ORP5 and ORP8: Sterol Sensors and Phospholipid Transfer Proteins at Membrane Contact Sites?
Abstract
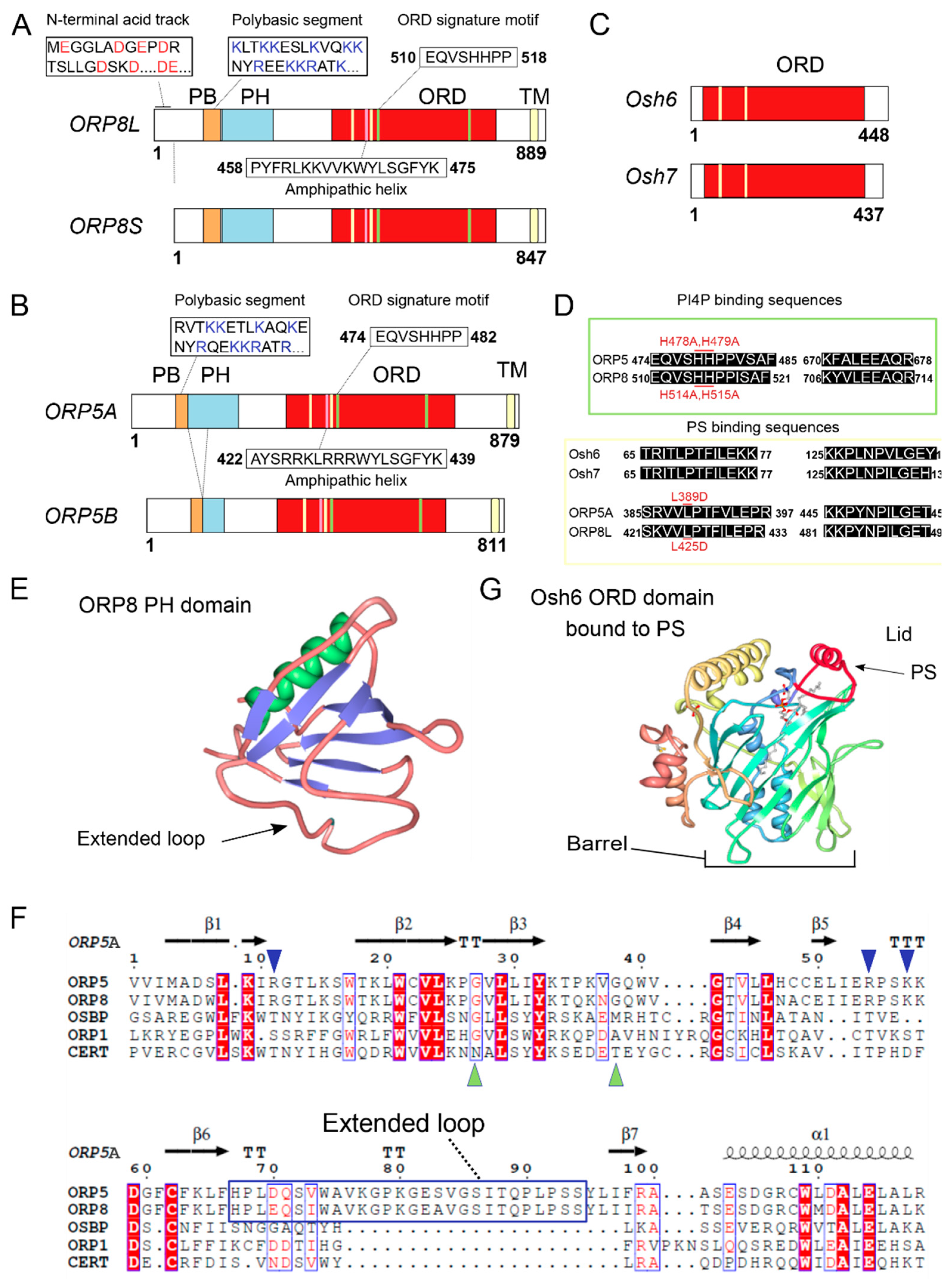
:1. Introduction
2. ORP5/8 as Oxysterol Sensors
3. ORP5/8 in the Control of Vesicular Trafficking
4. ORP5/8 as Phospholipid Transfer Proteins at Membrane Contact Sites
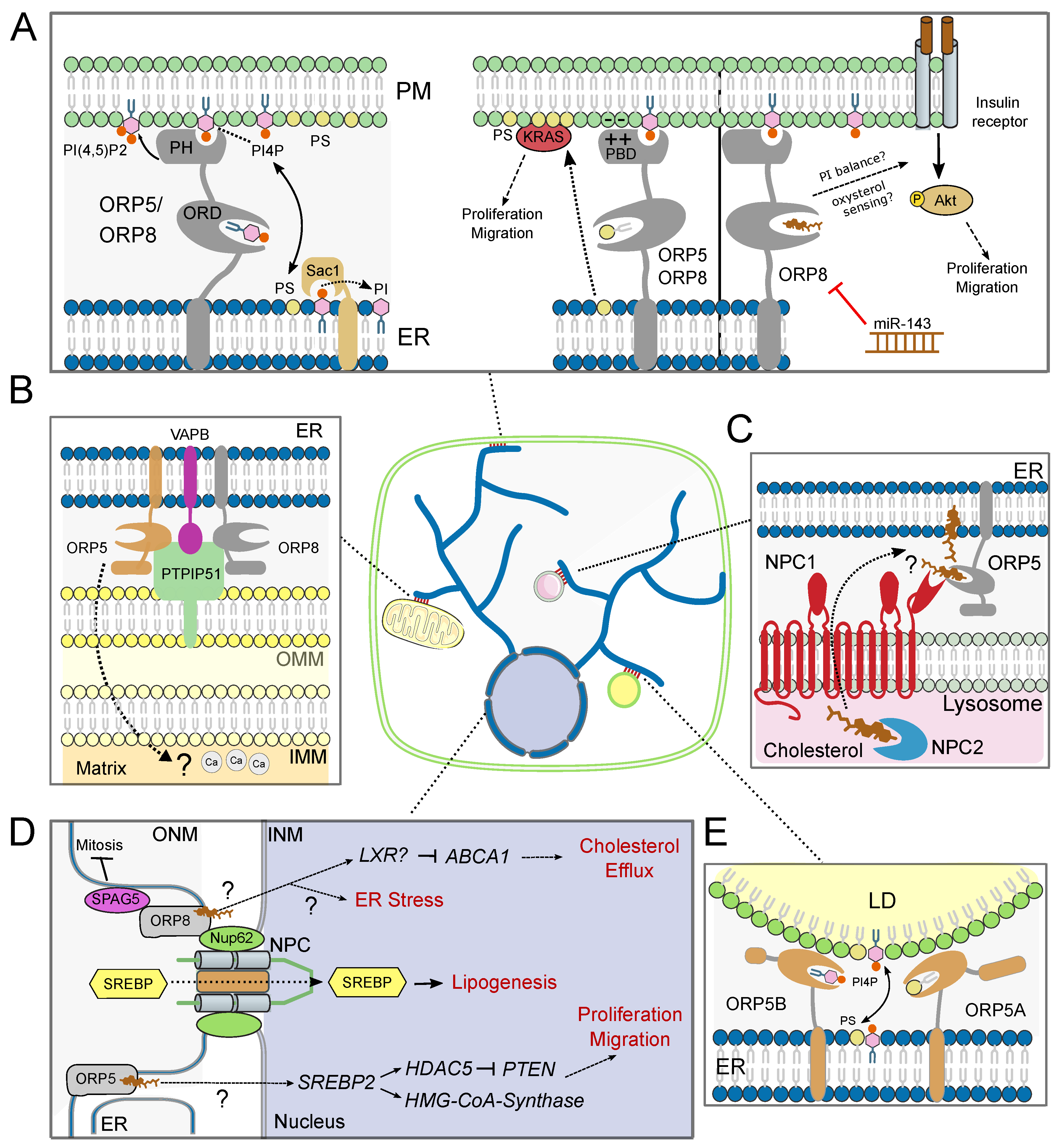
4.1. ORP5/8 at Endoplasmic Reticulum-Plasma Membrane Contact Sites
4.2. ORP5/8 at Endoplasmic Reticulum-Mitochondria Membrane Contact Sites
4.3. ORP5/8 at Endoplasmic Reticulum-Lipid Droplet Membrane Contact Sites
5. Conclusions and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Munro, S. Organelle identity and the organization of membrane traffic. Nature 2004, 6, 469–472. [Google Scholar] [CrossRef]
- Holthuis, J.C.; Menon, A.K. Lipid landscapes and pipelines in membrane homeostasis. Nature 2014, 510, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Lorizate, M.; Kräusslich, H.-G. Role of Lipids in Virus Replication. Cold Spring Harb. Perspect. Boil. 2011, 3, a004820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- A Kandutsch, A.; Shown, E.P. Assay of oxysterol-binding protein in a mouse fibroblast, cell-free system. Dissociation constant and other properties of the system. J. Boil. Chem. 1981, 256, 13068–13073. [Google Scholar]
- Taylor, F.R.; Kandutsch, A.A. Oxysterol binding protein. Chem. Phys. Lipids 1985, 38, 187–194. [Google Scholar] [CrossRef]
- Pietrangelo, A.; Ridgway, N.D. Bridging the molecular and biological functions of the oxysterol-binding protein family. Cell. Mol. Life Sci. 2018, 75, 3079–3098. [Google Scholar] [CrossRef] [PubMed]
- Jaworski, C.; Moreira, E.; Li, A.; Lee, R.; Rodriguez, I.R. A Family of 12 Human Genes Containing Oxysterol-Binding Domains. Genomics 2001, 78, 185–196. [Google Scholar] [CrossRef] [Green Version]
- Laitinen, S.; Olkkonen, V.M.; Ehnholm, C.; Ikonen, E. Family of human oxysterol binding protein (OSBP) homologues. A novel member implicated in brain sterol metabolism. J. Lipid Res. 1999, 40. [Google Scholar]
- Lehto, M.; Laitinen, S.; Chinetti, G.; Johansson, M.; Ehnholm, C.; Staels, B.; Ikonen, E.; Olkkonen, V.M. The OSBP-related protein family in humans. J. Lipid Res. 2001, 42, 1203–1213. [Google Scholar]
- Lehto, M.; Olkkonen, V.M. The OSBP-related proteins: A novel protein family involved in vesicle transport, cellular lipid metabolism, and cell signalling. Biochim. Biophys. Acta (BBA) - Mol. Cell Boil. Lipids 2003, 1631, 1–11. [Google Scholar] [CrossRef]
- Beh, C.T.; Cool, L.; Phillips, J.; Rine, J. Overlapping functions of the yeast oxysterol-binding protein homologues. Genetics 2001, 157, 1117–1140. [Google Scholar] [PubMed]
- Schroepfer, G.J. Oxysterols: Modulators of cholesterol metabolism and other processes. Physiol. Rev. 2000, 80, 361–554. [Google Scholar] [CrossRef] [PubMed]
- Olkkonen, V.M.; Levine, T.P. Oxysterol binding proteins: in more than one place at one time? Biochem. Cell Boil. 2004, 82, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Helle, S.C.; Kanfer, G.; Kolar, K.; Lang, A.; Michel, A.H.; Kornmann, B. Organization and function of membrane contact sites. Biochim. Biophys. Acta (BBA) - Bioenerg. 2013, 1833, 2526–2541. [Google Scholar] [CrossRef] [Green Version]
- Jain, A.; Holthuis, J.C. Membrane contact sites, ancient and central hubs of cellular lipid logistics. Biochim. Biophys. Acta (BBA) - Bioenerg. 2017, 1864, 1450–1458. [Google Scholar] [CrossRef]
- Ridgway, N.D.; Dawson, P.A.; Ho, Y.K.; Brown, M.S.; Goldstein, J.L. Translocation of oxysterol binding protein to Golgi apparatus triggered by ligand binding. J. Cell Boil. 1992, 116, 307–319. [Google Scholar] [CrossRef] [Green Version]
- Im, Y.J.; Raychaudhuri, S.; Prinz, W.A.; Hurley, J.H. Structural mechanism for sterol sensing and transport by OSBP-related proteins. Nature 2005, 437, 154–158. [Google Scholar] [CrossRef] [Green Version]
- Raychaudhuri, S.; Im, Y.J.; Hurley, J.H.; Prinz, W.A. Nonvesicular sterol movement from plasma membrane to ER requires oxysterol-binding protein–related proteins and phosphoinositides. J. Cell Boil. 2006, 173, 107–119. [Google Scholar] [CrossRef]
- De Saint-Jean, M.; Delfosse, V.; Douguet, D.; Chicanne, G.; Payrastre, B.; Bourguet, W.; Antonny, B.; Drin, G. Osh4p exchanges sterols for phosphatidylinositol 4-phosphate between lipid bilayers. J. Cell Boil. 2011, 195, 965–978. [Google Scholar] [CrossRef] [Green Version]
- Mesmin, B.; Bigay, J.; Von Filseck, J.M.; Lacas-Gervais, S.; Drin, G.; Antonny, B. A Four-Step Cycle Driven by PI(4)P Hydrolysis Directs Sterol/PI(4)P Exchange by the ER-Golgi Tether OSBP. Cell 2013, 155, 830–843. [Google Scholar] [CrossRef] [Green Version]
- Von Filseck, J.M.; Vanni, S.; Mesmin, B.; Antonny, B.; Drin, G. A phosphatidylinositol-4-phosphate powered exchange mechanism to create a lipid gradient between membranes. Nat. Commun. 2015, 6, 6671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, X.; Kumar, J.; Ferguson, C.; Schulz, T.A.; Ong, Y.S.; Hong, W.; Prinz, W.A.; Parton, R.; Brown, A.J.; Yang, H. A role for oxysterol-binding protein–related protein 5 in endosomal cholesterol trafficking. J. Cell Boil. 2011, 192, 121–135. [Google Scholar] [CrossRef] [Green Version]
- Chung, J.; Torta, F.; Masai, K.; Lucast, L.; Czapla, H.; Tanner, L.B.; Narayanaswamy, P.; Wenk, M.R.; Nakatsu, F.; De Camilli, P. PI4P/phosphatidylserine countertransport at ORP5- and ORP8-mediated ER-plasma membrane contacts. Science 2015, 349, 428–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sohn, M.; Korzeniowski, M.; Zewe, J.P.; Wills, R.C.; Hammond, G.R.V.; Humpolíčková, J.; Vrzal, L.; Chalupska, D.; Veverka, V.; Fairn, G.D.; et al. PI(4,5)P2 controls plasma membrane PI4P and PS levels via ORP5/8 recruitment to ER–PM contact sites. J. Cell Boil. 2018, 217, 1797–1813. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Fairn, G.D. Both the PH domain and N-terminal region of oxysterol-binding protein related protein 8S are required for localization to PM-ER contact sites. Biochem. Biophys. Res. Commun. 2018, 496, 1088–1094. [Google Scholar] [CrossRef] [PubMed]
- Ghai, R.; Du, X.; Wang, H.; Dong, J.; Ferguson, C.; Brown, A.J.; Parton, R.; Wu, J.-W.; Yang, H. ORP5 and ORP8 bind phosphatidylinositol-4, 5-biphosphate (PtdIns(4,5)P2) and regulate its level at the plasma membrane. Nat. Commun. 2017, 8, 757. [Google Scholar] [CrossRef]
- Raychaudhuri, S.; Prinz, W.A. The diverse functions of oxysterol-binding proteins. Annu. Rev. Cell Dev. Boil. 2010, 26, 157–177. [Google Scholar] [CrossRef] [Green Version]
- Yan, D.; Mäyränpää, M.I.; Wong, J.; Perttilä, J.; Lehto, M.; Jauhiainen, M.; Kovanen, P.T.; Ehnholm, C.; Brown, A.J.; Olkkonen, V.M. OSBP-related Protein 8 (ORP8) SuppressesABCA1Expression and Cholesterol Efflux from Macrophages. J. Boil. Chem. 2007, 283, 332–340. [Google Scholar] [CrossRef] [Green Version]
- Koga, Y.; Ishikawa, S.; Nakamura, T.; Masuda, T.; Nagai, Y.; Takamori, H.; Hirota, M.; Kanemitsu, K.; Baba, Y.; Baba, H. Oxysterol binding protein-related protein-5 is related to invasion and poor prognosis in pancreatic cancer. Cancer Sci. 2008, 99, 2387–2394. [Google Scholar] [CrossRef]
- Jordan, S.D.; Krüger, M.; Willmes, D.M.; Redemann, N.; Wunderlich, F.T.; Brönneke, H.S.; Merkwirth, C.; Kashkar, H.; Olkkonen, V.M.; Böttger, T.; et al. Obesity-induced overexpression of miRNA-143 inhibits insulin-stimulated AKT activation and impairs glucose metabolism. Nature 2011, 13, 434–446. [Google Scholar] [CrossRef]
- Béaslas, O.; Metso, J.; Nissila, E.; Laurila, P.-P.; Kaiharju, E.; Batchu, K.C.; Kaipiainen, L.; Mäyränpää, M.I.; Yan, D.; Gylling, H.; et al. Osbpl8 Deficiency in Mouse Causes an Elevation of High-Density Lipoproteins and Gender-Specific Alterations of Lipid Metabolism. PLOS ONE 2013, 8, e58856. [Google Scholar] [CrossRef] [PubMed]
- Galmes, R.; Houcine, A.; Van Vliet, A.; Agostinis, P.; Jackson, C.L.; Giordano, F. ORP5/ORP8 localize to endoplasmic reticulum–mitochondria contacts and are involved in mitochondrial function. EMBO Rep. 2016, 17, 800–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robert, X.; Gouet, P. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res. 2014, 42, W320–W324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Massey, J.B. Membrane and protein interactions of oxysterols. Curr. Opin. Lipidol. 2006, 17, 296–301. [Google Scholar] [CrossRef] [Green Version]
- Chang, T.-Y.; Chang, C.C.; Ohgami, N.; Yamauchi, Y. Cholesterol Sensing, Trafficking, and Esterification. Annu. Rev. Cell Dev. Boil. 2006, 22, 129–157. [Google Scholar] [CrossRef]
- Beh, C.T.; Rine, J. A role for yeast oxysterol-binding protein homologs in endocytosis and in the maintenance of intracellular sterol-lipid distribution. J. Cell Sci. 2004, 117, 2983–2996. [Google Scholar] [CrossRef] [Green Version]
- Maeda, K.; Anand, K.; Chiapparino, A.; Kumar, A.; Poletto, M.; Kaksonen, M.; Gavin, A.-C. Interactome map uncovers phosphatidylserine transport by oxysterol-binding proteins. Nature 2013, 501, 257–261. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, Y.; Li, H.; Chieu, H.K.; Munn, A.L.; Yang, H. AAA ATPases regulate membrane association of yeast oxysterol binding proteins and sterol metabolism. EMBO J. 2005, 24, 2989–2999. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Duan, W.; Munn, A.L.; Yang, H. Molecular characterization of Osh6p, an oxysterol binding protein homolog in the yeast Saccharomyces cerevisiae. FEBS J. 2005, 272, 4703–4715. [Google Scholar] [CrossRef]
- Suchanek, M.; Hynynen, R.; Wohlfahrt, G.; Lehto, M.; Johansson, M.; Saarinen, H.; Radzikowska, A.; Thiele, C.; Olkkonen, V.M. The mammalian oxysterol-binding protein-related proteins (ORPs) bind 25-hydroxycholesterol in an evolutionarily conserved pocket. Biochem. J. 2007, 405, 473–480. [Google Scholar] [CrossRef] [Green Version]
- Zhou, T.; Li, S.; Zhong, W.; Vihervaara, T.; Béaslas, O.; Perttilä, J.; Luo, W.; Jiang, Y.; Lehto, M.; Olkkonen, V.M.; et al. OSBP-Related Protein 8 (ORP8) Regulates Plasma and Liver Tissue Lipid Levels and Interacts with the Nucleoporin Nup62. PLOS ONE 2011, 6, e21078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Kampen, E.; Béaslas, O.; Hildebrand, R.B.; Lammers, B.; Van Berkel, T.J.C.; Olkkonen, V.M.; Van Eck, M. Orp8 Deficiency in Bone Marrow-Derived Cells Reduces Atherosclerotic Lesion Progression in LDL Receptor Knockout Mice. PLOS ONE 2014, 9, e109024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vihervaara, T.; Käkelä, R.; Liebisch, G.; Tarasov, K.; Schmitz, G.; Olkkonen, V.M. Modification of the lipidome in RAW264.7 macrophage subjected to stable silencing of oxysterol-binding proteins. Biochimie 2013, 95, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Fan, J.; Chena, N. A Novel Regulator of Type II Diabetes: MicroRNA-143. Trends Endocrinol. Metab. 2018, 29, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Blumensatt, M.; Greulich, S.; De Wiza, D.H.; Mueller, H.; Maxhera, B.; Rabelink, M.J.; Hoeben, R.C.; Akhyari, P.; Al-Hasani, H.; Ruige, J.B.; et al. Activin A impairs insulin action in cardiomyocytes via up-regulation of miR-143. Cardiovasc. Res. 2013, 100, 201–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blumensatt, M.; Wronkowitz, N.; Wiza, C.; Cramer, A.; Mueller, H.; Rabelink, M.J.; Hoeben, R.C.; Eckel, J.; Sell, H.; Ouwens, D.M. Adipocyte-derived factors impair insulin signaling in differentiated human vascular smooth muscle cells via the upregulation of miR-143. Biochim. Biophys. Acta (BBA) - Mol. Basis Dis. 2014, 1842, 275–283. [Google Scholar] [CrossRef] [Green Version]
- Zhong, W.; Zhou, Y.; Li, J.; Mysore, R.; Luo, W.; Li, S.; Chang, M.-S.; Olkkonen, V.M.; Yan, D. OSBP-related protein 8 (ORP8) interacts with Homo sapiens sperm associated antigen 5 (SPAG5) and mediates oxysterol interference of HepG2 cell cycle. Exp. Cell Res. 2014, 322, 227–235. [Google Scholar] [CrossRef]
- Li, J.; Zheng, X.; Lou, N.; Zhong, W.; Yan, D. Oxysterol binding protein-related protein 8 mediates the cytotoxicity of 25-hydroxycholesterol. J. Lipid Res. 2016, 57, 1845–1853. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Zhang, L.; Fan, Y.; Zhang, D.; Qin, L.; Dong, S.; Li, G. Oxysterol-Binding Protein-Related Protein 8 Inhibits Gastric Cancer Growth Through Induction of ER Stress, Inhibition of Wnt Signaling, and Activation of Apoptosis. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 2017, 25, 799–808. [Google Scholar] [CrossRef]
- Zhang, Z.; Gao, W.; Zhou, L.; Chen, Y.; Qin, S.; Zhang, L.; Liu, J.; He, Y.; Lei, Y.; Chen, H.-N.; et al. Repurposing Brigatinib for the Treatment of Colorectal Cancer Based on Inhibition of ER-phagy. Theranostics 2019, 9, 4878–4892. [Google Scholar] [CrossRef]
- Ishikawa, S.; Nagai, Y.; Masuda, T.; Koga, Y.; Nakamura, T.; Imamura, Y.; Takamori, H.; Hirota, M.; Funakosi, A.; Fukushima, M.; et al. The role of oxysterol binding protein-related protein 5 in pancreatic cancer. Cancer Sci. 2009, 101, 898–905. [Google Scholar] [CrossRef] [PubMed]
- Lippincott-Schwartz, J.; Phair, R. Lipids and cholesterol as regulators of traffic in the endomembrane system. Annu. Rev. Biophys. 2010, 39, 559–578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Thiele, C.; Huttner, W.B. Cholesterol is Required for the Formation of Regulated and Constitutive Secretory Vesicles from the trans-Golgi Network. Traffic 2000, 1, 952–962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Runz, H.; Miura, K.; Weiss, M.; Pepperkok, R. Sterols regulate ER-export dynamics of secretory cargo protein ts-O45-G. EMBO J. 2006, 25, 2953–2965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ridsdale, A.; Denis, M.; Gougeon, P.-Y.; Ngsee, J.K.; Presley, J.F.; Zha, X. Cholesterol Is Required for Efficient Endoplasmic Reticulum-to-Golgi Transport of Secretory Membrane Proteins. Mol. Boil. Cell 2006, 17, 1593–1605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nabi, I.R.; Le, P.U. Caveolae/raft-dependent endocytosis. J. Cell Boil. 2003, 161, 673–677. [Google Scholar] [CrossRef]
- Kozminski, K.G.; Alfaro, G.; Dighe, S.; Beh, C.T. Homologues of Oxysterol-Binding Proteins Affect Cdc42p- and Rho1p-Mediated Cell Polarization in Saccharomyces cerevisiae. Traffic 2006, 7, 1224–1242. [Google Scholar] [CrossRef]
- Béaslas, O.; Vihervaara, T.; Li, J.; Laurila, P.-P.; Yan, D.; Olkkonen, V.M. Silencing of OSBP-related protein 8 (ORP8) modifies the macrophage transcriptome, nucleoporin p62 distribution, and migration capacity. Exp. Cell Res. 2012, 318, 1933–1945. [Google Scholar] [CrossRef]
- Schulz, T.A.; Choi, M.-G.; Raychaudhuri, S.; Mears, J.A.; Ghirlando, R.; Hinshaw, J.E.; Prinz, W.A. Lipid-regulated sterol transfer between closely apposed membranes by oxysterol-binding protein homologues. J. Cell Boil. 2009, 187, 889–903. [Google Scholar] [CrossRef] [Green Version]
- Von Filseck, J.M.; Opi, A.; Delfosse, V.; Vanni, S.; Jackson, C.L.; Bourguet, W.; Drin, G.; Čopič, A. Phosphatidylserine transport by ORP/Osh proteins is driven by phosphatidylinositol 4-phosphate. Science 2015, 349, 432–436. [Google Scholar] [CrossRef]
- Sohn, M.; Ivanova, P.; Brown, H.A.; Tóth, D.; Varnai, P.; Kim, Y.J.; Balla, T. Lenz-Majewski mutations in PTDSS1 affect phosphatidylinositol 4-phosphate metabolism at ER-PM and ER-Golgi junctions. Proc. Natl. Acad. Sci. USA 2016, 113, 4314–4319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steenbergen, R.; Nanowski, T.S.; Beigneux, A.; Kulinski, A.; Young, S.G.; Vance, J.E. Disruption of the Phosphatidylserine Decarboxylase Gene in Mice Causes Embryonic Lethality and Mitochondrial Defects*. J. Boil. Chem. 2005, 280, 40032–40040. [Google Scholar] [CrossRef] [Green Version]
- Tian, S.; Ohta, A.; Horiuchi, H.; Fukuda, R. Oxysterol-binding protein homologs mediate sterol transport from the endoplasmic reticulum to mitochondria in yeast. J. Boil. Chem. 2018, 293, 5636–5648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pulli, I.; Lassila, T.; Pan, G.; Yan, D.; Olkkonen, V.M.; Törnquist, K. Oxysterol-binding protein related-proteins (ORPs) 5 and 8 regulate calcium signaling at specific cell compartments. Cell Calcium 2018, 72, 62–69. [Google Scholar] [CrossRef] [Green Version]
- Nowycky, M.C.; Thomas, A.P. Intracellular calcium signaling. J. Cell Sci. 2002, 115, 3715–3716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santulli, G.; Nakashima, R.; Yuan, Q.; Marks, A.R. Intracellular calcium release channels: An update. J. Physiol. 2017, 595, 3041–3051. [Google Scholar] [CrossRef] [Green Version]
- Du, X.; Zhou, L.; Aw, Y.C.; Mak, H.Y.; Xu, Y.; Rae, J.; Wang, W.; Zadoorian, A.; Hancock, S.E.; Osborne, B.; et al. ORP5 localizes to ER–lipid droplet contacts and regulates the level of PI(4)P on lipid droplets. J. Cell Boil. 2019, 219, 219. [Google Scholar] [CrossRef] [Green Version]
- Renne, M.F.; Emerling, B.M. ORP5 regulates PI(4)P on the lipid droplet: Novel players on the monolayer. J. Cell Boil. 2020, 219, 219. [Google Scholar] [CrossRef] [Green Version]
- Dong, R.; Saheki, Y.; Swarup, S.; Lucast, L.; Harper, J.W.; De Camilli, P. Endosome-ER Contacts Control Actin Nucleation and Retromer Function through VAP-Dependent Regulation of PI4P. Cell 2016, 166, 408–423. [Google Scholar] [CrossRef] [Green Version]
- Zhao, K.; Ridgway, N.D. Oxysterol-Binding Protein-Related Protein 1L Regulates Cholesterol Egress from the Endo-Lysosomal System. Cell Rep. 2017, 19, 1807–1818. [Google Scholar] [CrossRef] [Green Version]
- Maekawa, M.; Fairn, G.D. Complementary probes reveal that phosphatidylserine is required for the proper transbilayer distribution of cholesterol. J. Cell Sci. 2015, 128, 1422–1433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- E Kattan, W.; Chen, W.; Ma, X.; Lan, T.H.; Van Der Hoeven, D.; Van Der Hoeven, R.; Hancock, J.F. Targeting plasma membrane phosphatidylserine content to inhibit oncogenic KRAS function. Life Sci. Alliance 2019, 2, e201900431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, N.C.; Girik, V.; Nunes-Hasler, P. ORP5 and ORP8: Sterol Sensors and Phospholipid Transfer Proteins at Membrane Contact Sites? Biomolecules 2020, 10, 928. https://doi.org/10.3390/biom10060928
Santos NC, Girik V, Nunes-Hasler P. ORP5 and ORP8: Sterol Sensors and Phospholipid Transfer Proteins at Membrane Contact Sites? Biomolecules. 2020; 10(6):928. https://doi.org/10.3390/biom10060928
Chicago/Turabian StyleSantos, Nina Criado, Vladimir Girik, and Paula Nunes-Hasler. 2020. "ORP5 and ORP8: Sterol Sensors and Phospholipid Transfer Proteins at Membrane Contact Sites?" Biomolecules 10, no. 6: 928. https://doi.org/10.3390/biom10060928
APA StyleSantos, N. C., Girik, V., & Nunes-Hasler, P. (2020). ORP5 and ORP8: Sterol Sensors and Phospholipid Transfer Proteins at Membrane Contact Sites? Biomolecules, 10(6), 928. https://doi.org/10.3390/biom10060928




