Development of Biodegradable Agar-Agar/Gelatin-Based Superabsorbent Hydrogel as an Efficient Moisture-Retaining Agent
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
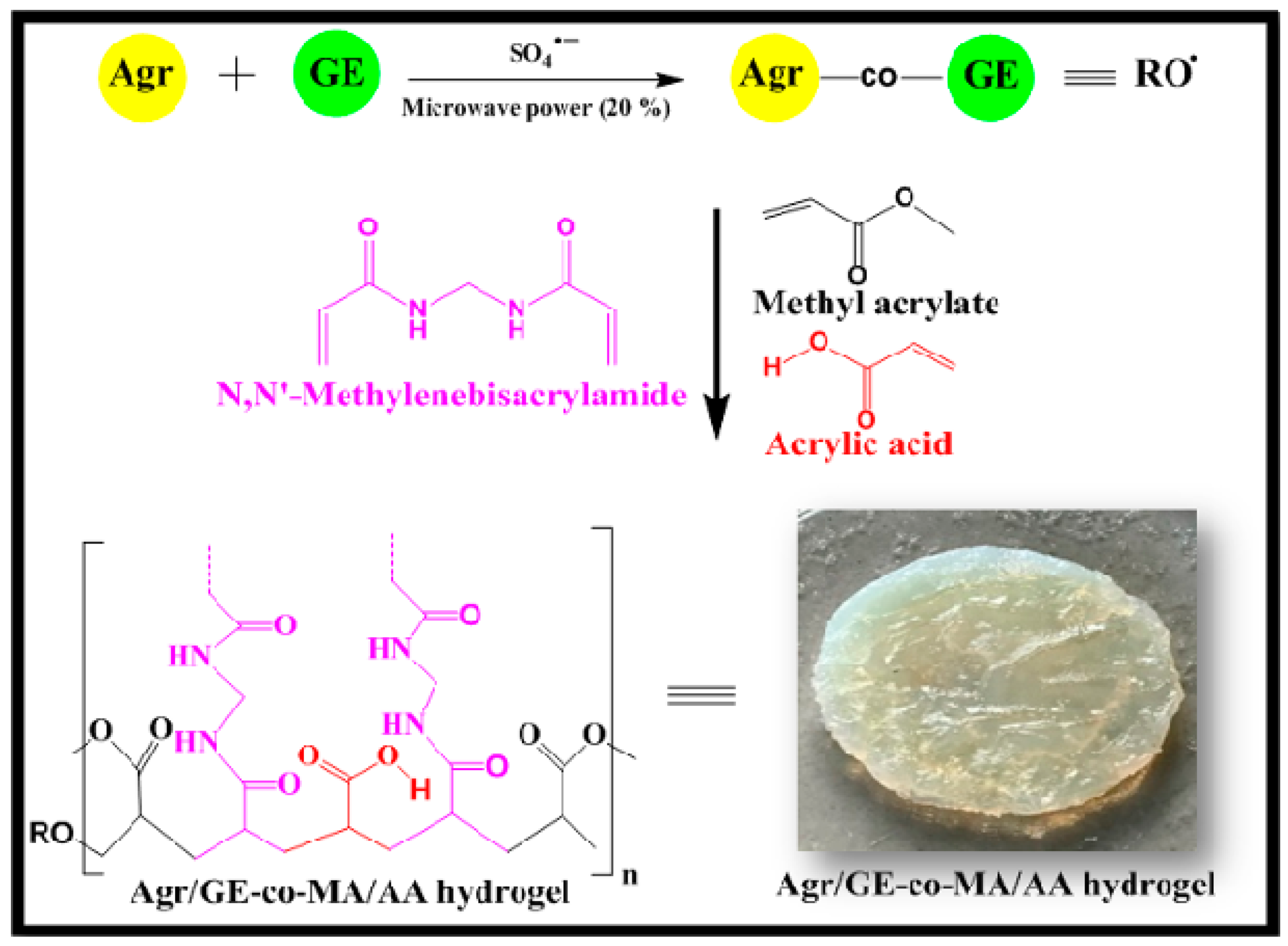
2.2. Synthesis of Agar-Agar/Gelatin Copolymerized Methyl Acrylate/Acrylic Acid Hydrogel
2.3. Characterization
2.4. Swelling Studies of Agar-Agar/Gelatin Copolymerized Methyl Acrylate/Acrylic Acid Hydrogel
2.5. Water Retention Study
3. Results and Discussions
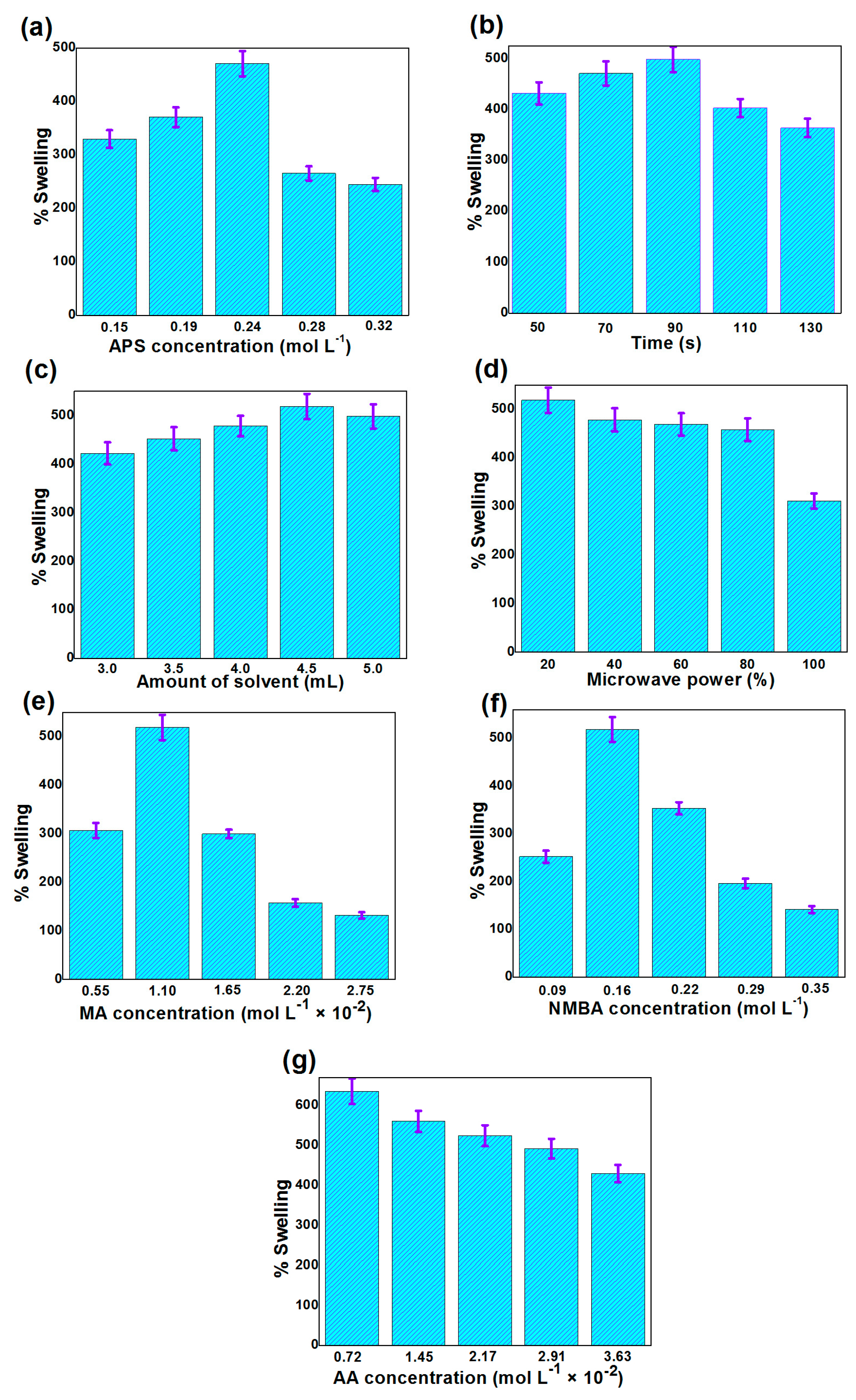
3.1. Optimization of Various Reaction Factors for the Swelling of Agar-Agar/Gelatin Copolymerized Methyl Acrylate/Acrylic Acid Hydrogel
3.2. Characterization
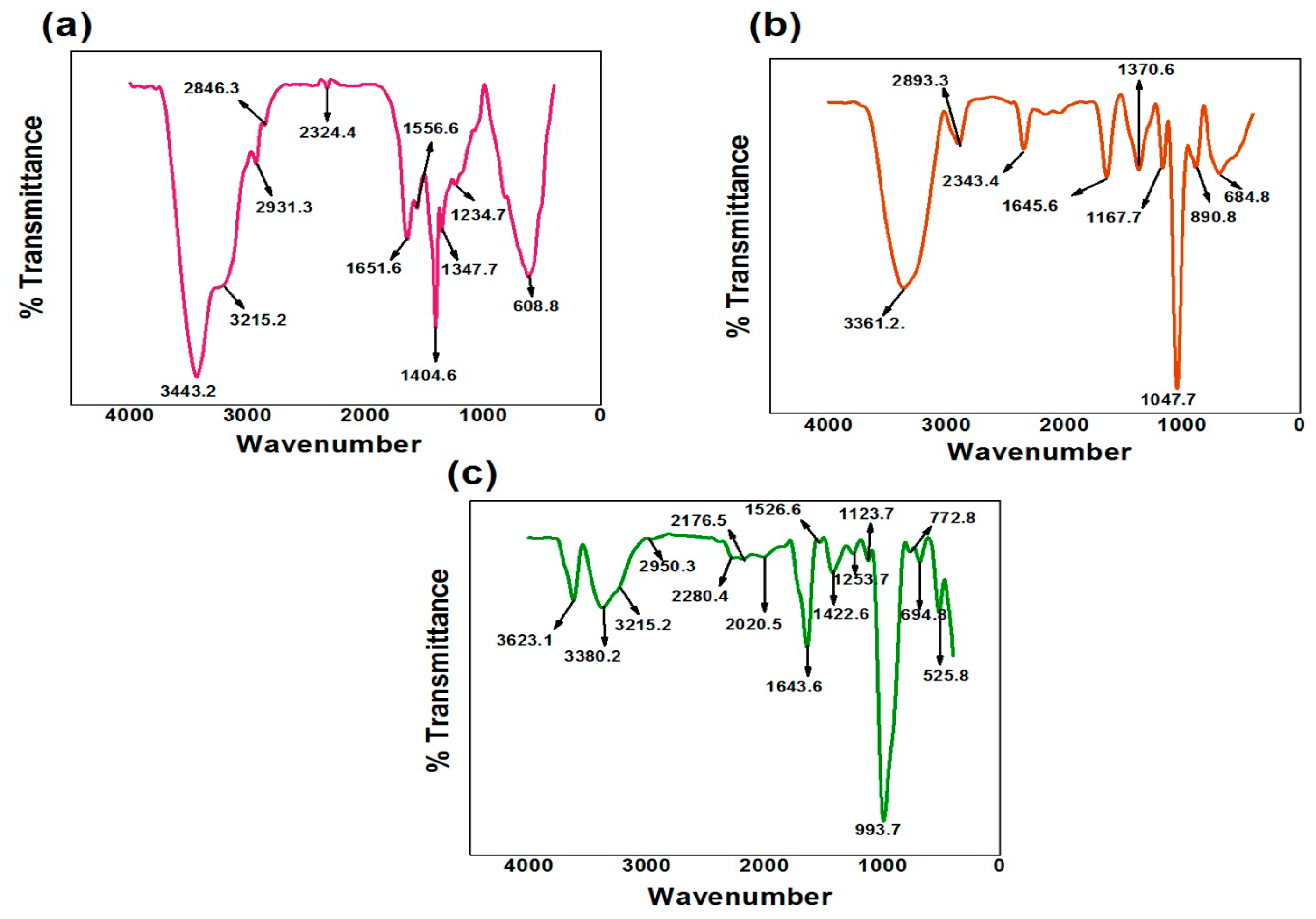
3.2.1. Fourier Transform Infrared Analysis
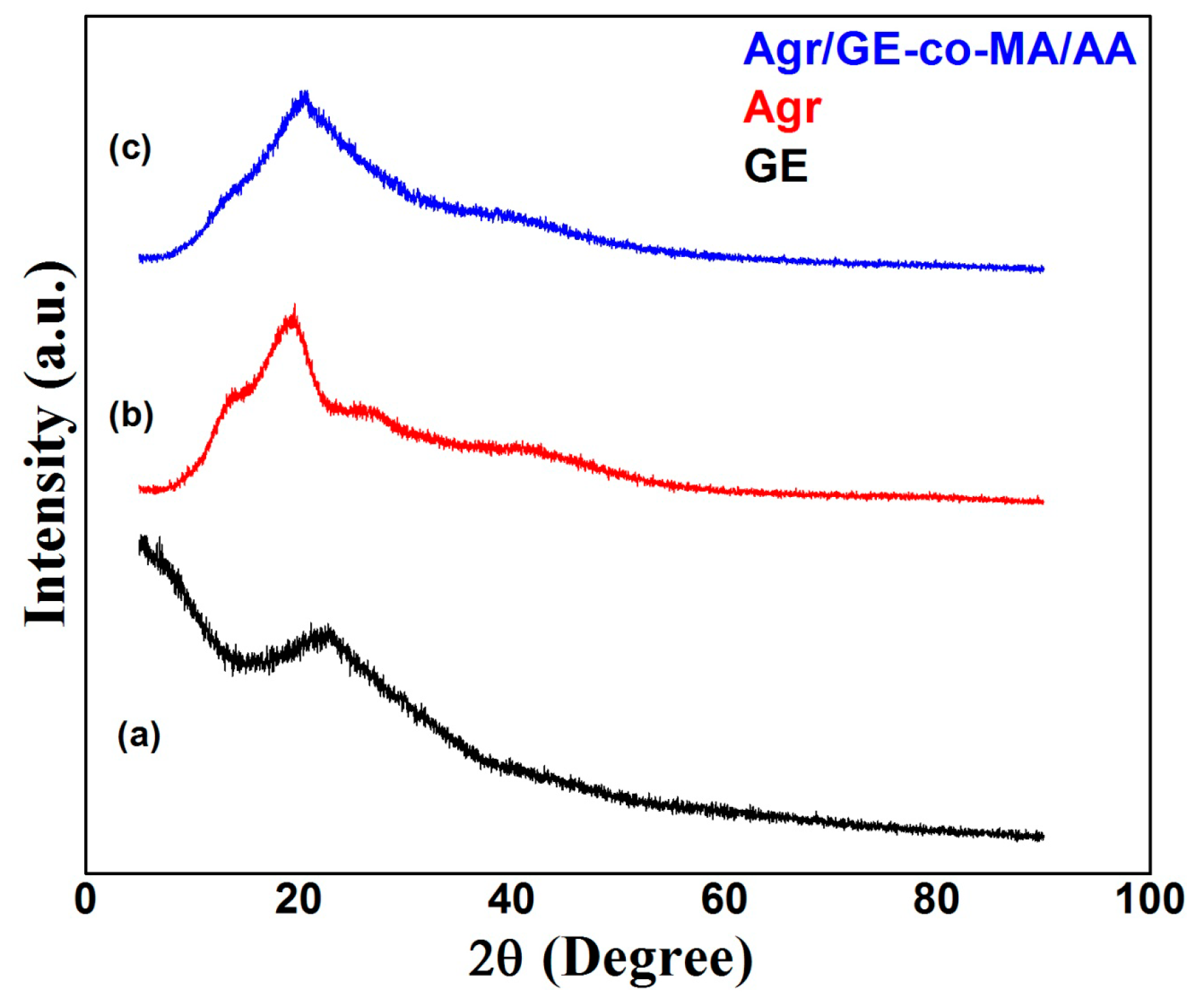
3.2.2. X-ray Diffraction
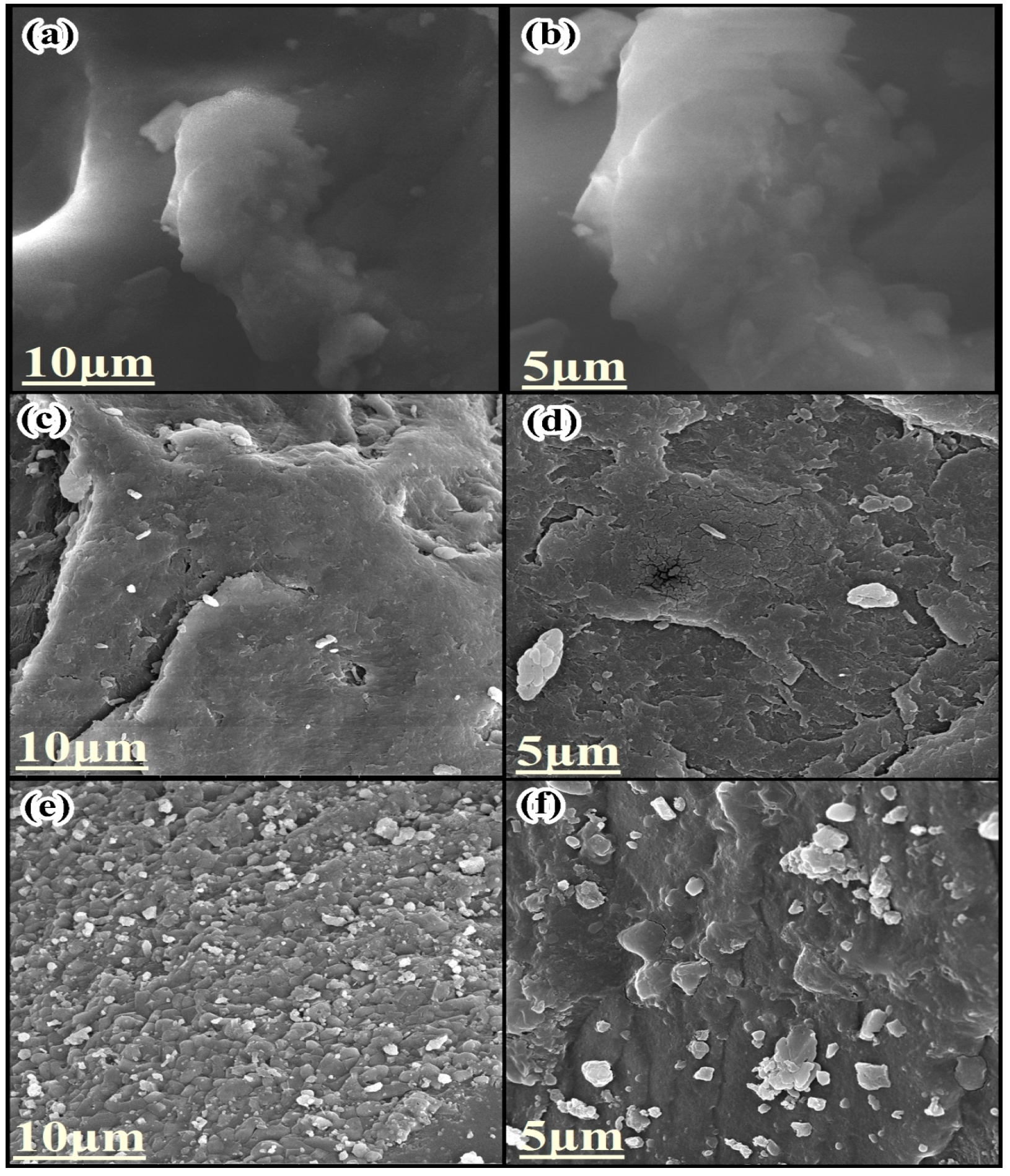
3.2.3. Field Emission Scanning Electron Microscope
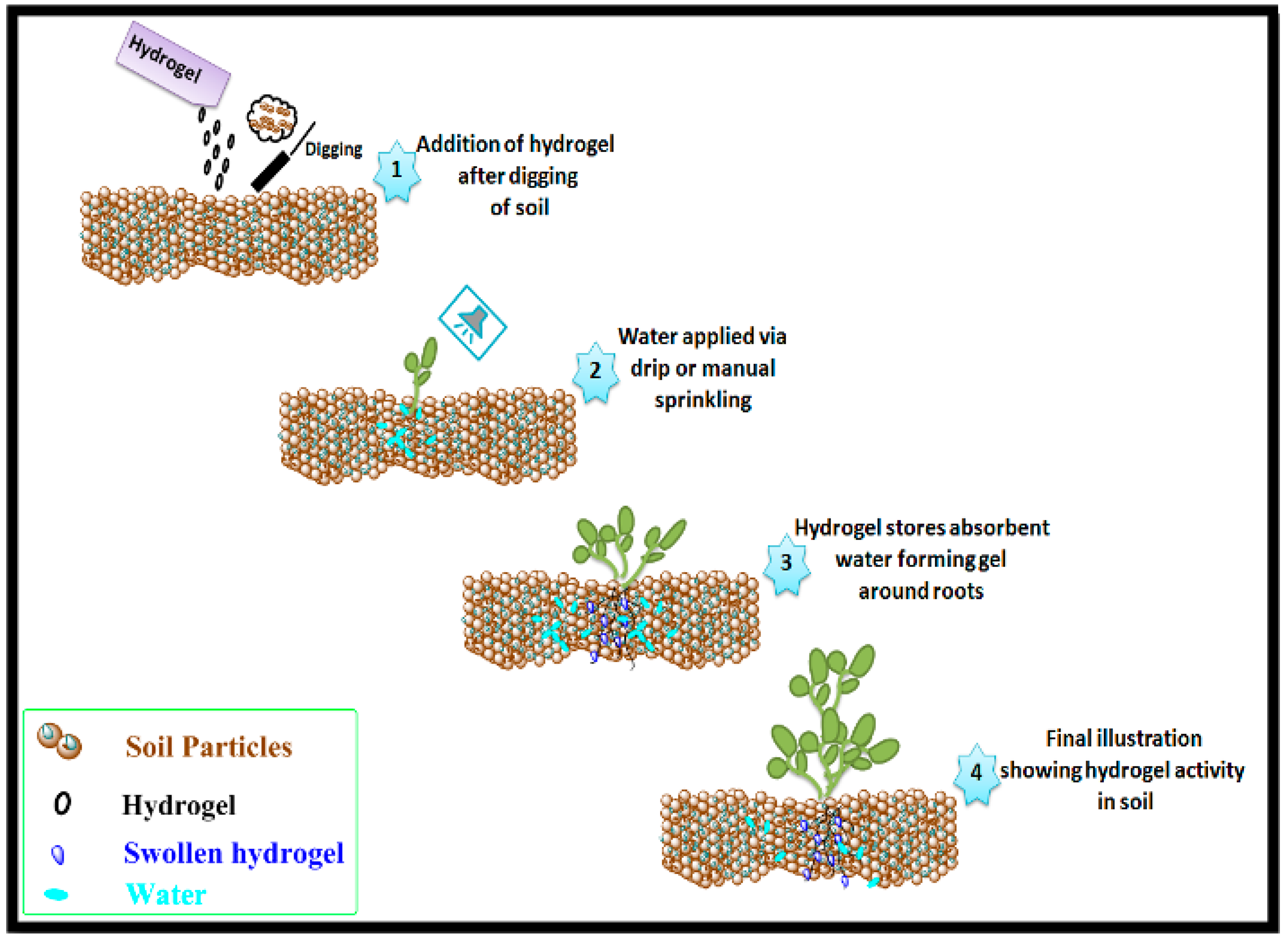
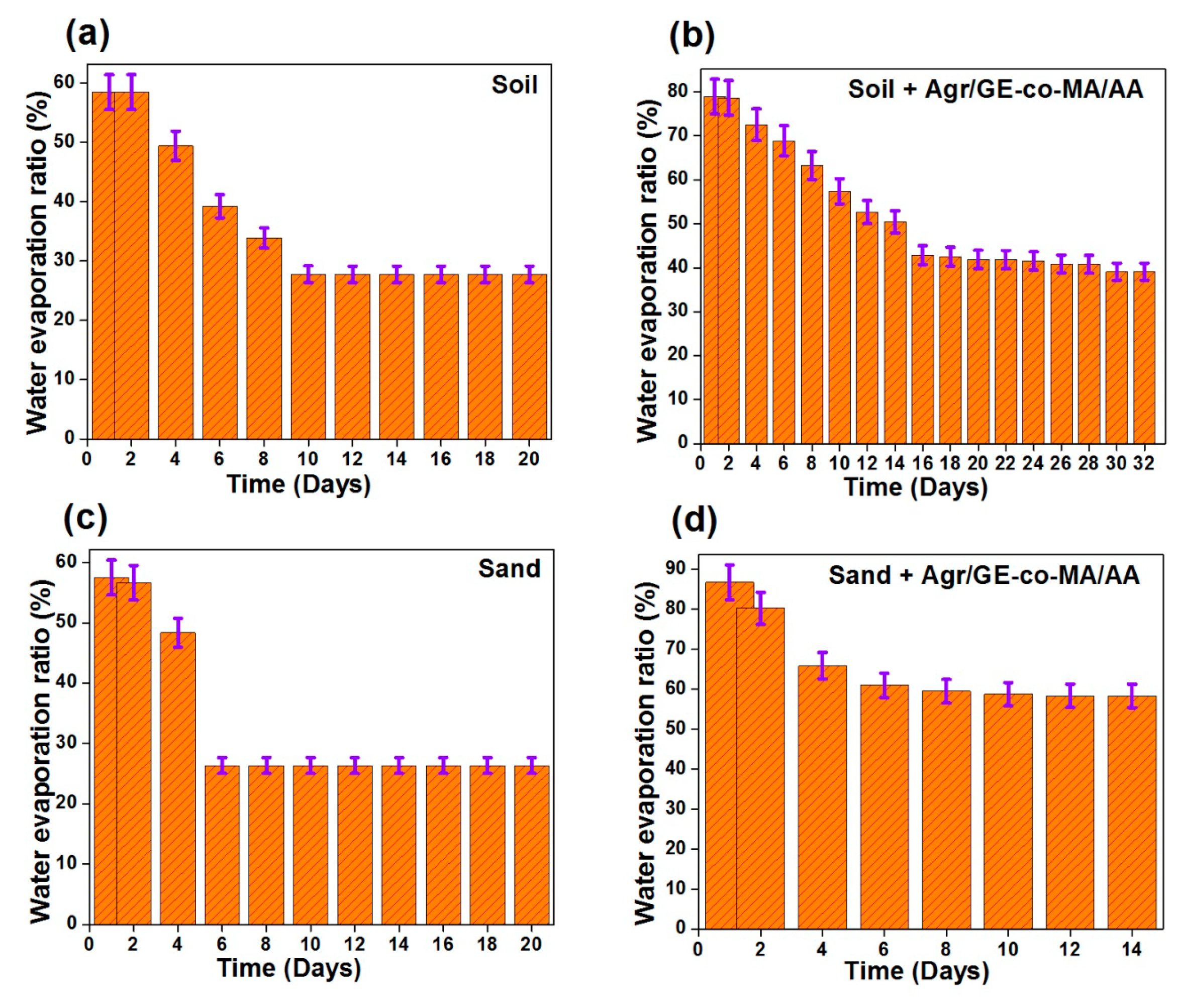
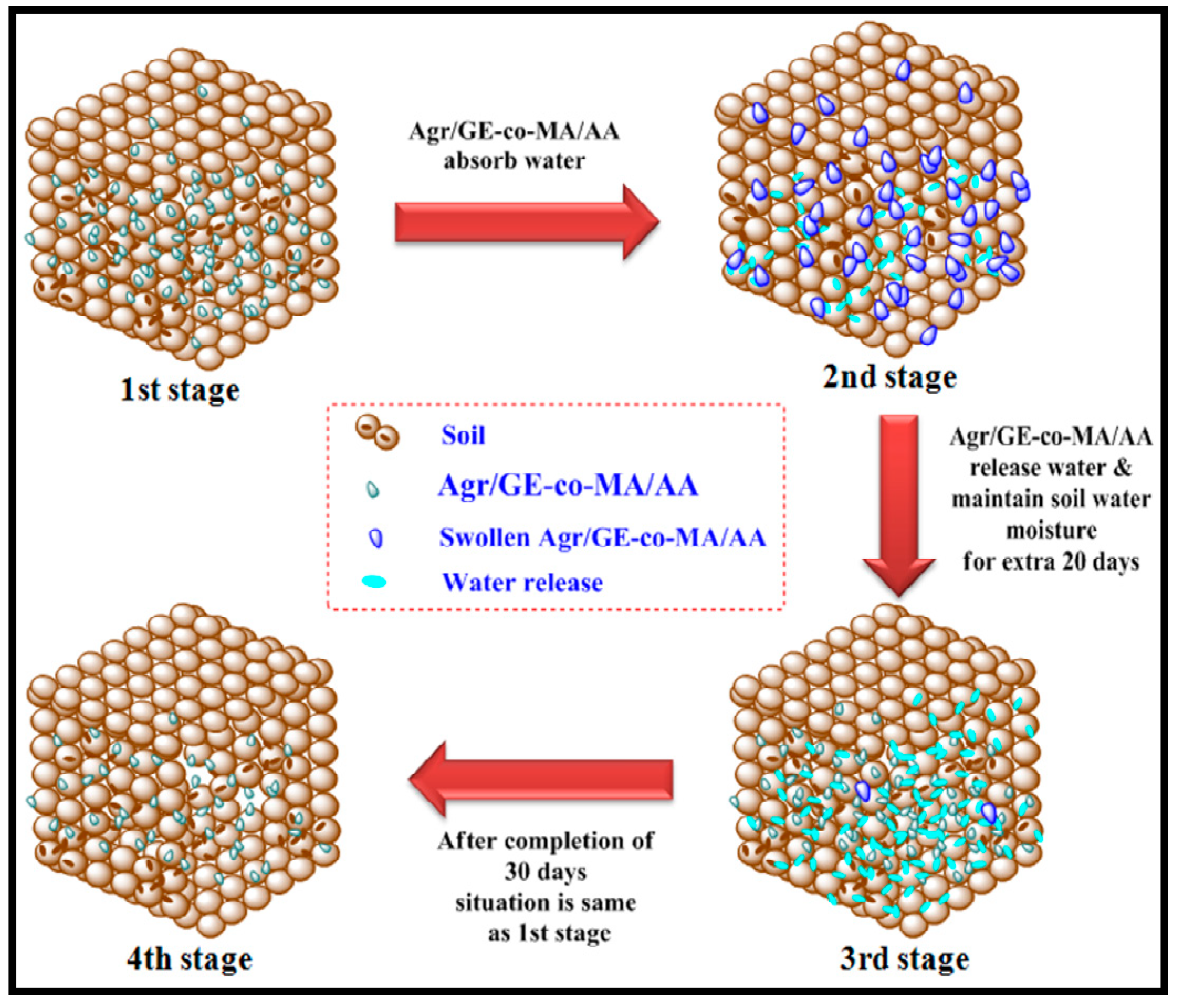
3.3. Water Retention Properties of Agar-Agar/Gelatin Copolymerized Methyl Acrylate/Acrylic Acid Hydrogel in Soil and Sand
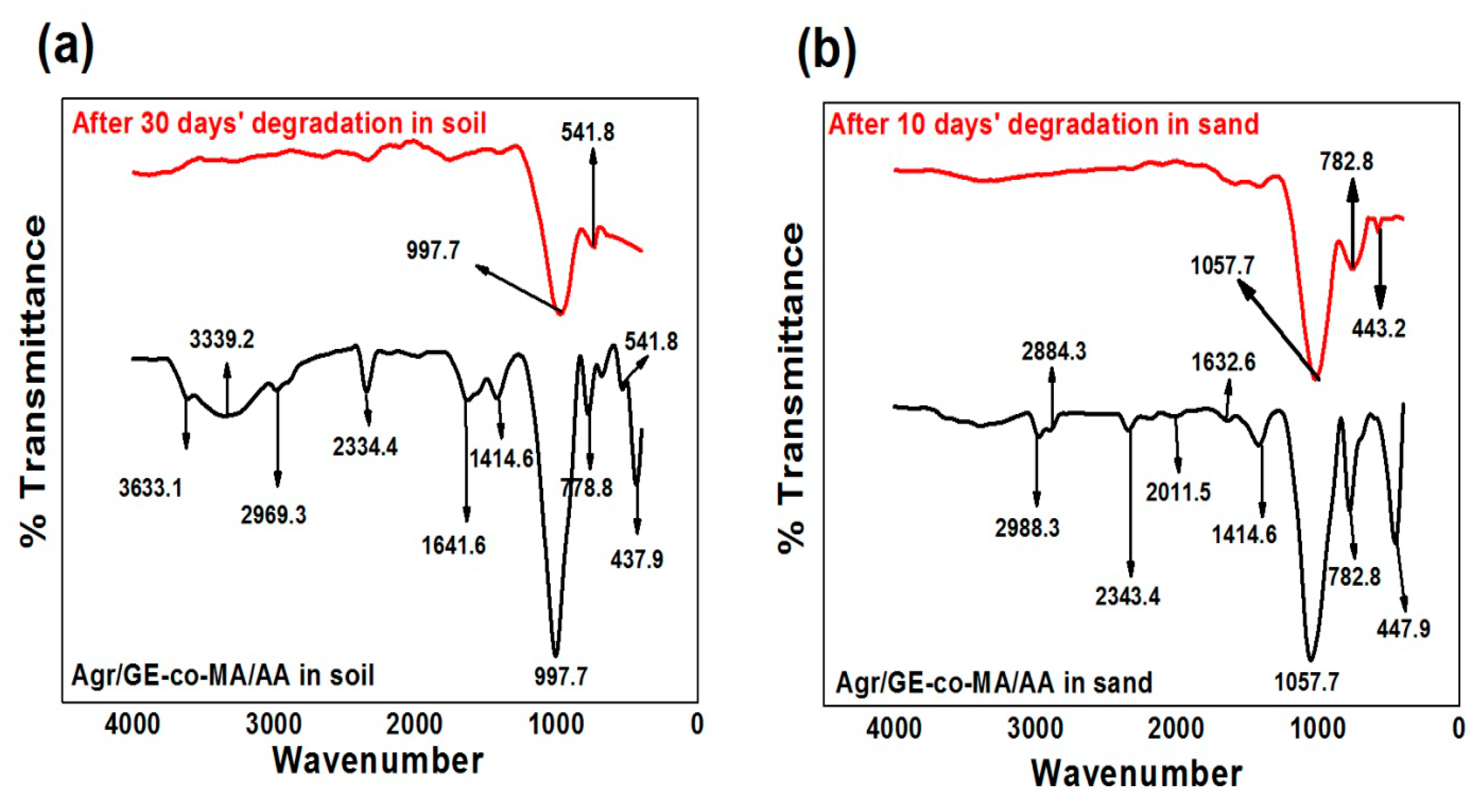
3.4. Confirmation of Agar-Agar/Gelatin Copolymerized Methyl Acrylate/Acrylic Acid Hydrogel Biodegradability via Fourier Transform Infrared Study
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nada, W.M.; Blumenstein, O. Characterization and impact of newly synthesized superabsorbent hydrogel nanocomposite on water retention characteristics of sandy soil and grass seedling growth. Int. J. Soil Sci. 2015, 10, 153–165. [Google Scholar] [CrossRef]
- Rasool, A.; Ata, S.; Islam, A.; Khan, R.U. Fabrication of novel carrageenan based stimuli responsive injectable hydrogels for controlled release of cephradine. RSC Adv. 2019, 9, 12282–12290. [Google Scholar] [CrossRef]
- Zhang, H.; Luan, Q.; Huang, Q.; Tang, H.; Huang, F.; Li, W.; Wan, C.; Liu, C.; Xu, J.; Guo, P. A facile and efficient strategy for the fabrication of porous linseed gum/cellulose superabsorbent hydrogels for water conservation. Carbohydr. Polym. 2017, 157, 1830–1836. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.; Liu, M. Preparation and properties of coated nitrogen fertilizer with slow release and water retention. Ind. Eng. Chem. Res. 2006, 45, 8610–8616. [Google Scholar] [CrossRef]
- Verma, M.K.; Pandey, P.; De, N. Characterization of water retention and release capacity of innovative nano clay polymer composite superabsorbent. J. Pharmacogn. Phytochem. 2017, 6, 42–48. [Google Scholar]
- Montesano, F.F.; Parente, A.; Santamaria, P.; Sannino, A.; Serio, F. Biodegradable superabsorbent hydrogel increaseswater retention properties of growing media and plant growth. Agric. Agric. Sci. Procedia 2015, 4, 451–458. [Google Scholar] [CrossRef]
- Valdez-Alegría, C.J.; Fuentes-Rivas, R.M.; García-Rivas, J.L.; Zavala Arce, R.E.; Núñez, J.; de la Luz, M.; García-Gaitán, B. Synthesis of Chitosan-Polyvinyl Alcohol Biopolymers to Eliminate Fluorides from Water. Biomolecules 2020, 10, 156. [Google Scholar] [CrossRef]
- Rizzi, V.; Romanazzi, F.; Gubitosa, J.; Fini, P.; Romita, R.; Agostiano, A.; Petrella, A.; Cosma, P. Chitosan Film as Eco-Friendly and Recyclable Bio-Adsorbent to Remove/Recover Diclofenac, Ketoprofen, and their Mixture from Wastewater. Biomolecules 2019, 9, 571. [Google Scholar] [CrossRef]
- Sakiyama, T.; Chu, C.-H.; Fujii, T.; Yano, T. Preparation of a polyelectrolyte complex gel from chitosan and κ-carrageenan and its pH-sensitive swelling. J. Appl. Polym. Sci. 1993, 50, 2021–2025. [Google Scholar] [CrossRef]
- Yoshida, M.; Asano, M.; Kumakura, M. A new temperature-sensitive hydrogel with α-amino acid group as side chain of polymer. Eur. Polym. J. 1989, 25, 1197–1202. [Google Scholar] [CrossRef]
- Hogari, K.; Ashiya, F. Advances in Superabsorbent Polymers; American Chemical Society: Washington, DC, USA, 1994; p. 2639. [Google Scholar]
- Zhang, J.; Chen, H.; Li, P.; Wang, A. Study on Superabsorbent Composite, 14: Preparation of Poly (acrylic acid)/Organo-attapulgite Composite Hydrogels and Swelling Behaviors in Aqueous Electrolyte Solution. Macromol. Mater. Eng. 2006, 291, 1529–1538. [Google Scholar] [CrossRef]
- Austin, M.E.; Bondari, K. Hydrogel as a Field Medium Amendment for Blueberry Plants. HortScience 1992, 27, 973–974. [Google Scholar] [CrossRef]
- Liu, C.; Lei, F.; Li, P.; Jiang, J.; Wang, K. Borax Crosslinked Fenugreek Galactomannan Hydrogel as Potential Water-retaining Agent in Agriculture. Carbohydr. Polym. 2020, 236, 116100. [Google Scholar] [CrossRef] [PubMed]
- Hasija, V.; Sharma, K.; Kumar, V.; Sharma, S.; Sharma, V. Green synthesis of agar/Gum Arabic based superabsorbent as an alternative for irrigation in agriculture. Vacuum 2018, 157, 458–464. [Google Scholar] [CrossRef]
- Thombare, N.; Mishra, S.; Siddiqui, M.Z.; Jha, U.; Singh, D.; Mahajan, G.R. Design and development of guar gum based novel, superabsorbent and moisture retaining hydrogels for agricultural applications. Carbohydr. Polym. 2018, 185, 169–178. [Google Scholar] [CrossRef]
- Zohuriaan-Mehr, M.J.; Pourjavadi, A.; Salimi, H.; Kurdtabar, M. Protein-and homo poly (amino acid)-based hydrogels with super-swelling properties. Polym. Adv. Technol. 2009, 20, 655–671. [Google Scholar] [CrossRef]
- Petre, M.; Zarnea, G.; Adrian, P.; Gheorghiu, E. Biodegradation and bioconversion of cellulose wastes using bacterial and fungal cells immobilized in radiopolymerized hydrogels. Resour. Conserv. Recycl. 1999, 27, 309–332. [Google Scholar] [CrossRef]
- Ali, A.; Ahmed, S. Recent advances in edible polymer based hydrogels as a sustainable alternative to conventional polymers. J. Agric. Food Chem. 2018, 66, 6940–6967. [Google Scholar] [CrossRef]
- Sharma, R.; Bajpai, J.; Bajpai, A.K.; Acharya, S.; Shrivastava, R.B.; Shukla, S.K. Designing slow water-releasing alginate nanoreserviors for sustained irrigation in scanty rainfall areas. Carbohydr. Polym. 2014, 102, 513–520. [Google Scholar] [CrossRef]
- Sharma, K.; Kumar, V.; Kaith, B.S.; Kumar, V.; Som, S.; Kalia, S.; Swart, H.C. Synthesis, characterization and water retention study of biodegradable Gum ghatti-poly (acrylic acid–aniline) hydrogels. Polym. Degrad. Stab. 2015, 111, 20–31. [Google Scholar] [CrossRef]
- Thakur, S.; Pandey, S.; Arotiba, O.A. Development of a sodium alginate-based organic/inorganic superabsorbent composite hydrogel for adsorption of methylene blue. Carbohydr. Polym. 2016, 153, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Chen, Y.-H. Thermo and pH-responsive methylcellulose and hydroxypropyl methylcellulose hydrogels containing K2SO4 for water retention and a controlled-release water-soluble fertilizer. Sci. Total Environ. 2019, 655, 958–967. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Thakur, S.; Mamba, G.; Gupta, R.K.; Thakur, P.; Thakur, V.K. Graphite modified sodium alginate hydrogel composite for efficient removal of malachite green dye. Int. J. Biol. Macromol. 2020. [Google Scholar] [CrossRef]
- Alonso, G.J.; Rivera, J.L.A.; Mendoza, A.M.M.; Mendez, M.L.H. Effect of temperature and pH on swelling behavior of hydroxyethyl cellullose-acrylamide hydrogel. e-Polymers 2007, 7. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, Y. Relationship between water absorbency and reaction conditions in aqueous solution polymerization of polyacrylate superabsorbents. J. Appl. Polym. Sci. 2000, 75, 808–814. [Google Scholar] [CrossRef]
- Bhowmik, S.; Islam, J.M.M.; Debnath, T.; Muhammed, Y.; Miah, M.; Bhattacharjee, S.; Khan, M. Reinforcement of Gelatin-Based Nanofilled Polymer Biocomposite by Crystalline Cellulose from Cotton for Advanced Wound Dressing Applications. Polymers 2017, 2017, 222. [Google Scholar] [CrossRef]
- Samiey, B.; Ashoori, F. Adsorptive removal of methylene blue by agar: Effects of NaCl and ethanol. Chem. Cent. J. 2012, 6, 14. [Google Scholar] [CrossRef]
- Mbese, J.Z.; Ajibade, P.A. Preparation and Characterization of ZnS, CdS and HgS/Poly(methyl methacrylate) Nanocomposites. Polymers 2014, 6, 2332–2344. [Google Scholar] [CrossRef]
- Kakar, R.; Tripathi, D.; Chandel, S.; Sultanpuri, A. Distribution of micronutrient cations in relation to soil properties in Saproon Valley of Solan district in North Western Himalayas. Ann. Plant Soil Res. 2018, 20, 143–147. [Google Scholar]
- El-Rehim, H.A.; Hegazy, E.-S.A.; El-Mohdy, H.L. Radiation synthesis of hydrogels to enhance sandy soils water retention and increase plant performance. J. Appl. Polym. Sci. 2004, 93, 1360–1371. [Google Scholar] [CrossRef]
- Mittal, H.; Fosso-Kankeu, E.; Mishra, S.B.; Mishra, A.K. Biosorption potential of Gum ghatti-g-poly (acrylic acid) and susceptibility to biodegradation by B. subtilis. Int. J. Biol. Macromol. 2013, 62, 370–378. [Google Scholar] [CrossRef] [PubMed]
| S.N.* | Analysis of Media | Absorbent Material | Water Evaporation Ratio (Days) | Reference |
|---|---|---|---|---|
| 1. | Soil | Alginate nanoparticles | 11 | [20] |
| 2. | Sandy soil | Fenugreek galactomannan-borax hydrogel | 11.5 | [14] |
| 3. | Alluvial soil | Nano clay polymer composite | 13 | [5] |
| 4. | Soil | Double-coated, slow-release and water-retention urea fertilizer | 17.3 | [4] |
| 5. | Clay soil | IPN hydrogel | 27 | [21] |
| 6. | Soil | Agar/Ga-Cl-poly(AA) | 28 | [15] |
| 7. | Sand | Agr/GE-co-MA/AA | 10 | Present work |
| 8. | Soil | Agr/GE-co-MA/AA | 30 | Present work |
| S. N. | Initiator (APS) (mol L−1) | Reaction Time (s) | Solvent (mL) | Microwave power (%) | Monomer (MA) (mol L−1) | Crosslinker (NMBA) (mol L−1) | Monomer (AA) (mol L−1) | % Swelling |
|---|---|---|---|---|---|---|---|---|
| 1. | 0.15 | 70 | 5.0 | 20 | 1.10 × 10−2 | 0.16 | -- | 329.6 |
| 2. | 0.19 | 70 | 5.0 | 20 | 1.10 × 10−2 | 0.16 | -- | 370.5 |
| 3. | 0.24 | 70 | 5.0 | 20 | 1.10 × 10−2 | 0.16 | -- | 470.7 |
| 4. | 0.28 | 70 | 5.0 | 20 | 1.10 × 10−2 | 0.16 | -- | 265.3 |
| 5. | 0.32 | 70 | 5.0 | 20 | 1.10 × 10−2 | 0.16 | -- | 244.9 |
| 6. | 0.24 | 50 | 5.0 | 20 | 1.10 × 10−2 | 0.16 | -- | 431.8 |
| 7. | 0.24 | 70 | 5.0 | 20 | 1.10 × 10−2 | 0.16 | -- | 470.7 |
| 8. | 0.24 | 90 | 5.0 | 20 | 1.10 × 10−2 | 0.16 | -- | 498.3 |
| 9. | 0.24 | 110 | 5.0 | 20 | 1.10 × 10−2 | 0.16 | -- | 402.9 |
| 10. | 0.24 | 130 | 5.0 | 20 | 1.10 × 10−2 | 0.16 | -- | 363.7 |
| 11. | 0.24 | 90 | 3.0 | 20 | 1.10 × 10−2 | 0.16 | -- | 422.2 |
| 12. | 0.24 | 90 | 3.5 | 20 | 1.10 × 10−2 | 0.16 | -- | 452.2 |
| 13. | 0.24 | 90 | 4.0 | 20 | 1.10 × 10−2 | 0.16 | -- | 478.5 |
| 14. | 0.24 | 90 | 4.5 | 20 | 1.10 × 10−2 | 0.16 | -- | 518.7 |
| 15. | 0.24 | 90 | 5.0 | 20 | 1.10 × 10−2 | 0.16 | -- | 498.3 |
| 16. | 0.24 | 90 | 4.5 | 20 | 1.10 × 10−2 | 0.16 | -- | 518.7 |
| 17. | 0.24 | 90 | 4.5 | 40 | 1.10 × 10−2 | 0.16 | -- | 478.1 |
| 18. | 0.24 | 90 | 4.5 | 60 | 1.10 × 10−2 | 0.16 | -- | 468.8 |
| 19. | 0.24 | 90 | 4.5 | 80 | 1.10 × 10−2 | 0.16 | -- | 458.1 |
| 20. | 0.24 | 90 | 4.5 | 100 | 1.10 × 10−2 | 0.16 | -- | 311.1 |
| 21. | 0.24 | 90 | 4.5 | 20 | 0.55 × 10−2 | 0.16 | -- | 306.8 |
| 22. | 0.24 | 90 | 4.5 | 20 | 1.10 × 10−2 | 0.16 | -- | 518.7 |
| 23. | 0.24 | 90 | 4.5 | 20 | 1.65 × 10−2 | 0.16 | -- | 299.6 |
| 24. | 0.24 | 90 | 4.5 | 20 | 2.20 × 10−2 | 0.16 | -- | 157.6 |
| 25. | 0.24 | 90 | 4.5 | 20 | 2.75 × 10−2 | 0.16 | -- | 132.2 |
| 26. | 0.24 | 90 | 4.5 | 20 | 1.10 × 10−2 | 0.09 | -- | 252.4 |
| 27. | 0.24 | 90 | 4.5 | 20 | 1.10 × 10−2 | 0.16 | -- | 518.7 |
| 28. | 0.24 | 90 | 4.5 | 20 | 1.10 × 10−2 | 0.22 | -- | 353.9 |
| 29. | 0.24 | 90 | 4.5 | 20 | 1.10 × 10−2 | 0.29 | -- | 196.4 |
| 30. | 0.24 | 90 | 4.5 | 20 | 1.10 × 10−2 | 0.35 | -- | 141.5 |
| 31. | 0.24 | 90 | 4.5 | 20 | 1.10 × 10−2 | 0.16 | 0.72 × 10−2 | 636.1 |
| 32. | 0.24 | 90 | 4.5 | 20 | 1.10 × 10−2 | 0.16 | 1.45 × 10−2 | 560.7 |
| 33. | 0.24 | 90 | 4.5 | 20 | 1.10 × 10−2 | 0.16 | 2.17 × 10−2 | 525.0 |
| 34. | 0.24 | 90 | 4.5 | 20 | 1.10 × 10−2 | 0.16 | 2.91 × 10−2 | 492.7 |
| 35. | 0.24 | 90 | 4.5 | 20 | 1.10 × 10−2 | 0.16 | 3.63 × 10−2 | 430.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaudhary, J.; Thakur, S.; Sharma, M.; Gupta, V.K.; Thakur, V.K. Development of Biodegradable Agar-Agar/Gelatin-Based Superabsorbent Hydrogel as an Efficient Moisture-Retaining Agent. Biomolecules 2020, 10, 939. https://doi.org/10.3390/biom10060939
Chaudhary J, Thakur S, Sharma M, Gupta VK, Thakur VK. Development of Biodegradable Agar-Agar/Gelatin-Based Superabsorbent Hydrogel as an Efficient Moisture-Retaining Agent. Biomolecules. 2020; 10(6):939. https://doi.org/10.3390/biom10060939
Chicago/Turabian StyleChaudhary, Jyoti, Sourbh Thakur, Minaxi Sharma, Vijai Kumar Gupta, and Vijay Kumar Thakur. 2020. "Development of Biodegradable Agar-Agar/Gelatin-Based Superabsorbent Hydrogel as an Efficient Moisture-Retaining Agent" Biomolecules 10, no. 6: 939. https://doi.org/10.3390/biom10060939
APA StyleChaudhary, J., Thakur, S., Sharma, M., Gupta, V. K., & Thakur, V. K. (2020). Development of Biodegradable Agar-Agar/Gelatin-Based Superabsorbent Hydrogel as an Efficient Moisture-Retaining Agent. Biomolecules, 10(6), 939. https://doi.org/10.3390/biom10060939








