A Comprehensive Review of the Phytochemical, Pharmacological, and Toxicological Properties of Tribulus terrestris L.
Abstract
1. Introduction
2. Chemical Composition
3. Pharmacological Properties
3.1. Pharmacokinetic Properties of TT Main Compounds
3.2. Antioxidant Activity
3.3. Sexual Disorders
3.3.1. In Vitro Experiments
3.3.2. Preclinical Experimental Studies (Animal Models)
3.3.3. Clinical Trials
3.4. Antibacterial Activity
3.5. Antihyperglycemic Effect
3.5.1. In Vitro Determinations
3.5.2. Preclinical Studies
3.5.3. Clinical Studies
3.6. Anti-Inflammatory Properties
3.6.1. In Vitro Studies
3.6.2. In Vivo Studies
3.7. Action on the Central Nervous System
3.8. Toxicological Studies
3.8.1. In Vitro Studies
3.8.2. Preclinical Experimental Studies (Animal Models)
3.8.3. Case Reports
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pokrywka, A.; Obmiński, Z.; Malczewska-Lenczowska, J.; Fijałek, Z.; Turek-Lepa, E.; Grucza, R. Insights into Supplements with Tribulus Terrestris used by Athletes. J. Hum. Kinet. 2014, 41, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Neychev, V.; Mitev, V. Pro-sexual and androgen enhancing effects of Tribulus terrestris L.: Fact or Fiction. J. Ethnopharmacol. 2016, 179, 345–355. [Google Scholar] [CrossRef]
- Dinchev, D.; Janda, B.; Evstatieva, L.; Oleszek, W.; Aslani, M.R.; Kostova, I. Distribution of steroidal saponins in Tribulus terrestris from different geographical regions. Phytochemistry 2008, 69, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Lazarova, I.; Ivanova, A.; Mechkarova, P.; Peev, D.; Valyovska, N. Intraspecific variability of biologically active compounds of different populations of Tribulus terrestris L. (Zygophyllaceae) in South Bulgaria. Biotechnol. Biotechnol. Equip. 2011, 25, 2352–2356. [Google Scholar] [CrossRef]
- GamalEl Din, S.F. Role of Tribulus terrestris in Male Infertility: Is It Real or Fiction? J. Diet. Suppl. 2018, 15, 1010–1013. [Google Scholar] [CrossRef]
- Kostova, I.; Dinchev, D. Saponins in Tribulus terrestris—Chemistry and bioactivity. Phytochem. Rev. 2005, 4, 111–137. [Google Scholar] [CrossRef]
- Hashim, S.; Bakht, T.; Bahadar Marwat, K.; Jan, A. Medicinal properties, phytochemistry and pharmacology of Tribulus terrestris L. (Zygophyllaceae). Pakistan J. Bot. 2014, 46, 399–404. [Google Scholar]
- Chhatre, S.; Nesari, T.; Kanchan, D.; Somani, G.; Sathaye, S. Phytopharmacological overview of Tribulus terrestris. Pharmacogn. Rev. 2014, 8, 45–51. [Google Scholar] [CrossRef]
- Yanala, S.R.; Sathyanarayana, D.; Kannan, K. A recent phytochemical review—Fruits of Tribulus terrestris linn. J. Pharm. Sci. Res. 2016, 8, 132–140. [Google Scholar]
- Sivapalan, S.R. Biological and pharmacological studies of Tribulus terrestris Linn- A review. Int. J. Multidiscip. Res. Dev. 2016, 3, 257–265. [Google Scholar]
- Zhu, W.; Du, Y.; Meng, H.; Dong, Y.; Li, L. A review of traditional pharmacological uses, phytochemistry, and pharmacological activities of Tribulus terrestris. Chem. Cent. J. 2017, 11, 1–16. [Google Scholar] [CrossRef]
- Azam, F.; Munier, S.; Abbas, G. A review on advancements in ethnomedicine and phytochemistry of Tribulus terrestris – a plant with multiple health benefits. Int. J. Biosci. 2019, 14, 21–37. [Google Scholar]
- Sanagoo, S.; Sadeghzadeh Oskouei, B.; Gassab Abdollahi, N.; Salehi-Pourmehr, H.; Hazhir, N.; Farshbaf-Khalili, A. Effect of Tribulus terrestris L. on sperm parameters in men with idiopathic infertility: A systematic review. Complement. Ther. Med. 2019, 42, 95–103. [Google Scholar] [CrossRef]
- Semerdjieva, I.B.; Zheljazkov, V.D. Chemical Constituents, Biological Properties, and Uses of Tribulus terrestris: A Review. Nat. Prod. Commun. 2019, 14, 1–26. [Google Scholar] [CrossRef]
- Meena, P.; Anand, A.; Vishal, K. A comprehensive overview of Gokshura (Tribulus terrestris Linn.). J. Ayurveda Integr. Med. Sci. 2019, 4, 205–211. [Google Scholar]
- De Combarieu, E.; Fuzzati, N.; Lovati, M.; Mercalli, E. Furostanol saponins from Tribulus terrestris. Fitoterapia 2003, 74, 583–591. [Google Scholar] [CrossRef]
- Ganzera, M.; Bedir, E.; Khan, I.A. Determination of steroidal saponins in Tribulus terrestris by reversed-phase high-performance liquid chromatography and evaporative light scattering detection. J. Pharm. Sci. 2001, 90, 1752–1758. [Google Scholar] [CrossRef]
- Sarvin, B.; Stekolshchikova, E.; Rodin, I.; Stavrianidi, A.; Shpigun, O. Optimization and comparison of different techniques for complete extraction of saponins from T. terrestris. J. Appl. Res. Med. Aromat. Plants 2018, 8, 75–82. [Google Scholar] [CrossRef]
- Kostova, I.; Dinchev, D.; Rentsch, G.H.; Dimitrov, V.; Ivanova, A. Two new sulfated furostanol saponins from Tribulus terrestris. Zeitschrift fur Naturforsch. - Sect. C J. Biosci. 2002, 57, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.W.; Tan, C.H.; Jiang, S.H.; Zhu, D.Y. Terrestrinins A and B, two new steroid saponins from Tribulus terrestris. J. Asian Nat. Prod. Res. 2003, 5, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Skhirtladze, A.; Nebieridze, V.; Benidze, M.; Kemertelidze, E.; Ganzera, M. Furostanol glycosides from the roots of Tribulus terrestris L. Bull. Georg. Natl. Acad. Sci. 2017, 11, 122–126. [Google Scholar]
- Zhang, C.; Wang, S.; Guo, F.; Ma, T.; Zhang, L.; Sun, L.; Wang, Y.; Zhang, X. Analysis of variations in the contents of steroidal saponins in Fructus Tribuli during stir-frying treatment. Biomed. Chromatogr. 2020, 34, e4794. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Wang, F.; Zhao, Y.; Sun, X.; Kang, L.; Fan, Z.; Qiao, L.; Yan, R.; Liu, S.; Ma, B. Rapid Characterization of Constituents in Tribulus terrestris from Different Habitats by UHPLC/Q-TOF MS. J. Am. Soc. Mass Spectrom. 2017, 28, 2302–2318. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.F.; Wang, B.B.; Zhao, Y.; Wang, F.X.; Sun, Y.; Guo, R.J.; Song, X.B.; Xin, H.L.; Sun, X.G. Furostanol and Spirostanol Saponins from Tribulus terrestris. Molecules 2016, 21, 429. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.J.; Xu, T.H.; Liu, Y.; Xie, S.X.; Si, Y.S.; Xu, D.M. Two new steroidal glucosides from Tribulus terrestris L. J. Asian Nat. Prod. Res. 2009, 11, 548–553. [Google Scholar] [CrossRef]
- Bedir, E.; Khan, I.A. New steroidal glycosides from the fruits of Tribulus terrestris. J. Nat. Prod. 2000, 63, 1699–1701. [Google Scholar] [CrossRef]
- Hammoda, H.M.; Ghazy, N.M.; Harraz, F.M.; Radwan, M.M.; ElSohly, M.A.; Abdallah, I.I. Chemical constituents from Tribulus terrestris and screening of their antioxidant activity. Phytochemistry 2013, 92, 153–159. [Google Scholar] [CrossRef]
- Ivanova, A.; Serly, J.; Dinchev, D.; Ocsovszki, I.; Kostova, I.; Molnar, J. Screening of some saponins and phenolic components of Tribulus terrestris and Smilax excelsa as MDR modulators. In Vivo (Brooklyn). 2009, 23, 545–550. [Google Scholar]
- Miles, C.O.; Wilkins, A.L.; Munday, S.C.; Flåøyen, A.; Holland, P.T.; Smith, B.L. Identification of Insoluble Salts of the β-d-Glucuronides of Episarsasapogenin and Epismilagenin in the Bile of Lambs with Alveld and Examination of Narthecium ossifragum, Tribulus terrestris, and Panicum miliaceum for Sapogenins. J. Agric. Food Chem. 1993, 41, 914–917. [Google Scholar] [CrossRef]
- Burda, N.Y.; Zhuravel, I.O.; Dababneh, M.F.; Fedchenkova, Y.A. Analysis of diosgenin and phenol compounds in Tribulus terrestris L. Pharmacia 2019, 66, 41–44. [Google Scholar] [CrossRef]
- Vaidya, V.; Kondalkar, P.; Shinde, M.; Gotmare, S. HPTLC fingerprinting for simultaneous quantification of harmine, kaempferol, diosgenin and oleic acid in the fruit extract of Tribulus terrestris and its formulation. Int. J. Pharm. Sci. Res. 2018, 9, 3066–3074. [Google Scholar]
- Deepak, M.; Dipankar, G.; Prashanth, D.; Asha, M.K.; Amit, A.; Venkataraman, B.V. Tribulosin and β-sitosterol-D-glucoside, the anthelmintic principles of Tribulus terrestris. Phytomedicine 2002, 9, 753–756. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.X.; Chen, H.S.; Liu, W.Y.; Gu, Z.B.; Liang, H.Q. Two sapogenins from Tribulus terrestris. Phytochemistry 1998, 49, 199–201. [Google Scholar] [CrossRef]
- Wu, T.S.; Shi, L.S.; Kuo, S.C. Alkaloids and other constituents from Tribulus terrestris. Phytochemistry 1999, 50, 1411–1415. [Google Scholar] [CrossRef]
- Ma, Y.; Guo, Z.; Wang, X. Tribulus terrestris extracts alleviate muscle damage and promote anaerobic performance of trained male boxers and its mechanisms: Roles of androgen, IGF-1, and IGF binding protein-3. J. Sport Heal. Sci. 2017, 6, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.H.; Kim, D.W.; Curtis-Long, M.J.; Park, C.; Son, M.; Kim, J.Y.; Yuk, H.J.; Lee, K.W.; Park, K.H. Cinnamic acid amides from Tribulus terrestris displaying uncompetitive a-glucosidase inhibition. Eur. J. Med. Chem. 2016, 114, 201–208. [Google Scholar] [CrossRef]
- Li, J.X.; Shi, Q.; Xiong, Q.B.; Prasain, J.K.; Tezuka, Y.; Hareyama, T.; Wang, Z.T.; Tanaka, K.; Namba, T.; Kadota, S. Tribulusamide A and B, new hepatoprotective lignanamides from the fruits of Tribulus terrestris: Indications of cytoprotective activity in murine hepatocyte culture. Planta Med. 1998, 64, 628–631. [Google Scholar] [CrossRef]
- Bhutani, S.P.; Chibber, S.S.; Seshadri, T.R. Flavonoids of the fruits and leaves of Tribulus terrestris: Constitution of tribuloside. Phytochemistry 1969, 8, 299–303. [Google Scholar] [CrossRef]
- Tian, C.; Chang, Y.; Zhang, Z.; Wang, H.; Xiao, S.; Cui, C.; Liu, M. Extraction technology, component analysis, antioxidant, antibacterial, analgesic and anti-inflammatory activities of flavonoids fraction from Tribulus terrestris L. leaves. Heliyon 2019, 5, e02234. [Google Scholar] [CrossRef]
- Tian, C.; Zhang, Z.; Wang, H.; Guo, Y.; Zhao, J.; Liu, M. Extraction technology, component analysis, and in vitro antioxidant and antibacterial activities of total flavonoids and fatty acids from Tribulus terrestris L. fruits. Biomed. Chromatogr. 2019, 33, 4. [Google Scholar] [CrossRef]
- Louveaux, A.; Jay, M.; Hadi, O.T.M.E.; Roux, G. Variability in flavonoid compounds of four Tribulus species: Does it play a role in their identification by desert locust Schistocerca gregaria? J. Chem. Ecol. 1998, 24, 1465–1481. [Google Scholar] [CrossRef]
- Kumar, A. Comparative and quantitative determination of quercetin and other flavonoids in North Indian populations of Tribulus terrestris Linn, by HPLC. Int. J. Pharma Bio Sci. 2012, 3, 69–79. [Google Scholar]
- Kang, S.Y.; Jung, H.W.; Nam, J.H.; Kim, W.K.; Kang, J.S.; Kim, Y.H.; Cho, C.W.; Cho, C.W.; Park, Y.K.; Bae, H.S. Effects of the Fruit Extract of Tribulus terrestris on Skin Inflammation in Mice with Oxazolone-Induced Atopic Dermatitis through Regulation of Calcium Channels, Orai-1 and TRPV3, and Mast Cell Activation. Evidence-based Complement. Altern. Med. 2017, 2017, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Tosun, F.; Tanker, M.; Tosun, A. Alkaloids of Tribulus terrestris L. Growing in Turkey. Fabad J. Pharm. Sci. 1994, 19, 149–151. [Google Scholar]
- Bourke, C.; Stevens, G.; Carrigan, M. Locomotor effects in sheep of alkaloids identified in Australian Tribulus terrestris. Aust. Vet. J. 1992, 69, 163–165. [Google Scholar] [CrossRef]
- Ranjithkumar, R.; Alhadidi, Q.; Shah, Z.A.; Ramanathan, M. Tribulusterine Containing Tribulus terrestris Extract Exhibited Neuroprotection Through Attenuating Stress Kinases Mediated Inflammatory Mechanism: In Vitro and In Vivo Studies. Neurochem. Res. 2019, 44, 1228–1242. [Google Scholar] [CrossRef]
- Ko, H.J.; Ahn, E.K.; Oh, J.S. N-trans-q-caffeoyl tyramine isolated from Tribulus terrestris exerts anti-inflammatory effects in lipopolysaccharide-stimulated RAW 264.7 cells. Int. J. Mol. Med. 2015, 36, 1042–1048. [Google Scholar] [CrossRef]
- Lee, H.H.; Ahn, E.K.; Hong, S.S.; Oh, J.S. Anti-inflammatory effect of Tribulusamide D isolated from Tribulus terrestris in lipopolysaccharide-stimulated RAW264.7 macrophages. Mol. Med. Rep. 2017, 16, 4421–4428. [Google Scholar] [CrossRef]
- Zhang, X.; Wei, N.; Huang, K.; Tan, Y.; Jin, D. A new feruloyl amide derivative from the fruits of Tribulus terrestris, Nat. Prod. Res. 2012, 26, 1922–1925. [Google Scholar] [CrossRef]
- Javaid, A.; Anjum, F.; Akhtar, N. Molecular Characterization of Pyricularia oryzae and its Management by Stem Extract of Tribulus terrestris. Int. J. Agric. Biol. 2019, 1256–1262. [Google Scholar]
- Vadakkan, K.; Vijayanand, S.; Hemapriya, J.; Gunasekaran, R. Quorum sensing inimical activity of Tribulus terrestris against gram negative bacterial pathogens by signalling interference. 3 Biotech 2019, 9, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Guo, Z.; Li, J.; Ito, Y.; Sun, W.; Heart, N. A new quantitation method of protodioscin by HPLC–ESI-MS/MS in rat plasma and its application to the pharmacokinetic study. Steroids 2016, 106, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, J.; Ito, Y.; Sun, W. Simultaneous quantification of five steroid saponins from Dioscorea zingiberensis C.H.Wright in rat plasma by HPLC- MS/MS and its application to the pharmacokinetic studies. Steroids 2015, 1, 16–24. [Google Scholar]
- Tang, Y.N.; Pang, Y.X.; He, X.C.; Zhang, Y.Z.; Zhang, J.Y.; Zhao, Z.Z.; Yi, T.; Chen, H.B. UPLC-QTOF-MS identification of metabolites in rat biosamples after oral administration of Dioscorea saponins: A comparative study. J. Ethnopharmacol. 2015, 165, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Bors, W.; Heller, W.; Michel, C.; Saran, M. Flavonoids as antioxidants: Determination of radical-scavenging efficiencies. Methods Enzymol. 1990, 186, 343–355. [Google Scholar] [PubMed]
- Aboul-Enein, H.; Kruk, I.; Kladna, A.; Lichszteld, K.; Michalska, T. Scavenging effects of phenolic sompounds on reactive oxygen species. Biopolymers 2007, 86, 222–230. [Google Scholar] [CrossRef]
- Durgawale, P.P.; Datkhile, K.D. Study of Polyphenol Content and Anti- Oxidative Potential of Tribulus terrestris Dry Fruit Extract. Int. J. Pharmacogn. Phytochem. Res. 2017, 9, 716–721. [Google Scholar]
- Zheleva-Dimitrova, D.; Obreshkova, D.; Nedialkov, P. Antioxidant activity of Tribulus terrestris - a natural product in infertility therapy. Int. J. Pharm. Pharm. Sci. 2012, 4, 508–511. [Google Scholar]
- Dutt-Roy, R.; Kayalvizhi, E.; Chandrasekhar, M. Evaluation of the antidepressant activity of Tribulus terrestris in diabetic depression in rat model. Int. J. Pharm. Sci. Res. 2017, 8, 5392–5399. [Google Scholar]
- Pamela C., C.; Harvey, R.A.; Ferrier, D.R. Lippincott Biochimie Ilustrată; Ediția a 4; București: Editura Medicală Callisto: București, Romania, 2010. [Google Scholar]
- Marnett, L.J. Lipid peroxidation - DNA damage by malondialdehyde. Mutat. Res. 1999, 424, 83–95. [Google Scholar] [CrossRef]
- Amin, A.; Lotfy, M.; Shafiullah, M.; Adeghate, E. The protective effect of Tribulus terrestris in diabetes. Ann. N. Y. Acad. Sci. 2006, 1084, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Khaleghi, S.; Bakhtiari, M.; Asadmobini, A.; Esmaeili, F. Tribulus terrestris Extract Improves Human Sperm Parameters In Vitro. J. Evidence-Based Complement. Altern. Med. 2017, 22, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Kam, S.C.; Do, J.M.; Choi, J.H.; Jeon, B.T.; Roh, G.S.; Hyun, J.S. In Vivo and in Vitro Animal Investigation of the Effect of a Mixture of Herbal Extracts from Tribulus terrestris and Cornus officinalis on Penile Erection. J. Sex. Med. 2012, 9, 2544–2551. [Google Scholar] [CrossRef] [PubMed]
- Pavin, N.F.; Izaguirry, A.P.; Soares, M.B.; Spiazzi, C.C.; Mendez, A.S.L.; Leivas, F.G.; dos Santos Brum, D.; Cibin, F.W.S. Tribulus terrestris Protects against Male Reproductive Damage Induced by Cyclophosphamide in Mice. Oxid. Med. Cell. Longev. 2018, 2018, 1–9. [Google Scholar] [CrossRef]
- Yin, L.; Wang, Q.; Wang, X.; Song, L.-N. Effects of Tribulus terrestris saponins on exercise performance in overtraining rats and the underlying mechanisms. Can. J. Physiol. Pharmacol. 2016, 94, 1193–1201. [Google Scholar] [CrossRef]
- Ghanbari, A.; Moradi, M.; Raoofi, A.; Falahi, M.; Seydi, S. Tribulus terrestris Hydroalcoholic Extract Administration Effects on Reproductive Parameters and Serum Level of Glucose in Diabetic Male Rats. Int. J. Mophol. 2016, 34, 796–803. [Google Scholar] [CrossRef]
- Haghmorad, D.; Mahmoudi, M.B.; Haghighi, P.; Alidadiani, P.; Shahvazian, E.; Tavasolian, P.; Hosseini, M.; Mahmoudi, M. Improvement of fertility parameters with Tribulus Terrestris and Anacyclus Pyrethrum treatment in male rats. Int. Braz. J. Urol. 2019, 45, 1043–1054. [Google Scholar] [CrossRef]
- Oliveira, N.N.P.M.; Félix, M.A.R.; Pereira, T.C.S.; Rocha, L.G.P.; Miranda, J.R.; Zangeronimo, M.G.; Pinto, J.E.B.P.; Bertolucci, S.K.V.; Sousa, R.V.D. Sperm quality and testicular histomorphometry of wistar rats supplemented with extract and fractions of fruit of Tribulus terrestris L. Brazilian Arch. Biol. Technol. 2015, 58, 891–897. [Google Scholar] [CrossRef]
- Gauthaman, K.; Adaikan, P.G.; Prasad, R.N.V. Aphrodisiac properties of Tribulus Terrestris extract (Protodioscin) in normal and castrated rats. Life Sci. 2002, 71, 1385–1396. [Google Scholar] [CrossRef]
- Kamenov, Z.; Fileva, S.; Kalinov, K.; Jannini, E.A. Evaluation of the efficacy and safety of Tribulus terrestris in male sexual dysfunction—A prospective, randomized, double-blind, placebo-controlled clinical trial. Maturitas 2017, 99, 20–26. [Google Scholar] [CrossRef]
- Santos, C.A.; Reis, L.O.; Destro-saade, R.; Luiza-reis, A.; Fregonesi, A. Tribulus terrestris versus placebo in the treatment of erectile dysfunction: A prospective, randomized, double-blind study. Actas Urol. Esp. 2014, 38, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Milasius, K.; Dadeliene, R.; Skernevicius, J. The influence of the Tribulus terrestris extract on the parameters of the functional preparedness and athletes’ organism homeostasis. Fiziol. Zh. 2009, 55, 89–96. [Google Scholar] [PubMed]
- Iacono, F.; Prezioso, D.; Illiano, E.; Romeo, G.; Ruffo, A.; Amato, B. Sexual asthenia: Tradamixina versus Tadalafil 5 mg daily. BMC Surg. 2012, 12, S23. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, G.A.; Ita, J.R.; Garza, R.G.; Castilla-Cortazar, I. Insulin-like growth factor-1 deficiency and metabolic syndrome. J. Transl. Med. 2016, 14, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Neychev, V.K.; Mitev, V.I. The aphrodisiac herb Tribulus terrestris does not influence the androgen production in young men. J. Ethnopharmacol. 2005, 101, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Rogerson, S.; Riches, C.J.; Jennings, C.; Weatherby, R.P.; Meir, R.A.; Marshall-Gradisnik, S.M. The effect of five weeks of Tribulus terrestris supplementation on muscle strength and body composition during preseason training in elite rugby league players. J. Strength Cond. Res. 2007, 21, 348–353. [Google Scholar]
- Banihani, S.A. Testosterone in males as enhanced by onion (Allium cepa l.). Biomolecules 2019, 9, 75. [Google Scholar] [CrossRef]
- Phillips, O.A.; Mathew, K.T.; Oriowo, M.A. Antihypertensive and vasodilator effects of methanolic and aqueous extracts of Tribulus terrestris in rats. J. Ethnopharmacol. 2006, 104, 351–355. [Google Scholar] [CrossRef]
- Malavige, L.; Levy, J. Erectile dysfunction and diabetes mellitus. J. Sex. Med. 2009, 6, 1232–1247. [Google Scholar] [CrossRef]
- Vale, F.B.C.; Zanolla Dias de Souza, K.; Rezende, C.R.; Geber, S. Efficacy of Tribulus Terrestris for the treatment of premenopausal women with hypoactive sexual desire disorder: A randomized double-blinded, placebo-controlled trial. Gynecol. Endocrinol. 2018, 34, 442–445. [Google Scholar] [CrossRef]
- Fatima, L.; Sultana, A. Efficacy of Tribulus terrestris L. (fruits) in menopausal transition symptoms: A randomized placebo controlled study. Adv. Integr. Med. 2017, 4, 56–65. [Google Scholar] [CrossRef]
- Akhtari, E.; Raisi, F.; Keshavarz, M.; Hosseini, H.; Sohrabvand, F.; Bioos, S.; Kamalinejad, M.; Ghobadi, A. Tribulus terrestris for treatment of sexual dysfunction in women: Randomized double-blind placebo—Controlled study. Daru 2014, 22, 40. [Google Scholar] [CrossRef] [PubMed]
- World Anti-Doping Agency (WADA). Endogenous Anabolic Androgenic Steroids Measurement and Reporting. WADA Tech. Doc. – TD2016EAAS 2016, 1–16. [Google Scholar]
- Tag, H.; Abdelazek, H.; Mahoud, Y.; El-Shenawy, N. Efficay of Tribulus terrestris extract and metformin on fertility indices and oxidative stress of testicular tissue in streptozotocin-induced diabetic male rats. African J. Pharm. Pharmacol. 2015, 9, 861–874. [Google Scholar]
- Zhang, H.; Tong, W.T.; Zhang, C.R.; Li, J.L.; Meng, H.; Yang, H.G.; Chen, M. Gross saponin of Tribulus terrestris improves erectile dysfunction in type 2 diabetic rats by repairing the endothelial function of the penile corpus cavernosum. Diabetes, Metab. Syndr. Obes. Targets Ther. 2019, 12, 1705–1716. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.J. Biological Activity of Saponins Isolated from Tribulus terrestris ( Fruit ) on Growth of Some Bacteria. Tikrit J. Pure Sci. 2008, 13, 3. [Google Scholar]
- Jindal, A.; Kumar, P.; Gautam, K. Evaluation of antibiotic potential of alkaloids of Tribulus terrestris L. against some pathogenic microorganisms. Int. J. Green Pharm. 2013, 7, 102–105. [Google Scholar] [CrossRef]
- Soleimanpour, S.; Sedighinia, F.S.; Safipour Afshar, A.; Zarif, R.; Ghazvini, K. Antibacterial activity of Tribulus terrestris and its synergistic effect with Capsella bursa-pastoris and Glycyrrhiza glabra against oral pathogens: An in-vitro study. Avicenna J. phytomedicine 2015, 5, 210–217. [Google Scholar]
- Batoei, S.; Mahboubi, M.; Yari, R. Antibacterial activity of Tribulus terrestris methanol extract against clinical isolates of Escherichia coli. Herba Pol. 2016, 62, 57–66. [Google Scholar] [CrossRef]
- Recio, M.C.; Rios, J.L.; Villar, A. Antimicrobial activity of selected plants employed in the Spanish Mediterranean area. Part II. Phyther. Res. 1989, 3, 77–80. [Google Scholar] [CrossRef]
- Kianbakht, S.; Jahaniani, F. Evaluation of Antibacterial Activity of Tribulus terrestris L. Growing in Iran. Iran. J. Pharmacol. Ther. 2003, 2, 22–24. [Google Scholar]
- Böttger, S.; Hofmann, K.; Melzig, M.F. Saponins can perturb biologic membranes and reduce the surface tension of aqueous solutions: A correlation? Bioorganic Med. Chem. 2012, 20, 2822–2828. [Google Scholar] [CrossRef] [PubMed]
- Bangham, A.; Horne, R. Action of saponin on biological cell membranes. Nature 1962, 4858, 952–953. [Google Scholar] [CrossRef] [PubMed]
- Ercan, P.; El, S.N. Inhibitory effects of chickpea and Tribulus terrestris on lipase, alpha-amylase and alpha-glucosidase. Food Chem. 2016, 205, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Ponnusamy, S.; Ravindran, R.; Zinjarde, S.; Bhargava, S.; Ravi Kumar, A. Evaluation of traditional Indian antidiabetic medicinal plants for human pancreatic amylase inhibitory effect in vitro. Evidence-based Complement. Altern. Med. 2011, 2011, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Qu, W.; Zhong, S. Inhibitory effects of saponins from Tribulus terrestris on alpha-glucosidase in small intestines of rats. Zhongguo Zhong Yao Za Zhi 2006, 31, 910–913. [Google Scholar]
- El-Tantawy, W.H.; Hassanin, L.A. Hypoglycemic and hypolipidemic effects of alcoholic extract of Tribulus alatus in streptozotocin-induced diabetic rats: A comparative study with T. terrestris (Caltrop). Indian J. Exp. Biol. 2007, 45, 785–790. [Google Scholar]
- Lamba, H.S.; Bhargava, C.S.; Thakur, M.; Bhargava, S. α-glucosidase and aldose reductase inhibitory activity in vitro and antidiabetic activity in vivo of Tribulus terrestris L. (dunal). Int. J. Pharm. Pharm. Sci. 2011, 3, 270–272. [Google Scholar]
- El-Shaibany, A.; Al-Habori, M.; Al-Tahami, B.; Al-Massarani, S. Anti-hyperglycaemic activity of Tribulus terrestris L aerial part extract in glucose-loaded normal rabbits. Trop. J. Pharm. Res. 2015, 14, 2263–2268. [Google Scholar] [CrossRef]
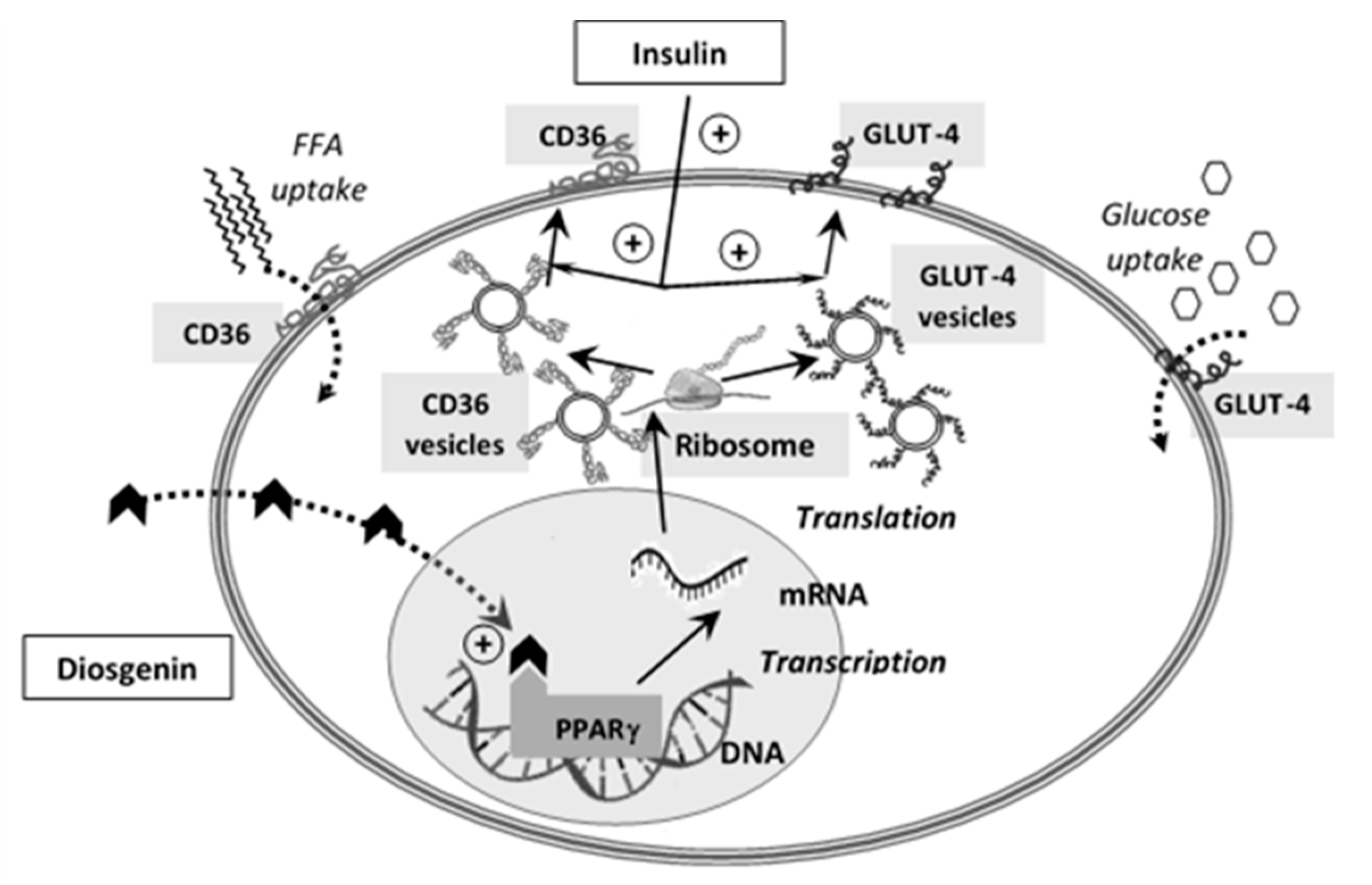
- Kalailingam, P.; Kannaian, B.; Tamilmani, E.; Kaliaperumal, R. Efficacy of natural diosgenin on cardiovascular risk, insulin secretion, and beta cells in streptozotocin (STZ)-induced diabetic rats. Phytomedicine 2014, 21, 1154–1161. [Google Scholar] [CrossRef]
- Tharaheswari, M.; Jayachandra Reddy, N.; Kumar, R.; Varshney, K.C.; Kannan, M.; Sudha Rani, S. Trigonelline and diosgenin attenuate ER stress, oxidative stress-mediated damage in pancreas and enhance adipose tissue PPARγ activity in type 2 diabetic rats. Mol. Cell. Biochem. 2014, 396, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Soldatov, V.O.; Shmykova, E.A.; Pershina, M.A.; Ksenofontov, A.O.; Zamitsky, Y.M. Imidazoline receptors agonists: Possible mechanisms of endothelioprotection. Res. Results Pharmacol. 2018, 4, 11–19. [Google Scholar] [CrossRef]
- Samani, N.B.; Jokar, A.; Soveid, M.; Heydari, M.; Mosavat, S.H. Efficacy of the Hydroalcoholic Extract of Tribulus terrestris on the Serum Glucose and Lipid Profile of Women With Diabetes Mellitus: A Double-Blind Randomized Placebo-Controlled Clinical Trial. J. Evidence-Based Complement. Altern. Med. 2016, 21, NP91–NP97. [Google Scholar] [CrossRef] [PubMed]
- Ramteke, R.; Thakar, A.; Trivedi, A.; Patil, P. Clinical efficacy of Gokshura-Punarnava Basti in the management of microalbuminuria in diabetes mellitus. AYU (An Int. Q. J. Res. Ayurveda) 2012, 33, 537–541. [Google Scholar] [CrossRef]
- Karakas, M.; Schäfer, S.; Appelbaum, S.; Ojeda, F.; Kuulasmaa, K.; Brückmann, B.; Berisha, F.; Schulte-Steinberg, B.; Jousilahti, P.; Blankenberg, S.; et al. Testosterone levels and type 2 diabetes—No correlation with age, differential predictive value in men and women. Biomolecules 2018, 8, 76. [Google Scholar] [CrossRef]
- Navarro, G.; Allard, C.; Xu, W.; Mauvais-Jarvis, F. The role of androgens in metabolism, obesity, and diabetes in males and females. Obesity 2015, 23, 713–719. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, J.C.; Min, J.S.; Kim, M.J.; Kim, J.A.; Kor, M.H.; Yoo, H.S.; Ahn, J.K. Aqueous extract of Tribulus terrestris Linn induces cell growth arrest and apoptosis by down-regulating NF-κB signaling in liver cancer cells. J. Ethnopharmacol. 2011, 136, 197–203. [Google Scholar] [CrossRef]
- Hong, C.H.; Hur, S.K.; Oh, O.J.; Kim, S.S.; Nam, K.A.; Lee, S.K. Evaluation of natural products on inhibition of inducible cyclooxygenase (COX-2) and nitric oxide synthase (iNOS) in cultured mouse macrophage cells J. Ethnopharmacol. 2002, 83, 153–159. [Google Scholar] [CrossRef]
- Ghareeb, D.A.; ElAhwany, A.M.D.; El-Mallawany, S.M.; Saif, A.A. In vitro screening for anti-acetylcholiesterase, Anti-oxidant, Anti-glucosidase, Anti-inflammatory and anti-bacterial effect of three traditional medicinal plants. Biotechnol. Biotechnol. Equip. 2014, 28, 1155–1164. [Google Scholar] [CrossRef]
- Mohammed, M.S.; Alajmi, M.F.; Alam, P.; Khalid, H.S.; Mahmoud, A.M.; Ahmed, W.J. Chromatographic finger print analysis of anti-inflammatory active extract fractions of aerial parts of Tribulus terrestris by HPTLC technique. Asian Pac. J. Trop. Biomed. 2014, 4, 203–208. [Google Scholar] [CrossRef]
- Qiu, M.; An, M.; Bian, M.; Yu, S.; Liu, C.; Liu, Q. Terrestrosin D from Tribulus terrestris attenuates bleomycin-induced inflammation and suppresses fibrotic changes in the lungs of mice. Pharm. Biol. 2019, 57, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Bourke, C.A. Staggers in sheep associated with the ingestion of Tribulus terrestris. Aust. Vet. J. 1984, 61, 360–363. [Google Scholar] [CrossRef] [PubMed]
- Chauhdary, Z.; Saleem, U.; Ahmad, B.; Shah, S.; Shah, M.A. Neuroprotective evaluation of Tribulus terrestris L. In aluminum chloride induced Alzheimer’s disease. Pak. J. Pharm. Sci. 2019, 32, 805–816. [Google Scholar] [PubMed]
- Song, S.; Fajol, A.; Chen, Y.; Ren, B.; Shi, S. Anticonvulsive effects of protodioscin against pilocarpine-induced epilepsy. Eur. J. Pharmacol. 2018, 833, 237–246. [Google Scholar] [CrossRef]
- Abudayyak, M.; Jannuzzi, A.T.; Özhan, G.; Alpertunga, B. Investigation on the toxic potential of Tribulus terrestris in vitro. Pharm. Biol. 2015, 53, 469–476. [Google Scholar] [CrossRef]
- Aslani, M.; Movassaghi, A.; Mohri, M.; Pedram, M.; Abavisani, A. Experimental Tribulus terrestris poisoning in sheep: Clinical, laboratory and pathological findings. Vet. Res. Commun. 2003, 27, 53–62. [Google Scholar] [CrossRef]
- Gandhi, S.; Srinivasan, B.P.; Akarte, A.S. Potential nephrotoxic effects produced by steroidal saponins from hydro alcoholic extract of Tribulus terrestris in STZ-induced diabetic rats. Toxicol. Mech. Methods 2013, 23, 548–557. [Google Scholar] [CrossRef]
- Bourke, C.A. A novel nigrostriatal dopaminergic disorder in sheep affected by Tribulus terrestris staggers. Res. Vet. Sci. 1987, 43, 347–350. [Google Scholar] [CrossRef]
- Hemalatha, S.; Hari, R. Acute and subacute toxicity studies of the saponin rich butanol extracts of Tribulus terrestris fruits in wistar rats. Int. J. Pharm. Sci. Rev. Res. 2014, 27, 307–313. [Google Scholar]
- Talasaz, A.H.; Abbasi, M.R.; Abkhiz, S.; Dashti-Khavidaki, S. Tribulus terrestris-induced severe nephrotoxicity in a young healthy male. Nephrol. Dial. Transplant. 2010, 25, 3792–3793. [Google Scholar] [CrossRef]
- Campanelli, M.; De Thomasis, R.; Tenaglia, R.L. Priapism caused by “Tribulus terrestris”. Int. J. Impot. Res. 2015, 28, 39–40. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.; Lazar, I.; Nadasdy, G.M.; Nadasdy, T.; Satoskar, A.A. Acute kidney injury and hyperbilirubinemia in a young male after ingestion of Tribulus terrestris. Clin. Nephrol. 2015, 83, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Selvaraju, R.; Thiruppathi, G.; Raman, R.G.; Dhakshanamoorthy, D. Estimation of Essential and Trace Elements in the Medicinal Plant Tribulus Terrestris By Icp-Oes and Flame Photometric Techniques. Rom. J. Biol. Plant Biol. 2015, 56, 65–75. [Google Scholar]
- Hassa, L.; Umar, K.; Umar, Z. Antinutritive factors in Tribulus terrestris (Linn.) leaves and predicted calcium and zinc bioavailability. J. Trop. Biosci. 2007, 7, 33–36. [Google Scholar]
- U.S. National Library of Medicine. Available online: https://chem.nlm.nih.gov/chemidplus/ (accessed on 23 April 2020).


| Year of the Review | Main Topic | Years Surveyed | Limitations | Reference |
|---|---|---|---|---|
| 2005 | Phytochemistry and pharmacology | <2004 | [6] | |
| 2014 | TT supplements | NS | [1] | |
| 2014 | Phytochemistry and pharmacology | short review | [7] | |
| 2014 | Phytochemistry and pharmacology | NS | short review | [8] |
| 2016 | Analysis of human and animal evidence | 1968–2015 | [2] | |
| 2016 | Phytochemistry | NS | Only the composition of fruits was discussed | [9] |
| 2016 | Phytochemistry and pharmacology | NS | [10] | |
| 2017 | Phytochemistry and pharmacology | NS | [11] | |
| 2018 | Male infertility | short review | [5] | |
| 2019 | Phytochemistry and ethnomedicine | NS | brief presentation of constituents | [12] |
| 2019 | Male infertility | NS | [13] | |
| 2019 | Phytochemistry and pharmacology | 1965–2017 | [14] | |
| 2020 | Phytochemistry and pharmacology | NS | the review is based mostly on Ayurvedic preparation The pharmacological effects are briefly presented | [15] |
| Compound | Chemical Formula | Plant Part | Conc. mg/100 g | Plant Origin | References |
|---|---|---|---|---|---|
| Furostanol Saponins | |||||
| Protodioscin | C51H84O22 | aerial parts | 109–1530 | Bulgaria | [3,4,16,17] |
| leaves | 1000–1330 | ||||
| stem | 19–27 | ||||
| fruits | 240–500 | ||||
| aerial parts | 340–1000 | Turkey | [3] | ||
| fruits | 10–60 | ||||
| aerial parts | 220–790 | Greece | [3] | ||
| aerial parts | 420–990 | Macedonia | [3] | ||
| aerial parts | 200 | Serbia | [3] | ||
| aerial parts | 560 | Georgia | [3] | ||
| aerial parts | 3 | Vietnam | [3] | ||
| fruits | 1 | ||||
| fruits | 63–89 | China | [17] | ||
| stem | 24 | India | [17] | ||
| aerial parts | 190 | Russia | [18] | ||
| Neoprotodioscin | C51H86O22 | aerial parts | NS | Bulgaria | [16] |
| Prototribestin | C45H73NaO20S | aerial parts | 130–2200 | Bulgaria | [3,4,16,19] |
| fruits | 21–28 | ||||
| leaves | 700 | ||||
| stems | 40 | ||||
| aerial parts, | 310–1000 | Turkey | [3] | ||
| fruits | 17–65 | ||||
| aerial parts | 220–790 | Greece | [3] | ||
| aerial parts | 420–990 | Macedonia | [3] | ||
| [3] | |||||
| aerial parts | 170 | Serbia | [3] | ||
| aerial parts | 240 | Georgia | [3] | ||
| Neoprototribestin | C45H75NaO20S | aerial parts | NS | Bulgaria | [16] |
| Terestrinin A | C33H48O9 | fruits | NS | China | [20] |
| Terestrinin B | C60H95O30 | root | NS | Georgia | [21] |
| fruits | NS | China | [20] | ||
| Terrestrinin D | C33H50O10 | fruits | 5.6 | China | [22,23] |
| Terestrinin J-T | whole plant | NS | China | [24] | |
| Terestroside A | root | NS | Georgia | [21] | |
| Terrestrosin K | C51H82O24 | fruits | 1.27 | China | [22] |
| Terrestrosin I | C51H84O25 | whole plant | NS | China | [23,24] |
| fruits | |||||
| Tribufuroside D | C45H74O21 | fruits | NS | China | [23,25] |
| Tribufuroside E | C45H74O21 | fruits | NS | China | [23,25] |
| Tribulosaponin A | C51H84O21 | fruits | NS | China | [26] |
| Polianthoside D | C56H92O29 | root | NS | Georgia | [21] |
| fruits | 59.6 | China | [22] | ||
| Spirostanol Saponins | |||||
| Dioscin | C45H72O16 | aerial parts | NS | Egypt | [27] |
| aerial parts | 60 | Russia | [18] | ||
| fruits, leaves, stem | 10–43 | Bulgaria | [3,4,28] | ||
| aerial parts | 6–13 | Turkey | [3] | ||
| fruits | 1–2 | ||||
| aerial parts | 26–31 | Greece | [3] | ||
| aerial parts | 13–15 | Macedonia | [3] | ||
| aerial parts | 87 | Serbia | [3] | ||
| aerial parts | 8 | Georgia | [3] | ||
| Tribestin | C39H61NaO14S | aerial parts | 2–220 | Bulgaria | [3,28] |
| fruits | 0.9–3.4 | ||||
| leaves | 62 | ||||
| aerial parts | 6.8–28 | Turkey | [3] | ||
| fruits | 0.5–1 | ||||
| aerial parts | 24 | Greece | [3] | ||
| aerial parts | 7.3–10 | Macedonia | [3] | ||
| aerial parts | 210 | Serbia | [3] | ||
| aerial parts | 6 | Georgia | [3] | ||
| Diosgenin | C27H42O3 | NS | NS | China | [29] |
| NS | NS | Ukraine | [30] | ||
| fruits | 86 | India | [31] | ||
| Tribulosin | C55H90O25 | aerial parts | 0.1–7.7 | Bulgaria | [3] |
| fruits | 2.6 | ||||
| leaves | 0.8 | ||||
| stem | 1.7 | ||||
| aerial parts | 0.03–1.7 | Turkey | [3] | ||
| fruits | 0.14 | ||||
| aerial parts | 1.3–2.4 | Greece | [3] | ||
| aerial parts | 0.68 | Macedonia | [3] | ||
| aerial parts | 2.24 | Serbia | [3] | ||
| aerial parts | 0.56 | Georgia | [3] | ||
| aerial parts | 22 | Vietnam | [3] | ||
| fruits | 420 | ||||
| fruits | 1 | India | [3] | ||
| leaves | 644 | ||||
| stem | 185 | ||||
| whole plant | NS | India | [32] | ||
| Tigogenin | C27H44O3 | fruits | 0.05 | China | [22,29,33] |
| Terestrinin U | whole plant | NS | China | [24] | |
| Gitogenin | C27H44O4 | NS | NS | China | [33] |
| Hecogenin | C27H42O4 | fruits | NS | Taiwan | [34] |
| fruits | 0.4 | China | [22] | ||
| Agovoside A | fruits | NS | China | [20] | |
| Prosapogenin B | aerial parts | NS | Egypt | [27] | |
| 25R-5a-Spirost-3,6,12-trione | C27H39O5 | NS | NS | China | [33] |
| 25R-Spirost-4-ene-3,12-dione | C27H40O4 | NS | NS | China | [33,35] |
| 25R-Spirost-4-ene-3,6,12-trione | C27H38O6 | NS | NS | China | [33,35] |
| Cinnamic Acid Amides | |||||
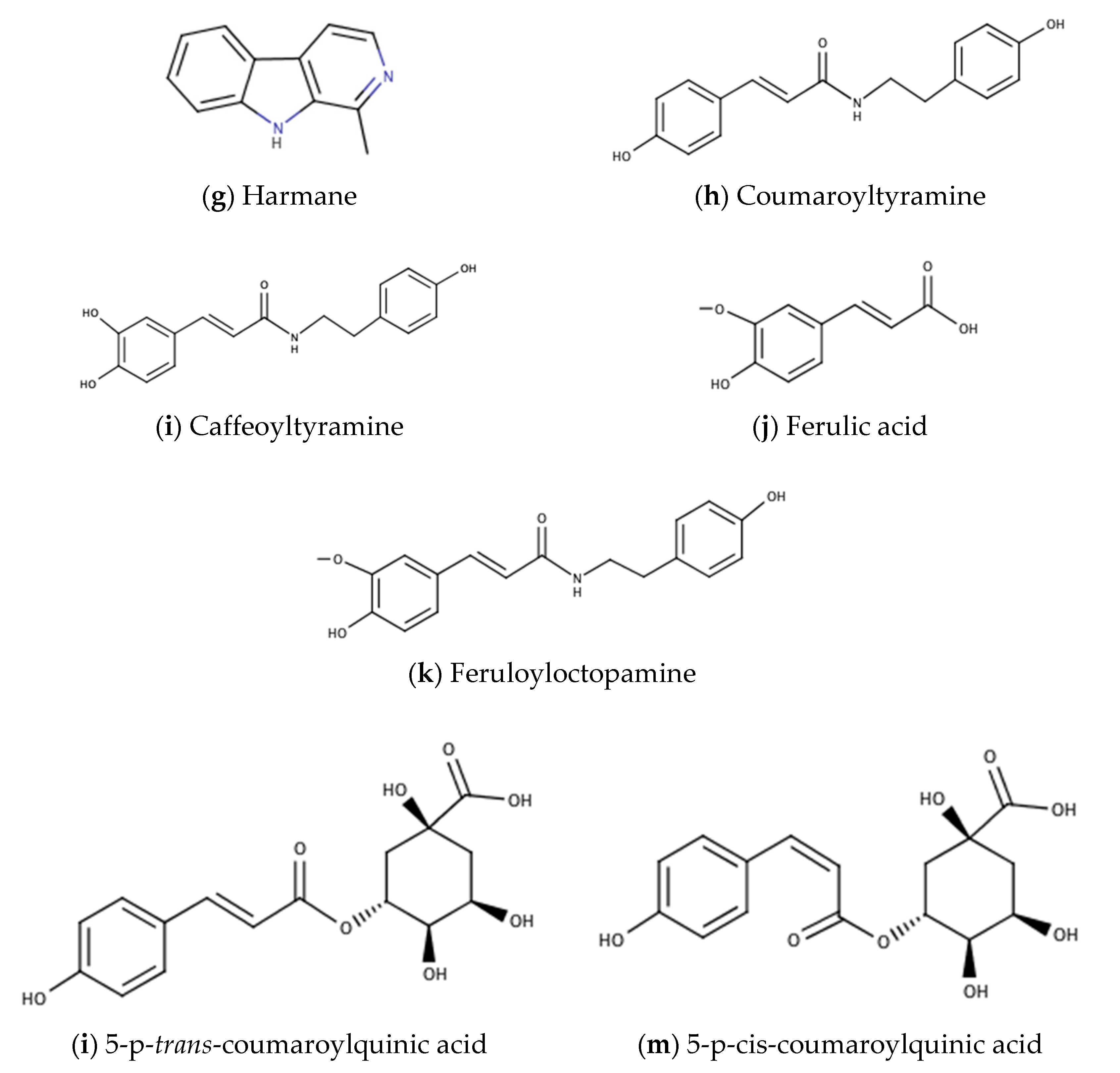
| Coumaroyltyramine | C17H17NO3 | fruits | NS | Taiwan | [34,36,37] |
| fruits | NS | China | |||
| Ferulic acid | fruits | NS | Taiwan | [34] | |
| Feruloyloctopamine | C18H19NO5 | fruits | NS | China | [36] |
| Quinic Acid Derivatives | |||||
| 5-p-cis-coumaroylquinic acid | C16H18O8 | aerial parts | NS | Egypt | [27] |
| 5-p-trans-coumaroylquinic acid | aerial parts | NS | Egypt | [27] | |
| 4,5-Di-p-trans-coumaroylquinic acid | aerial parts | NS | Egypt | [27] | |
| 4,5-Di-p-cis-coumaroylquinic acid | aerial parts | NS | Egypt | [27] | |
| Flavonoids | |||||
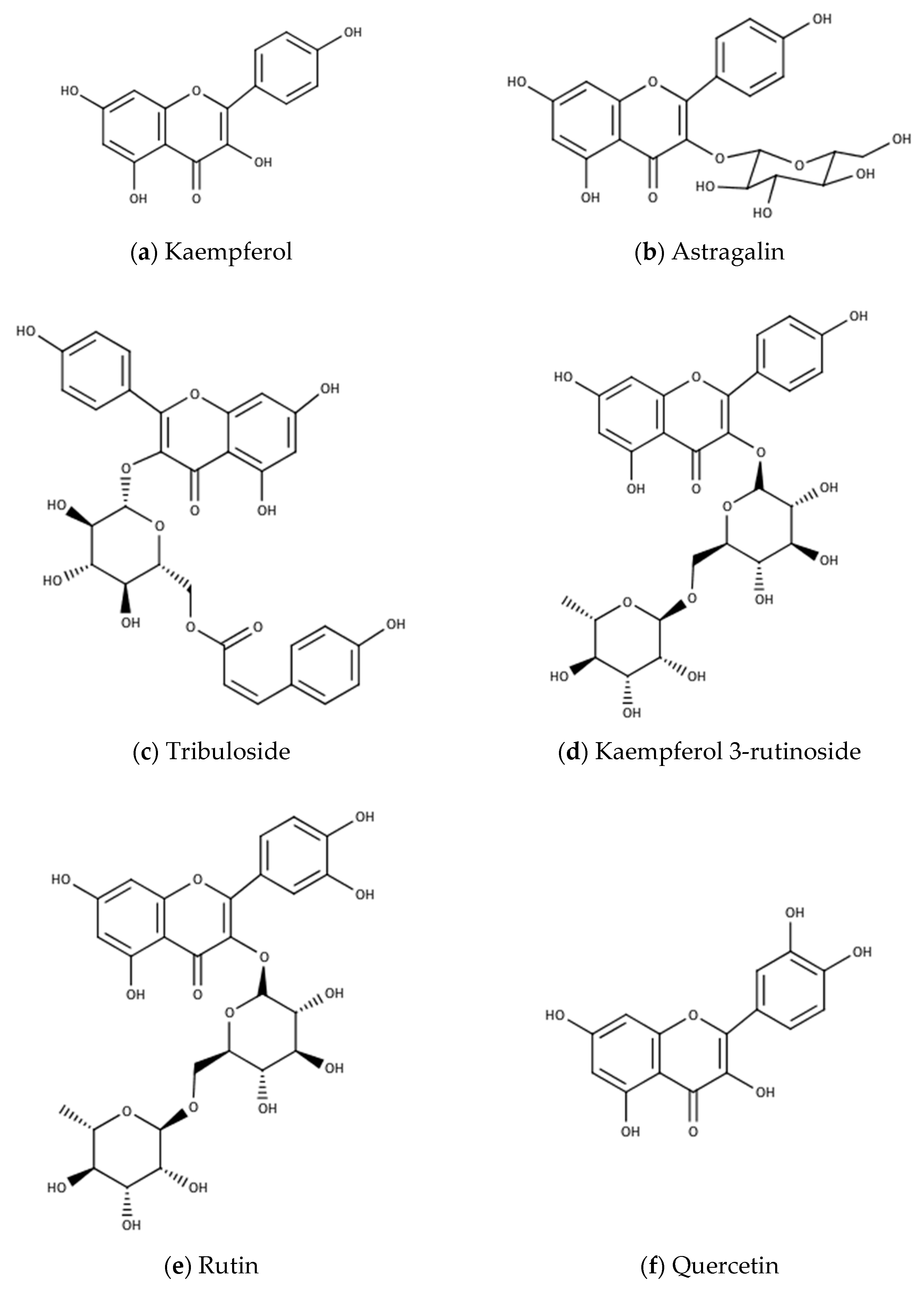
| Tribuloside | C30H26O13 | leaves, fruits | NS | India | [38] |
| Kaempferol | C15H10O6 | leaves, fruits | 18 | India | [31,38] |
| Astragalin (kaempferol 3-glucoside) | C21H20O11 | leaves, fruits | NS | India | [38] |
| Kaempferol 3-rutinoside | C27H30O15 | leaves, fruits | NS | India | [38] |
| Kaempferol-3- gentiobioside | C27H30O16 | fruits leaves | NS | China | [39,40] |
| Rutin | C27H30O16 | leaves | NS | Mauritania | [4,41,42,43] |
| fruits, leaves | NS | India | |||
| fruits, leaves | 70–250 | Bulgaria | |||
| fruits | NS | Korea | |||
| NS | NS | Ukraine | [30] | ||
| Quercetin | C15H10O7 | fruits, leaves | NS | India | [42] |
| Quercetin-3-O-arabinosyl galactoside Isorhamnetin-3-glucoside | C26H28O16 | fruits leaves | NS | China | [39,40] |
| Quercetin-3-O-sophoroside-7-O-glucoside | C33H40O21 | leaves | NS | China | [39] |
| Quercetin-3- gentiobioside | C27H30O17 | fruits, leaves | NS | China | [39,40] |
| Quercetin 3,7-diglucoside | C27H30O17 | fruits, leaves | NS | China | [39,40] |
| Isoquercitrin | C21H20O12 | fruits, leaves | NS | China | [39,40] |
| Luteolin-7-O-β-D- glucoside | C30H18O11 | leaves | NS | China | [39] |
| Isorhamnetin-3-glucoside | C22H22O12 | leaves | NS | China | [39] |
| Apiotribosides A-D | roots | NS | Georgia | [21] | |
| Alkaloids | |||||
| Harmine | C13H12N2O | fruits | 14 | India | [31] |
| fruits, stem, leaves, roots | NS | Turkey | [44] | ||
| Harmane | C12H10N2 | fruits, stem, leaves, roots | NS | Turkey | [44] |
| aerial parts | NS | Australia | [45] | ||
| Harmalol | C12H12N2O | fruits, stem, leaves, roots | NS | Turkey | [44] |
| Harmaline | C13H14N2O | stem, leaves, roots | NS | Turkey | [44] |
| Norharmane | C11H8N2 | aerial parts | NS | Australia | [45] |
| Tribulusterine | C16H12N2O2 | fruits | NS | Taiwan | [34] |
| not specified | NS | India | [46] | ||
| n-Caffeoyltyramine | fruits | NS | Korea | [36,47] | |
| fruits | China | ||||
| Perlolyrine | C16H12N2O2 | not specified | NS | India | [46] |
| Amides and Lignanamides | |||||
| Terrestribisamide | C13 H18NO5 | fruits | NS | Taiwan | [34] |
| Tribulusamide A | C36H36N2O8 | fruits | NS | China | [37] |
| Tribulusamide B | C36H34N2O9 | fruits | NS | China | [37] |
| Tribulusamide D | C17H15NO5 | fruits | NS | Korea | [48] |
| Tribulusamide C | C18H15NO6 | fruits | NS | China | [49] |
| Fatty Acids and Fatty Acid Esters | |||||
| Oleic acid | C18H34O2 | stem | NS | Pakistan | [50] |
| Palmitic acid | C16H32O2 | stem | NS | Pakistan | [50] |
| 6,9,12,15-Docosatetraenoic acid, methyl ester | C23H38O2 | stem | NS | Pakistan | [50] |
| Pentadecanoic acid, 14-methyl-, methyl ester | C17H34O2 | stem | NS | Pakistan | [50] |
| 9,12-Octadecadienoic acid, methyl ester (E,E)- | C19H34O2 | stem | NS | Pakistan | [50] |
| Phytosterols | |||||
| β-sistosterol-D-glucoside | C35H60O6 | whole plant | NS | India | [32] |
| Stigmasterol | C29H48O | stem | NS | Pakistan | [50] |
| Other Compounds | |||||
| ß-1, 5-O-dibenzoyl ribofuranose | C19H18O7 | roots | NS | India | [51] |
| 1,3-Benzenedicarboxylic acid, bis(2-ethylhexyl) ester | C24H38O4 | stem | NS | Pakistan | [50] |
| Apiol | C12H14O4 | stem | NS | Pakistan | [50] |
| Octacosane | C28H58 | stem | NS | Pakistan | [50] |
| Heptacosane | C27H56 | stem | NS | Pakistan | [50] |
| Herbal Drug and Subjects | Assay/Parameters | Outcome of Treated Group | Study Design Evaluation | Reference |
|---|---|---|---|---|
| In Vitro Studies | ||||
| Organ bath study of the corpus cavernosum from | Relaxation level | Concentration-dependent relaxation response | Part of the plant: NO | Kam et al. (2012) |
| male rabbits | Origin: NO | [64] | ||
| Phytochemical analysis: NO | ||||
| Control group: NO | ||||
| Appropriate Statistical analysis: YES | ||||
| Human sperm from 40 healthy volunteers | Motility analysis | Motility ↑ * after 60 minutes of incubation | Part of the plant: NO | Khaleghi et al. (2017) |
| TT extract | Sperm viability analysis | Viability ↑ * in a dose-dependent manner after 120 minutes of incubation | Origin: YES | [63] |
| Determination of DNA fragmentation | No effect on DNA fragmentation of human sperm in vitro | Phytochemical analysis: NO | ||
| Control group: YES | ||||
| Appropriate statistical analysis: YES | ||||
| In Vivo Animal Studies | ||||
| Male adult Sprague Dawley rats, castrated and normal | Sexual behavior studies: MF, IF, ML, IL, EL, PEI | Treatment of castrated rats (with testosterone or TT extract) showed increase in prostate weight and ICP that were statistically significant | Part of the plant: NCS | Gauthaman et al. (2002) |
| TT extract | ICP | Mild to moderate improvement of sexual behavior parameters | Origin: YES | [70] |
| Phytochemical analysis: NCS | ||||
| Control group: YES | ||||
| Positive control group: YES | ||||
| Appropriate statistical analysis: YES | ||||
| Male Sprague Dawley rats | ICP | ICP concentration-dependent increase in TT treated group* | Part of the plant: NCS | Kam et al. (2012) |
| TT extract, Cornus officinalis extract and a mixture of both | cAMP, cGMP in corpus cavernosum | cAMP ↑* in the group treated with the mixture | Origin: YES | [64] |
| cGMP no significant difference as compared with the control | Phytochemical analysis: NO | |||
| Control group: YES | ||||
| Positive control group: NO | ||||
| Appropriate statistical analysis: YES | ||||
| -Male rats | Morphometric analysis | Testicular weight ↑* | Origin: YES | Oliveira et al. (2015) |
| TT fruit extract and fractions | Gonadosomatic index | Gonadosomatic index increased in the group supplemented with ethanolic extract | Part of the plant: YES | [69] |
| Sperm quality analysis: motility, | -Nuclear, cytoplasmic, and individual volume of Leydig cells increased in supplementation with hexanic and aqueous fractions | Phytochemical analysis: NO | ||
| sperm count, | The extract influenced the spermatogenesis | Control group: YES | ||
| morphology, viability | Positive control group: NO | |||
| Appropriate statistical analysis: YES | ||||
| Male Wistar rats with STZ-induced diabetes (55 mg/kg) | Sperm characteristics, morphology | TT restored antioxidant enzyme activity in testis | Part of the plant: YES | Tag et al. (2015) |
| TT fruit extract | Body and genital organ weight | Improved lipid profile content in serum | Origin: YES | [85] |
| Serum testosterone, FSH, LPO level in testicular homogenate | TT treatment decreased testis tubular damage and restored it to normal morphology. | Phytochemical analysis: YES (identification reactions) | ||
| Activity of testicular SOD | Control group: YES | |||
| Testicular CAT activity | Positive control group: YES | |||
| GPx, GST | Appropriate statistical analysis: YES | |||
| Male Wistar rats with STZ-induced diabetes (50 mg/kg) | Testosterone | Sperm motility, sperm count, percentage of sperms with normal morphology ↑* | Part of the plant: YES | Ghanbari et al. (2016) |
| TT seed extract | Sperm analysis: morphology, count and motility | Testosterone ↑* | Origin: NO | [67] |
| Phytochemical analysis: NO | ||||
| Control group: YES | ||||
| Positive control group: NO | ||||
| Appropriate statistical analysis: YES | ||||
| Male Sprague Dawley rats | Time to exhaustion of over trained rats | Performance (time to exhaustion) ↑* | Origin: YES | Yin et al. (2016) |
| TT fruit extract (saponins >70%) | Serum testosterone, corticosterone, AR, IGF-1R in liver, gastrocnemius, and soleus | Increase in body weights, relative weights, and protein levels of gastrocnemius | Part of the plant: YES | [66] |
| Testosterone ↑* | Phytochemical analysis: YES (UHPLC-Q-TOF/MS) | |||
| AR ↑* | Control group: YES | |||
| IGF-1R ↓# | Appropriate Statistical analysis: YES | |||
| Adult male Swiss albino mice | SOD, CAT, GPx, | SOD, CAT, GST ↓# | Part of the plant: YES | Pavin et al. (2018) |
| TT fruit extract | GR, GST, GSH, 17β-HSD | GPx ↑# | Origin: YES | [65] |
| Plasma testosterone | 17β-HSD activity in treated group was not statistically significant different as compared with the control group | Phytochemical analysis: YES (UHPLC-Q-TOF/MS) | ||
| Semen analysis: | Testosterone ↑ | Control group: YES | ||
| motility, vigor, membrane integrity | Motility ↑# | Positive control group: YES | ||
| Histology of testes | No significant modifications in testicular architecture | Appropriate statistical analysis: YES | ||
| Male Wistar rats | Sperm analysis: sperm count, viability, motility | Testosterone, LH ↑* | Part of the plant: YES | Haghmorad |
| TT flower extract and | Serum testosterone, LH, FSH levels | All the treatment groups had higher number of Leydig, spermatogonia and spermatid cells | Origin: YES | et al. (2019) |
| Anacyclus Pyrethrum dried root extract | Histological analysis of Leydig and Sertoli cells, spermatogonia, and spermatid cell numbers measure | Phytochemical analysis: NO | [68] | |
| Control group: YES | ||||
| Positive control group: NO | ||||
| Appropriate statistical analysis: YES | ||||
| Sprague Dawley rats with type 2 diabetes induced with high-fat and high-sugar feeding and STZ (30 mg/kg) | ICP, MAP | ICP, ICP/MAP ↑ * | Part of the plant: NCS | Zhang et al. (2019) |
| Gross saponins of TT (GSTT) | eNOS expression level | Nitric oxide ↑* | Origin: YES | [86] |
| Nitric oxide level | ROS ↓* | Phytochemical analysis: NCS | ||
| cAMP expression level | No significant difference between the GSTT group and the sildenafil group in increasing cGMP levels | Control group: YES | ||
| ROS levels | Positive control group: YES | |||
| Appropriate statistical analysis: YES | ||||
| Clinical Studies | ||||
| 20–36-Year-old men | Testosterone, androstenedione, LH levels in the serum were measured before and after treatment (24, 72, 240, 408, and 576 h) | No significant difference between TT supplemented groups and the control in the serum testosterone, androstenedione, and LH | Part of the plant: YES | Neychev and Mitev (2005) |
| TT extract | Origin: YES | [76] | ||
| Phytochemical analysis or standardization: YES | ||||
| Placebo group: YES | ||||
| Randomization: YES | ||||
| Double-blind: NCS | ||||
| Appropriate statistical analysis: YES | ||||
| Australian elite male rugby league players | Strength, fat free mass | No significant changes | Part of the plant: NCS | Rogerson et al. (2007) |
| Urinary T/E ratio | No changes in urinary T/E ratio | Origin: YES | [77] | |
| Phytochemical analysis or standardization: YES | ||||
| Placebo group: YES | ||||
| Randomization: YES | ||||
| Double-blind: YES | ||||
| Appropriate statistical analysis: YES | ||||
| 20–22-Year-old athletes | CK, testosterone | CK ↑* | Part of the plant: NCS | Milasius et al. (2009) |
| TT capsules | Anaerobic alactic muscular power | Testosterone ↑* during the first half (10 days) of the experiment | Origin: NCS | [73] |
| Anaerobic alactic glycolytic power | Anaerobic alactic muscular power ↑* | Phytochemical analysis or standardization: NCS | ||
| Anaerobic alactic glycolytic power ↑* | Placebo group: YES | |||
| Randomization: NO | ||||
| Double-blind: NO | ||||
| Appropriate statistical analysis: YES | ||||
| Double-blind, randomized trial | IIEF, SQolM, | IIEF ↑* | Part of the plant: NCS | Iacono et al. (2012) |
| Male patients > sixty years with | Testosterone levels after 60 days of treatment, | SQolM ↑* | Origin: NCS | [74] |
| reduced libido, with or without erectile dysfunction (ED) | Side effects | TT level increased | Phytochemical analysis or standardization: NCS | |
| Treatment with “Tradamixina”, tadalafil | No side effects (headache, | Placebo group: NO | ||
| nasopharyngitis, | Randomization: YES | |||
| back pain, | Double-blind: YES | |||
| dizziness, | Appropriate statistical analysis: NO | |||
| dyspepsia) were observed | ||||
| Prospective, randomized, double-blind, placebo controlled study | IIEF and serum testosterone were obtained before randomization and after 30 days of study | No effects as compared with the placebo | Part of the plant: NO | Santos et al. (2014) |
| Healthy men, spontaneously complaining of ED, ≥40 years of age | Origin: NO | [72] | ||
| TT extract | Phytochemical analysis or standardization: NO | |||
| Placebo group: YES Randomization: YES | ||||
| Double-blind: YES | ||||
| Appropriate statistical analysis: YES | ||||
| Randomized, double-blind, placebo controlled clinical trial study | FSFI score | FSFI ↑* | Part of the plant: YES | Akhtari et al. (2014)[83] |
| Women with hypoactive sexual desire disorder | Origin: YES | |||
| TT leaves extract | Phytochemical analysis or standardization: NCS | |||
| Placebo group: YES | ||||
| Randomization: YES | ||||
| Double-blind: YES | ||||
| Appropriate statistical analysis: YES | ||||
| Prospective, randomized, double-blind, placebo controlled clinical trial | IIEF score | IIEF score ↑* | Part of the plant: YES | Kamenov et al. (2017) |
| Male with mild to moderate ED | GEQ responses | GEQ responses ↑* | Origin: YES | [71] |
| TT product: Tribestan®, | Phytochemical analysis or standardization: YES | |||
| 12-Week treatment period | Placebo group: YES | |||
| Randomization: YES | ||||
| Double-blind: YES | ||||
| Appropriate statistical analysis: YES | ||||
| Single-blind, placebo controlled, parallel study | MRS | Severity of menopausal transition sympt. ↓* | Part of the plant: YES | Fatima and Sultana (2017) |
| Perimenopausal women | Severity of menopausal transition symptoms | MRS ↓* | Origin: YES | [82] |
| TT fruit extract | Phytochemical analysis or standardization: NCS | |||
| Placebo group: YES | ||||
| Randomization: YES | ||||
| Double-blind: NO (single-blind) | ||||
| Appropriate statistical analysis: YES | ||||
| Prospective, randomized, double-blind, placebo controlled trial, | FSFI score | FSFI ↑* | Part of the plant: NCS | Vale et al. (2018) |
| Premenopausal women with diminished libido | QS-F score | QS-F ↑* | Origin: YES | [81] |
| TT extract | Serum testosterone | Serum testosterone ↑* | Phytochemical analysis or standardization: NCS | |
| Placebo group: YES | ||||
| Randomization: YES | ||||
| Double-blind: YES | ||||
| Appropriate statistical analysis: YES | ||||
| Herbal Drug and Subjects | Assay/Parameters | Outcome of Treated Group | Study Design Evaluation | Reference |
|---|---|---|---|---|
| In Vitro Studies | ||||
| TT fruit extract | α-Glucosidase | Activity inhibition on all tested enzymes | Part of the plant: YES | Lamba et al. (2011) |
| Aldose reductase | Origin: YES | [99] | ||
| Phytochemical analysis: NCS | ||||
| Control group: YES | ||||
| Positive control group: YES | ||||
| Appropriate statistical analysis: YES | ||||
| TT seeds | α-Amylase | Concentration- inhibition of enzyme activity | Part of the plant: YES | Ponnusamy et al. (2011) |
| Kinetic studies. | Origin: YES | [96] | ||
| Phytochemical analysis: YES (identification reactions, GC/MS) | ||||
| Positive control: YES | ||||
| Appropriate statistical analysis: YES | ||||
| TT leaves | Lipase | Activity inhibition on all tested enzymes | Part of the plant: YES | Ercan and El (2016) |
| α-Amylase | Origin: YES | [95] | ||
| α-Glucosidase | Phytochemical analysis: YES (spectrophotometric) | |||
| Positive control: YES | ||||
| Appropriate statistical analysis: YES | ||||
| In Vivo Animal Studies | ||||
| Male Swiss albino rats with STZ-induced diabetes (55 mg/kg) | BW, BG, Hb, HbA1c, TG, TC, HDL, LDL-c | BW ↑* | Part of the plant: YES | El-Tantawy and Hassanin (2007) |
| TT aerial part extract | Histopathological analysis of the pancreas | BG ↓* after 2,4, and 6 h | Origin: YES | [98] |
| HbA1c returned to the normal values | Phytochemical analysis: NO | |||
| HDL ↑* | Control group: YES | |||
| TG, TC, LDL-c ↓* | Positive control group: YES | |||
| Histological structure was less affected as compared with the control group | Appropriate statistical analysis: YES | |||
| Wistar rats with STZ-induced diabetes (50 mg/kg) | BG, BW, HbA1c, INS, GLG | BG ↓* | Part of the plant: YES | Lamba et al. (2011) |
| TT fruit extract | Urinary albumin levels | BW ↑* | Origin: YES | [99] |
| HbA1c, GLG ↑ | Phytochemical analysis: NCS | |||
| Control group: YES | ||||
| Positive control group: YES | ||||
| Appropriate statistical analysis: YES | ||||
| Male Wistar rats with STZ-induced diabetes (40 mg/kg) | BG | BG, PT, APTT, TC, TG, LDL, ALT, AST, ALP, glucose-6-phosphatas, fructose-1, 6-bisphosphatase, LPO ↓ * | Control group: YES | Kalailingam et al (2014) |
| Diosgenin | HbA1c | HDL, SOD, CAT, GSH ↑ * | Positive control group: NO | [101] |
| TC, TG, HDL, LDL, AST, ALP | Appropriate statistical analysis: YES | |||
| PT, APTT | ||||
| Hepatic glucose-6-phosphatase, fructose-1, 6-bisphosphatase SOD, CAT, GSH, LPO | ||||
| Male Sprague Dawley rats with type 2 diabetes induced with high-fat diet (HFD) + STZ (35 mg/kg) | BG, INS, BW | BG ↓ *, INS ↑ *, BW ↑ * | Control group: YES | Tharaheswari et al. (2014) |
| Diosgenin | FFA, TNF-α, IL-6, leptin | FFA, TNF-α, IL-6, leptin ↓ * | Positive control group: NO | [102] |
| HOMA-IR, HOMA-B, QUICKI | HOMA-IR, HOMA-B, QUICKI – improved values | Appropriate statistical analysis: YES | ||
| In tissue homogenate were determined: LPO, GSH, SOD, CAT, GPx | Increased adipose tissue mass | |||
| Histopathological analysis of pancreas | Enhanced PPARc expression | |||
| Quantification of adipose PPAR γ | Good interaction of diosgenin with PPAR γ | |||
| Glucose-loaded normal rabbits, | FBG at 30 min, 1, 2, 3 h after dosing | FBG ↓* at 2 hours | Part of the plant: YES | El-Shaibany et al. (2015) |
| TT aerial parts extract | Acute toxicity study | No toxicity | Origin: YES | [100] |
| Phytochemical analysis: YES (TLC) | ||||
| Control group: YES | ||||
| Positive control group: YES | ||||
| Appropriate Statistical analysis: YES | ||||
| Male Wistar rats with STZ-induced diabetes (55 mg/kg) | BG | BG ↓* | Part of the plant: YES | Tag et al. (2015) |
| TT fruit extract | INS | INS ↑* | Origin: YES | [85] |
| Phytochemical analysis: YES (identification reactions) | ||||
| Control group: YES | ||||
| Positiv control group: YES | ||||
| Appropriate Statistical analysis: YES | ||||
| Sprague Dawley rats with type 2 diabetes induced with high-fat and high-sugar feeding and STZ (30 mg/kg) | BG | BG ↓ | Part of the plant: NO | Zhang et al. (2019) |
| Gross saponins of TT | BW | No significant differences in BW | Origin: YES | [86] |
| Phytochemical analysis: NCS | ||||
| Control group: YES | ||||
| Positive control group: YES | ||||
| Appropriate statistical analysis: YES | ||||
| Clinical Studies | ||||
| 100 Patients suffering from DM with microalbuminuria | BG | BG ↓ * | Part of the plant: NCS | Ramteke et al. (2012) |
| Ayurvedic preparation with TT | BP | BP ↓ * | Origin: NCS | [105] |
| Urine albumin | Urine albumin ↓* | Phtochemical analysis or standardization: NO | ||
| Placebo group: NO | ||||
| Randomization: YES | ||||
| Double-blind: NCS | ||||
| Appropriate statistical analysis: YES | ||||
| Double-blind randomized placebo controlled clinical trial | FBG, BG 2-hour postprandial HbA1c | BG ↓* | Part of the plant: NCS | Samani et al. (2016)[104] |
| Ninety-eight women with diabetes mellitus type 2 | TG, TC, LDL, HDL | TC, LDL ↓* | Origin: YES | |
| TT extract | HbA1c, TG, HDL - no significant differences as compared with the placebo | Phtochemical analysis or standardization: YES | ||
| Placebo group: YES | ||||
| Randomization: YES | ||||
| Double-blind: YES | ||||
| Appropriate statistical analysis: YES | ||||
| Compound | Toxicological Information |
|---|---|
| Diosgenin | Oral LD50 (rat) > 8 g/kg |
| Intraperitoneal LD50 (rat) 4872 mg/kg | |
| Oral LD50 (mouse) > 8 g/kg | |
| Intraperitoneal LD50 (mouse) 3564 mg/kg | |
| Dioscin | Subcutaneous LD50 (mouse) >300 mg/kg |
| Oral TDLo (rat) 1050 mg/kg/1W (intermittent) | |
| Oral TDLo (mouse):400 mg/kg/10D (intermittent) | |
| Tigogenin | Intraperitoneal LDLo (rat):10 mg/kg |
| Harmine | Intramuscular TDLo (man):3 mg/kg |
| Intravenous LDLo (cat) 10 mg/kg | |
| Subcutaneous LDLo (frog) 300 mg/kg | |
| Subcutaneous LD50 (mouse) 243 mg/kg | |
| Intravenous LDLo (mouse) 50 mg/kg | |
| Subcutaneous LD50 (rat) 200 mg/kg | |
| Harmane | Intraperitoneal LD50 (mouse) 50 mg/kg |
| Interperitoneal TDLo (rat) 1 mg/kg | |
| Intraperitoneal LD50 (rabbit) 200 mg/kg | |
| Harmaline | Subcutaneous LD50 (rat) 120 mg/kg |
| Subcutaneous LD50 (mouse) 120 mg/kg | |
| Intraperitoneal TDLo (rat) 4 mg/kg | |
| Norharmane | Oral TDLo (rat) 1050 mg/kg/6W (continuous) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ștefănescu, R.; Tero-Vescan, A.; Negroiu, A.; Aurică, E.; Vari, C.-E. A Comprehensive Review of the Phytochemical, Pharmacological, and Toxicological Properties of Tribulus terrestris L. Biomolecules 2020, 10, 752. https://doi.org/10.3390/biom10050752
Ștefănescu R, Tero-Vescan A, Negroiu A, Aurică E, Vari C-E. A Comprehensive Review of the Phytochemical, Pharmacological, and Toxicological Properties of Tribulus terrestris L. Biomolecules. 2020; 10(5):752. https://doi.org/10.3390/biom10050752
Chicago/Turabian StyleȘtefănescu, Ruxandra, Amelia Tero-Vescan, Ancuța Negroiu, Elena Aurică, and Camil-Eugen Vari. 2020. "A Comprehensive Review of the Phytochemical, Pharmacological, and Toxicological Properties of Tribulus terrestris L." Biomolecules 10, no. 5: 752. https://doi.org/10.3390/biom10050752
APA StyleȘtefănescu, R., Tero-Vescan, A., Negroiu, A., Aurică, E., & Vari, C.-E. (2020). A Comprehensive Review of the Phytochemical, Pharmacological, and Toxicological Properties of Tribulus terrestris L. Biomolecules, 10(5), 752. https://doi.org/10.3390/biom10050752






