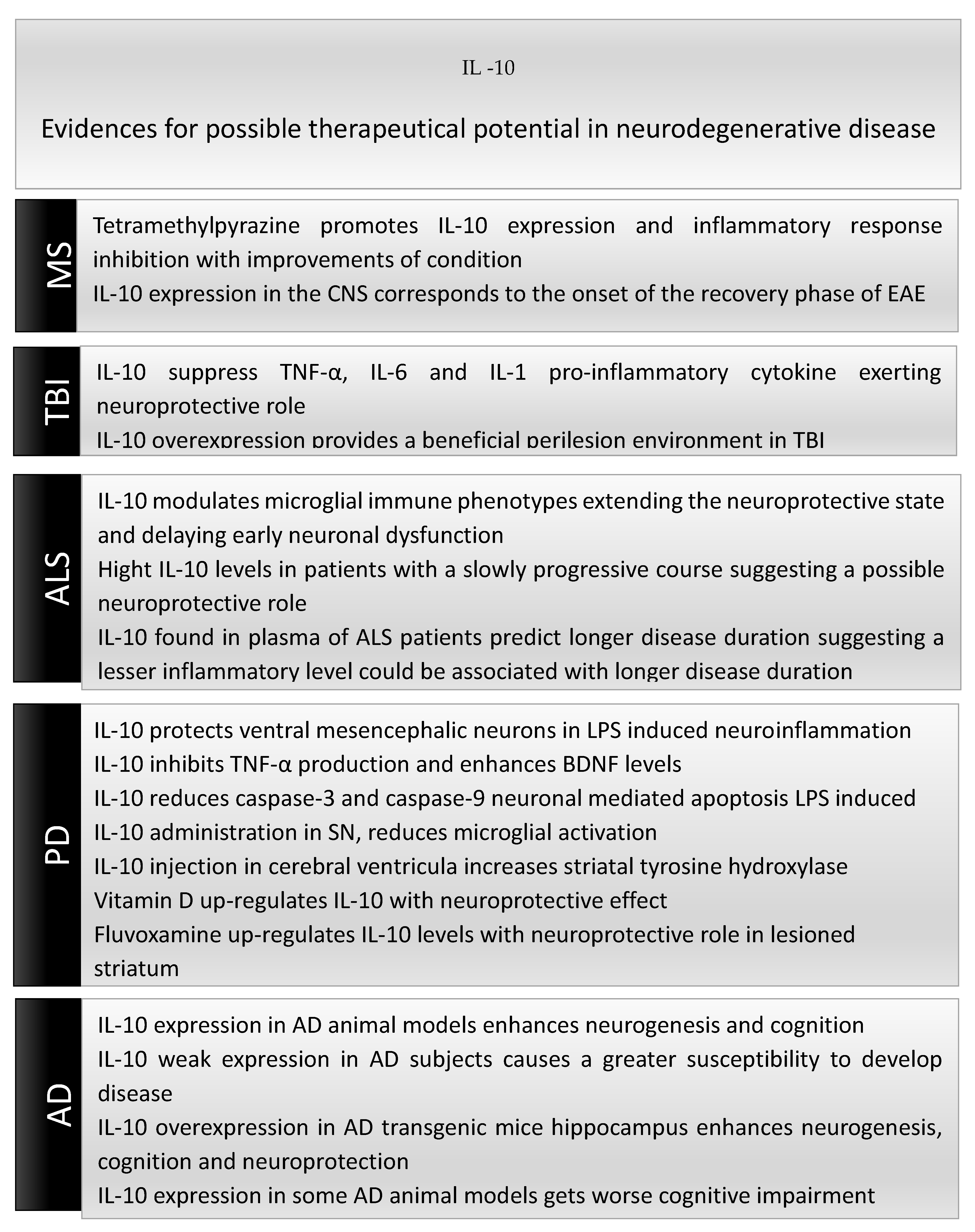
6. Therapeutic Potential of IL-10-Based Therapies in Neurodegenerative Diseases
7. Conclusions
Funding
Conflicts of Interest
References
- Mosser, D.M.; Zhang, X. Interleukin-10: New perspectives on an old cytokine. Immunol. Rev. 2008, 226, 205–218. [Google Scholar] [CrossRef]
- Fiorentino, D.F.; Bond, M.W.; Mosmann, T.R. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J. Exp. Med. 1989, 170, 2081–2095. [Google Scholar]
- Moore, K.W.; Vieira, P.; Fiorentino, D.F.; Trounstine, M.L.; Khan, T.A.; Mosmann, T.R. Homology of cytokine synthesis inhibitory factor (IL-10) to the Epstein-Barr virus gene BCRFI. Science 1990, 248, 1230–1234. [Google Scholar] [CrossRef] [PubMed]
- Roncarolo, M.G.; Gregori, S.; Battaglia, M.; Bacchetta, R.; Fleischhauer, K.; Levings, M.K. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol. Rev. 2006, 212, 28–50. [Google Scholar] [CrossRef] [PubMed]
- Patterson, Z.R.; Holahan, M.R. Understanding the neuroinflammatory response following concussion to develop treatment strategies. Front. Cell. Neurosci. 2012, 6, 58. [Google Scholar]
- Ziebell, J.M.; Morganti-Kossmann, M.C. Involvement of pro- and anti-inflammatory cytokines and chemokines in the pathophysiology of traumatic brain injury. Neurotherapeutics 2010, 7, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Strle, K.; Zhou, J.H.; Broussard, S.R.; Venters, H.D.; Johnson, R.W.; Freund, G.G.; Dantzer, R.; Kelley, K.W. IL-10 promotes survival of microglia without activating Akt. J. Neuroimmunol. 2002, 122, 9–19. [Google Scholar] [CrossRef]
- Eskdale, J.; Kube, D.; Tesch, H.; Gallagher, G. Mapping of the human IL10 gene and further characterization of the 5’ flanking sequence. Immunogenetics 1997, 46, 120–128. [Google Scholar] [CrossRef]
- Saraiva, M.; Christensen, J.R.; Veldhoen, M.; Murphy, T.L.; Murphy, K.M.; O’Garra, A. Interleukin-10 production by Th1 cells requires interleukin-12-induced STAT4 transcription factor and ERK MAP kinase activation by high antigen dose. Immunity 2009, 31, 209–219. [Google Scholar] [CrossRef]
- Itakura, E.; Huang, R.R.; Wen, D.R.; Paul, E.; Wünsch, P.H.; Cochran, A.J. IL-10 expression by primary tumor cells correlates with melanoma progression from radial to vertical growth phase and development of metastatic competence. Mod. Pathol. 2011, 24, 801–809. [Google Scholar] [CrossRef]
- Koya, T.; Matsuda, H.; Takeda, K.; Matsubara, S.; Miyahara, N.; Balhorn, A.; Dakhama, A.; Gelfand, E.W. IL-10-treated dendritic cells decrease airway hyperresponsiveness and airway inflammation in mice. J. Allergy Clin. Immunol. 2007, 119, 1241–1250. [Google Scholar] [CrossRef] [PubMed]
- Batista, M.L., Jr.; Lopes, R.D.; Seelaender, M.C.; Lopes, A.C. Anti-inflammatory effect of physical training in heart failure: Role of TNF-alpha and IL-10. Arq. Bras. Cardiol. 2009, 93, 643–651. [Google Scholar] [PubMed]
- de Waal Malefyt, R.; Abrams, J.; Bennett, B.; Figdor, C.G.; de Vries, J.E. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: An autoregulatory role of IL-10 produced by monocytes. J. Exp. Med. 1991, 174, 1209–1220. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.W.; de Waal Malefyt, R.; Coffman, R.L.; O’Garra, A. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 2001, 19, 683–765. [Google Scholar] [CrossRef] [PubMed]
- Ho, A.S.; Liu, Y.; Khan, T.A.; Hsu, D.H.; Bazan, J.F.; Moore, K.W. A receptor for interleukin-10 is related to interferon receptors. Proc. Natl. Acad. Sci. USA 1993, 901, 1267–1271. [Google Scholar] [CrossRef] [PubMed]
- Kotenko, S.V.; Krause, C.D.; Izotova, L.S.; Pollack, B.P.; Wu, W.; Pestka, S. Identification and functional characterization of a second chain of the interleukin-10 receptor complex. EMBO J. 1997, 16, 5894–5903. [Google Scholar] [CrossRef]
- Spencer, S.D.; Di Marco, F.; Hooley, J.; Pitts-Meek, S.; Bauer, M.; Ryan, A.M.; Sordat, B.; Gibbs, V.C.; Aguet, M. The orphan receptor CRF2–4 is an essential subunit of the interleukin 10 receptor. J. Exp. Med. 1998, 187, 571–578. [Google Scholar] [CrossRef]
- Schülke, S. Induction of Interleukin-10 producing dendritic Cells as a Tool to suppress allergen-specific T helper 2 responses. Front. Immunol. 2018, 9, 455. [Google Scholar] [CrossRef]
- Berti, F.C.B.; Pereira, A.P.L.; Cebinelli, G.C.M.; Trugilo, K.P.; Brajão de Oliveira, K. The role of interleukin 10 in human papilloma virus infection and progres-sion to cervical carcinoma. Cytokine Growth Factor Rev. 2017, 34, 1–13. [Google Scholar] [CrossRef]
- Rodig, S.J.; Meraz, M.A.; White, J.M.; Lampe, P.A.; Riley, J.K.; Arthur, C.D.; King, K.L.; Sheehan, K.C.F.; Yin, L.; Pennica, D.; et al. Disruption of the Jak1 gene demonstrates obligatory and nonredundant roles of the Jaks in cytokine-induced biologic responses. Cell 1998, 93, 373–383. [Google Scholar] [CrossRef]
- Berlato, C.; Cassatella, M.A.; Kinjyo, I.; Gatto, L.; Yoshimura, A.; Bazzoni, F. Involvement of suppressor of cytokine signaling-3 as a mediator of the inhib-itory effects of IL-10 on lipopolysaccharide-induced macrophage activation. J. Immunol. 2002, 168, 6404–6411. [Google Scholar] [CrossRef]
- Cheng, S.B.; Sharma, S. Interleukin-10: A pleiotropic regulator in pregnancy. Am. J. Reprod. Immunol. 2015, 73, 487–500. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.; Bradley, L.; Smith, A.; Foxwell, B. Signal transducer and activator of transcription 3 is the dominant mediator of the anti-inflammatory effects of IL-10 in human macrophages. J. Immunol. 2004, 172, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.S.; Chau, L.Y. Heme oxygenase-1 mediates the anti-inflammatory effect of interleukin-10 in mice. Nat. Med. 2002, 8, 240–246. [Google Scholar] [CrossRef]
- Otterbein, L.E.; Bach, F.H.; Alam, J.; Soares, M.; Tao Lu, H.; Wysk, M.; Davis, R.J.; Flavell, R.A.; Choi, A.M.K. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat. Med. 2000, 6, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Melillo, J.A.; Song, L.; Bhagat, G.; Blazquez, A.B.; Plumlee, C.R.; Lee, C.; Berin, C.; Reizis, B.; Schindler, C. Dendritic cell (DC)-specific targeting reveals Stat3 as a negative regulator of DC function. J. Immunol. 2010, 184, 2638–2645. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.M.; Sarma, U.; Willets, K.; Smallie, T.; Brennan, F.; Foxwell, B.M. Expression of constitutively active STAT3 can replicate the cytokinesuppressive activity of interleukin-10 in human primary macrophages. J. Biol. Chem. 2007, 282, 6965–6975. [Google Scholar] [CrossRef] [PubMed]
- Kortylewski, M.; Kujawski, M.; Wang, T.; Wei, S.; Zhang, S.; Pilon-Thomas, S.; Niu, G.; Kay, H.; Mulé, J.; Kerr, W.J.; et al. Inhibiting Stat3 signaling in the hematopoietic system elicits multicomponent antitumor immunity. Nat. Med. 2005, 11, 1314–1321. [Google Scholar] [CrossRef]
- Hu, D.; Wan, L.; Chen, M.; Caudle, Y.; LeSage, G.; Li, Q.; Yin, D. Essential role of IL-10/STAT3 in chronic stress-induced immune suppression. Brain Behav. Immun. 2014, 36, 118–127. [Google Scholar] [CrossRef]
- Olson, J.K.; Miller, S.D. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J. Immunol. 2004, 173, 3916–3924. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Dissing-Olesen, L.; MacVicar, B.A.; Stevens, B. Microglia: Dynamic mediators of synapse development and plasticity. Trends Immunol. 2015, 36, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.; Miller-Fleming, L.; Pais, T.F. Microglia and inflammation: Conspiracy, controversy or control? Cell. Mol. Life Sci. 2014, 71, 3969–3985. [Google Scholar] [CrossRef] [PubMed]
- Park, K.W.; Lee, H.G.; Jin, B.K.; Lee, Y.B. Interleukin-10 endogenously expressed in microglia prevents lipopolysaccharide-induced neurodegeneration in the rat cerebral cortex in vivo. Exp. Mol. Med. 2007, 39, 812–819. [Google Scholar] [CrossRef] [PubMed]
- Rasley, A.; Tranguch, S.L.; Rati, D.M.; Marriott, I. Murine glia express the immunosuppressive cytokine, interleukin-10, following exposure to Borrelia burgdorferi or Neisseria meningitidis. Glia 2006, 53, 583–592. [Google Scholar] [CrossRef]
- Cianciulli, A.; Dragone, T.; Calvello, R.; Porro, C.; Trotta, T.; Lofrumento, D.D.; Panaro, M.A. IL-10 plays a pivotal role in anti-inflammatory effects of resveratrol in activated microglia cells. Int. Immunopharmacol. 2015, 24, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Murgas, Y.M.; Skar, G.; Ramirez, D.; Beaver, M.; Snowden, J.N. IL-10 plays an important role in the control of inflammation but not in the bacterial burden in S. epidermidis CNS catheter infection. J. Neuroinflamm. 2016, 13, 271. [Google Scholar] [CrossRef]
- Martin, N.M.; Griffin, D.E. Interleukin-10 modulation of virus clearance and disease in mice with alphaviral encephalomyelitis. J. Virol. 2017, 92. [Google Scholar] [CrossRef] [PubMed]
- Peferoen, L.; Kipp, M.; van der Valk, P.; van Noort, J.M.; Amor, S. Oligodendrocytemicroglia cross-talk in the central nervous system. Immunology 2014, 141, 302–313. [Google Scholar] [CrossRef]
- Boyd, Z.S.; Kriatchko, A.; Yang, J.; Agarwal, N.; Wax, M.B.; Patil, R.V. Interleukin-10 receptor signaling through STAT-3 regulates the apoptosis of retinal ganglion cells in response to stress. Investig. Ophthalmol. Vis. Sci. 2003, 44, 5206–5211. [Google Scholar] [CrossRef]
- Milligan, E.D.; Penzkover, K.R.; Soderquist, R.G.; Mahoney, M.J. Spinal Interleukin-10 therapy to treat peripheral neuropathic pain. Neuromodulation 2012, 15, 520–526. [Google Scholar] [CrossRef]
- Martinez Doncel, A.; Rubio, A.; Arroyo, R.; de las Heras, V.V.; Martín, C.; Fernandez-Arquero, M.; de la Conchaa, E.G. Interleukin-10 polymorphisms in spanish multiple sclerosis patients. J. Neuroimmunol. 2002, 131, 168–172. [Google Scholar] [CrossRef]
- Myhr, K.M.; Vågnes, K.S.; Marøy, T.H.; Aarseth, J.H.; Nyland, H.I.; Vedeler, C.A. Interleukin-10 promoter polymorphisms in patients with multiple sclerosis. J. Neurol. Sci. 2002, 202, 93–97. [Google Scholar] [CrossRef]
- O’Neill, E.J.; Day, M.J.; Wraith, D.C. IL-10 is essential for disease protection following intranasal peptide administration in the C57BL/6 model of EAE. J. Neuroimmunol. 2006, 178, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Klose, J.; Schmidt, N.O.; Melms, A.; Dohi, M.; Miyazaki, J.; Bischof, F.; Greve, B. Suppression of experimental autoimmune encephalomyelitis by interleukin-10 transduced neural stem/progenitor cells. J. Neuroinflamm. 2013, 10, 17. [Google Scholar] [CrossRef] [PubMed]
- Yen, J.H.; Xu, S.; Park, Y.S.; Ganea, D.; Kim, K.C. Higher susceptibility to experimental autoimmune encephalomyelitis in Muc1-deficient mice is associated with increased Th1/Th17 responses. Brain Behav. Immun. 2013, 29, 70–81. [Google Scholar] [CrossRef]
- Hauser, S.L.; Waubant, E.; Arnold, D.L.; Vollmer, T.; Antel, J.; Fox, R.J.; Bar-Or, A.; Panzara, M.; Sarkar, N.; Agarwal, S.; et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N. Engl. J. Med. 2008, 358, 676–688. [Google Scholar] [CrossRef]
- Okada, Y.; Ochi, H.; Fujii, C.; Hashi, Y.; Hamatani, M.; Ashida, S.; Kawamura, K.; Kusaka, H.; Matsumoto, S.; Nakagawa, M.; et al. Signaling via toll-like receptor 4 and CD40 in B cells plays a regulatory role in the pathogenesis of multiple sclerosis through interleukin-10 production. J. Autoimmun. 2018, 88, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Lampropoulou, V.; Hoehlig, K.; Roch, T.; Neves, P.; Calderón Gómez, E.; Sweenie, C.H.; Hao, Y.; Freitas, A.A.; Steinhoff, U.; Anderton, S.M.; et al. TLR-activated B cells suppress T cell-mediated autoimmunity. J. Immunol. 2008, 180, 4763–4773. [Google Scholar] [CrossRef] [PubMed]
- Korniotis, S.; Gras, C.; Letscher, H.; Montandon, R.; Mégret, J.; Siegert, S.; Ezine, S.; Fallon, P.G.; Luther, S.A.; Fillatreau, S.; et al. Treatment of ongoing autoimmune encephalomyelitis with activated B-cell progenitors maturing into regulatory B cells. Nat. Commun. 2016, 7, 12134. [Google Scholar] [CrossRef] [PubMed]
- Yoshizaki, A.; Miyagaki, T.; DiLillo, D.J.; Matsushita, T.; Horikawa, M.; Kountikov, E.I.; Spolski, R.; Poe, J.C.; Leonard, W.J.; Tedder, T.F. Regulatory B cells control T-cell autoimmunity through IL-21-dependent cognate interactions. Nature 2012, 491, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.M.; Stillings, S.A.; Leclerc, J.L.; Phillips, H.; Edwards, N.J.; Robicsek, S.A.; Hoh, B.L.; Blackburn, S.; Sylvain Doré, S. Role of interleukin-10 in acute brain injuries. Front. Neurol. 2017, 8, 244. [Google Scholar] [CrossRef]
- Woodcock, T.; Morganti-Kossmann, M.C. The role of markers of inflammation in traumatic brain injury. Front. Neurol. 2013, 4, 18. [Google Scholar] [CrossRef]
- Li, Z.; Zeng, G.; Zheng, X.; Wang, W.; Ling, Y.; Tang, H.; Zhang, J. Neuroprotective effect of formononetin against TBI in rats via suppressing inflammatory reaction in cortical neurons. Biomed. Pharmacother. 2018, 106, 349–354. [Google Scholar] [CrossRef]
- Lagerstedt, L.; Egea-Guerrero, J.J.; Rodríguez-Rodríguez, A.; Bustamante, A.; Montaner, J.; El Rahal, A.; Andereggen, E.; Rinaldi, L.; Sarrafzadeh, A.; Schaller, K.; et al. Early measurement of interleukin-10 predicts the absence of CT scan lesions in mild traumatic brain injury. PLoS ONE 2018, 13, e0193278. [Google Scholar] [CrossRef]
- Schneider Soares, M.; Menezes de Souza, N.; Liborio Schwarzbold, M.; Paim Diaz, A.; Costa Nunes, J.; Hohl, A.; Walz, R. Interleukin-10 is an independent biomarker of severe traumatic brain injury prognosis. Neuroimmunomodulation 2012, 19, 377–385. [Google Scholar] [CrossRef]
- Kirchhoff, C.; Buhmann, S.; Bogner, V.; Stegmaier, J.; Leidel, B.A.; Braunstein, V.; Mutschler, W.; Biberthaler, P. Cerebrospinal IL-10 concentration is elevated in non-survivors as compared to survivors after severe traumatic brain injury. Eur. J. Med. Res. 2008, 13, 464–468. [Google Scholar] [PubMed]
- Peruzzaro, S.T.; Andrews, M.M.M.; Al-Gharaibeh, A.; Pupiec, O.; Resk, M.; Story, D.; Maiti, P.; Rossignol, J.; Dunbar, G.L. Transplantation of mesenchymal stem cells genetically engineered to overexpress interleukin-10 promotes alternative inflammatory response in rat model of traumatic brain injury. J. Neuroinflamm. 2019, 16, 2. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.B.; Irvin, C.W.; Tilva, K.R.; Mitchell, C.S. State of the field: Aninformatics-based systematic review of the SOD1-G93A Amyotrophic Lateral Sclerosis transgenic mouse model. Amyotroph. Lateral Scler. Front. Degener. 2015, 17, 1–14. [Google Scholar] [CrossRef]
- Gravel, M.; Béland, L.C.; Soucy, G.; Abdelhamid, E.; Rahimian, R.; Gravel, C.; Kriz, J. IL-10 controls early microglial phenotypes and disease onset in als caused by misfolded superoxide dismutase 1. J. Neurosci. 2016, 36, 1031–1048. [Google Scholar] [CrossRef] [PubMed]
- Ciaramella, A.; Spalletta, G.; Bizzoni, F.; Salani, F.; Caltagirone, C.; Bossù, P. Effect of age on surface molecules and cytokine expression in human dendritic cells. Cell. Immunol. 2011, 269, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Obál, I.; Klausz, G.; Mándi, Y.; Deli, M.; Siklós, L.; Engelhardt, J.I. Intraperitoneally administered IgG from patients with amyotrophic lateral sclerosis or from an immune-mediated goat model increase the levels of TNF-α, IL-6, and IL-10 in the spinal cord and serum of mice. J. Neuroinflamm. 2016, 13, 121. [Google Scholar] [CrossRef]
- Furukawa, T.; Matsui, N.; Fujita, K.; Nodera, H.; Shimizu, F.; Miyamoto, K.; Takahashi, Y.; Kanda, T.; Kusunoki, S.; Izumi, Y.; et al. CSF cytokine profile distinguishes multifocal motor neuropathy from progressive muscular atrophy. Neurol. Neuroimmunol. Neuroinflamm. 2015, 2, e138. [Google Scholar] [CrossRef] [PubMed]
- Su, F.; Bai, F.; Zhang, Z. Inflammatory cytokines and Alzheimer’s disease: A review from the perspective of genetic polymorphisms. Neurosci. Bull. 2016, 32, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Ehrhart, J.; Smith, A.J.; Kuzmin-Nichols, N.; Zesiewicz, T.A.; Jahan, I.; Shytle, R.D.; Kim, S.-H.; Sanberg, C.D.; Vu, T.H.; Gooch, C.L.; et al. Humoral factors in ALS patients during disease progression. J. Neuroinflamm. 2015, 12, 127. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Günther, R.; Akgün, K.; Hermann, A.; Ziemssen, T. Peripheral proinflammatory Th1/Th17 immune cell shift is linked to disease severity in amyotrophic lateral sclerosis. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Long, J.M.; Holtzman, D.M. Alzheimer disease: An update on pathobiology and treatment strategies. Cell 2019, 179, 312–339. [Google Scholar] [CrossRef]
- Cisbani, G.; Koppel, A.; Knezevic, D.; Suridjan, I.; Mizrahi, R.; Bazinet, R.P. Peripheral cytokine and fatty acid associations with neuroinflammation in AD and AMCI Patients: An exploratory study. Brain Behav. Immun. 2020, 87, 679–688. [Google Scholar] [CrossRef]
- Baune, B.T.; Ponath, G.; Rothermundt, M.; Roesler, A.; Berger, K. Association between cytokines and cerebral MRI changes in the aging brain. J. Geriatr. Psychiatry Neurol. 2009, 22, 23–34. [Google Scholar] [CrossRef]
- Kiyota, T.; Ingraham, K.L.; Swan, R.J.; Jacobsen, M.T.; Andrews, S.J.; Ikezu, T. AAV serotype 2/1-mediated gene delivery of anti-inflammatory interleukin-10 enhances neurogenesis and cognitive function in APP+PS1 mice. Gene Ther. 2012, 19, 724–733. [Google Scholar] [CrossRef]
- Michaud, J.P.; Rivest, S. Anti-inflammatory signaling in microglia exacerbates Alzheimer’s disease-related pathology. Neuron 2015, 85, 450–452. [Google Scholar] [CrossRef]
- Guillot-Sestier, M.V.; Doty, K.R.; Gate, D.; Rodriguez, J., Jr.; Leung, B.P.; Rezai-Zadeh, K.; Town, T. Il10 deficiency rebalances innate immunity to mitigate Alzheimer-like pathology. Neuron 2015, 85, 534–548. [Google Scholar] [CrossRef] [PubMed]
- Bai, F.; Xie, C.; Yuan, Y.; Shi, Y.; Zhang, Z. Promoter haplotypes of interleukin-10 gene linked to cortex plasticity in subjects with risk of Alzheimer’s disease. Neuroimage Clin. 2017, 17, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Mun, M.J.; Kim, J.H.; Choi, J.Y.; Jang, W.C. Genetic polymorphisms of interleukin genes and the risk of Alzheimer’s disease: An update meta-analysis. Meta Gene 2016, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Panaro, M.A.; Cianciulli, A. Current opinions and perspectives on the role of immune system in the pathogenesis of Parkinson’s disease. Curr. Pharm. Des. 2012, 18, 200–208. [Google Scholar] [CrossRef]
- Kwilasz, A.J.; Grace, P.M.; Serbedzija, P.; Maier, S.F.; Watkins, L.R. The therapeutic potential of interleukin-10 in neuroimmune diseases. Neuropharmacology 2015, 96, 55–69. [Google Scholar] [CrossRef]
- Karpenko, M.N.; Vasilishina, A.A.; Gromova, E.A.; Muruzheva, Z.M.; Bernadotte, A. Interleukin-1β, interleukin-1 receptor antagonist, interleukin-6, interleukin-10, and tumor necrosis factor-α levels in CSF and serum in relation to the clinical diversity of Parkinson’s disease. Cell. Immunol. 2018, 327, 77–82. [Google Scholar] [CrossRef]
- Rentzos, M.; Nikolaou, C.; Andreadou, E.; Paraskevas, G.P.; Rombos, A.; Zoga, M.; Tsoutsou, A.; Boufidou, F.; Kapaki, E.; Vassilopoulos, D. Circulating interleukin-10 and interleukin-12 in Parkinson’s disease. Acta Neurol. Scand. 2009, 119, 332–337. [Google Scholar] [CrossRef]
- Menza, M.; Dobkin, R.D.; Marin, H.; Mark, M.H.; Gara, M.; Bienfait, K.; Dicke, A.; Kusnekov, A. The role of inflammatory cytokines in cognition and other non-motor symptoms of Parkinson’s disease. Psychosomatics 2010, 51, 474–479. [Google Scholar]
- Li, D.; Song, X.; Huang, H.; Huang, H.; Ye, Z. Association of Parkinson’s disease-related pain with plasma interleukin-1, interleukin-6, interleukin-10, and tumour necrosis factor-α. Neurosci. Lett. 2018, 683, 181–184. [Google Scholar] [CrossRef]
- Chu, K.; Zhou, X.; Luo, B.Y. Cytokine gene polymorphisms and Parkinson’s disease: A meta-analysis. Can. J. Neurol. Sci. 2012, 39, 58–64. [Google Scholar]
- Pascale, E.; Passarelli, E.; Purcaro, C.; Vestri, A.R.; Fakeri, A.; Guglielmi, R.; Passarelli, F.; Meco, G. Lack of association between IL-1beta, TNF-alpha, and IL-10 gene polymorphisms and sporadic Parkinson’s disease in an Italian cohort. Acta Neurol. Scand. 2011, 124, 176–181. [Google Scholar] [CrossRef]
- Li, D.; He, Q.; Li, R.; Xu, X.; Chen, B.; Xie, A. Interleukin-10 promoter polymorphisms in Chinese patients with Parkinson’s disease. Neurosci. Lett. 2012, 513, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.Y.; Zhang, S.P.; Cao, C.; Loh, Y.P.; Cheng, Y. Aberrations in peripheral inflammatory cytokine levels in Parkinson disease: A systematic review and meta-analysis. JAMA Neurol. 2016, 73, 1316–1324. [Google Scholar] [CrossRef] [PubMed]
- Williams-Gray, C.H.; Wijeyekoon, R.; Yarnall, A.J.; Lawson, R.A.; Breen, D.P.; Evans, J.R.; Cummins, G.A.; Duncan, G.W.; Khoo, T.K.; Burn, D.J.; et al. Serum immune markers and disease progression in an incident Parkinson’s disease cohort (ICICLE-PD). Mov. Disord. 2016, 31, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.; Kim, H.J.; Kim, A.; Jang, M.; Kim, A.; Kim, Y.; Yoo, D.; Im, J.H.; Choi, J.H.; Jeon, B. Peripheral blood inflammatory markers in early Parkinson’s disease. J. Clin. Neurosci. 2018, 58, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Richwine, A.F.; Sparkman, N.L.; Dilger, R.N.; Buchanan, J.B.; Johnson, R.W. Cognitive deficits in interleukin-10-deficient mice after peripheral injection of lipopolysaccharide. Brain Behav. Immun. 2009, 23, 794–802. [Google Scholar] [CrossRef]
- Henderson, V.W. Alzheimer’s disease: Review of hormone therapy trials and implications for treatment and prevention after menopause. J. Steroid Biochem. Mol. Biol. 2014, 14, 99–106. [Google Scholar] [CrossRef]
- Maki, P.M.; Henderson, V.W. Cognition and the menopause transition. Menopause 2016, 23, 803–805. [Google Scholar] [CrossRef] [PubMed]
- Bagyinszky, E.; Giau, V.V.; Shim, K.; Suk, K.; An, S.S.A.; Kim, S. Role of inflammatory molecules in the Alzheimer’s disease progression and diagnosis. J. Neurol. Sci. 2017, 376, 242–254. [Google Scholar] [CrossRef]
- Wiciński, M.; Wódkiewicz, E.; Słupski, M.; Walczak, M.; Socha, M.; Malinowski, B.; Pawlak-Osińska4, K. Neuroprotective activity of sitagliptin via reduction of neuroinflammation beyond the incretin effect: Focus on Alzheimer’s disease. Bio. Med. Res. Int. 2018, 6091014. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, Z.; Peng, Y.P.; Qiu, Y.H. Interleukin-10 inhibits neuroinflammation-mediated apoptosis of ventral mesencephalic neurons via JAK-STAT3 pathway. Int. Immunopharmacol. 2017, 50, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Arimoto, T.; Choi, D.Y.; Lu, X.; Liu, M.; Nguyen, X.V.; Zheng, N.; Stewart, C.A.; Kim, H.C.; Bing, G. Interleukin-10 protects against inflammation-mediated degeneration of dopaminergic neurons in substantia nigra. Neurobiol. Aging 2007, 28, 894–906. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Lin, W.; Lin, P.; Zheng, M.; Lai, Y.; Chen, M.; Zhang, Y.; Chen, J.; Lin, X.; Lin, L.; et al. IL-10 produces a dual effect on OGD-induced neuronal apoptosis of cultured cortical neurons via the NF-κB pathway. Aging 2019, 11, 10796. [Google Scholar] [CrossRef]
- Schwenkgrub, J.; Joniec-Maciejak, I.; Sznejder-Pacholek, A.; Wawer, A.; Ciesielska, A.; Bankiewicz, K.; Członkowska, A.; Członkowski, A. Effect of human interleukin-10 on the expression of nitric oxide synthases in the MPTP-based model of Parkinson’s disease. Pharmacol. Rep. 2013, 65, 44–49. [Google Scholar] [CrossRef]
- Calvello, R.; Cianciulli, A.; Nicolardi, G.; De Nuccio, F.; Giannotti, L.; Salvatore, R.; Porro, C.; Trotta, T.; Panaro, M.A.; Lofrumento, D.D. Vitamin D treatment attenuates neuroinflammation and dopaminergic neurodegeneration in an animal model of Parkinson’s Disease, shifting M1 to M2 microglia responses. J. Neuroimmune Pharmacol. 2017, 12, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Dallé, E.; Daniels, W.M.; Mabandla, M.V. Fluvoxamine maleate normalizes striatal neuronal inflammatory cytokine activity in a Parkinsonian rat model associated with depression. Behav. Brain Res. 2017, 316, 189–196. [Google Scholar] [CrossRef]
- Ping, Z.; Xiaomu, W.; Xufang, X.; Liang, S. Vinpocetine regulates levels of circulating TLRs in Parkinson’s disease patients. Neurol. Sci. 2019, 40, 113–120. [Google Scholar] [CrossRef]
- Hosseini, A.; Estiri, H.; Akhavan Niaki, H.; Alizadeh, A.; Abdolhossein Zadeh, B.; Ghaderian, S.M.H.; Farjadfar, A.; Fallah, A. Multiple sclerosis gene therapy with recombinant viral vectors: Overexpression of IL-4, leukemia inhibitory factor, and IL-10 in Wharton’s jelly stem cells used in EAE mice model. Cell J. 2017, 19, 361–374. [Google Scholar]
- Zorzella-Pezavento, S.F.; Chiuso-Minicucci, F.; França, T.G.; Ishikawa, L.L.; da Rosa, L.C.; Colavite, P.M.; Balbino, B.; Marques, C.; Ikoma, M.R.V.; Masson, A.P.; et al. pVAXhsp65 vaccination primes for high IL-10 production and decreases experimental encephalomyelitis severity. J. Immunol. Res. 2017, 6257958. [Google Scholar] [CrossRef]
- Bai, X.Y.; Wang, X.F.; Zhang, L.S.; Du, P.C.; Cao, Z.; Hou, Y. Tetramethylpyrazine ameliorates experimental autoimmune encephalomyelitis by modulating the inflammatory response. Biochem. Biophys. Res. Commun. 2018, 503, 1968–1972. [Google Scholar] [CrossRef]
- Pérez-de Puig, I.; Miró, F.; Salas-Perdomo, A.; Bonfill-Teixidor, E.; Ferrer-Ferrer, M.; Márquez-Kisinousky, L.; Planas, A.M. IL-10 deficiency exacerbates the brain inflammatory response to permanent ischemia without preventing resolution of the lesion. J. Cereb. Blood Flow Metab. 2013, 33, 1955–1966. [Google Scholar] [CrossRef] [PubMed]
- Joniec-Maciejak, A.; Ciesielska, A.; Wawer, A.; Sznejder-Pachołek, A.; Schwenkgrub, J.; Cudna, A.; Hadaczek, P.; Bankiewicz, K.B.; Członkowska, A.; Członkowski, A. The influence of AAV2-mediated gene transfer of human IL-10 on neurodegeneration and immune response in a murine model of Parkinson’s disease. Pharmacol. Rep. 2014, 66, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarty, P.; Li, A.; Ceballos-Diaz, C.; Eddy, J.A.; Funk, C.C.; Moore, B.; DiNunno, N.; Rosario, A.M.; Cruz, P.E.; Verbeeck, C.; et al. IL-10 alters immunoproteostasis in APP mice, increasing plaque burden and worsening cognitive behavior. Neuron 2015, 85, 519–533. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porro, C.; Cianciulli, A.; Panaro, M.A. The Regulatory Role of IL-10 in Neurodegenerative Diseases. Biomolecules 2020, 10, 1017. https://doi.org/10.3390/biom10071017
Porro C, Cianciulli A, Panaro MA. The Regulatory Role of IL-10 in Neurodegenerative Diseases. Biomolecules. 2020; 10(7):1017. https://doi.org/10.3390/biom10071017
Chicago/Turabian StylePorro, Chiara, Antonia Cianciulli, and Maria Antonietta Panaro. 2020. "The Regulatory Role of IL-10 in Neurodegenerative Diseases" Biomolecules 10, no. 7: 1017. https://doi.org/10.3390/biom10071017
APA StylePorro, C., Cianciulli, A., & Panaro, M. A. (2020). The Regulatory Role of IL-10 in Neurodegenerative Diseases. Biomolecules, 10(7), 1017. https://doi.org/10.3390/biom10071017






