Differential Anti-Proliferative and Anti-Migratory Activities of Ursolic Acid, 3-O-Acetylursolic Acid and Their Combination Treatments with Quercetin on Melanoma Cells
Abstract
1. Introduction
2. Materials and Methods
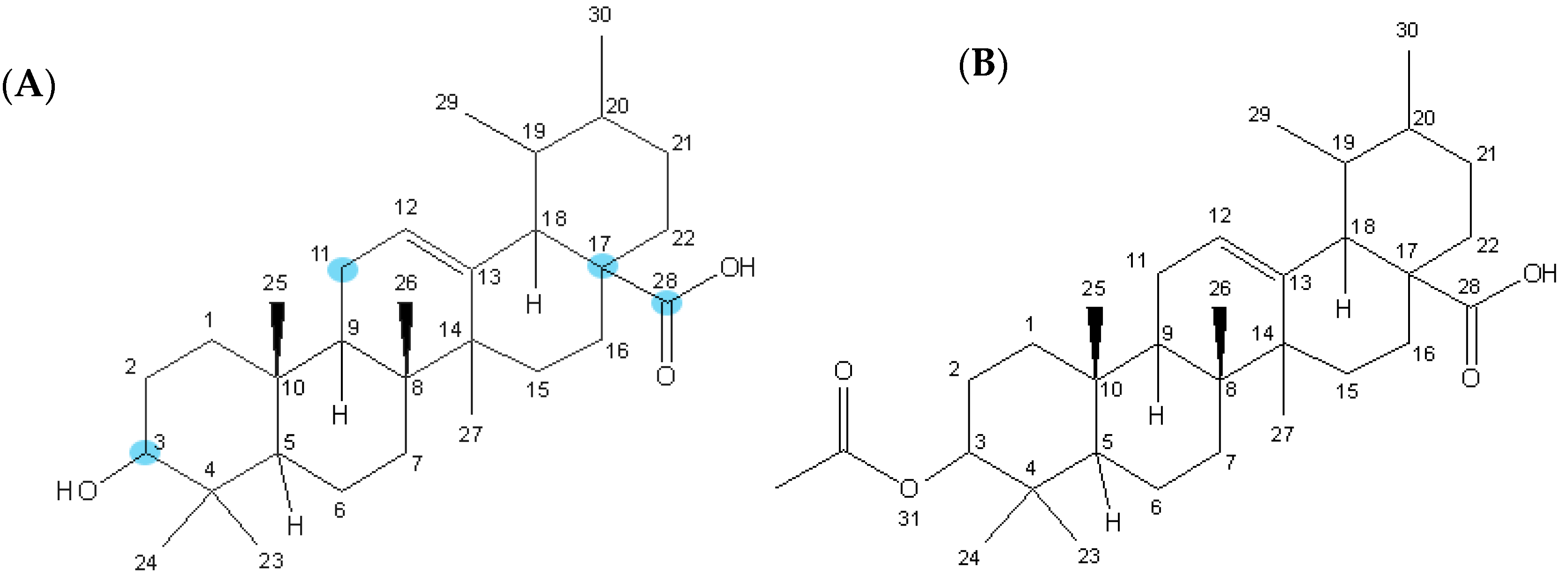
2.1. Materials
2.2. Cell Lines
2.3. Sulforhodamine B (SRB) Assay
2.4. Cell Cycle Analysis
2.5. Apoptosis Induction Analysis by Flow Cytometry
2.6. Caspase-3/7 Activity Assay
2.7. Phase-Contrast and Fluorescence Microscopy
2.8. Western Blot Analysis
2.9. Scratch Assay for Anti-Migratory Activity
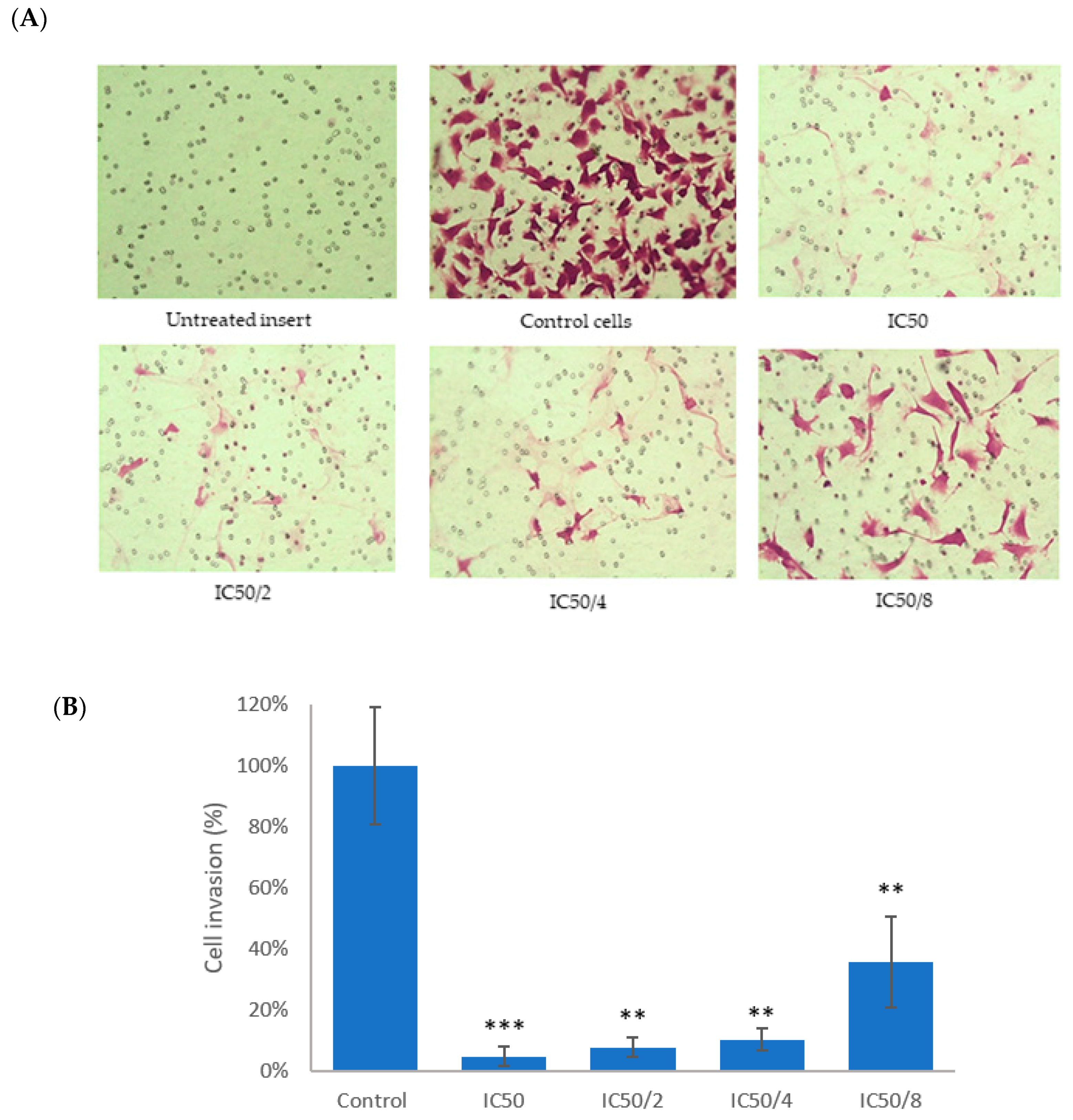
2.10. Transwell© Assay for Anti-Invasive Activity
2.11. Statistical Analysis
3. Results
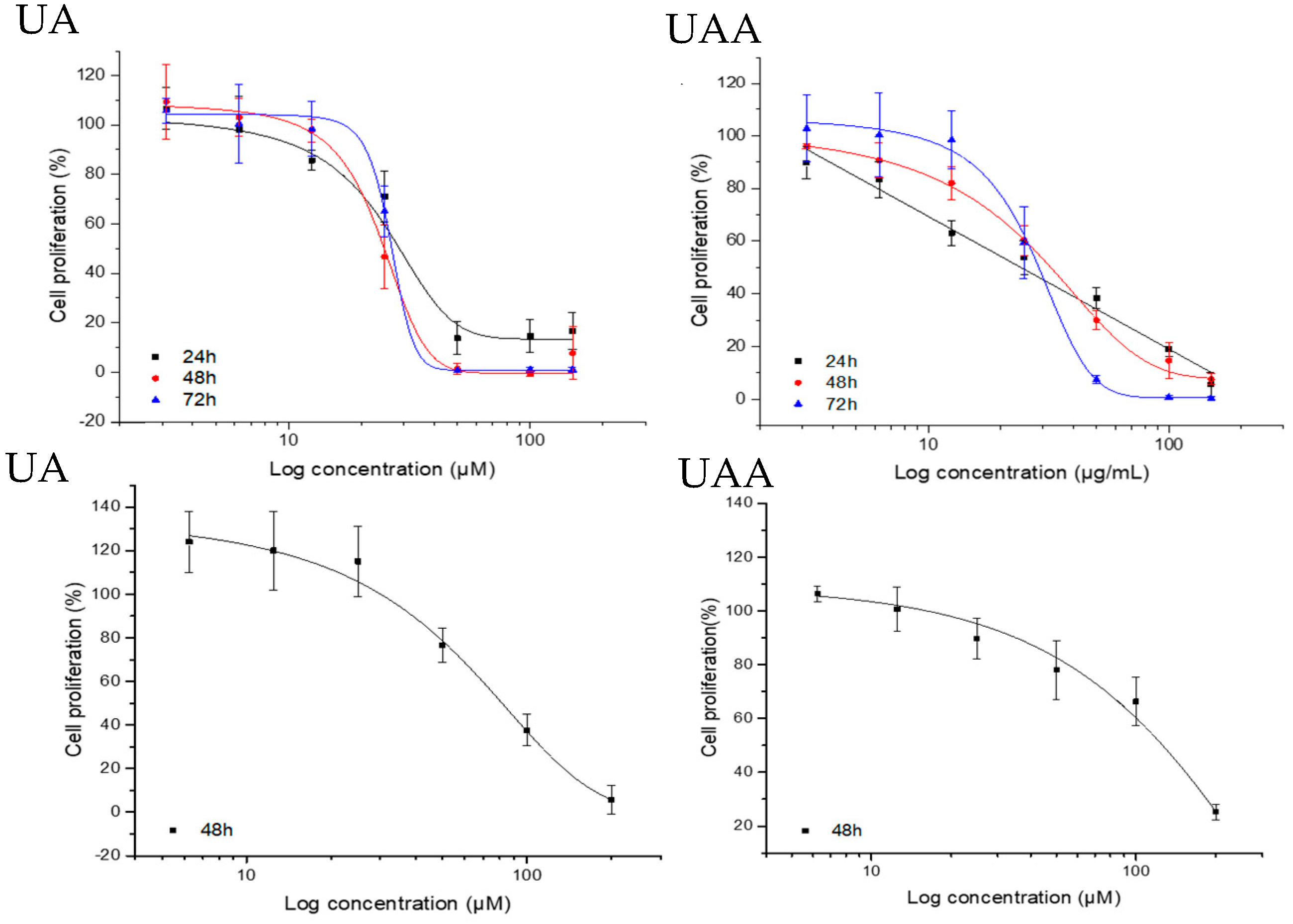
3.1. Effects on A375 Human Melanoma Cells Proliferation and Selectivity Towards Adult Human Dermal Fibroblasts
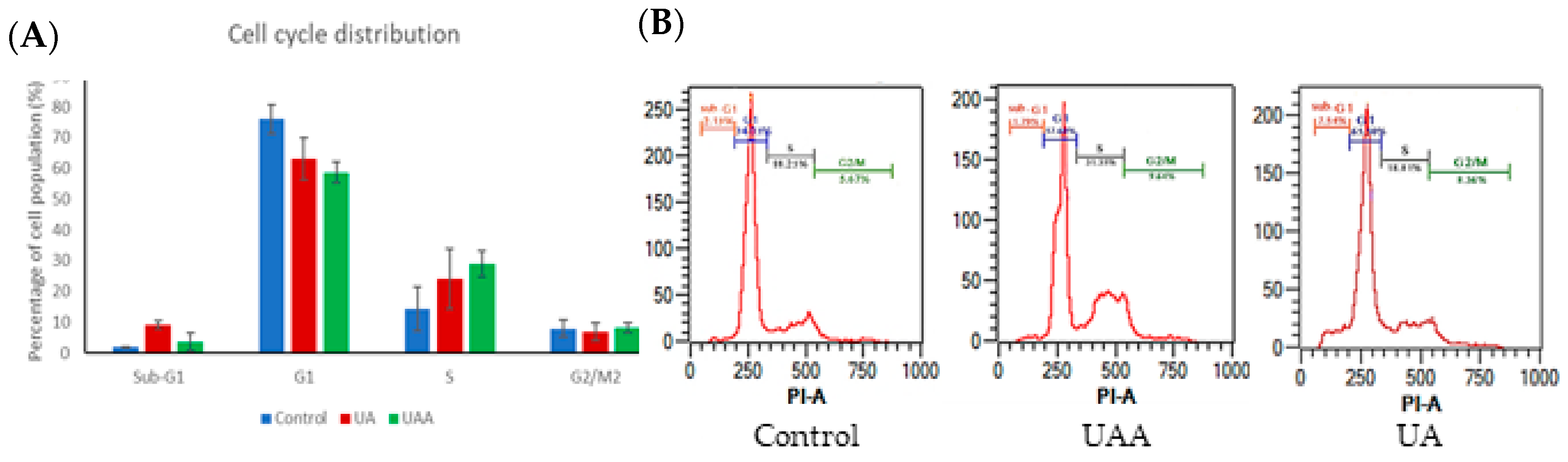
3.2. Effects on A375 Cell Cycle Distribution
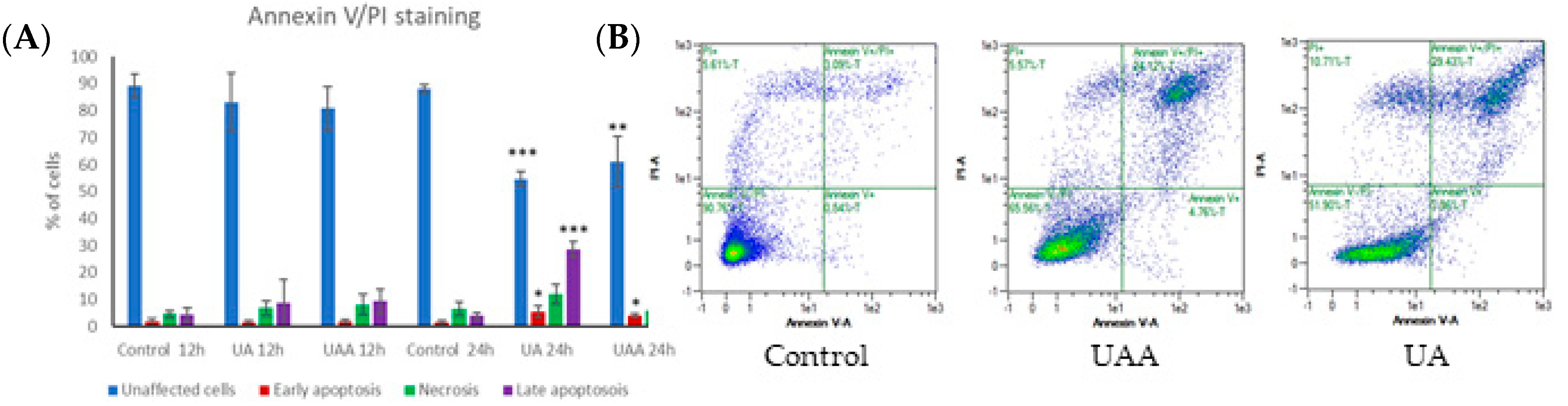
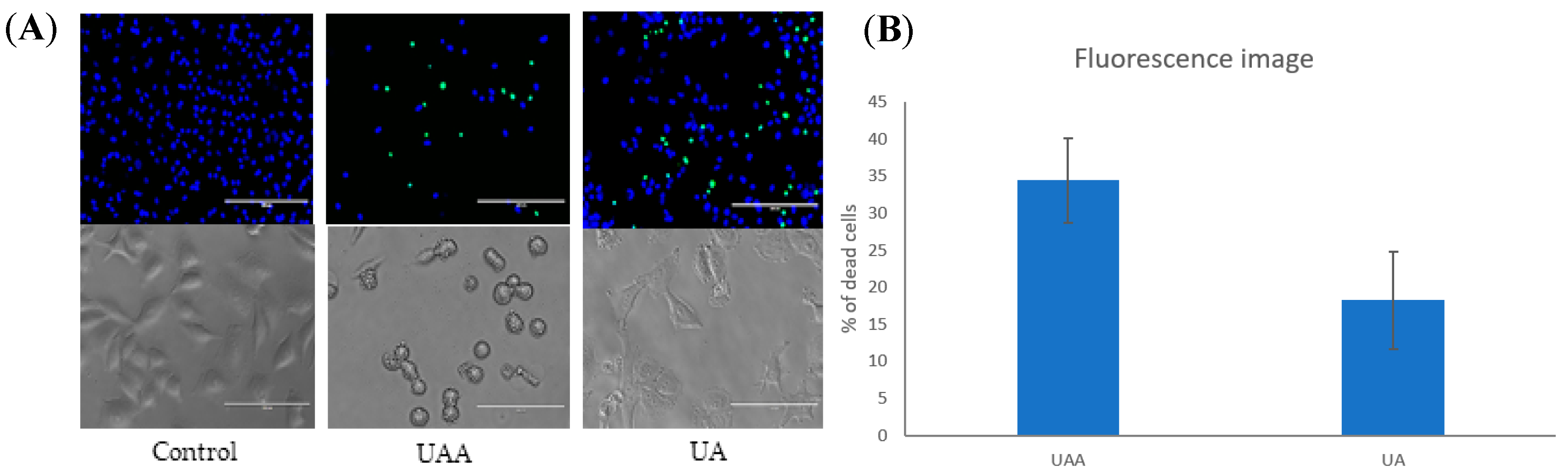
3.3. Induction of Apoptosis According to Annexin V-FITC/Propidium Iodide (PI) Staining
3.4. Changes in Cell Morphology Associated with the Treatment
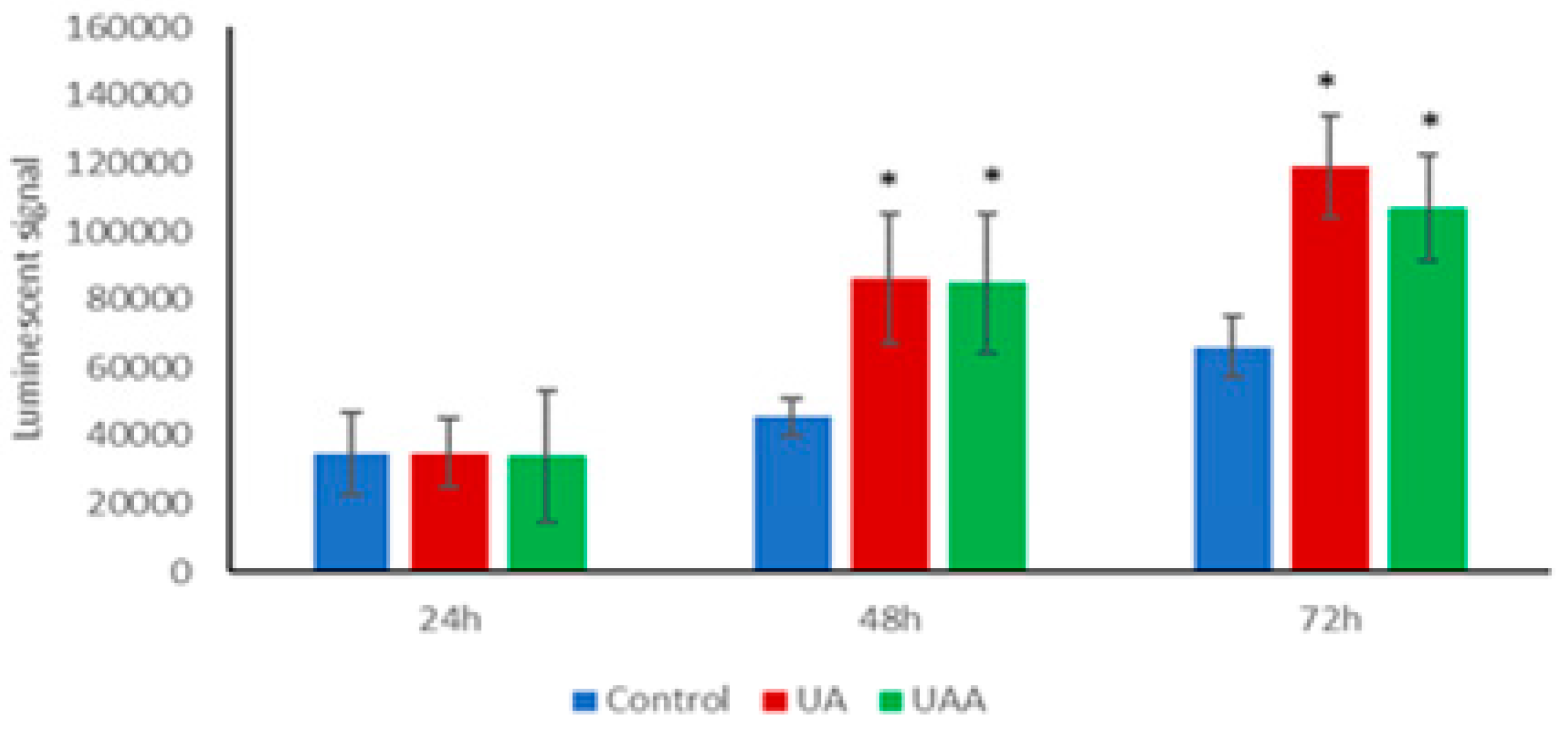
3.5. Induction of Caspase-3/7 Activity
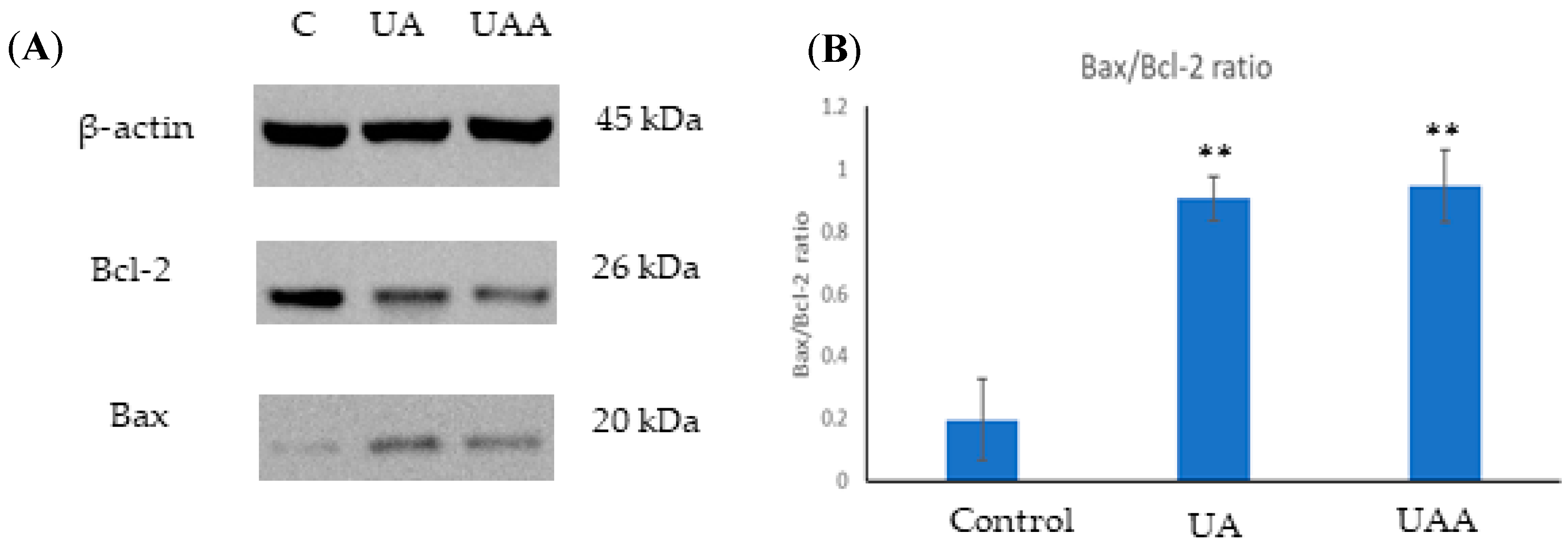
3.6. Bax and Bcl-2 Expression in the Presence of Ursolic Acid and Its Acetate
3.7. Effects of the Combination Treatments
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chudzik, M.; Korzonek-Szlacheta, I.; Król, W. Triterpenes as potentially cytotoxic compounds. Molecules 2015, 20, 1610–1625. [Google Scholar] [CrossRef]
- Shan, J.-Z.; Xuan, Y.-Y.; Zheng, S.; Dong, Q.; Zhang, S.-Z. Ursolic acid inhibits proliferation and induces apoptosis of HT-29 colon cancer cells by inhibiting the EGFR/MAPK pathway. J. Zhejiang Univ. Sci. B 2009, 10, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, L.; Wang, B.; Xiang, J. Effects of ursolic acid on the proliferation and apoptosis of human ovarian cancer cells. J. Huazhong Univ. Sci. Technol. 2009, 29, 761–764. [Google Scholar] [CrossRef] [PubMed]
- Kassi, E.; Sourlingas, T.G.; Spiliotaki, M.; Papoutsi, Z.; Pratsinis, H.; Aligiannis, N.; Moutsatsou, P. Ursolic acid triggers apoptosis and Bcl-2 downregulation in MCF-7 breast cancer cells. Cancer Investig. 2009, 27, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, M.K.; Rajendran, P.; Li, F.; Nema, T.; Vali, S.; Abbasi, T.; Kapoor, S.; Sharma, A.; Kumar, A.P.; Ho, P.C.; et al. Ursolic acid inhibits multiple cell survival pathways leading to suppression of growth of prostate cancer xenograft in nude mice. J. Mol. Med. 2011, 89, 713–727. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, M.; Rabe, S.Z.T.; Balali-Mood, M.; Karimi, G.; Tabasi, N.; Riahi-Zanjani, B. Ursolic acid induced apoptotic cell death following activation of caspases in isolated human melanoma cells. Cell Biol. Int. 2015, 39, 230–236. [Google Scholar] [CrossRef]
- Harmand, P.-O.; Duval, R.; Delage, C.; Simon, A. Ursolic acid induces apoptosis through mitochondrial intrinsic pathway and caspase-3 activation in M4Beu melanoma cells. Int. J. Cancer 2005, 114, 1–11. [Google Scholar] [CrossRef]
- Lee, Y.H.; Wang, E.; Kumar, N.; Glickman, R.D. Ursolic acid differentially modulates apoptosis in skin melanoma and retinal pigment epithelial cells exposed to UV-VIS broadband radiation. Apoptosis 2014, 19, 816–828. [Google Scholar] [CrossRef]
- Strüh, C.M.; Jäger, S.; Kersten, A.; Schempp, C.M.; Scheffler, A.; Martin, S.F. Triterpenoids amplify anti-tumoral effects of mistletoe extracts on murine B16-F10 melanoma in vivo. PLoS ONE 2013, 8, e62148. [Google Scholar] [CrossRef]
- Wozniak, L.; Skapska, S.; Marszalek, K. Ursolic Acid—A Pentacyclic Triterpenoid with a Wide Spectrum of Pharmacological Activities. Molecules 2015, 20, 20614–20641. [Google Scholar] [CrossRef]
- Shao, J.-W.; Dai, Y.-C.; Xue, J.-P.; Wang, J.-C.; Lin, F.-P.; Guo, Y.-H. In vitro and in vivo anticancer activity evaluation of ursolic acid derivatives. Eur. J. Med. Chem. 2011, 46, 2652–2661. [Google Scholar] [CrossRef]
- Batra, A.; Sastry, V.G. Extraction of ursolic acid from Ocimum sanctum and synthesis of its novel derivatives: Effects on extracellular homocysteine, dihydrofolate reductase activity and proliferation of HepG2 human hepatoma cells. Pteridines 2013, 24. [Google Scholar] [CrossRef]
- Mazumder, K.; Tanaka, K.; Fukase, K. Cytotoxic activity of ursolic acid derivatives obtained by isolation and oxidative derivatization. Molecules 2013, 18, 8929–8944. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.M.; Cai, S.Q.; Cui, J.R.; Wang, R.Q.; Tu, P.F.; Hattori, M.; Daneshtalab, M. The cytotoxic activity of ursolic acid derivatives. Eur. J. Med. Chem. 2005, 40, 582–589. [Google Scholar] [CrossRef]
- Ciftci, H.I.; Radwan, M.O.; Ozturk, S.E.; Ulusoy, N.G.; Sozer, E.; Ellakwa, D.E.; Ocak, Z.; Can, M.; Ali, T.F.S.; Abd-Alla, H.I.; et al. Design, Synthesis and Biological Evaluation of Pentacyclic Triterpene Derivatives: Optimization of Anti-ABL Kinase Activity. Molecules 2019, 24, 3535. [Google Scholar] [CrossRef] [PubMed]
- AlQathama, A.; Bader, A.; Khondkar, P.; Gibbons, S.; Prieto, J.M. Bioguided Isolation of Cytotoxic Compounds Against Melanoma Cells from Carissa Spinarum L. In Proceedings of the 63rd International Congress and Annual Meeting of the Society for Medicinal Plant and Natural Product Research (GA2015), Budapest, Hungary, 23–27 August 2015. [Google Scholar]
- Ali, A.; Ahmed Zaki, M.; Parveen, A.; Ali, Z.; Khan, I.A. Bioassay guided isolation of mosquito biting deterrent compounds from Strumpfia maritima. Pest. Manag. Sci. 2020. [Google Scholar] [CrossRef] [PubMed]
- Caltagirone, S.; Rossi, C.; Poggi, A.; Ranelletti, F.O.; Natali, P.G.; Brunetti, M.; Aiello, F.B.; Piantelli, M. Flavonoids apigenin and quercetin inhibit melanoma growth and metastatic potential. Int. J. Cancer 2000, 87, 595–600. [Google Scholar] [CrossRef]
- Prasad, S.; Tyagi, A.K.; Aggarwal, B.B. Chemosensitization by Ursolic Acid: A New Avenue for Cancer Therapy. In Role of Nutraceuticals in Chemoresistance to Cancer; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Brito, A.F.; Ribeiro, M.; Abrantes, A.M.; Pires, A.S.; Teixo, R.J.; Tralhao, J.G.; Botelho, M.F. Quercetin in Cancer Treatment, Alone or in Combination with Conventional Therapeutics? Curr. Med. Chem. 2015, 22, 3025–3039. [Google Scholar] [CrossRef]
- Vanicha, V.; Kanyawim, K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat. Protoc. 2006, 1, 1112–1116. [Google Scholar] [CrossRef]
- Li, Y.-L.; Gan, G.-P.; Zhang, H.-Z.; Wu, H.-Z.; Li, C.-L.; Huang, Y.-P.; Liu, Y.-W.; Liu, J.-W. A flavonoid glycoside isolated from Smilax china L. rhizome in vitro anti-cancer effects on human cancer cell lines. J. Ethnopharmacol. 2007, 113, 115–124. [Google Scholar] [CrossRef]
- Biswas, R.; Mandal, S.K.; Dutta, S.; Bhattacharyya, S.S.; Boujedaini, N.; Khuda-Bukhsh, A.R. Thujone-rich fraction of Thuja occidentalis demonstrates major anti-cancer potentials: Evidences from in vitro studies on A375 cells. Evid. -Based Complement. Altern. Med. 2011, 2011, 568148. [Google Scholar] [CrossRef]
- Wang, H.; Ke, M.; Tian, Y.; Wang, J.; Li, B.; Wang, Y.; Dou, J.; Zhou, C. BF-30 selectively inhibits melanoma cell proliferation via cytoplasmic membrane permeabilization and DNA-binding in vitro and in B16F10-bearing mice. Eur. J. Pharmacol. 2013, 707, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Sun, J.; Cheng, J.; Djeu, J.Y.; Wei, S.; Sebti, S. Akt mediates Ras downregulation of RhoB, a suppressor of transformation, invasion, and metastasis. Mol. Cell. Biol. 2004, 24, 5565–5576. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.; Hayball, M. CalcuSyn for Windows, Multiple drug Dose-Effect Nalyser and Manual; Biosoft: Cambridge, UK, 1996; p. 68. [Google Scholar]
- Hsu, Y.-L.; Kuo, P.-L.; Lin, C.-C. Proliferative inhibition, cell-cycle dysregulation, and induction of apoptosis by ursolic acid in human non-small cell lung cancer A549 cells. Life Sci. 2004, 75, 2303–2316. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, F.; Yang, L.; Mei, Y.; Long, H.; Zhang, X.; Zhang, J.; Qimuge, S.; Su, X. Ursolic acid inhibits proliferation and induces apoptosis of cancer cells in vitro and in vivo. J. Biomed. Biotechnol. 2011, 2011, 419343. [Google Scholar] [CrossRef] [PubMed]
- Duval, R.E.; Harmand, P.O.; Jayat-Vignoles, C.; Cook-Moreau, J.; Pinon, A.; Delage, C.; Simon, A. Differential involvement of mitochondria during ursolic acid-induced apoptotic process in HaCaT and M4Beu cells. Oncol. Rep. 2008, 19, 145–149. [Google Scholar] [CrossRef][Green Version]
- Es-Saady, D.; Simon, A.; Jayat-Vignoles, C.; Chulia, A.J.; Delage, C. MCF-7 cell cycle arrested at G1 through ursolic acid, and increased reduction of tetrazolium salts. Anticancer Res. 1996, 16, 481–486. [Google Scholar]
- Pagliacci, M.C.; Spinozzi, F.; Migliorati, G.; Fumi, G.; Smacchia, M.; Grignani, F.; Riccardi, C.; Nicoletti, I. Genistein inhibits tumour cell growth in vitro but enhances mitochondrial reduction of tetrazolium salts: A further pitfall in the use of the MTT assay for evaluating cell growth and survival. Eur. J. Cancer 1993, 29, 1573–1577. [Google Scholar] [CrossRef]
- Beberok, A.; Wrzesniok, D.; Minecka, A.; Rok, J.; Delijewski, M.; Rzepka, Z.; Respondek, M.; Buszman, E. Ciprofloxacin-mediated induction of S-phase cell cycle arrest and apoptosis in COLO829 melanoma cells. Pharmacol. Rep. 2018, 70, 6–13. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, L.; Wu, S.; Teraishi, F.; Davis, J.J.; Jacob, D.; Fang, B. Induction of S-phase arrest and p21 overexpression by a small molecule 2[lsqb][lsqb]3-(2,3-dichlorophenoxy)propyl[rsqb] amino[rsqb]ethanol in correlation with activation of ERK. Oncogene 2004, 23, 4984–4992. [Google Scholar] [CrossRef]
- Pradhan, S.J.; Mishra, R.; Sharma, P.; Kundu, G.C. Quercetin and sulforaphane in combination suppress the progression of melanoma through the down-regulation of matrix metalloproteinase-9. Exp. Ther. Med. 2010, 1, 915–920. [Google Scholar] [CrossRef] [PubMed]
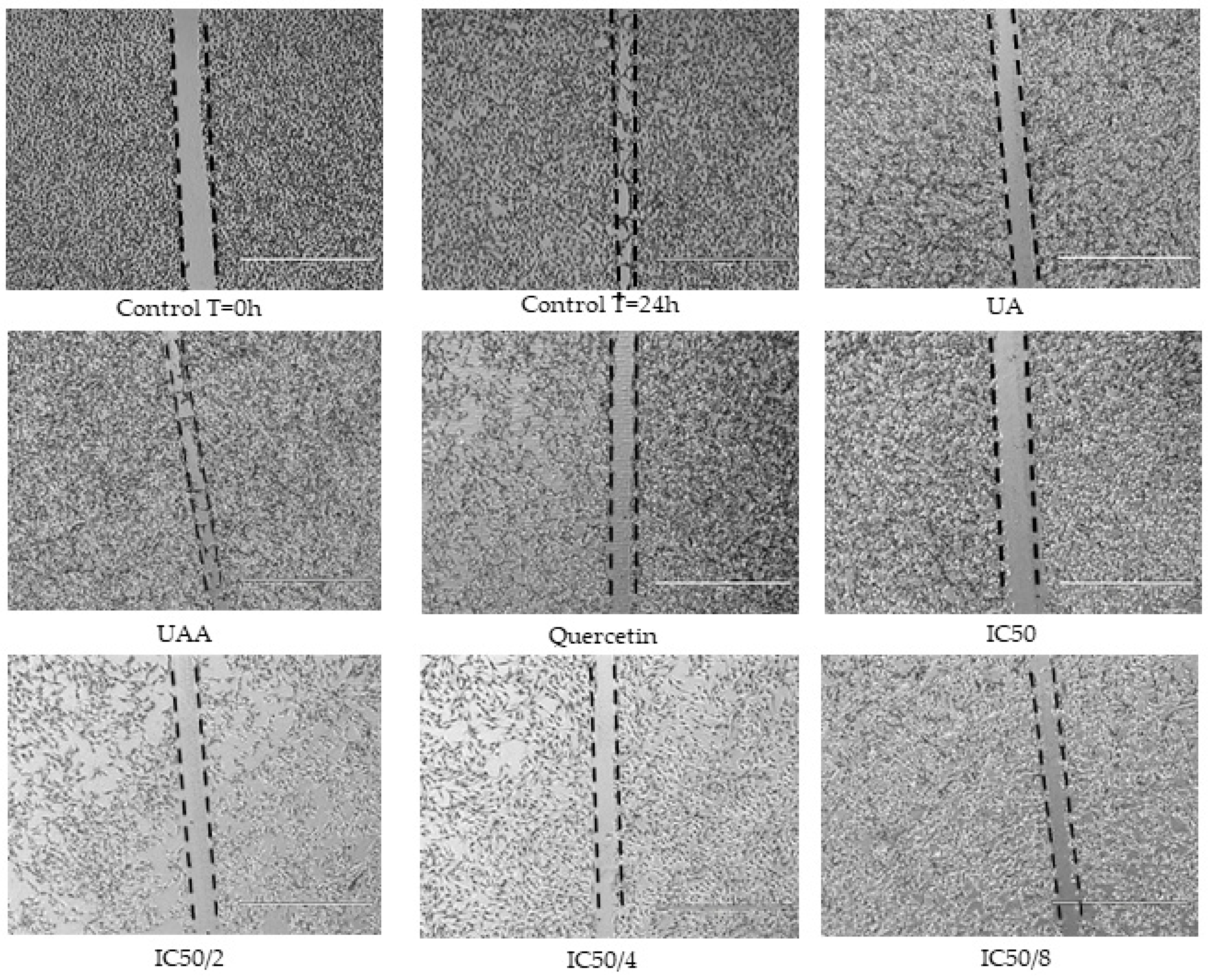
| Ratios | Quercetin µM | Ursolic Acid µM | Relative Migration | Combination Index | Relative Synergy 1 |
|---|---|---|---|---|---|
| n/a | 125 | 0 | 50% | n/a | n/a |
| n/a | 0 | 13.7 | 50% | n/a | n/a |
| IC50 | 125 | 13.7 | 24% | 0.90-1.10 | ± |
| IC50/2 | 65.5 | 6.85 | 34% | 0.7-0.85 | ++ |
| IC50/4 | 31.25 | 3.42 | 44% | 0.3-0.7 | +++ |
| IC50/8 | 15.62 | 1.71 | 52% | 0.3-0.7 | +++ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
AlQathama, A.; Shao, L.; Bader, A.; Khondkar, P.; Gibbons, S.; M Prieto, J. Differential Anti-Proliferative and Anti-Migratory Activities of Ursolic Acid, 3-O-Acetylursolic Acid and Their Combination Treatments with Quercetin on Melanoma Cells. Biomolecules 2020, 10, 894. https://doi.org/10.3390/biom10060894
AlQathama A, Shao L, Bader A, Khondkar P, Gibbons S, M Prieto J. Differential Anti-Proliferative and Anti-Migratory Activities of Ursolic Acid, 3-O-Acetylursolic Acid and Their Combination Treatments with Quercetin on Melanoma Cells. Biomolecules. 2020; 10(6):894. https://doi.org/10.3390/biom10060894
Chicago/Turabian StyleAlQathama, Aljawharah, Luying Shao, Ammar Bader, Proma Khondkar, Simon Gibbons, and Jose M Prieto. 2020. "Differential Anti-Proliferative and Anti-Migratory Activities of Ursolic Acid, 3-O-Acetylursolic Acid and Their Combination Treatments with Quercetin on Melanoma Cells" Biomolecules 10, no. 6: 894. https://doi.org/10.3390/biom10060894
APA StyleAlQathama, A., Shao, L., Bader, A., Khondkar, P., Gibbons, S., & M Prieto, J. (2020). Differential Anti-Proliferative and Anti-Migratory Activities of Ursolic Acid, 3-O-Acetylursolic Acid and Their Combination Treatments with Quercetin on Melanoma Cells. Biomolecules, 10(6), 894. https://doi.org/10.3390/biom10060894







