Chemoenzymatic Synthesis and Biological Evaluation for Bioactive Molecules Derived from Bacterial Benzoyl Coenzyme A Ligase and Plant Type III Polyketide Synthase
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Bacterial Strains
2.3. Site-Directed Mutagenesis
2.4. Protein Expression and Purification
2.5. Enzyme Reaction
2.6. Synthesis of Starter-CoA Derivatives
2.7. Synthesis of Acyl-NAC and Coenzyme-A Derivatives
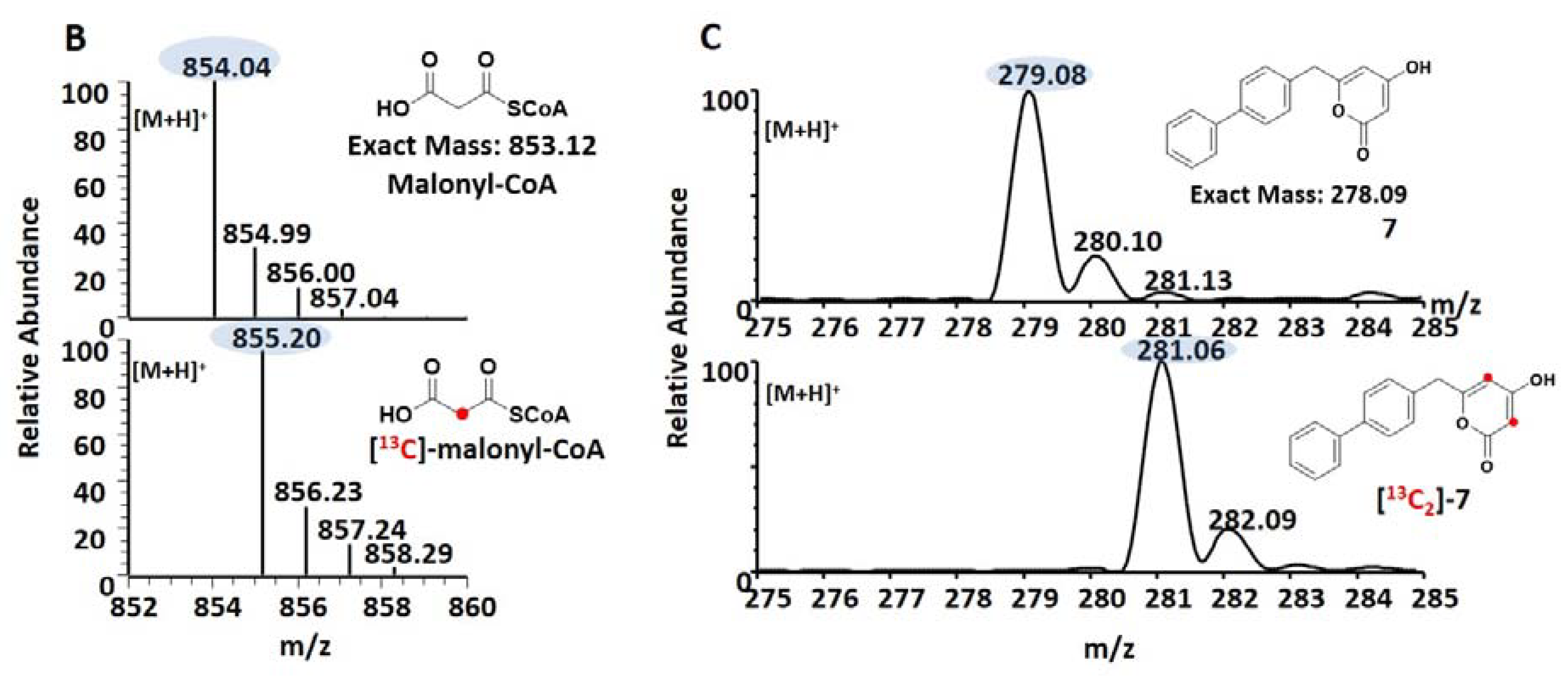
2.8. Synthesis of Malonyl-CoA and [2-13C]-malonyl-CoA
2.9. Kinetics Assay
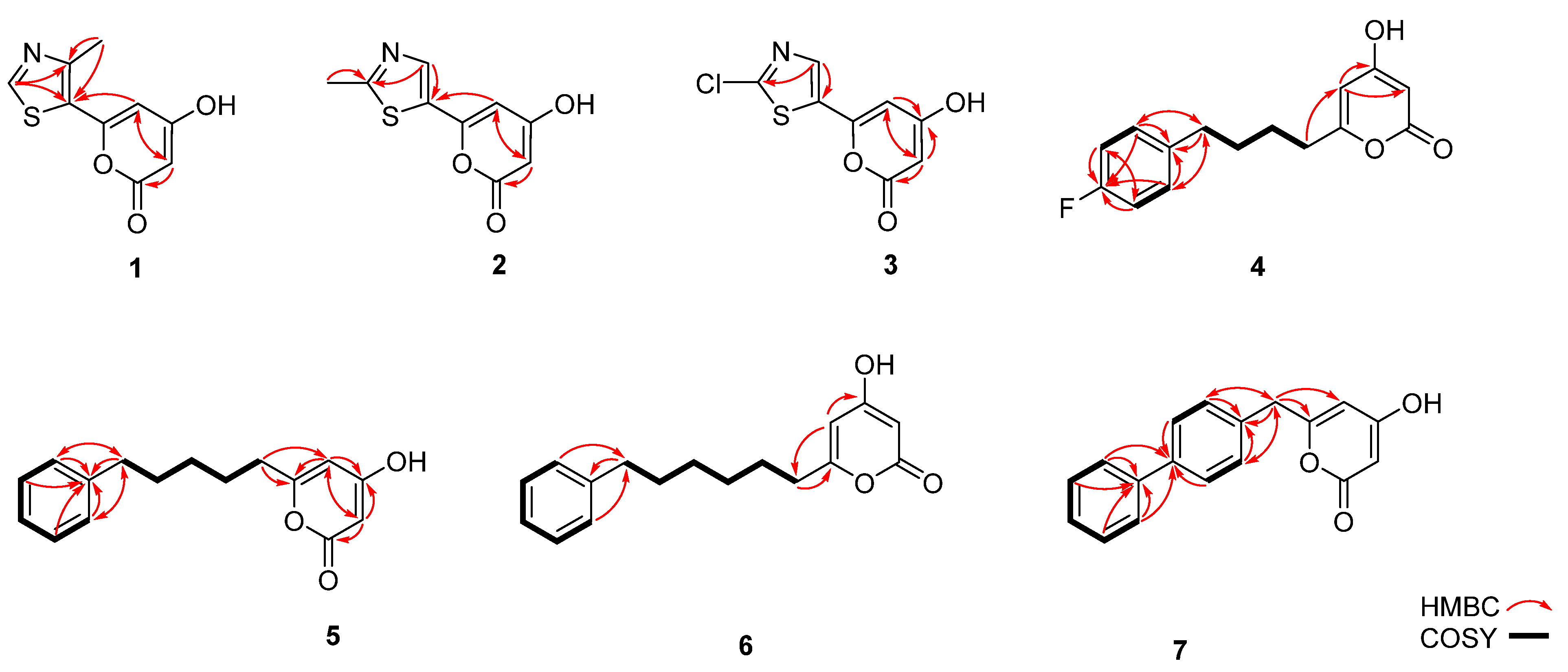
2.10. NMR Analysis
2.11. Crystallization and Data Collection
2.12. Structure Determination and Refinement
2.13. Cell Culture
2.14. Cell Viability Assay
2.15. Flow Cytometry Analysis
3. Results
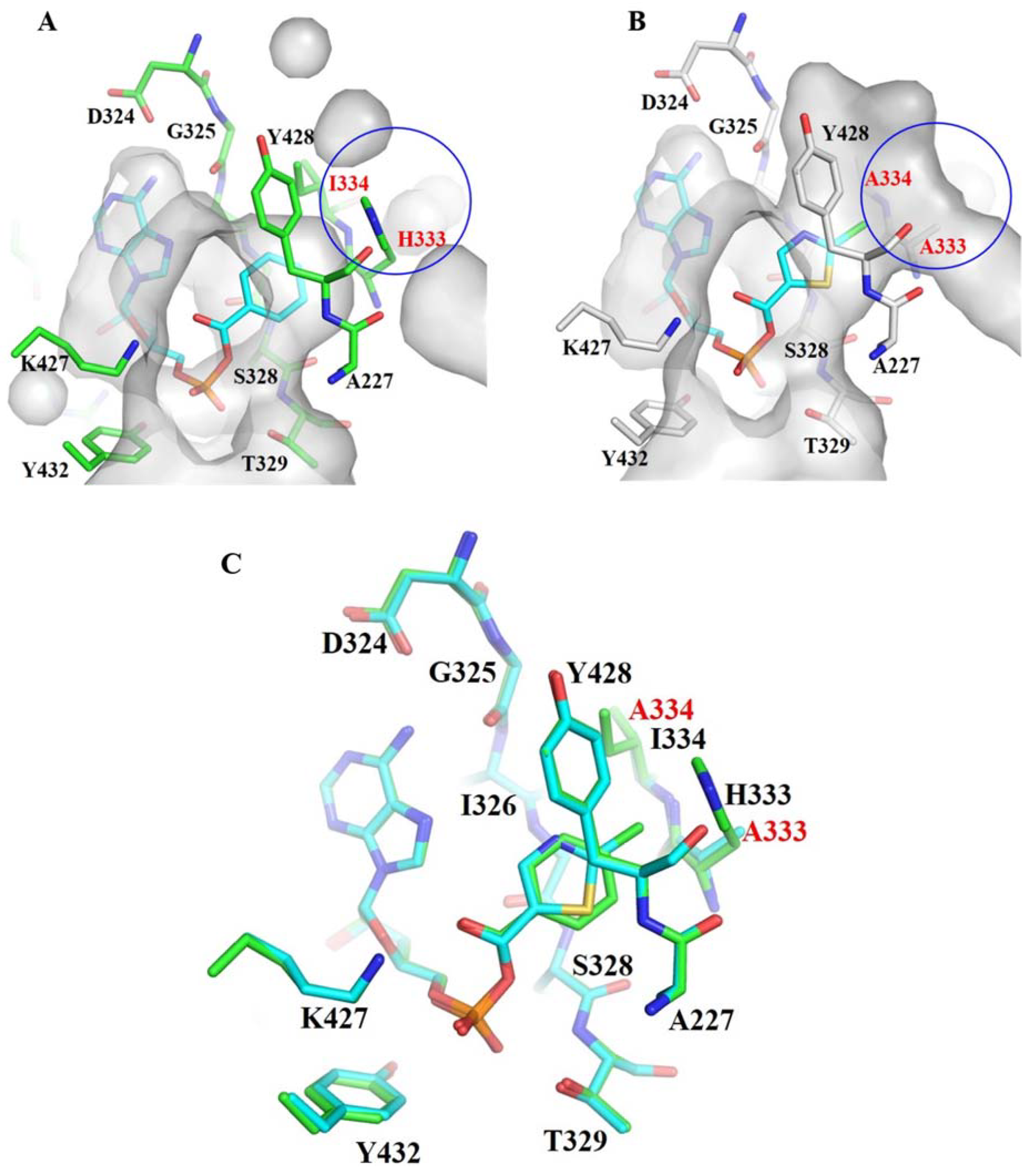
3.1. Structure-Based Engineering of Benzoate-Coenzyme A Ligase (BadA)
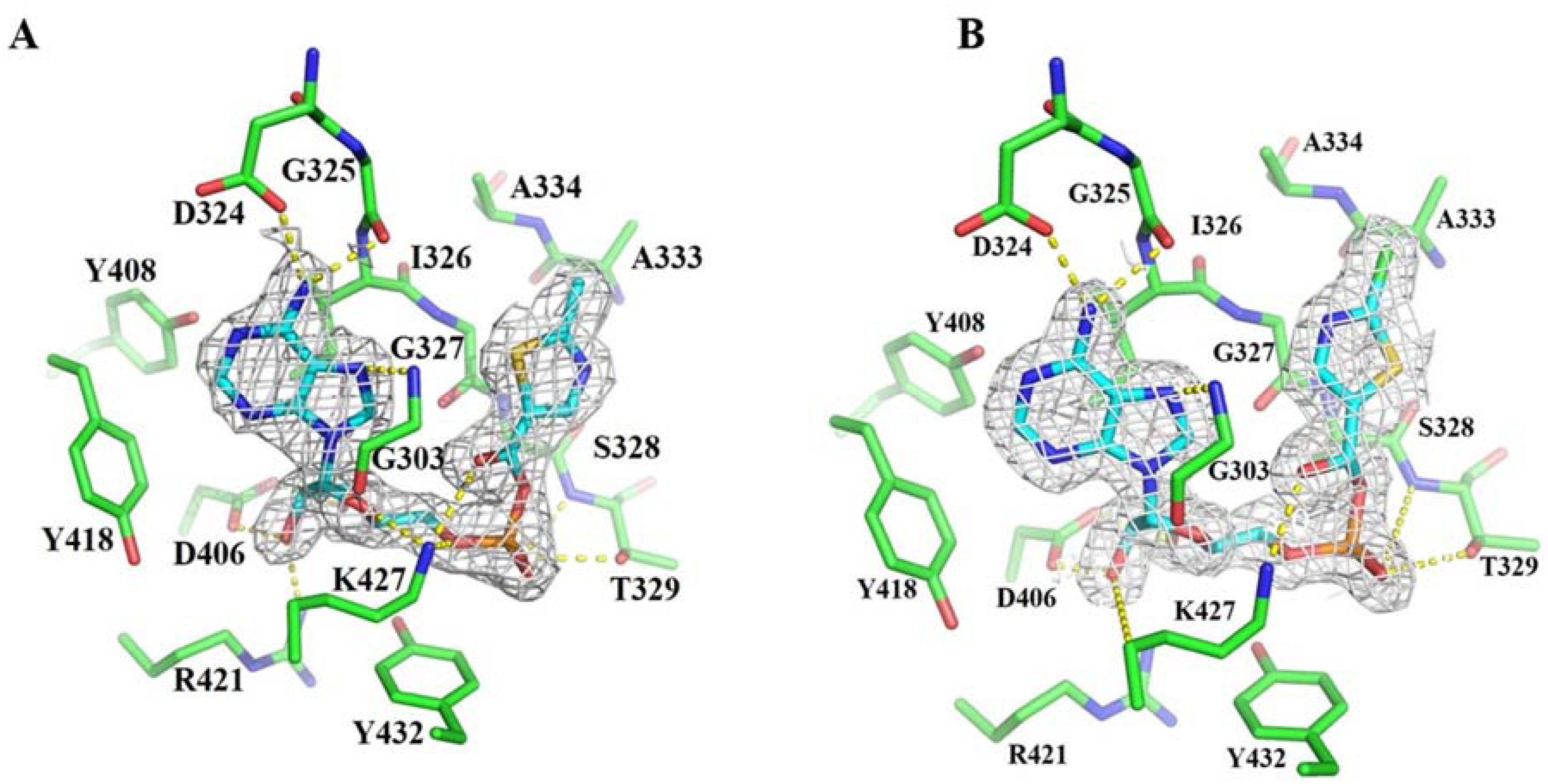
3.2. Crystal Structures of BadA-Mutant (H333A/I333A)
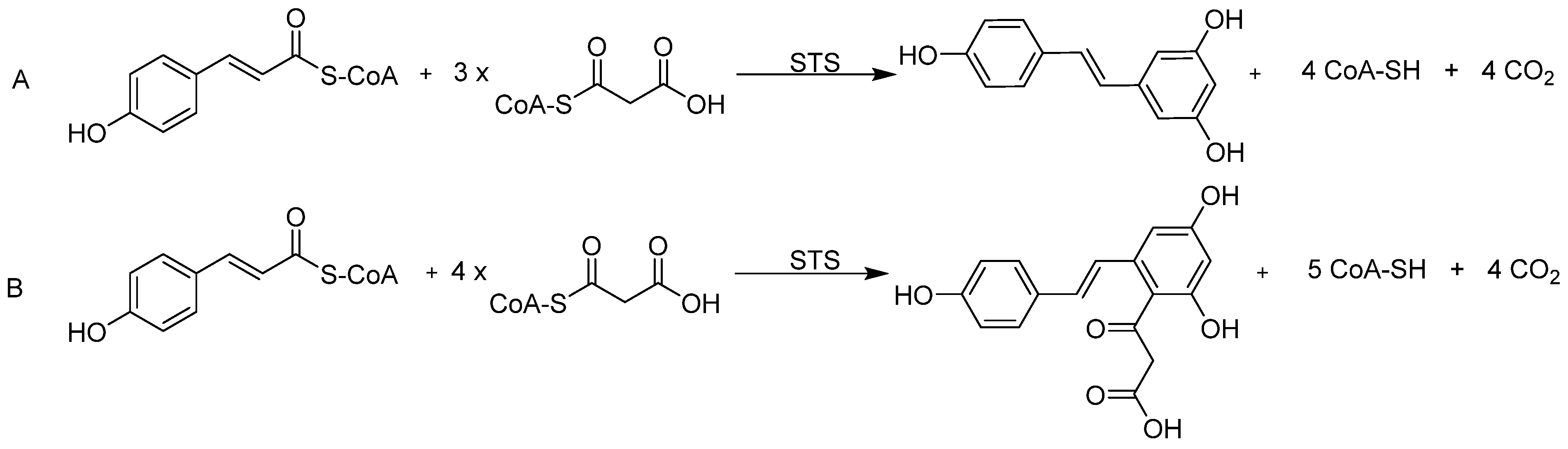
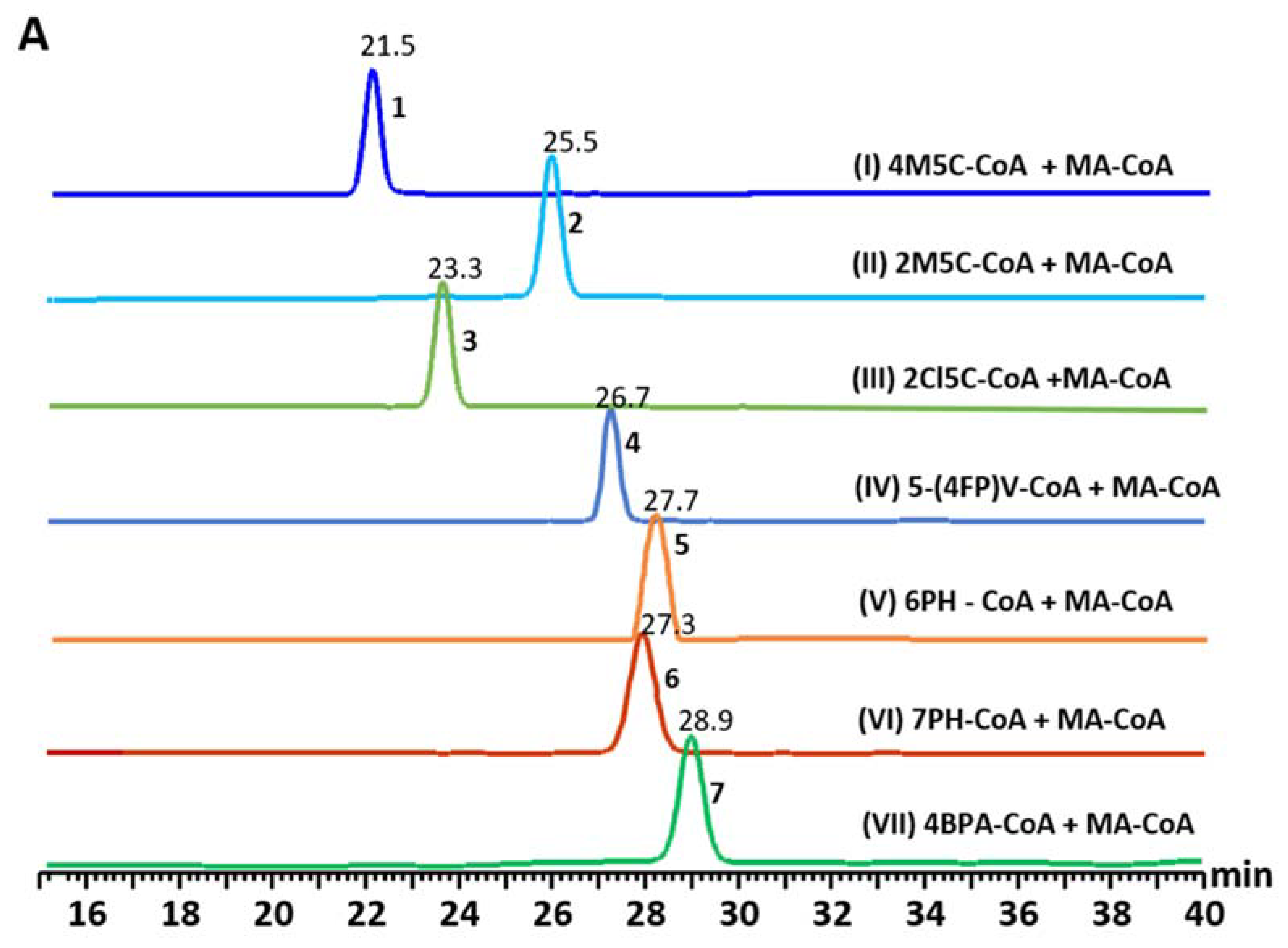
3.3. Precursor-Directed Biosynthesis of Novel Polyketide Products
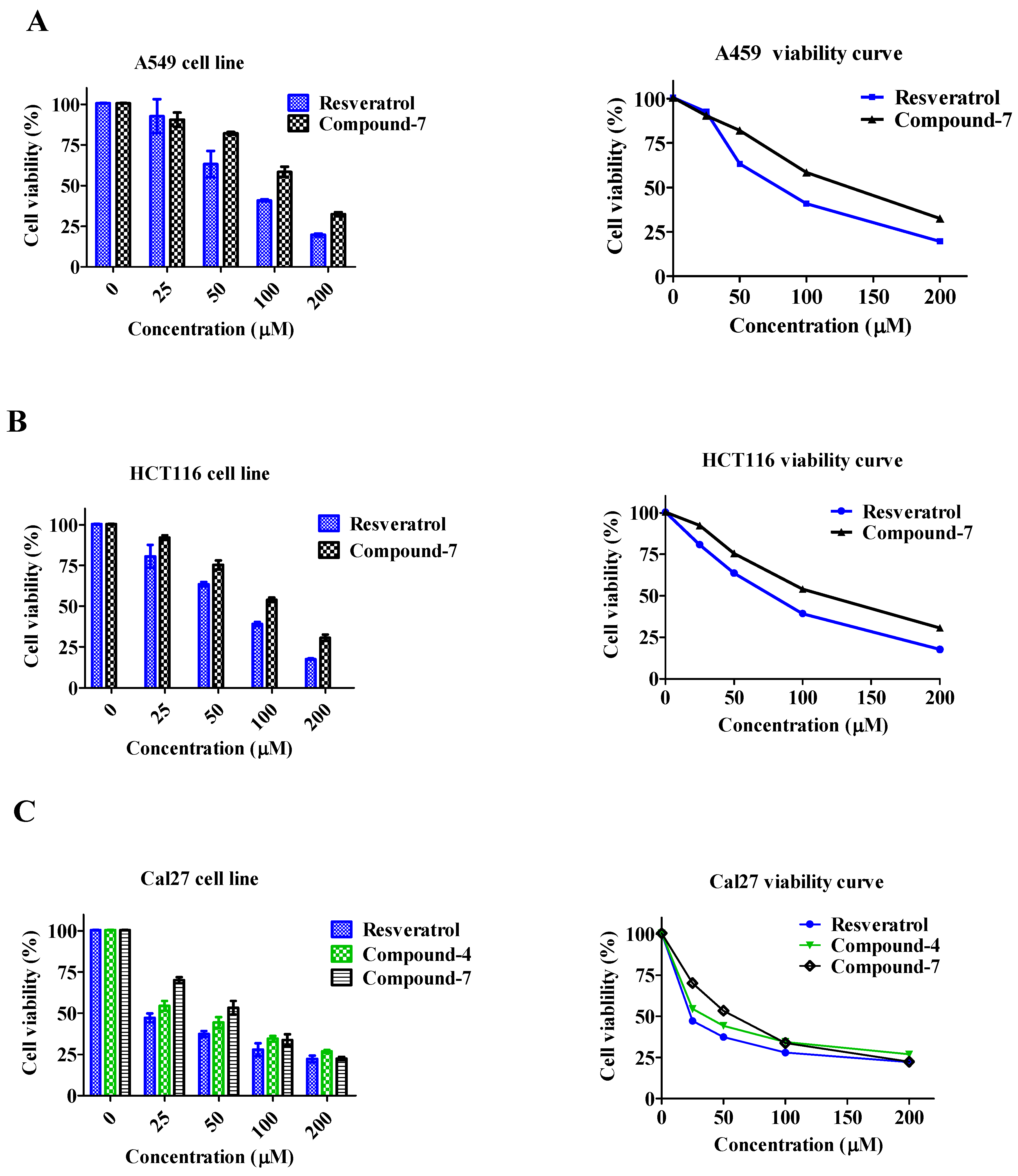
3.4. Cell Viability
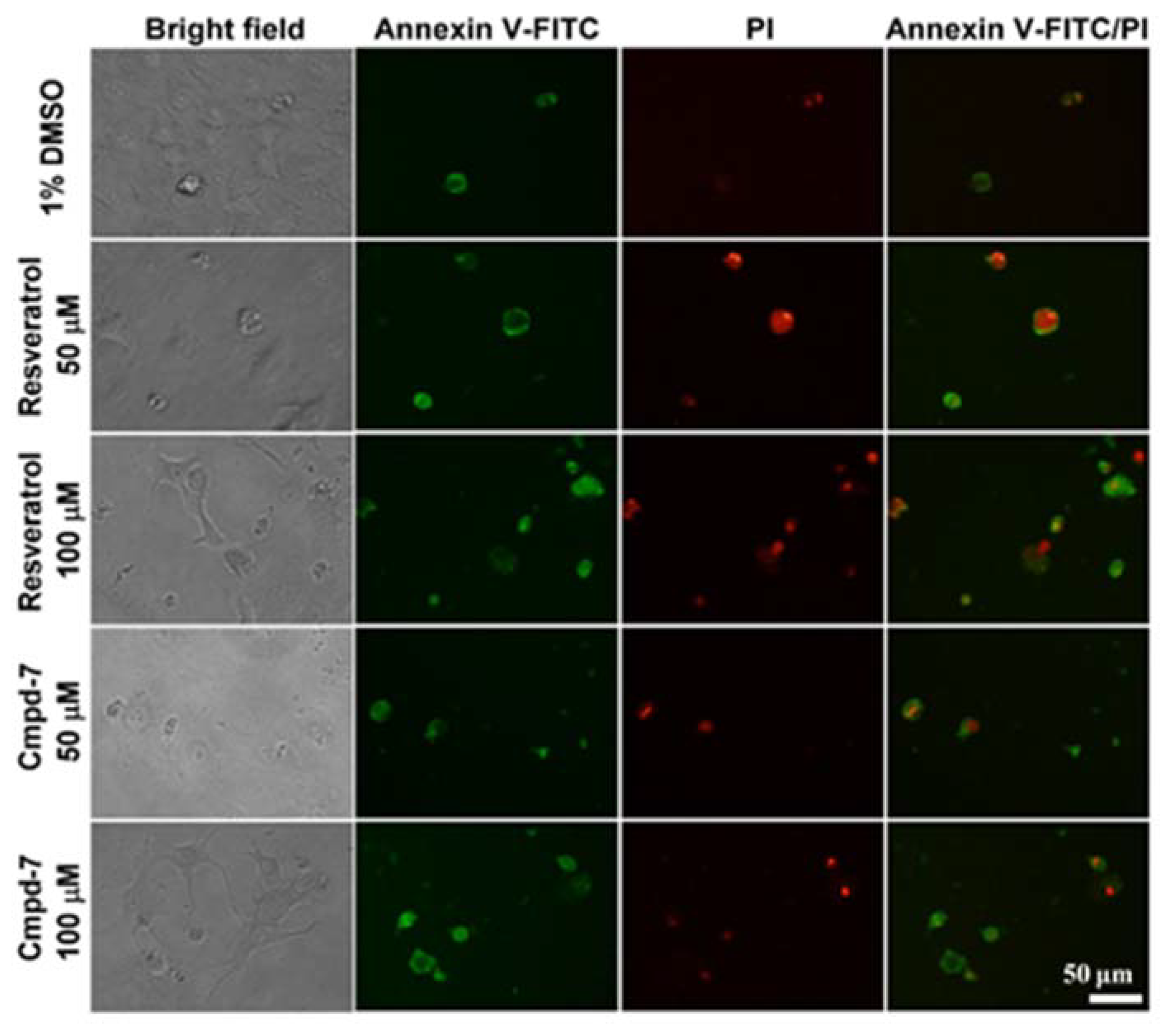
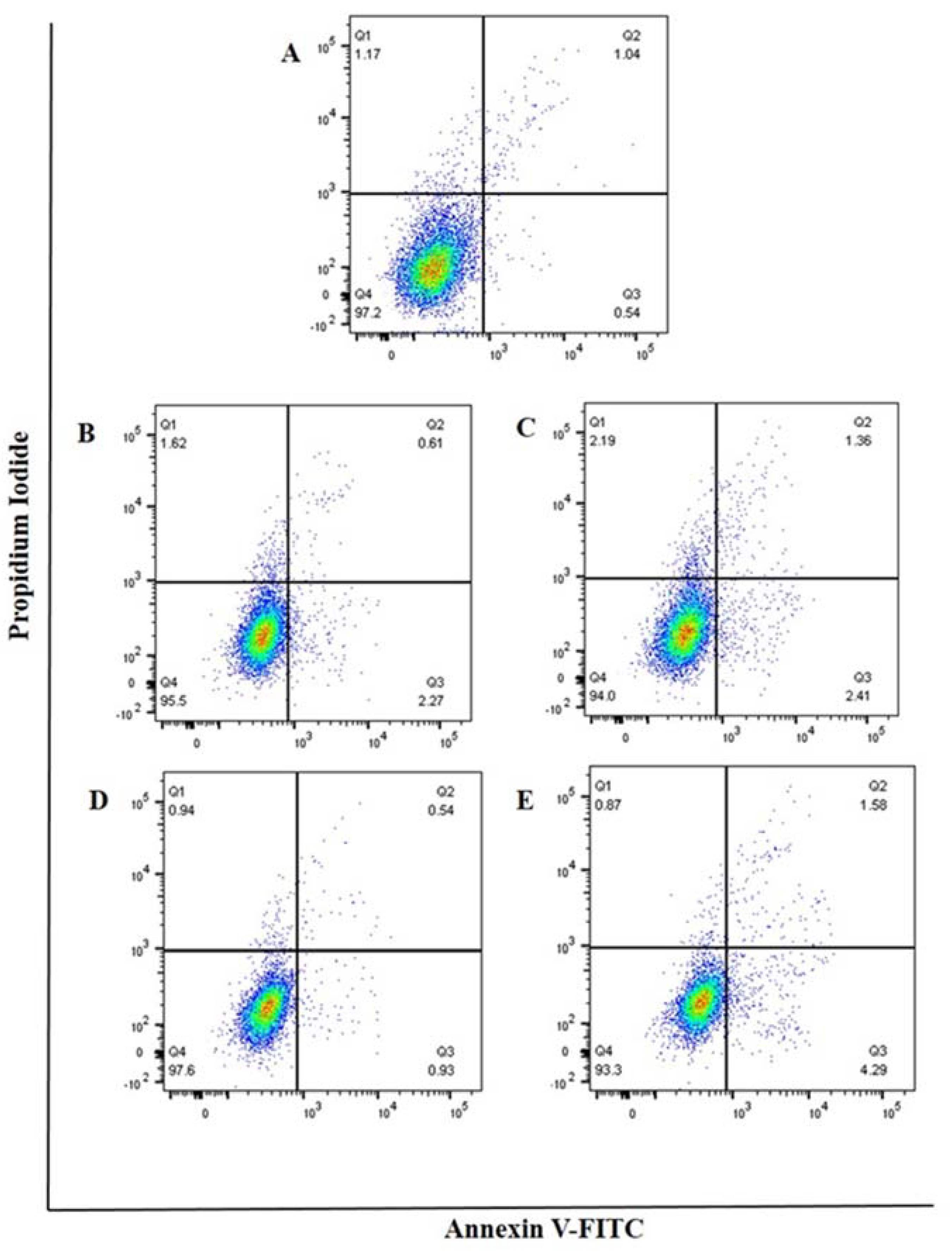
3.5. Cell Apoptosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Weissman, K.J.; Leadlay, P.F. Combinatorial biosynthesis of reduced polyketides. Nat. Rev. Microbiol. 2005, 3, 925–936. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.M.; Li, J.W.; Choi, J.W.; Zhou, H.; Lee, K.K.; Moorthie, V.A.; Xie, X.; Kealey, J.T.; Da Silva, N.A.; Vederas, J.C.; et al. Complete reconstitution of a highly reducing iterative polyketide synthase. Science 2009, 326, 589–592. [Google Scholar] [CrossRef] [PubMed]
- Tung, B.T.; Rodriguez-Bies, E.; Talero, E.; Gamero-Estevez, E.; Motilva, V.; Navas, P.; Lopez-Lluch, G. Anti-inflammatory effect of resveratrol in old mice liver. Exp. Gerontol. 2015, 64, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Xia, N.; Daiber, A.; Forstermann, U.; Li, H. Antioxidant effects of resveratrol in the cardiovascular system. Br. J. Pharmacol. 2017, 174, 1633–1646. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Lee, H.J.; Min, H.Y.; Park, E.J.; Lee, K.M.; Ahn, Y.H.; Cho, Y.J.; Pyee, J.H. Antibacterial and antifungal activity of pinosylvin, a constituent of pine. Fitoterapia 2005, 76, 258–260. [Google Scholar] [CrossRef]
- Li, T.L.; Spiteller, D.; Spencer, J.B. Identification of a pentaketide stilbene produced by a type III polyketide synthase from Pinus sylvestris and characterisation of free coenzyme A intermediates. Chembiochem 2009, 10, 896–901. [Google Scholar] [CrossRef]
- Morita, H.; Noguchi, H.; Schroder, J.; Abe, I. Novel polyketides synthesized with a higher plant stilbene synthase. Eur. J. Biochem. 2001, 268, 3759–3766. [Google Scholar] [CrossRef]
- Bhan, N.; Cress, B.F.; Linhardt, R.J.; Koffas, M. Expanding the chemical space of polyketides through structure-guided mutagenesis of Vitis vinifera stilbene synthase. Biochimie 2015, 115, 136–143. [Google Scholar] [CrossRef]
- Sales, J.M.; Resurreccion, A.V. Resveratrol in peanuts. Crit. Rev. Food Sci. Nutr. 2014, 54, 734–770. [Google Scholar] [CrossRef]
- Li, X.; Phillips, F.M.; An, H.S.; Ellman, M.; Thonar, E.J.; Wu, W.; Park, D.; Im, H.J. The action of resveratrol, a phytoestrogen found in grapes, on the intervertebral disc. Spine 2008, 33, 2586–2595. [Google Scholar] [CrossRef]
- Chávez, D.; Chai, H.-B.; Chagwedera, T.E.; Gao, Q.; Farnsworth, N.R.; Cordell, G.A.; Pezzuto, J.M.; Kinghorn, A.D. Novel stilbenes isolated from the root bark of Ekebergia benguelensis. Tetrahedron Lett. 2001, 42, 3685–3688. [Google Scholar] [CrossRef]
- Bishayee, A. Cancer prevention and treatment with resveratrol: From rodent studies to clinical trials. Cancer Prev. Res. Phila. Pa 2009, 2, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Fulda, S. Resveratrol and derivatives for the prevention and treatment of cancer. Drug Discov. Today 2010, 15, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Howitz, K.T.; Bitterman, K.J.; Cohen, H.Y.; Lamming, D.W.; Lavu, S.; Wood, J.G.; Zipkin, R.E.; Chung, P.; Kisielewski, A.; Zhang, L.L.; et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 2003, 425, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Abe, I. Engineered biosynthesis of plant polyketides: Structure-based and precursor-directed approach. Top. Curr. Chem. 2010, 297, 45–66. [Google Scholar] [CrossRef]
- Abe, I.; Oguro, S.; Utsumi, Y.; Sano, Y.; Noguchi, H. Engineered biosynthesis of plant polyketides: Chain length control in an octaketide-producing plant type III polyketide synthase. J. Am. Chem. Soc. 2005, 127, 12709–12716. [Google Scholar] [CrossRef]
- Abe, I.; Morita, H.; Oguro, S.; Noma, H.; Wanibuchi, K.; Kawahara, N.; Goda, Y.; Noguchi, H.; Kohno, T. Structure-based engineering of a plant type III polyketide synthase: Formation of an Unnatural nonaketide naphthopyrone. J. Am. Chem. Soc. 2007, 129, 5976–5980. [Google Scholar] [CrossRef]
- Morita, H.; Yamashita, M.; Shi, S.-P.; Wakimoto, T.; Kondo, S.; Kato, R.; Sugio, S.; Kohno, T.; Abe, I. Synthesis of unnatural alkaloid scaffolds by exploiting plant polyketide synthase. Proc. Natl. Acad. Sci. USA 2011, 108, 13504. [Google Scholar] [CrossRef]
- Abe, I. Novel applications of plant polyketide synthases. Curr. Opin. Chem. Biol. 2012, 16, 179–185. [Google Scholar] [CrossRef]
- Go, M.K.; Wongsantichon, J.; Cheung, V.W.N.; Chow, J.Y.; Robinson, R.C.; Yew, W.S. Synthetic Polyketide Enzymology: Platform for Biosynthesis of Antimicrobial Polyketides. ACS Catal. 2015, 5, 4033–4042. [Google Scholar] [CrossRef]
- Austin, M.B.; Bowman, M.E.; Ferrer, J.L.; Schroder, J.; Noel, J.P. An aldol switch discovered in stilbene synthases mediates cyclization specificity of type III polyketide synthases. Chem. Biol. 2004, 11, 1179–1194. [Google Scholar] [CrossRef] [PubMed]
- Verwoert, II.; Verbree, E.C.; van der Linden, K.H.; Nijkamp, H.J.; Stuitje, A.R. Cloning, nucleotide sequence, and expression of the Escherichia coli fabD gene, encoding malonyl coenzyme A-acyl carrier protein transacylase. J. Bacteriol. 1992, 174, 2851–2857. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Gai, Y.; Yin, L.; Wang, X.; Feng, C.; Feng, L.; Li, D.; Jiang, X.N.; Wang, D.C. Crystal structures of a Populus tomentosa 4-coumarate:CoA ligase shed light on its enzymatic mechanisms. Plant cell 2010, 22, 3093–3104. [Google Scholar] [CrossRef] [PubMed]
- Lyu, S.Y.; Liu, Y.C.; Chang, C.Y.; Huang, C.J.; Chiu, Y.H.; Huang, C.M.; Hsu, N.S.; Lin, K.H.; Wu, C.J.; Tsai, M.D.; et al. Multiple complexes of long aliphatic N-acyltransferases lead to synthesis of 2,6-diacylated/2-acyl-substituted glycopeptide antibiotics, effectively killing vancomycin-resistant enterococcus. J. Am. Chem. Soc. 2014, 136, 10989–10995. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.C.; Thuronyi, B.W.; Charkoudian, L.K.; Lowry, B.; Khosla, C.; Chang, M.C. Expanding the fluorine chemistry of living systems using engineered polyketide synthase pathways. Science 2013, 341, 1089–1094. [Google Scholar] [CrossRef] [PubMed]
- Otwinowski, Z.; Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 1997, 276, 307–326. [Google Scholar] [CrossRef]
- Kantardjieff, K.A.; Rupp, B. Matthews coefficient probabilities: Improved estimates for unit cell contents of proteins, DNA, and protein-nucleic acid complex crystals. Protein Sci. 2003, 12, 1865–1871. [Google Scholar] [CrossRef]
- Chen, V.B.; Arendall, W.B., 3rd; Headd, J.J.; Keedy, D.A.; Immormino, R.M.; Kapral, G.J.; Murray, L.W.; Richardson, J.S.; Richardson, D.C. MolProbity: All-atom structure validation for macromolecular crystallography. Acta Crystallogr. Sect. D Biol. Crystallogr. 2010, 66, 12–21. [Google Scholar] [CrossRef]
- Thornburg, C.K.; Wortas-Strom, S.; Nosrati, M.; Geiger, J.H.; Walker, K.D. Kinetically and crystallographically guided mutations of a benzoate CoA ligase (BadA) elucidate mechanism and expand substrate permissivity. Biochemistry 2015, 54, 6230–6242. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera--a visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Binkowski, T.A.; Naghibzadeh, S.; Liang, J. CASTp: Computed Atlas of Surface Topography of proteins. Nucleic Acids Res. 2003, 31, 3352–3355. [Google Scholar] [CrossRef]
- Bains, J.; Boulanger, M.J. Biochemical and structural characterization of the paralogous benzoate CoA ligases from Burkholderia xenovorans LB400: Defining the entry point into the novel benzoate oxidation (box) pathway. J. Mol. Biol. 2007, 373, 965–977. [Google Scholar] [CrossRef]
- Gulick, A.M.; Lu, X.; Dunaway-Mariano, D. Crystal structure of 4-chlorobenzoate:CoA ligase/synthetase in the unliganded and aryl substrate-bound states. Biochemistry 2004, 43, 8670–8679. [Google Scholar] [CrossRef] [PubMed]
- Hagmann, W.K. The many roles for fluorine in medicinal chemistry. J. Med. Chem. 2008, 51, 4359–4369. [Google Scholar] [CrossRef] [PubMed]
- Purser, S.; Moore, P.R.; Swallow, S.; Gouverneur, V. Fluorine in medicinal chemistry. Chem. Soc. Rev. 2008, 37, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sanchez-Rosello, M.; Acena, J.L.; del Pozo, C.; Sorochinsky, A.E.; Fustero, S.; Soloshonok, V.A.; Liu, H. Fluorine in pharmaceutical industry: Fluorine-containing drugs introduced to the market in the last decade (2001-2011). Chem. Rev. 2014, 114, 2432–2506. [Google Scholar] [CrossRef] [PubMed]
- Gribble, G.W. Biological activity of recently discovered halogenated marine natural products. Mar. Drugs 2015, 13, 4044–4136. [Google Scholar] [CrossRef]
- Walker, M.C.; Chang, M.C. Natural and engineered biosynthesis of fluorinated natural products. Chem. Soc. Rev. 2014, 43, 6527–6536. [Google Scholar] [CrossRef]
- Fang, J.; Hait, D.; Head-Gordon, M.; Chang, M.C.Y. Chemoenzymatic Platform for Synthesis of Chiral Organofluorines Based on Type II Aldolases. Angew. Chem. Int. Ed. Engl. 2019, 58, 11841–11845. [Google Scholar] [CrossRef]
- Thuronyi, B.W.; Chang, M.C. Synthetic biology approaches to fluorinated polyketides. Acc. Chem. Res. 2015, 48, 584–592. [Google Scholar] [CrossRef]
- Hong, H.; Spiteller, D.; Spencer, J.B. Incorporation of fluoroacetate into an aromatic polyketide and its influence on the mode of cyclization. Angew. Chem. Int. Ed. Engl. 2008, 47, 6028–6032. [Google Scholar] [CrossRef] [PubMed]
- Ji, Q.; Liu, X.; Fu, X.; Zhang, L.; Sui, H.; Zhou, L.; Sun, J.; Cai, J.; Qin, J.; Ren, J.; et al. Resveratrol inhibits invasion and metastasis of colorectal cancer cells via MALAT1 mediated Wnt/beta-catenin signal pathway. PLoS ONE 2013, 8, e78700. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.A.; Lien, H.M.; Kao, M.C.; Lo, U.G.; Lin, L.C.; Lin, C.J.; Chang, S.J.; Chen, C.C.; Hsieh, J.T.; Lin, H.; et al. Sensitization of Radioresistant Prostate Cancer Cells by Resveratrol Isolated from Arachis hypogaea Stems. PLoS ONE 2017, 12, e0169204. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhou, X.; Zhou, K. Resveratrol inhibits human nasopharyngeal carcinoma cell growth via blocking pAkt/p70S6K signaling pathways. Int. J. Mol. Med. 2013, 31, 621–627. [Google Scholar] [CrossRef]

| H333A/I334A-Apo | H333A/I334A-2Cl5C-AMP | H333A/I334A-2M5C-AMP | |
|---|---|---|---|
| PDB code | 6M2O | 6M2U | 6M2T |
| Wave length (Å) | 1.0 | 1.0 | 1.0 |
| Space group | P 1 21 1 | P 1 21 1 | P 1 21 1 |
| a,b, c (Å) | 59.1,95.3, 94.9 | 58.5, 94.6, 94.3 | 98, 95.1, 119.5 |
| α, β, γ (°) | 90, 105, 90 | 90, 104.9, 90 | 90, 110.5, 90 |
| Resolution range (Å) a | 29.52–1.57(1.63–1.57) | 29.8–1.70 (1.77–1.70) | 27.7–2.13 (2.22–2.13) |
| Rmerge (%) a,b | 2.8 (36.5) | 6.8 (73.2) | 5.1 (20.1) |
| 〈I/σ(I)〉 a | 31.6(3.1) | 33 (2.8) | 25.3 (6.6) |
| Completeness (%) a | 99.1 (98.3) | 99.9(99.9) | 100 (100.0) |
| Redundancy | 3.8(3.8) | 7.4 (6.7) | 3.8 (3.8) |
| Refinement | |||
| Resolution range (Å) a | 29.52–1.57 (1.57–1.52) | 29.8–1.70 (1.77–1.70) | 27.7–2.13 (2.22–2.13) |
| Rwork/Rfree (%) | 18.7/21.1 | 17/20.2 | 15.9/19.7 |
| R.m.s. deviations | |||
| Bond lengths (Å) | 0.187 | 0.171 | 0.160 |
| Bond angles (°) | 1.43 | 1.89 | 1.77 |
| No. reflections | 140471 | 105468 | 113090 |
| No. atoms | |||
| Protein | 1036 | 1036 | 2072 |
| Ligand/ion | 62 | 124 | |
| Water | 1180 | 1307 | 1421 |
| B-factors | |||
| Protein | 27.8 | 19.15 | 30.38 |
| Ligand/ion | 22.4 | 26.28 | |
| Water | 27.9 | 29.3 | 30.7 |
| Compounds | BadA Mutant (H333A/I334A) | Wild-Type BadA |
|---|---|---|
| 4-methylthiazole-2-carboxylic acid | + | - |
| 4-methylthiazole-5-carboxylic acid | + | - |
| 2-methylthiazole-4-carboxylic acid | + | - |
| 2-methylthiazole-5-carboxylic acid | + | - |
| 2-chloro-1,3-thiazole-5-carboxylic acid | + | - |
| 2-chloro-1,3-thiazole-4-carboxylic acid | + | - |
| 2-bromo-4-methylthiazole-5-carboxylic acid | + | - |
| 2-bromo-5-methylthiazole-4-carboxylic acid | + | - |
| 2-aminothiazole-4-carboxylic acid | + | - |
| 2-aminothiazole-5-carboxylic acid | + | - |
| 3-(p-tolyl)propionic acid | + | - |
| dihydrocinnamic acid | + | - |
| p-coumaric acid | + | - |
| Starter Unit | Product | Product Mass [M + H]+ | Kinetics | UVMax (nm) | |
|---|---|---|---|---|---|
| Km (μm) | kcat (min−1) | ||||
| 4-methylthiazole-5-carboxyl-CoA | 1 | 210.0218 | 3.82 | 0.16 | 310 |
| 2-methylthiazole-5-carboxyl-CoA | 2 | 210.0225 | 3.87 | 0.01 | 310 |
| 2-choloro-1,3-thiazole-5-carboxyl-CoA | 3 | 229.9681 | 12.77 | 0.19 | 310 |
| 5-(4-fluorophenyl) valeriyol-CoA | 4 | 263.1084 | 4.15 | 0. 40 | 280 |
| 6-phenylhexanoyl-CoA | 5 | 259.134 | 43.89 | 0. 40 | 285 |
| 7-phenylheptanoyl-CoA | 6 | 243.1475 | 29.42 | 0.27 | 285 |
| 4-biphenylacetyol-CoA | 7 | 279.1012 | 23.24 | 0.21 | 254 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adhikari, K.; Lo, I.-W.; Chen, C.-L.; Wang, Y.-L.; Lin, K.-H.; Zadeh, S.M.; Rattinam, R.; Li, Y.-S.; Wu, C.-J.; Li, T.-L. Chemoenzymatic Synthesis and Biological Evaluation for Bioactive Molecules Derived from Bacterial Benzoyl Coenzyme A Ligase and Plant Type III Polyketide Synthase. Biomolecules 2020, 10, 738. https://doi.org/10.3390/biom10050738
Adhikari K, Lo I-W, Chen C-L, Wang Y-L, Lin K-H, Zadeh SM, Rattinam R, Li Y-S, Wu C-J, Li T-L. Chemoenzymatic Synthesis and Biological Evaluation for Bioactive Molecules Derived from Bacterial Benzoyl Coenzyme A Ligase and Plant Type III Polyketide Synthase. Biomolecules. 2020; 10(5):738. https://doi.org/10.3390/biom10050738
Chicago/Turabian StyleAdhikari, Kamal, I-Wen Lo, Chun-Liang Chen, Yung-Lin Wang, Kuan-Hung Lin, Saeid Malek Zadeh, Rajesh Rattinam, Yi-Shan Li, Chang-Jer Wu, and Tsung-Lin Li. 2020. "Chemoenzymatic Synthesis and Biological Evaluation for Bioactive Molecules Derived from Bacterial Benzoyl Coenzyme A Ligase and Plant Type III Polyketide Synthase" Biomolecules 10, no. 5: 738. https://doi.org/10.3390/biom10050738
APA StyleAdhikari, K., Lo, I.-W., Chen, C.-L., Wang, Y.-L., Lin, K.-H., Zadeh, S. M., Rattinam, R., Li, Y.-S., Wu, C.-J., & Li, T.-L. (2020). Chemoenzymatic Synthesis and Biological Evaluation for Bioactive Molecules Derived from Bacterial Benzoyl Coenzyme A Ligase and Plant Type III Polyketide Synthase. Biomolecules, 10(5), 738. https://doi.org/10.3390/biom10050738







