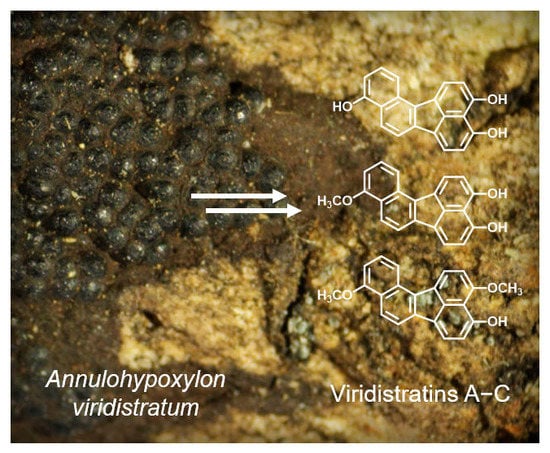
Viridistratins A−C, Antimicrobial and Cytotoxic Benzo[j]fluoranthenes from Stromata of Annulohypoxylon viridistratum (Hypoxylaceae, Ascomycota)
Abstract
1. Introduction
2. Materials and Methods
2.1. General
2.2. Fungal Material
2.3. Extraction and Isolation
2.4. Antimicrobial Activity Assay
2.5. Cytotoxicity Assay
2.6. Spectral Data
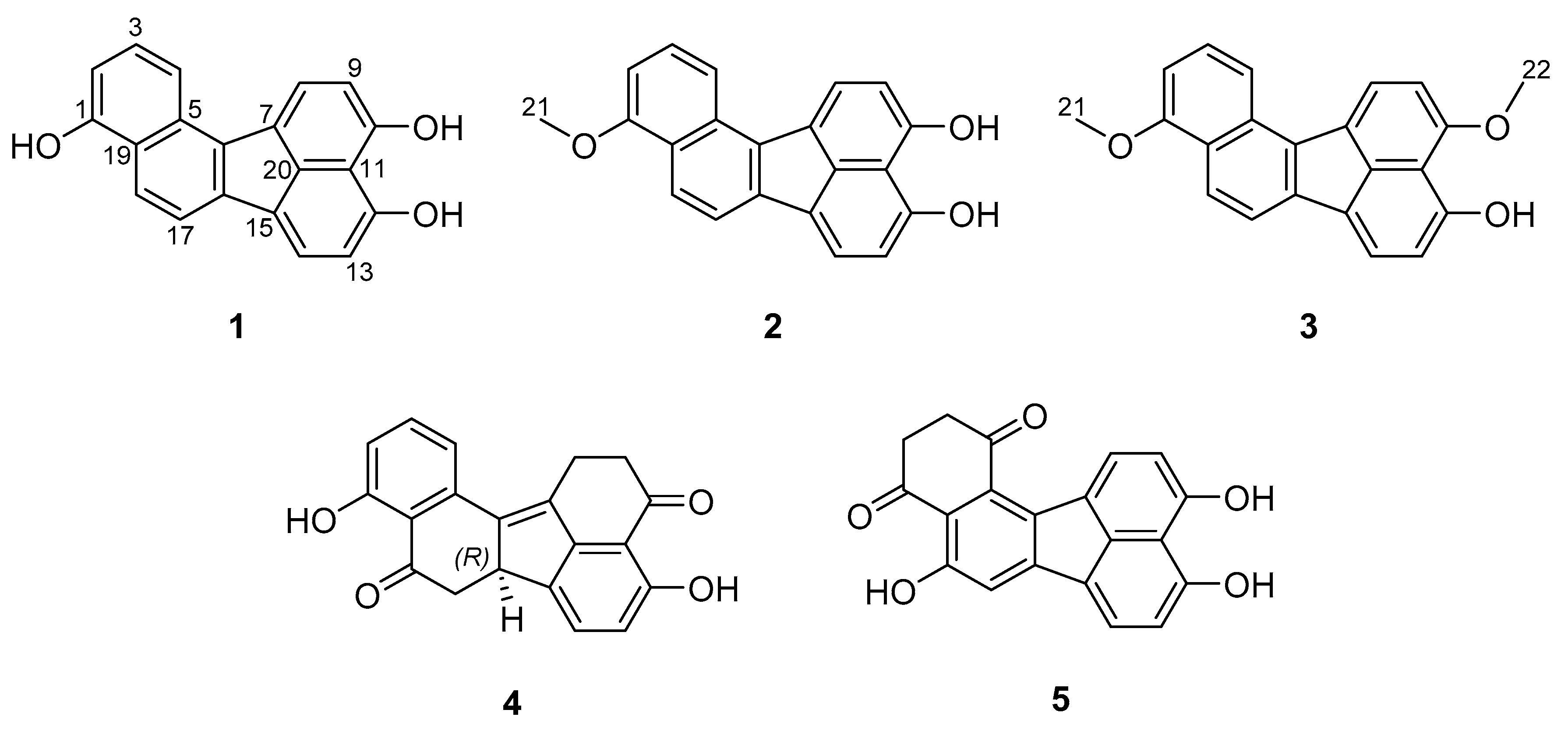
2.6.1. Viridistratin A (1)
2.6.2. Viridistratin B (2)
2.6.3. Viridistratin C (3)
3. Results
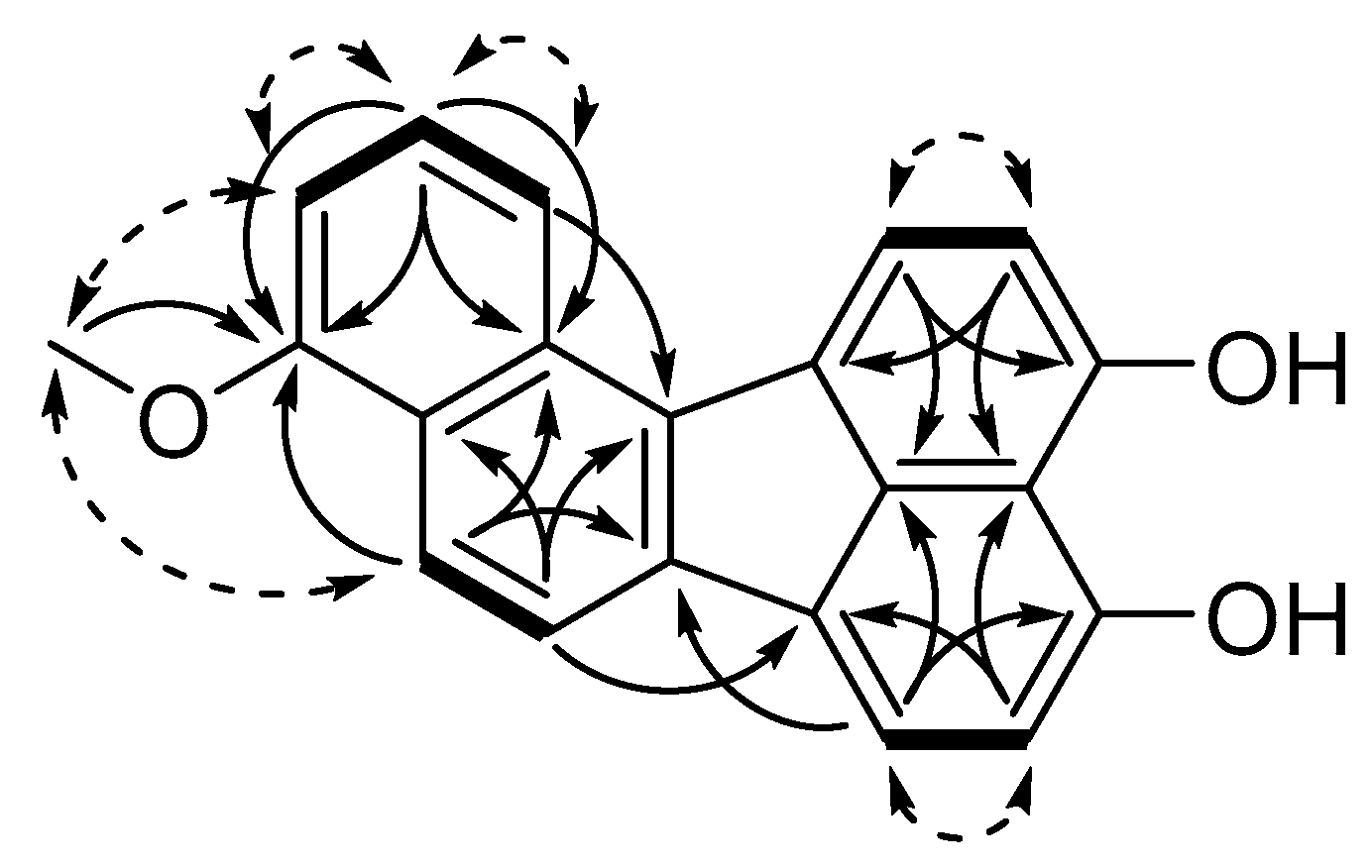
3.1. Structure Elucidation of Viridistratins A−C (1−3)
3.2. Antibacterial, Antifungal and Cytotoxic Activities of Compounds 1−5
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wendt, L.; Sir, E.B.; Kuhnert, E.; Heitkämper, S.; Lambert, C.; Hladki, A.I.; Romero, A.I.; Luangsa-ard, J.J.; Srikitikulchai, P.; Peršoh, D.; et al. Resurrection and emendation of the Hypoxylaceae, recognised from a multigene phylogeny of the Xylariales. Mycol. Prog. 2018, 17, 115–154. [Google Scholar] [CrossRef]
- Kuhnert, E.; Sir, E.B.; Lambert, C.; Hyde, K.D.; Hladki, A.I.; Romero, A.I.; Rohde, M.; Stadler, M. Phylogenetic and chemotaxonomic resolution of the genus Annulohypoxylon (Xylariaceae) including four new species. Fungal Divers. 2017, 85, 1–43. [Google Scholar] [CrossRef]
- Hsieh, H.-M.; Ju, Y.-M.; Rogers, J.D. Molecular phylogeny of Hypoxylon and closely related genera. Mycologia 2017, 97, 844–865. [Google Scholar] [CrossRef]
- Helaly, S.E.; Thongbai, B.; Stadler, M. Diversity of biologically active secondary metabolites from endophytic and saprotrophic fungi of the ascomycete order Xylariales. Nat. Prod. Rep. 2018, 35, 992–1014. [Google Scholar] [CrossRef] [PubMed]
- Stadler, M.; Læssøe, T.; Fournier, J.; Decock, C.; Schmieschek, B.; Tichy, H.-V.; Peršoh, D. A polyphasic taxonomy of Daldinia (Xylariaceae). Stud. Mycol. 2014, 77, 1–143. [Google Scholar] [CrossRef] [PubMed]
- Stadler, M.; Fournier, J.; Granmo, A.; Beltrán-Tejera, E. The “red Hypoxylons” of the temperate and subtropical Northern hemisphere. N. Am. Fungi 2008, 3, 73–125. [Google Scholar] [CrossRef]
- Quang, D.N.; Hashimoto, T.; Nomura, Y.; Wollweber, H.; Hellwig, V.; Fournier, J.; Stadler, M.; Asakawa, Y. Cohaerins A and B, azaphilones from the fungus Hypoxylon cohaerens, and comparison of HPLC-based metabolite profiles in Hypoxylon sect. Annulata. Phytochemistry 2005, 66, 797–809. [Google Scholar] [CrossRef]
- Surup, F.; Narmani, A.; Wendt, L.; Pfütze, S.; Kretz, R.; Becker, K.; Menbrivès, C.; Giosa, A.; Elliott, M.; Petit, C.; et al. Identification of fungal fossils and novel azaphilone pigments in ancient carbonised specimens of Hypoxylon fragiforme from forest soils of Châtillon-sur-Seine (Burgundy). Fungal Divers. 2018, 92, 345–356. [Google Scholar] [CrossRef]
- Daranagama, D.A.; Hyde, K.D.; Sir, E.B.; Thambugala, K.M.; Tian, Q.; Samarakoon, M.C.; McKenzie, E.H.C.; Jayasiri, S.C.; Tibpromma, S.; Bhat, J.D.; et al. Towards a natural classification and backbone tree for Graphostromataceae, Hypoxylaceae, Lopadostomataceae and Xylariaceae. Fungal Divers. 2018, 88, 1–165. [Google Scholar] [CrossRef]
- Wibberg, D.; Stadler, M.; Lambert, C.; Bunk, B.; Spröer, C.; Rückert, C.; Kalinowski, J.; Cox, R.J.; Kuhnert, E. High quality genome sequences of thirteen Hypoxylaceae (Ascomycota) strengthen the phylogenetic family backbone and enable the discovery of new taxa. Fungal Divers. 2020, in press. [Google Scholar] [CrossRef]
- Kuhnert, E.; Surup, F.; Halecker, S.; Stadler, M. Minutellins A–D, azaphilones from the stromata of Annulohypoxylon minutellum (Xylariaceae). Phytochemistry 2017, 137, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Sudarman, E.; Kuhnert, E.; Hyde, K.; Sir, E.; Surup, F.; Stadler, M. Truncatones A–D, benzo[j]fluoranthenes from Annulohypoxylon species (Xylariaceae, Ascomycota). Tetrahedron 2016, 72, 6450–6454. [Google Scholar] [CrossRef]
- Koyama, K.; Kuramochi, D.; Kinoshita, K.; Takahashi, K. Hypoxylonols A and B, novel reduced benzo[j]fluoranthene derivatives from the mushroom Hypoxylon truncatum. J. Nat. Prod. 2002, 65, 1489–1490. [Google Scholar] [CrossRef] [PubMed]
- Fukai, M.; Tsukada, M.; Miki, K.; Suzuki, T.; Sugita, T.; Kinoshita, K.; Takahashi, K.; Shiro, M.; Koyama, K. Hypoxylonols C-F, benzo[j]fluoranthenes from Hypoxylon truncatum. J. Nat. Prod. 2011, 75, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Helaly, S.E.; Ashrafi, S.; Teponno, R.B.; Bernecker, S.; Dababat, A.A.; Maier, W.; Stadler, M. Nematicidal cyclic lipodepsipeptides and a xanthocillin derivative from a phaeosphariaceous fungus parasitizing eggs of the plant parasitic nematode Heterodera filipjevi. J. Nat. Prod. 2018, 81, 2228–2234. [Google Scholar] [CrossRef]
- Sandargo, B.; Michehl, M.; Praditya, D.; Steinmann, E.; Stadler, M.; Surup, F. Antiviral meroterpenoid rhodatin and sesquiterpenoids rhodocoranes A-E from the Wrinkled Peach Mushroom, Rhodotus palmatus. Org. Lett. 2019, 21, 3286–3289. [Google Scholar] [CrossRef]
- Eisenman, H.C.; Casadevall, A. Synthesis and assembly of fungal melanin. Appl. Microbiol. Biotechnol. 2012, 93, 931–940. [Google Scholar] [CrossRef]
- Cox, R.J.; Simpson, T.J. Chapter 3 Fungal Type I Polyketide Synthases. In Methods in Enzymology; Elsevier Inc.: Amsterdam, The Netherlands, 2009; Volume 459, pp. 49–78. ISBN 9780123745910. [Google Scholar]
- Stadler, M.; Lambert, C.; Wibberg, D.; Kalinowski, J.; Cox, R.J.; Kolařík, M.; Kuhnert, E. Intragenomic polymorphisms in the ITS region of high-quality genomes of the Hypoxylaceae (Xylariales, Ascomycota). Mycol. Prog. 2020, 19, 235–245. [Google Scholar] [CrossRef]
- Quang, D.N.; Hashimoto, T.; Tanaka, M.; Baumgartner, M.; Stadler, M.; Asakawa, Y. Chemical constituents of the ascomycete Daldinia concentrica. J. Nat. Prod. 2002, 65, 1869–1874. [Google Scholar] [CrossRef]
- Gu, W.; Ge, H.M.; Song, Y.C.; Ding, H.; Zhu, H.L.; Zhao, X.A.; Tan, R.X. Cytotoxic benzo[j]fluoranthene metabolites from Hypoxylon truncatum IFB-18, an endophyte of Artemisia annua. J. Nat. Prod. 2007, 70, 114–117. [Google Scholar] [CrossRef]
- Lee, D.; Choi, P.; Hwang, B.S.; Kim, T.; Kim, Y.; Kim, J.-C.; Song, J.H.; Park, J.S.; Hwang, G.S.; Yamabe, N.; et al. Protective effect of hypoxylonol C and 4,5,4′,5′-tetrahydroxy-1,1′-binaphthyl isolated from Annulohypoxylon annulatum against streptozotocin-induced damage in INS-1 cells. Bioorg. Chem. 2019, 90, 103053. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Hwang, B.S.; Choi, P.; Kim, T.; Kim, Y.; Song, B.G.; Yamabe, N.; Hwang, G.S.; Kang, K.S.; Ham, J. Hypoxylonol F Isolated from Annulohypoxylon annulatum improves insulin secretion by regulating pancreatic β-cell metabolism. Biomolecules 2019, 9, 335. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Health & Human Services, Public Health Service Agency for Toxic Substances and Disease Registry. Toxicological Profile for Polycyclic Aromatic Hydrocarbons, U.S. Department of Health & Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry, Washington, D.C., August, 1985. J. Toxicol. Cutan. Ocul. Toxicol. 1999, 18, 141–147. [Google Scholar]
- Bhaganna, P.; Volkers, R.J.M.; Bell, A.N.W.; Kluge, K.; Timson, D.J.; McGrath, J.W.; Ruijssenaars, H.J.; Hallsworth, J.E. Hydrophobic substances induce water stress in microbial cells. Microb. Biotechnol. 2010, 3, 701–716. [Google Scholar] [CrossRef]
- Cray, J.A.; Stevenson, A.; Ball, P.; Bankar, S.B.; Eleutherio, E.C.A.; Ezeji, T.C.; Singhal, R.S.; Thevelein, J.M.; Timson, D.J.; Hallsworth, J.E. Chaotropicity: A key factor in product tolerance of biofuel-producing microorganisms. Curr. Opin. Biotechnol. 2015, 33, 228–259. [Google Scholar] [CrossRef]
| pos 1 | 1 | 2 | 3 | |||||
|---|---|---|---|---|---|---|---|---|
| δC, mult 2 | δH, mult 2 | δC, mult | δH, mult | δC, mult | δH, mult | |||
| 1 | 155.1, C | 156.8, C | 156.2, C | |||||
| 2 | 108.2, CH | 6.90, d (7.53) | 103.6, CH | 6.92, d (7.63) | 104.0, CH | 6.97, m | ||
| 3 | 128.1, CH | 7.42, t (2 × 7.96) | 127.5, CH | 7.52, t (2 × 8.09) | 127.9, CH | 7.56, t (2 × 7.96) | ||
| 4 | 116.4, CH | 8.22, d (8.39) | 116.8, CH | 8.29, d (8.39) | 116.6, CH | 8.30, d (8.39) | ||
| 5 | 132.5, C | 131.5, C | 130.9, C | |||||
| 6 | 133.5, C | 133.0, C | 131.6, C | |||||
| 7 | 129.9, C | 129.3, C | 129.2, C | |||||
| 8 | 127.4, CH | 8.45, m | 126.9, CH | 8.45, d (7.78) | 126.8, CH | 8.56, d (8.17) | ||
| 9 | 111.2, CH | 7.06, d (7.53) | 110.8, CH | 7.06, d (7.78) | 106.7, CH | 7.13, d (7.96) | ||
| 10 | 156.2, C | 155.7, C | 158.1, C | |||||
| 11 | 112.6, C | 112.1, C | 112.7, C | |||||
| 12 | 156.8, C | 156.3, C | 156.8, C | |||||
| 13 | 110.9, CH | 7.01, d (7.53) | 110.5, CH | 7.01, d (7.63) | 112.0, CH | 7.00, m | ||
| 14 | 124.3, CH | 8.06, br s | 123.8, CH | 8.05, m | 124.6, CH | 8.09, m | ||
| 15 | 129.1, C | 128.5, C | 126.7, C | |||||
| 16 | 137.9, C | 137.5, C | 137.3, C | |||||
| 17 | 119.0, CH | 8.06, m | 118.9, CH | 8.06, m | 119.2, CH | 8.09, m | ||
| 18 | 121.8, CH | 8.26, d (8.60) | 120.8, CH | 8.22, m | 120.7, CH | 8.17, d (8.60) | ||
| 19 | 125.4, C | 125.4, C | 124.5, C | |||||
| 20 | 135.2, C | 134.7, C | 134.1, C | |||||
| 21 | 55.5, CH3 | 4.04, s | 56.0, CH3 | 4.02, s | ||||
| 22 | 56.8, CH3 | 4.09, s | ||||||
| Test Organism | Minimum Inhibitory Concentration (MIC) (µg/mL) | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | Reference | |
| Bacillus subtilis | 33.3 | 16.7 | >66.7 | >66.7 | 66.7 | 8.3 1 |
| Staphylococcus aureus | 66.7 | 16.7 | >66.7 | >66.7 | >66.7 | 0.4 2 |
| Micrococcus luteus | 16.7 | 8.3 | 66.7 | 33.3 | 16.7 | 0.8 2 |
| Chromobacterium violaceum | 66.7 | 66.7 | >66.7 | >66.7 | >66.7 | 0.1 2 |
| Escherichia coli | >66.7 | >66.7 | >66.7 | >66.7 | >66.7 | 1.7 2 |
| Pseudomonas aeruginosa | >66.7 | >66.7 | >66.7 | >66.7 | >66.7 | 0.4 3 |
| Mycolicibacterium smegmatis | >66.7 | 33.3 | >66.7 | >66.7 | >66.7 | 3.3 4 |
| Candida albicans | >66.7 | >66.7 | >66.7 | >66.7 | >66.7 | 66.7 5 |
| Schizosaccharomyces pombe | 66.7 | 33.3 | >66.7 | >66.7 | >66.7 | 33.3 5 |
| Mucor hiemalis | 66.7 | 4.2 | >66.7 | >66.7 | 66.7 | 66.7 5 |
| Pichia anomala | 66.7 | 33.3 | >66.7 | >66.7 | >66.7 | 66.7 5 |
| Rhodotorula glutinis | 33.3 | 33.3 | >66.7 | >66.7 | 66.7 | 16.7 5 |
| Cell Line | Cytotoxicity (IC50) (µM) | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | Reference 1 | ||
| L929 | mouse fibroblasts | 12.7 | 17.2 | 61.0 | 10.4 | 16.3 | 0.00006 |
| KB 3.1 | human endocervical adenocarcinoma (AC) | 28.3 | 17.2 | 85.4 | 44.0 | 30.1 | 0.00079 |
| PC-3 | human prostate AC | 23.7 | 9.9 | n.d. | 44.0 | 25.6 | 0.00008 |
| SK-OV-3 | human ovary AC | 56.7 | 7.3 | n.d. | 66.0 | 33.1 | 0.00034 |
| MCF-7 | human breast AC | 9.7 | 5.1 | n.d. | 8.8 | 7.8 | 0.00012 |
| A431 | human squamous AC | 8.7 | 1.1 | n.d. | 5.7 | 16.3 | 0.00005 |
| A549 | human lung carcinoma | 20.0 | 1.4 | n.d. | 17.0 | 27.1 | 0.00008 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Becker, K.; Wessel, A.-C.; Luangsa-ard, J.J.; Stadler, M. Viridistratins A−C, Antimicrobial and Cytotoxic Benzo[j]fluoranthenes from Stromata of Annulohypoxylon viridistratum (Hypoxylaceae, Ascomycota). Biomolecules 2020, 10, 805. https://doi.org/10.3390/biom10050805
Becker K, Wessel A-C, Luangsa-ard JJ, Stadler M. Viridistratins A−C, Antimicrobial and Cytotoxic Benzo[j]fluoranthenes from Stromata of Annulohypoxylon viridistratum (Hypoxylaceae, Ascomycota). Biomolecules. 2020; 10(5):805. https://doi.org/10.3390/biom10050805
Chicago/Turabian StyleBecker, Kevin, Anna-Charleen Wessel, J. Jennifer Luangsa-ard, and Marc Stadler. 2020. "Viridistratins A−C, Antimicrobial and Cytotoxic Benzo[j]fluoranthenes from Stromata of Annulohypoxylon viridistratum (Hypoxylaceae, Ascomycota)" Biomolecules 10, no. 5: 805. https://doi.org/10.3390/biom10050805
APA StyleBecker, K., Wessel, A.-C., Luangsa-ard, J. J., & Stadler, M. (2020). Viridistratins A−C, Antimicrobial and Cytotoxic Benzo[j]fluoranthenes from Stromata of Annulohypoxylon viridistratum (Hypoxylaceae, Ascomycota). Biomolecules, 10(5), 805. https://doi.org/10.3390/biom10050805